Input-Output Budgets of Nutrients in Adjacent Norway Spruce and European Beech Monocultures Recovering from Acidification
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Water Chemistry
2.3. Data Handling
2.4. Statistical Analysis
3. Results
3.1. Atmospheric Deposition Chemistry
3.2. Soil Solution Chemistry
3.2.1. Anion Concentrations
3.2.2. pH, Al Concentrations and Al Speciation
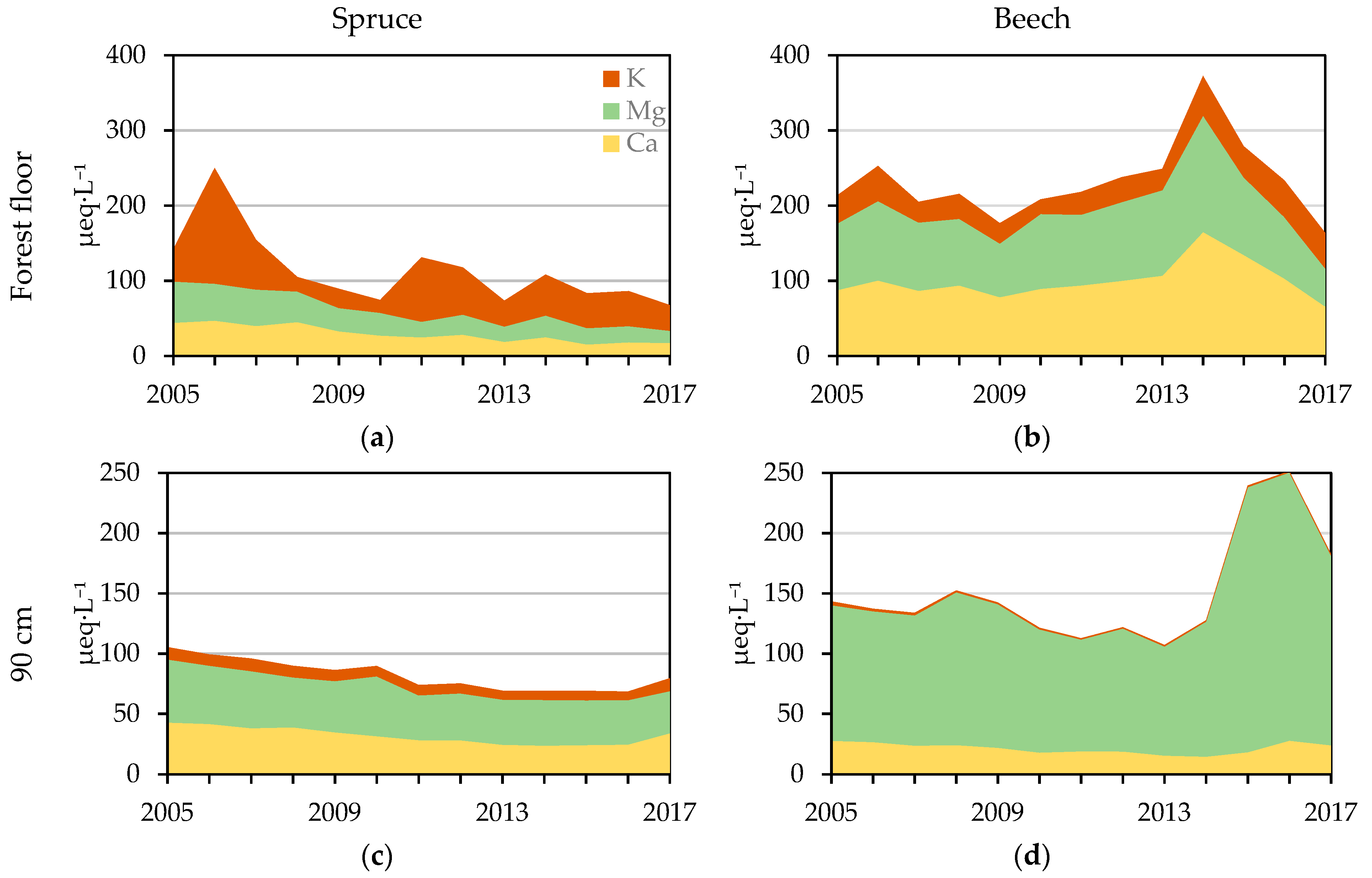
3.2.3. NH4+, Na+, K+, Mg2+, Ca2+, and the Bc/Alin Ratio
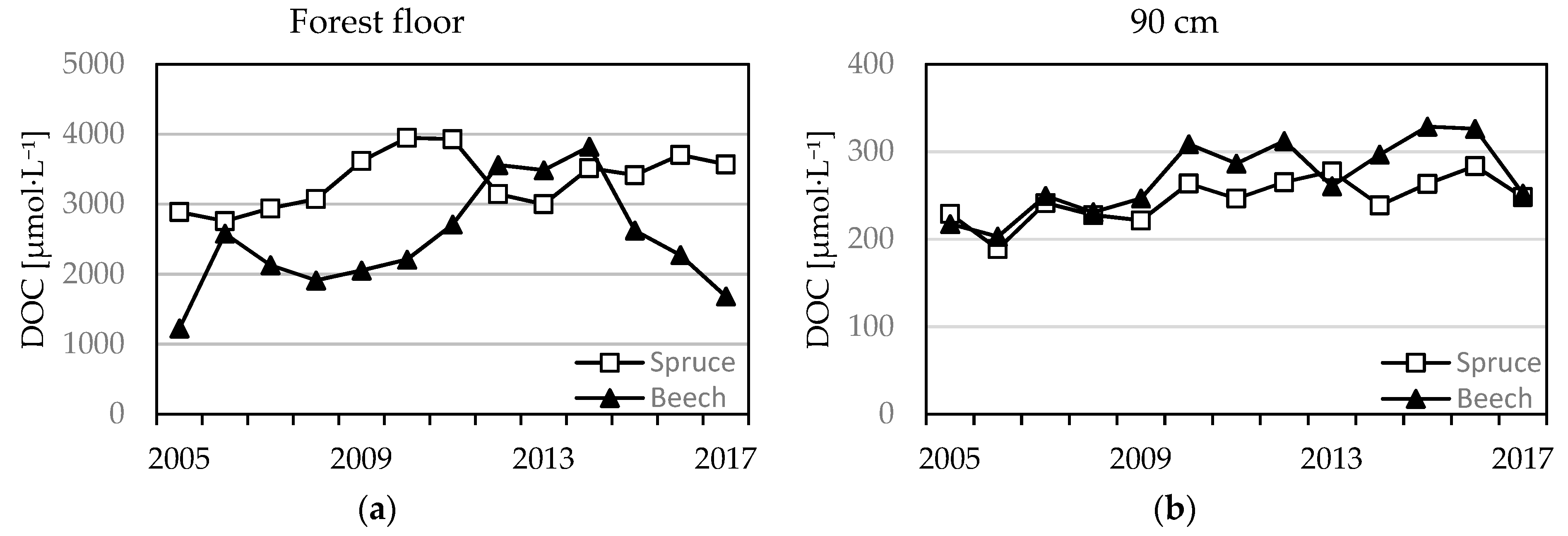
3.2.4. DOC, DON Concentrations, and the DOC/DON Ratio
3.3. Water Balance
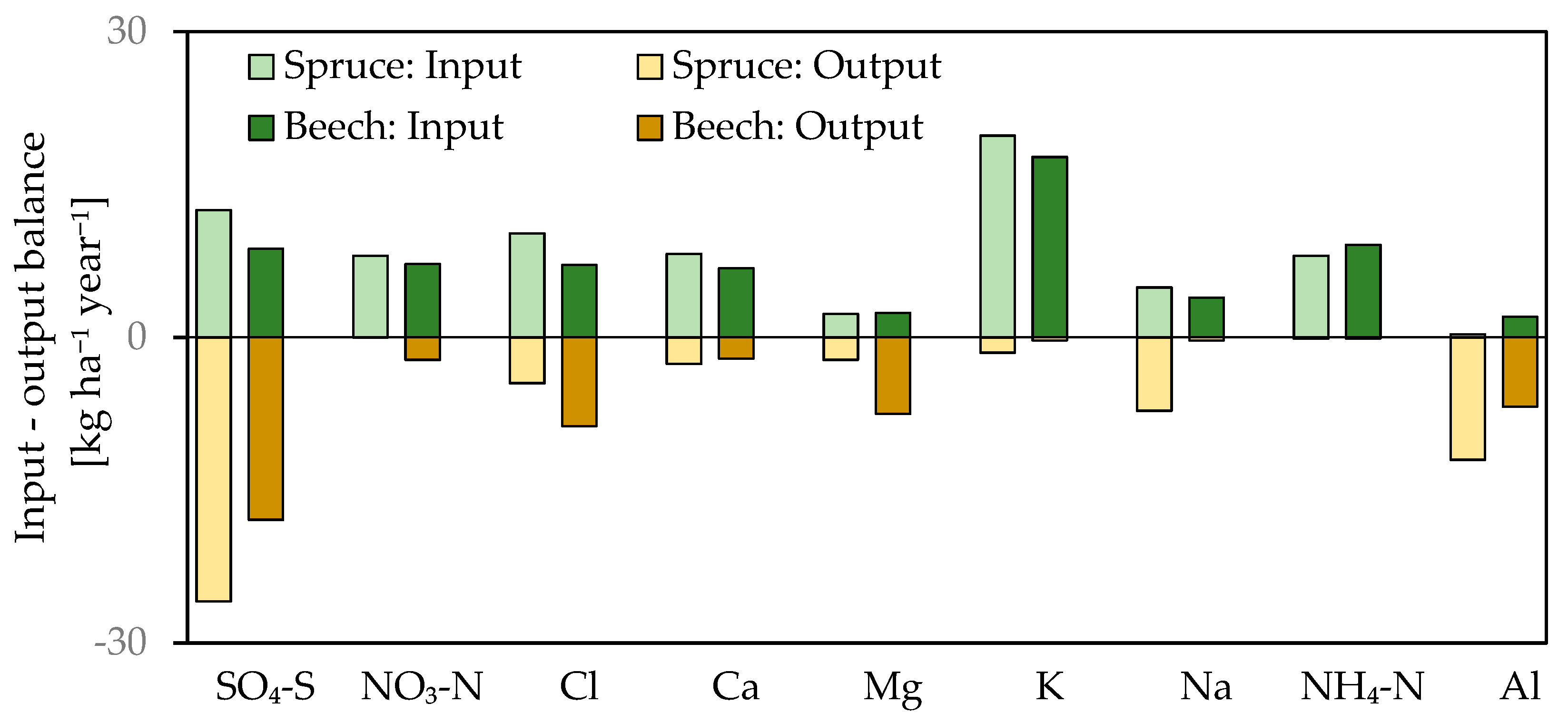
3.4. Mass Balance
4. Discussion
4.1. Water Fluxes
4.2. Deposition
4.3. S, Cl, DOC, and pH
4.4. N Budget
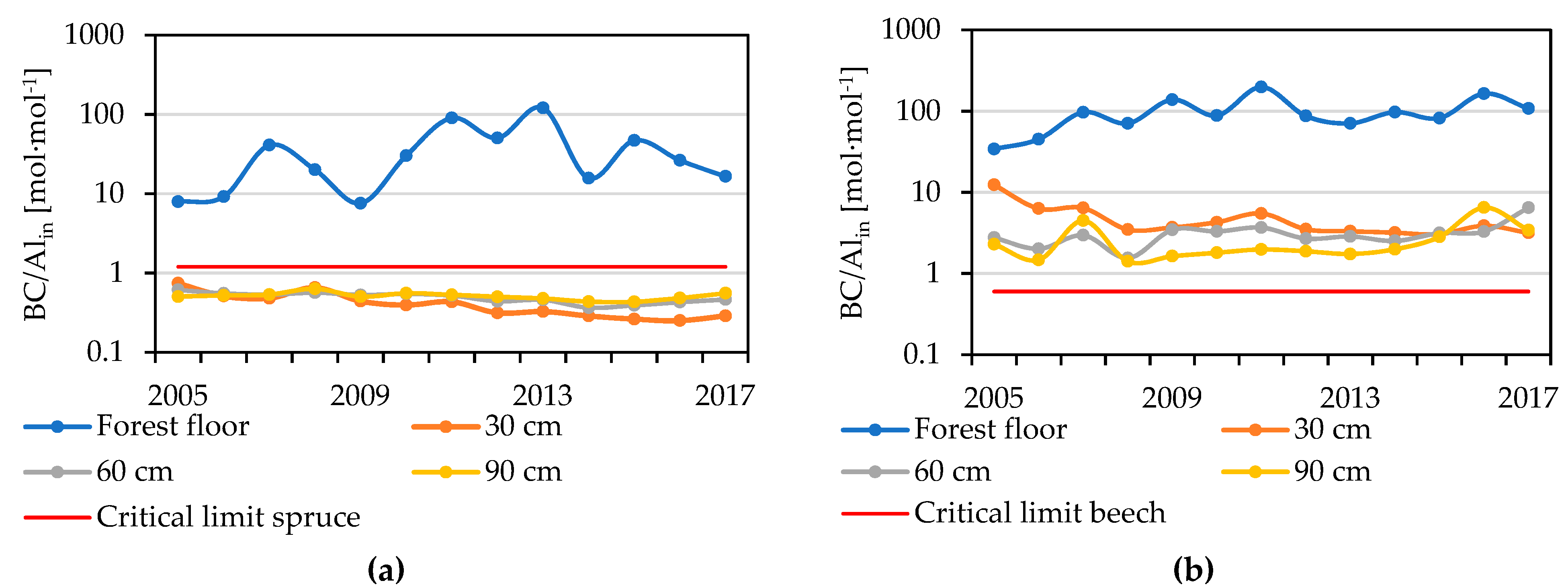
4.5. Base Cations, Aluminium and BC/Alin
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fowler, D.; Smith, R.; Muller, J.; Cape, J.N.; Sutton, M.; Erisman, J.W.; Fagerli, H. Long term trends in sulphur and nitrogen deposition in Europe and the cause of non-linearities. Water Air Soil Pollut. Focus 2007, 7, 41–47. [Google Scholar] [CrossRef]
- Waldner, P.; Marchetto, A.; Thimonier, A.; Schmitt, M.; Rogora, M.; Granke, O.; Mues, V.; Hansen, K.; Pihl Karlsson, G.; Žlindra, D.; et al. Detection of temporal trends in atmospheric deposition of inorganic nitrogen and sulphate to forests in Europe. Atmos. Environ. 2014, 95, 363–374. [Google Scholar] [CrossRef]
- de Wit, H.A.; Eldhuset, T.D.; Mulder, J. Dissolved Al reduces Mg uptake in Norway spruce forest: Results from a long-term field manipulation experiment in Norway. For. Ecol. Manag. 2010, 259, 2072–2082. [Google Scholar] [CrossRef]
- Dise, N.B.; Wright, R.F. Forest Ecology and Management Nitrogen leaching from European forests in relation to nitrogen deposition. For. Ecol. Manag. 1995, 71, 153–169. [Google Scholar] [CrossRef]
- Alewell, C.; Manderscheid, B.; Gerstberger, P.; Matzner, E. Effects of reduced atmospheric deposition on soil solution chemistry and elemental contents of spruce needles in NE—Bavaria, Germany. J. Plant Nutr. Soil Sci. 2000, 163, 509–516. [Google Scholar] [CrossRef]
- Monteith, D.T.; Stoddard, J.L.; Evans, C.D.; de Wit, H.A.; Forsius, M.; Høgåsen, T.; Wilander, A.; Skjelkvåle, B.L.; Jeffries, D.S.; Vuorenmaa, J.; et al. Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 2007, 450, 537–540. [Google Scholar] [CrossRef]
