Fabrication of Biomass-Derived Carbon Aerogels with High Adsorption of Oils and Organic Solvents: Effect of Hydrothermal and Post-Pyrolysis Processes
Abstract
:1. Introduction
2. Results and Discussion
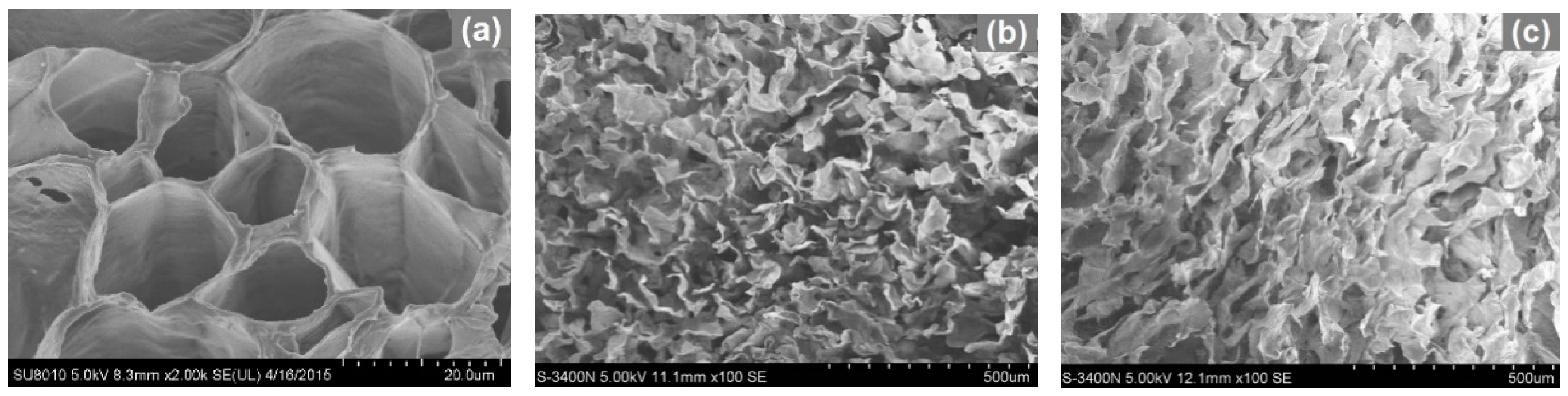