- Oulehle, F.; Chuman, T.; Hruška, J.; Krám, P.; McDowell, W.H.; Myška, O.; Navrátil, T.; Tesař, M. Recovery from acidification alters concentrations and fluxes of solutes from Czech catchments. Biogeochemistry 2017, 132, 251–272. [Google Scholar] [CrossRef]
- Evans, C.D.; Jones, T.G.; Burden, A.; Ostle, N.; Zieliński, P.; Cooper, M.D.A.; Peacock, M.; Clark, J.M.; Oulehle, F.; Cooper, D.; et al. Acidity controls on dissolved organic carbon mobility in organic soils. Glob. Change Biol. 2012. [Google Scholar] [CrossRef]
- Oulehle, F.; Chuman, T.; Majer, V.; Hruška, J. Chemical recovery of acidified Bohemian lakes between 1984 and 2012: the role of acid deposition and bark beetle induced forest disturbance. Biogeochemistry 2013, 116, 83–101. [Google Scholar] [CrossRef]
- Martinson, L.; Lamersdorf, N.; Warfvinge, P. The Solling roof revisited—slow recovery from acidification observed and modeled despite a decade of “clean-rain” treatment. Environ. Pollut. 2005, 135, 293–302. [Google Scholar] [CrossRef]
- Johnson, J.; Pannatier, E.G.; Carnicelli, S.; Cecchini, G.; Clarke, N.; Cools, N.; Hansen, K.; Meesenburg, H.; Nieminen, T.M.; Pihl-Karlsson, G.; et al. The response of soil solution chemistry in European forests to decreasing acid deposition. Glob. Change Biol. 2018, 24, 3603–3619. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.; Sanders, T.G.M.; Bolte, A.; Bussotti, F.; Dirnböck, T.; Johnson, J.; Peñuelas, J.; Pollastrini, M.; Prescher, A.-K.; Sardans, J.; et al. Responses of forest ecosystems in Europe to decreasing nitrogen deposition. Environ. Pollut. 2019, 244, 980–994. [Google Scholar] [CrossRef]
- Verstraeten, A.; Neirynck, J.; Genouw, G.; Cools, N.; Roskams, P.; Hens, M. Impact of declining atmospheric deposition on forest soil solution chemistry in Flanders, Belgium. Atmos. Environ. 2012, 62, 50–63. [Google Scholar] [CrossRef]
- Iost, S.; Rautio, P.; Lindroos, A.-J. Spatio-temporal Trends in Soil Solution Bc/Al and N in Relation to Critical Limits in European Forest Soils. Water. Air. Soil Pollut. 2012, 223, 1467–1479. [Google Scholar] [CrossRef]
- Augusto, L.; De Schrijver, A.; Vesterdal, L.; Smolander, A.; Prescott, C.; Ranger, J. Influences of evergreen gymnosperm and deciduous angiosperm tree species on the functioning of temperate and boreal forests. Biol. Rev. 2015, 90, 444–466. [Google Scholar] [CrossRef] [PubMed]
- Augusto, L.; Ranger, J.; Ponette, Q.; Rapp, M. Relationships between forest tree species, stand production and stand nutrient amount. Ann. For. Sci. 2000, 57, 313–324. [Google Scholar] [CrossRef]