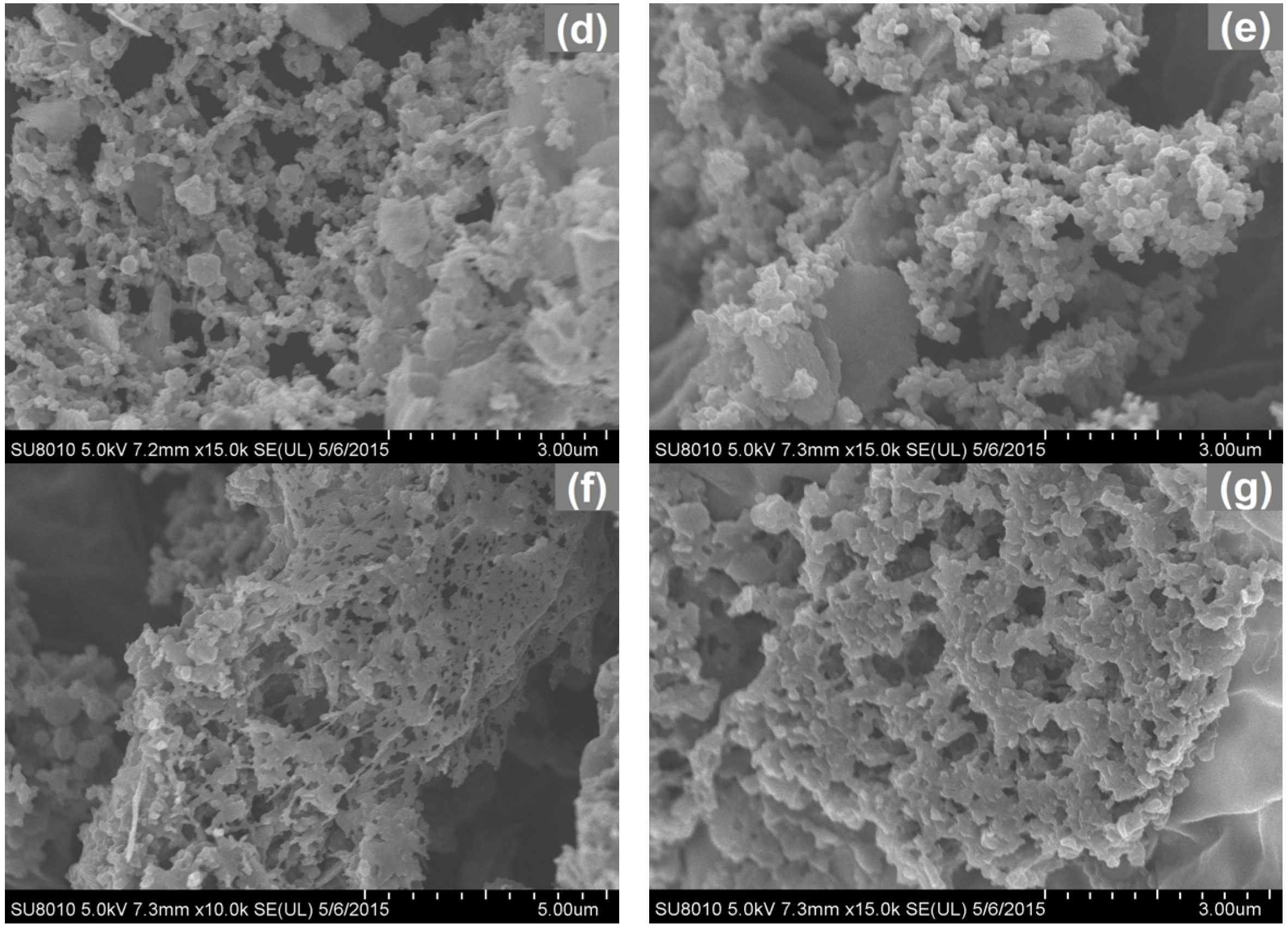
2.1. Effect of Hydrothermal Treatments and Carbonization