- De Schrijver, A.; Geudens, G.; Augusto, L.; Staelens, J.; Mertens, J.; Wuyts, K.; Gielis, L.; Verheyen, K. The effect of forest type on throughfall deposition and seepage flux: A review. Oecologia 2007, 153, 663–674. [Google Scholar] [CrossRef]
- Augusto, L.; Ranger, J.; Binkley, D.; Rothe, A. Impact of several common tree species of European temperate forests on soil fertility. Ann. For. Sci. 2002, 59, 233–253. [Google Scholar] [CrossRef]
- Oulehle, F.; Hruška, J. Tree species (Picea abies and Fagus sylvatica) effects on soil water acidification and aluminium chemistry at sites subjected to long-term acidification in the Ore Mts., Czech Republic. J. Inorg. Biochem. 2005, 99, 1822–1829. [Google Scholar] [CrossRef]
- Rothe, A.; Kreutzer, K. Influence of tree species composition on soil and soil solution properties in two mixed spruce-beech stands with contrasting history in Southern Germany. Plant Soil 2002, 240, 47–56. [Google Scholar] [CrossRef]
- Berger, T.W.; Untersteiner, H.; Toplitzer, M.; Neubauer, C. Nutrient fluxes in pure and mixed stands of spruce (Picea abies) and beech (Fagus sylvatica). Plant Soil 2009, 322, 317–342. [Google Scholar] [CrossRef]
- Vanguelova, E.I.; Benham, S.; Pitman, R.; Moffat, A.J.; Broadmeadow, M.; Nisbet, T.; Durrant, D.; Barsoum, N.; Wilkinson, M.; Bochereau, F.; et al. Chemical fluxes in time through forest ecosystems in the UK - Soil response to pollution recovery. Environ. Pollut. 2010, 158, 1857–1869. [Google Scholar] [CrossRef] [PubMed]
- Meesenburg, H.; Ahrends, B.; Fleck, S.; Wagner, M.; Fortmann, H.; Scheler, B.; Klinck, U.; Dammann, I.; Eichhorn, J.; Mindrup, M.; et al. Long-term changes of ecosystem services at Solling, Germany: Recovery from acidification, but increasing nitrogen saturation? Ecol. Indic. 2016, 65, 103–112. [Google Scholar] [CrossRef]
- Oulehle, F.; Hofmeister, J.; Hruška, J. Modeling of the long-term effect of tree species (Norway spruce and European beech) on soil acidification in the Ore Mountains. Ecol. Model. 2007, 204, 359–371. [Google Scholar] [CrossRef]
- Oulehle, F.; Růžek, M.; Tahovská, K.; Bárta, J.; Myška, O. Carbon and Nitrogen Pools and Fluxes in Adjacent Mature Norway Spruce and European Beech Forests. Forests 2016, 7, 282. [Google Scholar] [CrossRef]
- Dambrine, E.; Kinkor, V.; Jehlička, J.; Gelhaye, D. Fluxes of dissolved mineral elements through a forest ecosystem submitted to extremely high atmopsheric pollution inputs (Czech Republic). Ann. Sci. For. 1993, 50, 147–157. [Google Scholar] [CrossRef]
- Dambrine, E.; Probst, A.; Viville, D.; Biron, P.; Belgrand, M.C.; Paces, T.; Novak, M.; Buzek, F.; Cerny, J.; Groscheova, H. Spatial Variability and Long-Term Trends in Mass Balance of N and S in Central European Forested Catchments. In Carbon and Nitrogen Cycling in European Forest Ecosystems; Schulze, E.-D., Ed.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2000; pp. 405–418. ISBN 978-3-642-57219-7. [Google Scholar]
- Oulehle, F.; Evans, C.D.; Hofmeister, J.; Krejčí, R.; Tahovská, K.; Persson, T.; Cudlín, P.; Hruška, J. Major changes in forest carbon and nitrogen cycling caused by declining sulphur deposition. Glob. Change Biol. 2011, 17, 3115–3129. [Google Scholar] [CrossRef]
- Røgeberg, E.J.S.; Henriksen, A. Automatic method for fractionation and determination of aluminium species in fresh waters. Vaten 1985, 41, 48–53. [Google Scholar]
- LaZerte, B.D.; Chun, C.; Evans, D.; Tomassinl, F. Measurement of Aqueous Aluminum Species: Comparison of Dialysis and Ion-Exchange Techniques. Environ. Sci. Technol. 1988, 22, 1106–1108. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Schecher, W.D. Aluminium in the environment. In Metal Ions in Biological Systems; Sigel, H., Sigel, A., Eds.; Marcel Dekker: New York, NY, USA, 1988; pp. 59–122. [Google Scholar]
- Sverdrup, H.; Warfvinge, P. The Effect of Soil Acidification on the Growth of Trees, Grass and Herbs as Expressed by the (Ca+Mg+K)/Al Ratio; Reports in Ecology and Environmental Engineering, Report 2: 1993, KF-Sigma; Department of Chemistry Engineering, Lund University: Lund, Sweden, 1993. [Google Scholar]
- Kučera, J.; Brito, P.; Jiménez, M.S.; Urban, J. Direct Penman–Monteith parameterization for estimating stomatal conductance and modeling sap flow. Trees - Struct. Funct. 2017, 31, 873–885. [Google Scholar] [CrossRef]
- Hirsch, R.M.; Slack, J.R.; Smith, R.A. Techniques of trend analysis for monthly water quality data. Water Resour. Res. 1982, 18, 107–121. [Google Scholar] [CrossRef]
- Sen, K.P. Estimates of the Regression Coefficient Based on Kendall’ s Tau Pranab Kumar Sen. J. Am. Stat. Assoc. 1968, 63, 1379–1389. [Google Scholar] [CrossRef]
- Falkengren-Grerup, U. Effect of Stemflow on Beech Forest Soils and Vegetation in Southern Sweden. J. Appl. Ecol. 1989, 26, 341. [Google Scholar] [CrossRef]
- Vesterdal, L.; Elberling, B.; Christiansen, J.R.; Callesen, I.; Schmidt, I.K. Soil respiration and rates of soil carbon turnover differ among six common European tree species. For. Ecol. Manag. 2012, 264, 185–196. [Google Scholar] [CrossRef]