on Microstructure
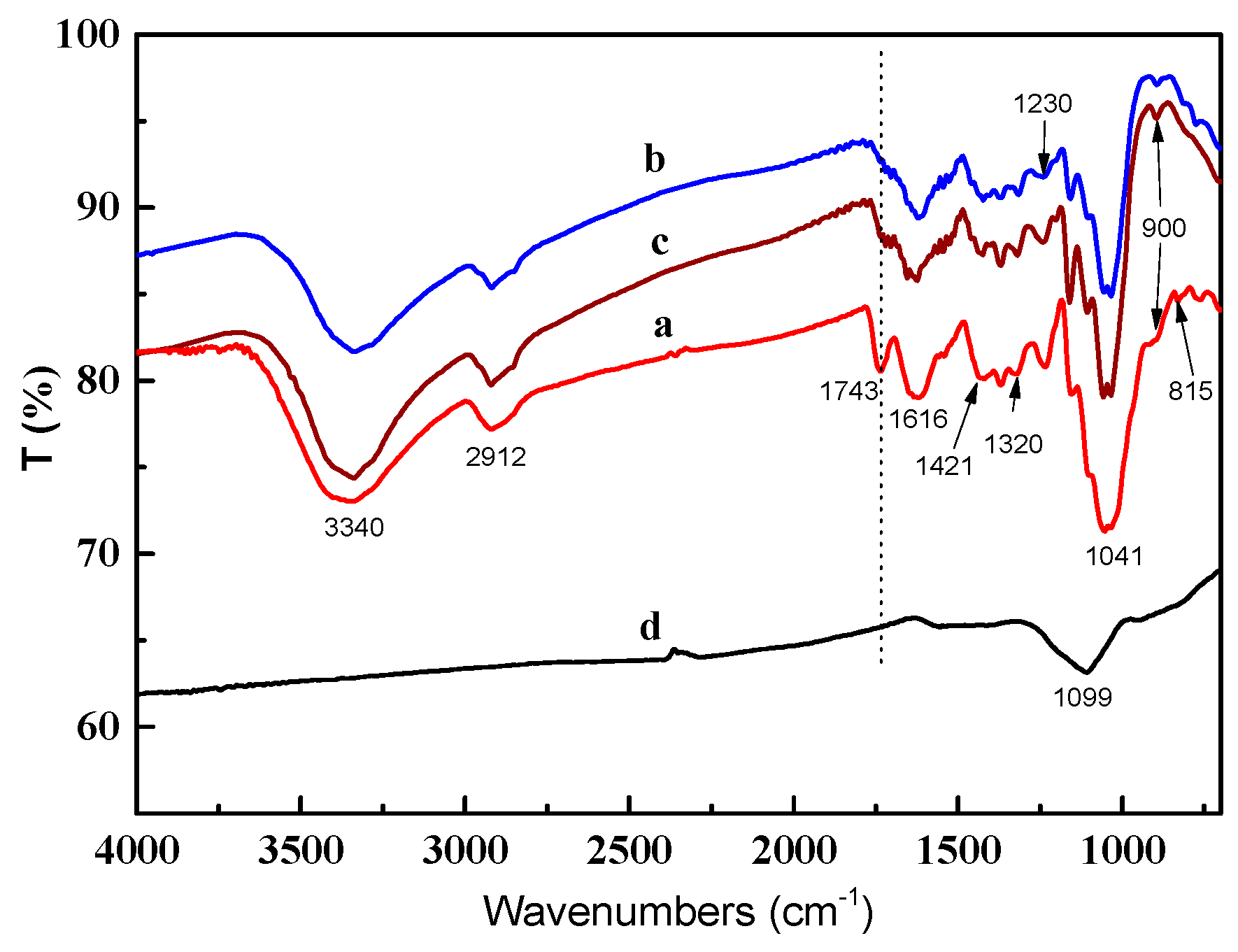
2.2. FT-IR Spectra
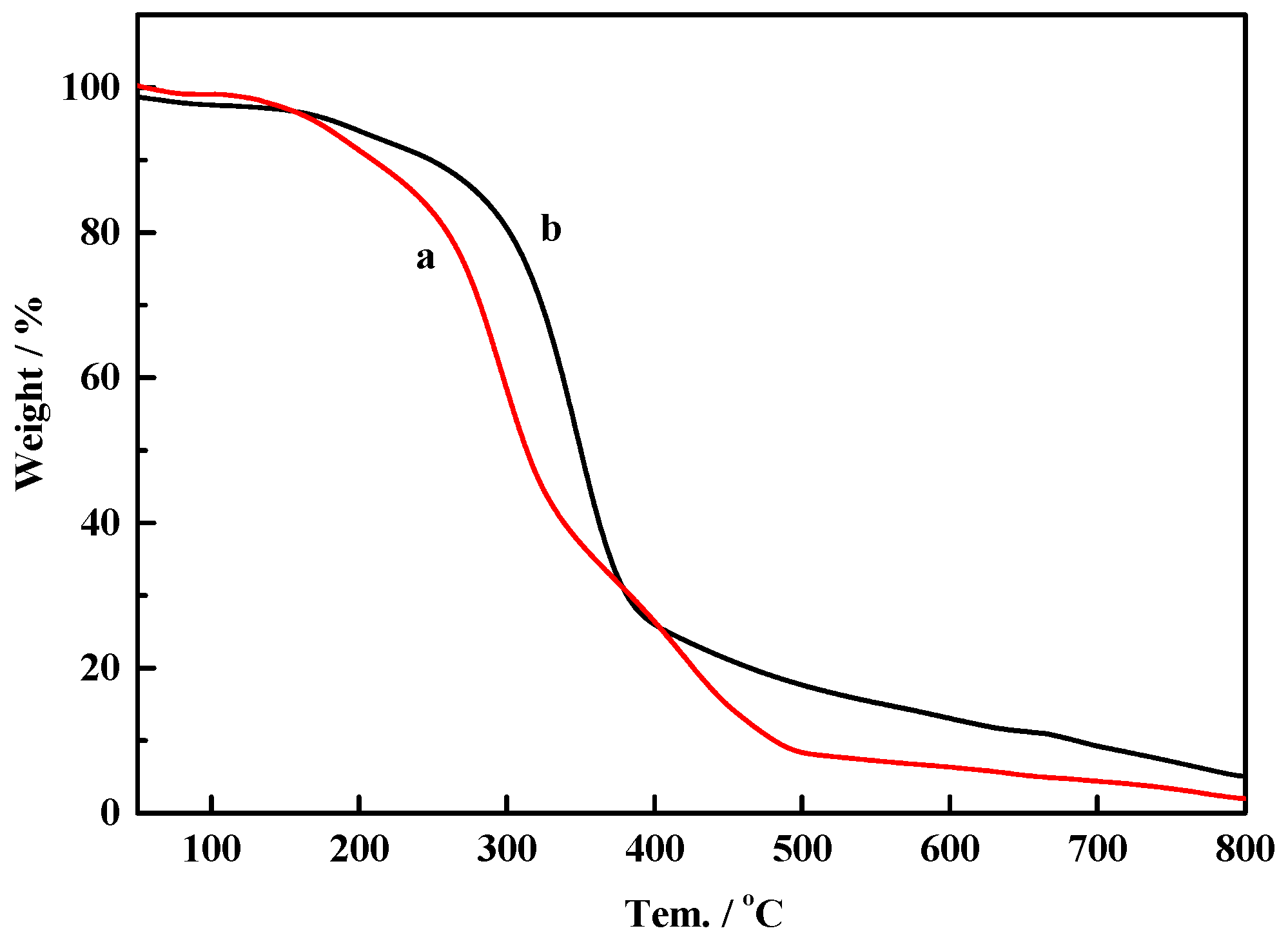