- Berger, T.W.; Inselsbacher, E.; Mutsch, F.; Pfeffer, M. Nutrient cycling and soil leaching in eighteen pure and mixed stands of beech (Fagus sylvatica) and spruce (Picea abies). For. Ecol. Manag. 2009, 258, 2578–2592. [Google Scholar] [CrossRef]
- Oulehle, F.; Hofmeister, J.; Cudlín, P.; Hruška, J. The effect of reduced atmospheric deposition on soil and soil solution chemistry at a site subjected to long-term acidification, Nacetín, Czech Republic. Sci. Total Environ. 2006, 370, 532–544. [Google Scholar] [CrossRef]
- Engardt, M.; Simpson, D.; Schwikowski, M.; Granat, L. Deposition of sulphur and nitrogen in Europe 1900–2050. Model calculations and comparison to historical observations. Tellus B Chem. Phys. Meteorol. 2017, 69, 1328945. [Google Scholar] [CrossRef]
- Balestrini, R.; Tagliaferri, A. Atmospheric deposition and canopy exchange processes in alpine forest ecosystems (northern Italy). Atmos. Environ. 2001, 35, 6421–6433. [Google Scholar] [CrossRef]
- Rothe, A.; Huber, C.; Kreutzer, K.; Weis, W. Deposition and soil leaching in stands of Norway spruce and European beech: Results from the Höglwald research in comparison with other European case studies. Plant Soil 2002, 240, 33–45. [Google Scholar] [CrossRef]
- Van Breemen, N. Soil Acidification and Alkalinization. In Soil Acidity; Springer: Berlin/Heidelberg, Germany, 1991; pp. 1–7. ISBN 978-3-642-74444-0. [Google Scholar]
- Prechtel, A.; Alewell, C.; Armbruster, M.; Bittersohl, J.; Cullen, J.M.; Evans, C.D.; Helliwell, R.; Kopáček, J.; Marchetto, A.; Matzner, E.; et al. Response of sulphur dynamics in European catchments to decreasing sulphate deposition. Hydrol. Earth Syst. Sci. 2001, 5, 311–326. [Google Scholar] [CrossRef]
- Svensson, T.; Lovett, G.M.; Likens, G.E. Is chloride a conservative ion in forest ecosystems? Biogeochemistry 2012, 107, 125–134. [Google Scholar] [CrossRef]
- Redon, P.-O.; Abdelouas, A.; Bastviken, D.; Cecchini, S.; Nicolas, M.; Thiry, Y. Chloride and Organic Chlorine in Forest Soils: Storage, Residence Times, And Influence of Ecological Conditions. Environ. Sci. Technol. 2011, 45, 7202–7208. [Google Scholar] [CrossRef] [PubMed]
- Oulehle, F.; Jones, T.G.; Burden, A.; Cooper, M.D.A.; Lebron, I.; Zieliński, P.; Evans, C.D. Soil-solution partitioning of DOC in acid organic soils: Results from a UK field acidification and alkalization experiment. Eur. J. Soil Sci. 2013, 64, 787–796. [Google Scholar] [CrossRef]
- Hruška, J.; Krám, P.; McDowell, W.H.; Oulehle, F. Increased Dissolved Organic Carbon (DOC) in Central European Streams is Driven by Reductions in Ionic Strength Rather than Climate Change or Decreasing Acidity. Environ. Sci. Technol. 2009, 43, 4320–4326. [Google Scholar] [CrossRef]
- Perakis, S.S.; Hedin, L.O. Nitrogen loss from unpolluted South American forests mainly via dissolved organic compounds. Nature 2002, 415, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Aber, J.D.; Nadelhoffer, K.J.; Steudler, P.; Melillo, J.M. Nitrogen Saturation in Northern Forest Ecosystems. BioScience 1989, 39, 378–386. [Google Scholar] [CrossRef]
- Gundersen, P.; Emmett, B.A.; Kjønaas, O.J.; Koopmans, C.J.; Tietema, A. Impact of nitrogen deposition on nitrogen cycling in forests: A synthesis of NITREX data. For. Ecol. Manag. 1998, 101, 37–55. [Google Scholar] [CrossRef]
- Gundersen, P.; Schmidt, I.K.; Raulund-Rasmussen, K. Leaching of nitrate from temperate forests - effects of air pollution and forest management. Environ. Rev. 2006, 14, 1–57. [Google Scholar] [CrossRef]
- Corre, M.D.; Brumme, R.R.; Veldkamp, E.; Beese, F.O. Changes in nitrogen cycling and retention processes in soils under spruce forests along a nitrogen enrichment gradient in Germany. Glob. Change Biol. 2007, 13, 1509–1527. [Google Scholar] [CrossRef]
- Joseph, G.; Henry, H.A.L. Soil nitrogen leaching losses in response to freeze–thaw cycles and pulsed warming in a temperate old field. Soil Biol. Biochem. 2008, 40, 1947–1953. [Google Scholar] [CrossRef]
- Verstraeten, A.; Verschelde, P.; De Vos, B.; Neirynck, J.; Cools, N.; Roskams, P.; Hens, M.; Louette, G.; Sleutel, S.; De Neve, S. Increasing trends of dissolved organic nitrogen (DON) in temperate forests under recovery from acidification in Flanders, Belgium. Sci. Total Environ. 2016, 553, 107–119. [Google Scholar] [CrossRef]
- Watmough, S.A.; Aherne, J.; Alewell, C.; Arp, P.; Bailey, S.; Clair, T.; Dillon, P.; Duchesne, L.; Eimers, C.; Fernandez, I.; et al. Sulphate, Nitrogen and Base Cation Budgets at 21 Forested Catchments in Canada, the United States and Europe. Environ. Monit. Assess. 2005, 109, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Cronan, C.S.; Grigal, D.F. Use of Calcium/Aluminum Ratios as Indicators of Stress in Forest Ecosystems. J. Environ. Qual. 1995, 24, 209. [Google Scholar] [CrossRef]
- Hansen, K.; Vesterdal, L.; Bastrup-Birk, A.; Bille-Hansen, J. Are Indicators for Critical Load Exceedance Related to Forest Condition? Water. Air. Soil Pollut. 2007, 183, 293–308. [Google Scholar] [CrossRef]
- Hruška, J.; Laudon, H.; Johnson, C.E.; Köhler, S.; Bishop, K. Acid/base character of organic acids in a boreal stream during snowmelt. Water Resour. Res. 2001, 37, 1043–1056. [Google Scholar] [CrossRef]
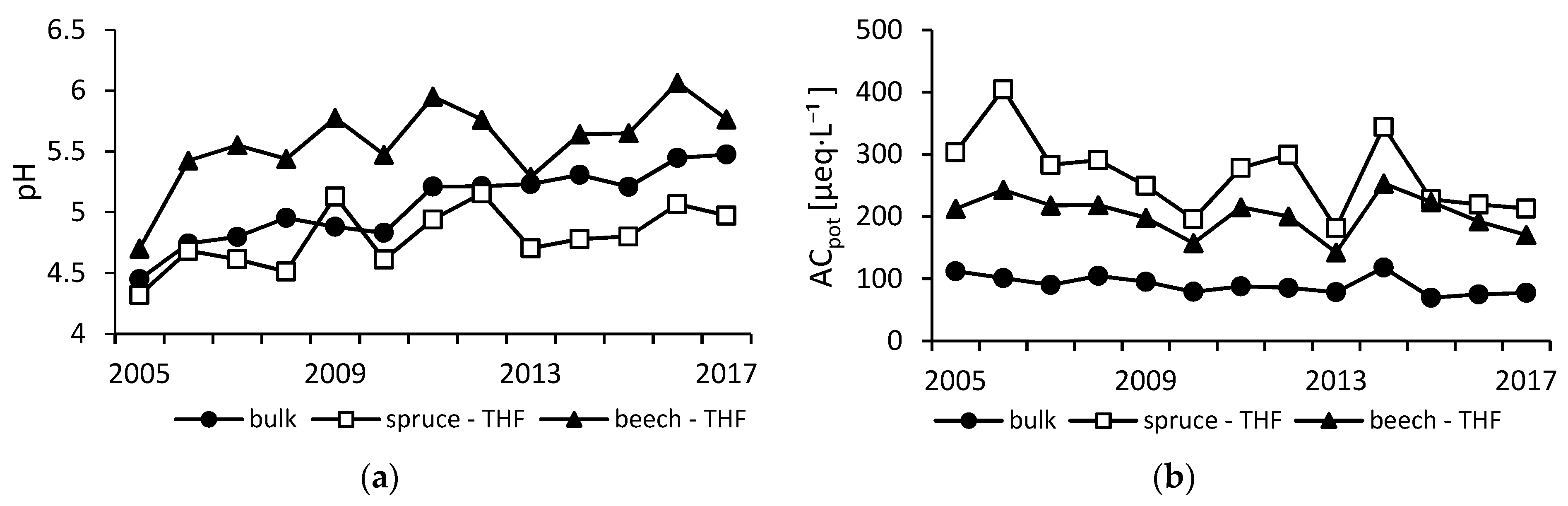
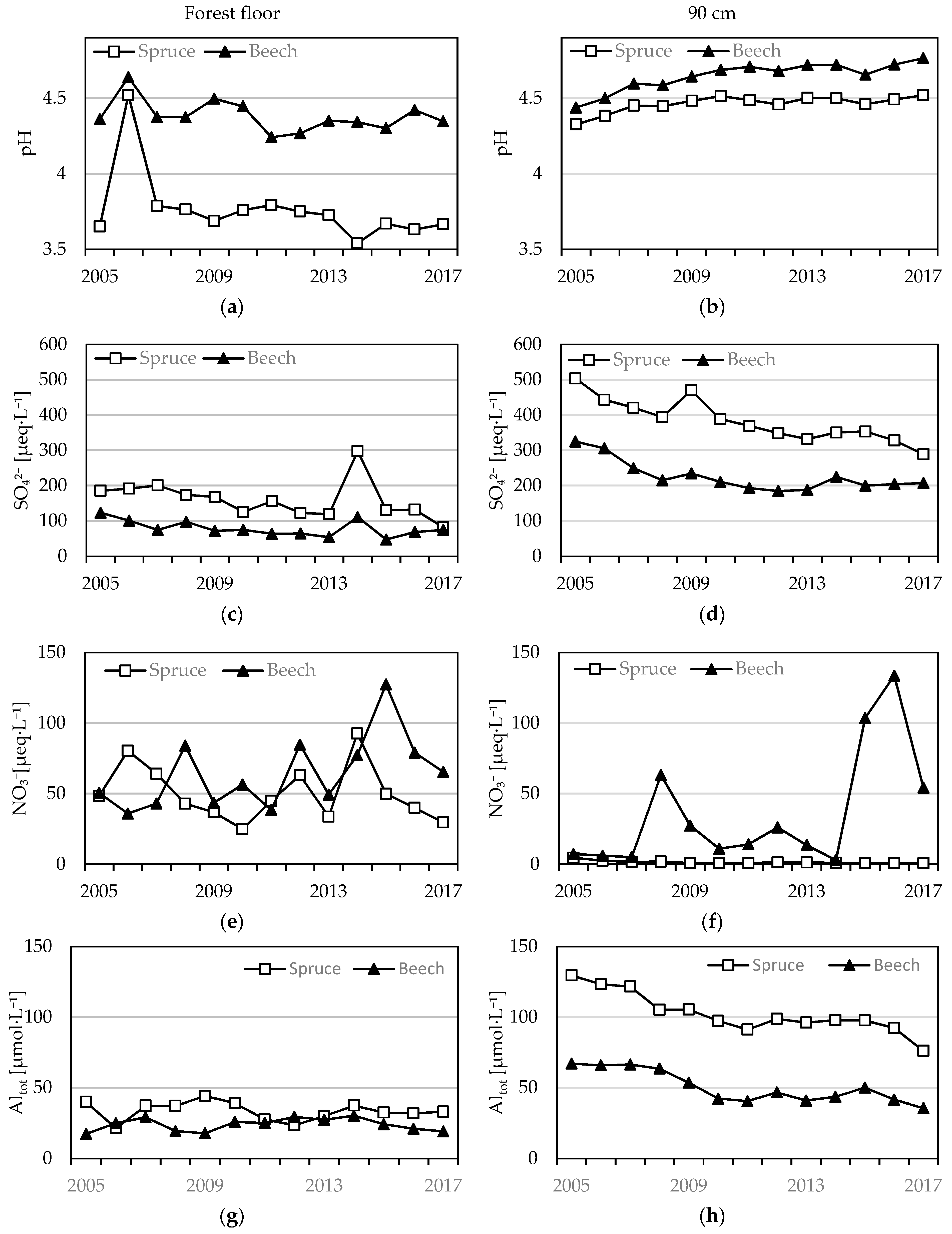
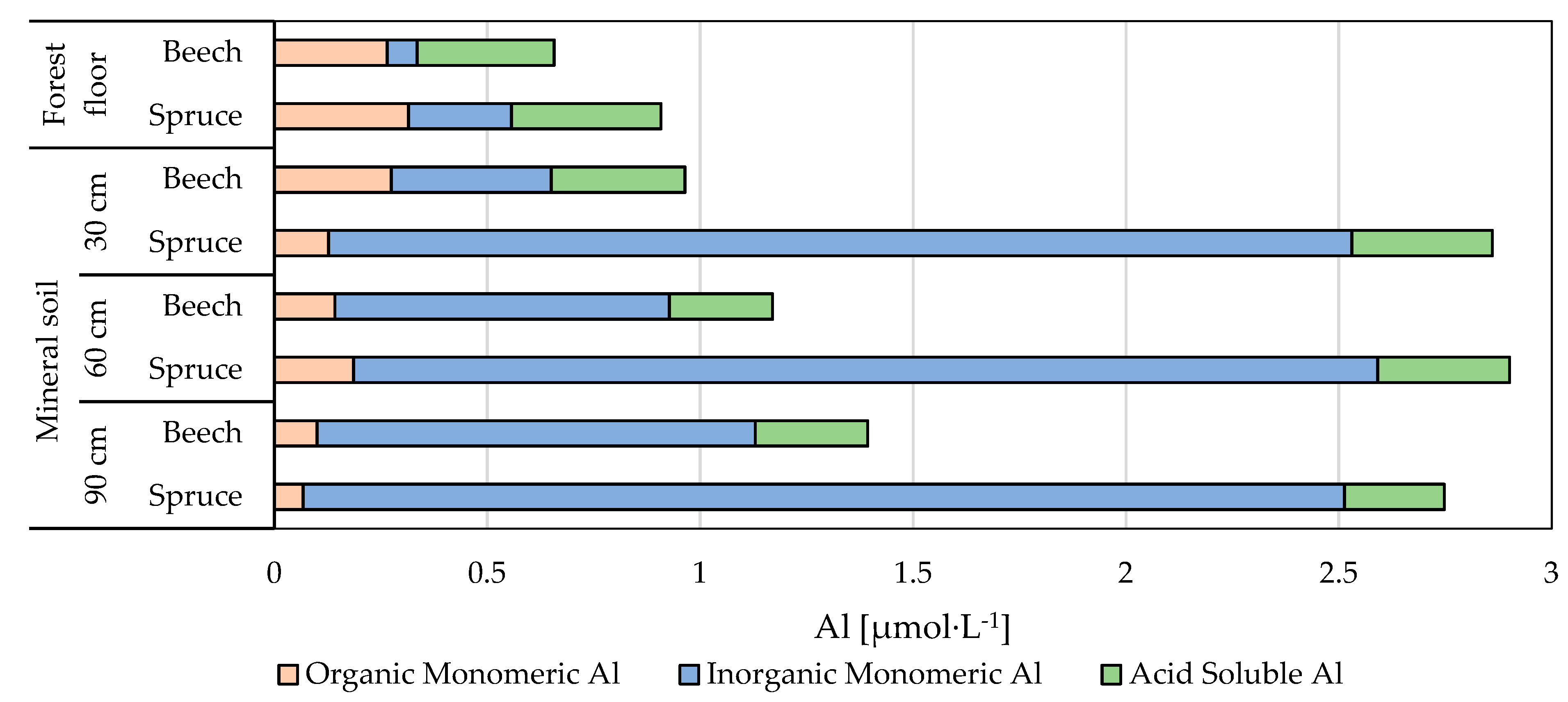
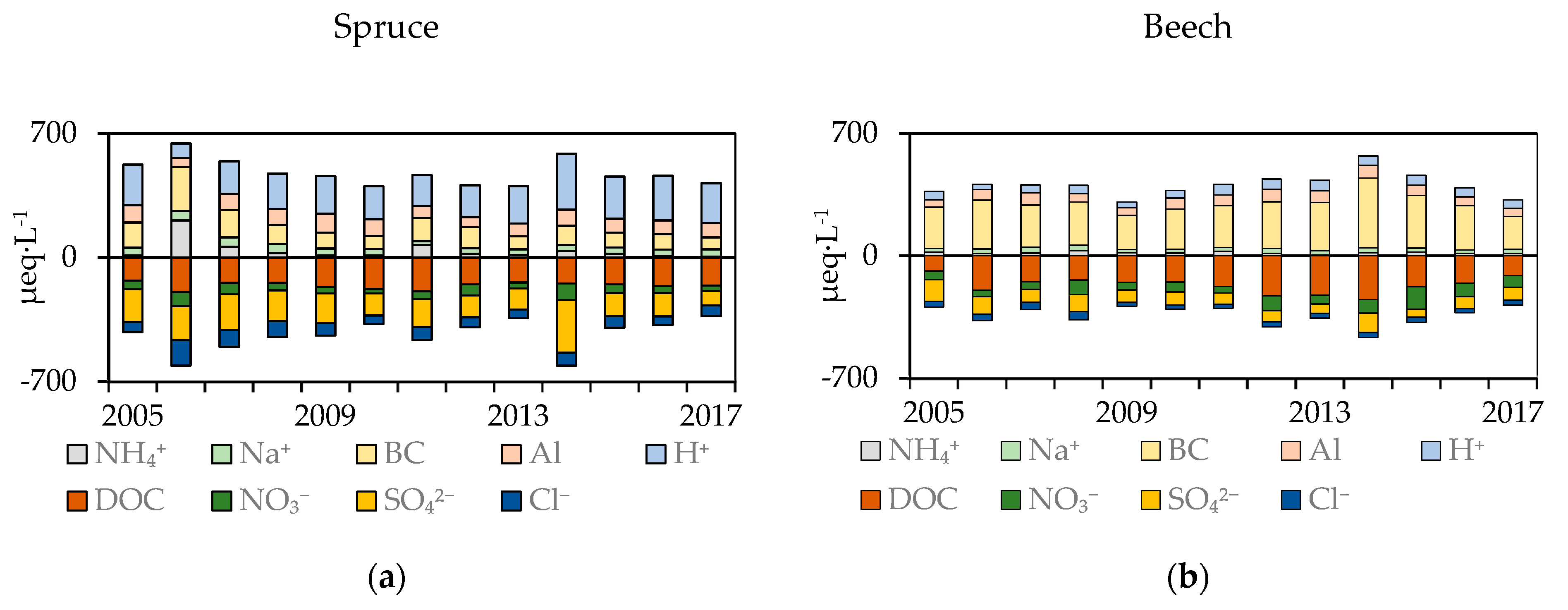
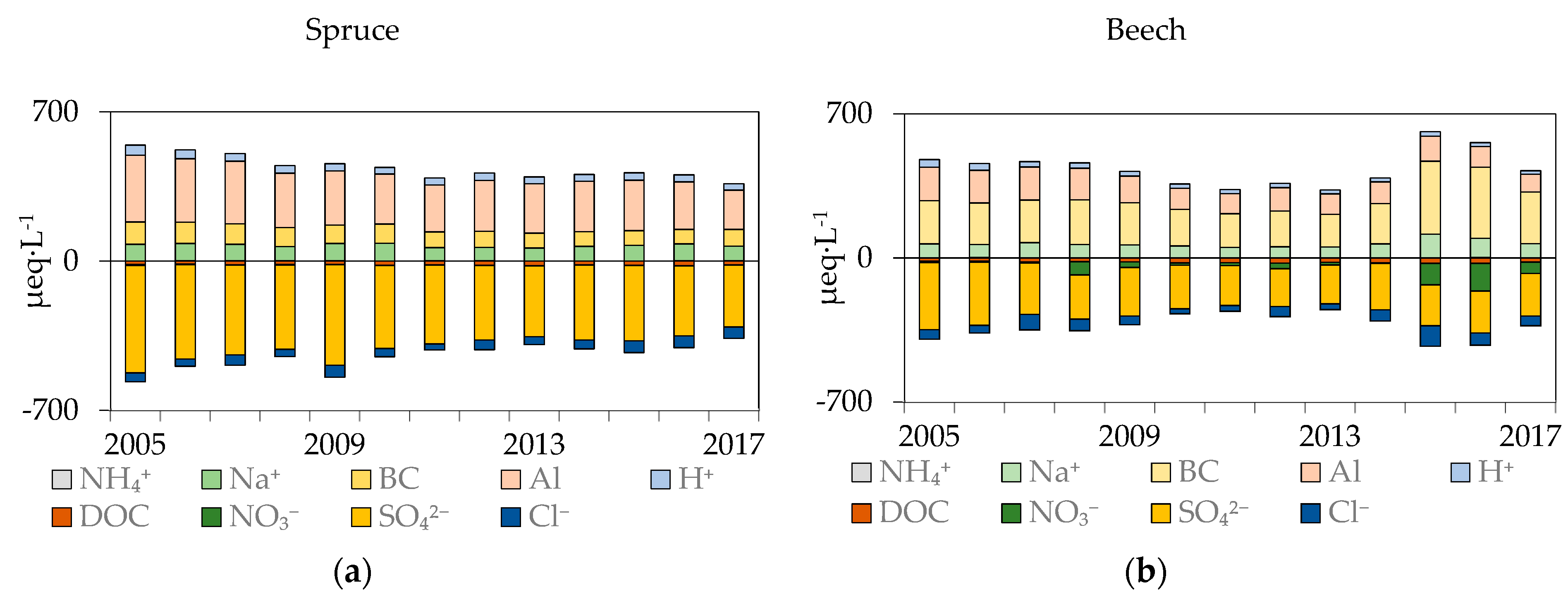
| Plot | SO42− | NO3− | Cl | DOC | NH4+ | BC | pH | Altot | BC/Alin | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Slope | Mean | Slope | Mean | Slope | Mean | Slope | Mean | Slope | Mean | Slope | Mean | Slope | Mean | Slope | Mean | Slope | |
| BULK | 28 | −1.1 * | 29 | 11 | 165 | 45 | 31 | 5.06 | +0.07 *** | |||||||||
| THF | ||||||||||||||||||
| Spruce | 147 | 97 | −3.0 ** | 48 | 1067 | 95 | 190 | 4.79 | +0.04 * | |||||||||
| Beech | 79 | 78 | 29 | 435 | 280 | 148 | 5.59 | +0.04 * | ||||||||||
| Forest floor | ||||||||||||||||||
| Spruce | 160 | −6.6 * | 50 | 69 | 3355 | +63 * | 32 | 115 | −7.6 * | 3.73 | −0.01 * | 33 | 42.0 | |||||
| Beech | 78 | 65 | 30 | 2519 | 17 | 259 | 4.38 | 24 | 101.6 | +4.69 * | ||||||||
| Mineral soil | ||||||||||||||||||
| 30 cm | ||||||||||||||||||
| Spruce | 400 | −11.9 ** | 2 | −0.1 ** | 27 | 445 | 1 | 68 | −6.2 *** | 4.43 | +0.01 ** | 109 | −3.4 *** | 0.4 | −0.03 *** | |||
| Beech | 89 | 28 | 37 | 875 | −30 *** | 2 | 152 | −5.5 * | 4.53 | 36 | 5.0 | −0.26 * | ||||||
| 60 cm | ||||||||||||||||||
| Spruce | 424 | −12.5 *** | 2 | 38 | 610 | 1 | 84 | −3.5 *** | 4.32 | +0.01 ** | 110 | −1.9 *** | 0.5 | −0.02 ** | ||||
| Beech | 166 | −8.2 *** | 52 | 37 | 392 | −6 * | 3 | 199 | 4.62 | +0.02 *** | 41 | −1.4 ** | 3.0 | |||||
| 90 cm | ||||||||||||||||||
| Spruce | 386 | −13.9 *** | 2 | −0.1 *** | 44 | 245 | +4 * | 1 | 82 | −3.4 *** | 4.46 | +0.01 ** | 103 | −3.2 *** | 0.5 | |||
| Beech | 228 | −7.6 *** | 36 | 50 | 272 | +9 *** | 1 | 220 | 4.64 | +0.02 *** | 51 | −2.5 ** | 2.6 | +0.09 * |
| Horizon (mm) | Spruce | Beech |
|---|---|---|
| Bulk | 1122 ± 117 | 1122 ± 117 |
| THF (STEM) | 756 ± 109 | 721 ± 79 (220 ± 21) |
| Organic horizon | 632 ± 110 | 834 ± 91 |
| 30 cm | 520 ± 113 | 742 ± 93 |
| 60 cm | 462 ± 120 | 591 ± 101 |
| 90 cm | 430 ± 130 | 511 ± 101 |
| Actual transpiration (modelled average 2005–2017) | 207 ± 33 | 345 ± 27 |
| Actual transpiration (measured in 2017) | 165 | 337 |
| Spruce | Na | K | Ca | Mg | Al | S-SO4 | N-NH4 | N-NO3 | DON | DOC | BC | Cl |
| kg·ha−1·year−1 | ||||||||||||
| Bulk | 2.5 | 1.4 | 2.2 | 0.6 | 0.1 | 4.3 | 5.9 | 3.8 | – | 19.0 | 4.2 | 4.1 |
| THF | 4.9 | 19.8 | 8.2 | 2.3 | 0.3 | 12.5 | 8.0 | 8.0 | 3.2 | 85.0 | 30.3 | 10.2 |
| Forest Floor | ||||||||||||
| 5.6 | 12.5 | 3.6 | 2.3 | 5.7 | 14.5 | 3.4 | 0.3 | 6.9 | 253.2 | 18.4 | 12.8 | |
| Mineral Soil | ||||||||||||
| 30 cm | 10.8 | 1.2 | 1.5 | 2.9 | 15.1 | 32.2 | 0.1 | 0.0 | 1.0 | 29.1 | 5.6 | 4.7 |
| 60 cm | 10.3 | 0.8 | 2.1 | 3.1 | 13.4 | 29.6 | 0.1 | 0.0 | 1.0 | 33.5 | 6.0 | 6.0 |
| 90 cm | 7.2 | 1.5 | 2.6 | 2.2 | 12.0 | 25.9 | 0.1 | 0.0 | 0.4 | 12.7 | 6.3 | 6.8 |
| Beech | Na | K | Ca | Mg | Al | S-SO4 | N-NH4 | N-NO3 | DON | DOC | BC | Cl |
| kg·ha−1·year−1 | ||||||||||||
| Bulk | 2.5 | 1.4 | 2.2 | 0.6 | 0.1 | 4.3 | 5.9 | 3.8 | 19.0 | 4.2 | 4.1 | |
| THF | 3.1 | 14.5 | 5.7 | 2.1 | 0.2 | 7.0 | 7.8 | 6.3 | 2.3 | 34.6 | 22.3 | 5.7 |
| STEM | 0.8 | 3.2 | 1.1 | 0.3 | 0.1 | 1.7 | 1.3 | 0.9 | 0.7 | 11.1 | 4.6 | 1.4 |
| Forest floor | ||||||||||||
| 4.3 | 10.9 | 14.8 | 8.6 | 5.1 | 8.6 | 1.8 | 6.4 | 6.9 | 234.8 | 34.4 | 7.8 | |
| Mineral soil | ||||||||||||
| 30 cm | 8.3 | 0.9 | 3.1 | 6.8 | 7.0 | 10.0 | 0.2 | 2.4 | 2.6 | 78.2 | 10.8 | 9.0 |
| 60 cm | 7.0 | 0.7 | 3.1 | 8.0 | 6.3 | 14.6 | 0.2 | 4.0 | 1.4 | 26.7 | 11.8 | 8.2 |
| 90 cm | 7.6 | 0.3 | 2.1 | 7.5 | 6.8 | 17.9 | 0.1 | 2.2 | 1.0 | 15.8 | 9.9 | 8.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Růžek, M.; Myška, O.; Kučera, J.; Oulehle, F. Input-Output Budgets of Nutrients in Adjacent Norway Spruce and European Beech Monocultures Recovering from Acidification. Forests 2019, 10, 68. https://doi.org/10.3390/f10010068
Růžek M, Myška O, Kučera J, Oulehle F. Input-Output Budgets of Nutrients in Adjacent Norway Spruce and European Beech Monocultures Recovering from Acidification. Forests. 2019; 10(1):68. https://doi.org/10.3390/f10010068
Chicago/Turabian StyleRůžek, Michal, Oldřich Myška, Jiří Kučera, and Filip Oulehle. 2019. "Input-Output Budgets of Nutrients in Adjacent Norway Spruce and European Beech Monocultures Recovering from Acidification" Forests 10, no. 1: 68. https://doi.org/10.3390/f10010068
APA StyleRůžek, M., Myška, O., Kučera, J., & Oulehle, F. (2019). Input-Output Budgets of Nutrients in Adjacent Norway Spruce and European Beech Monocultures Recovering from Acidification. Forests, 10(1), 68. https://doi.org/10.3390/f10010068