2.3. Thermal Analysis
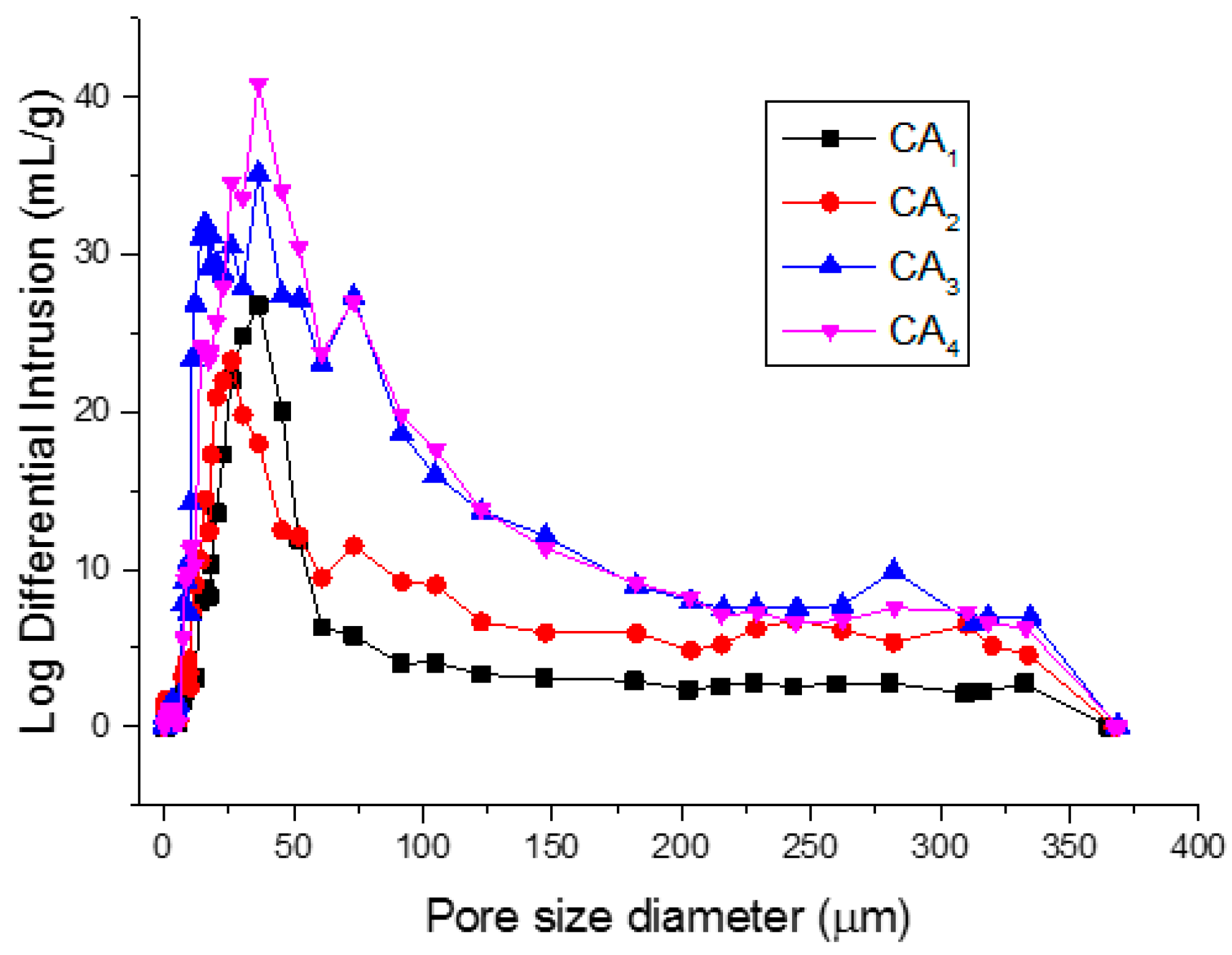
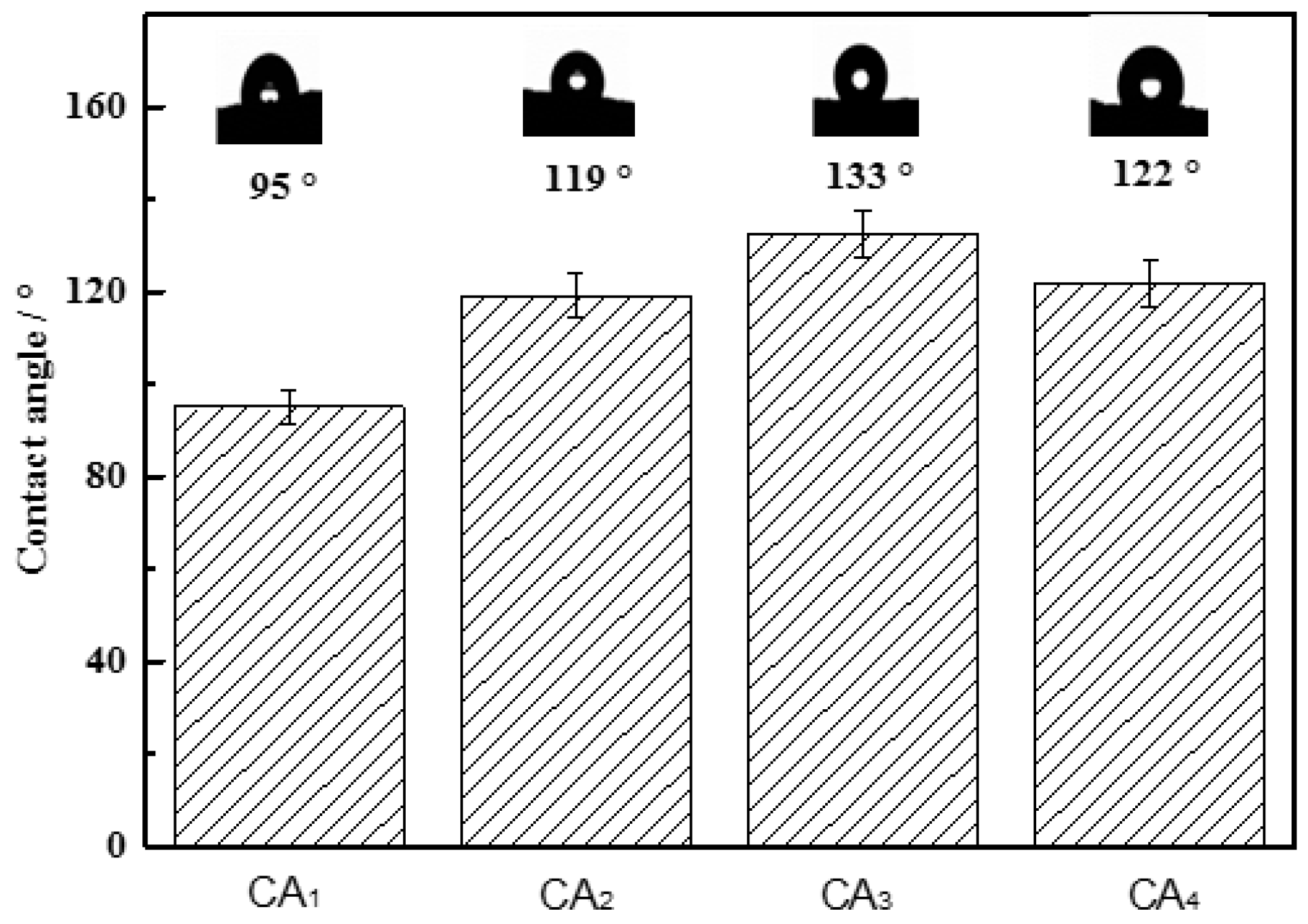
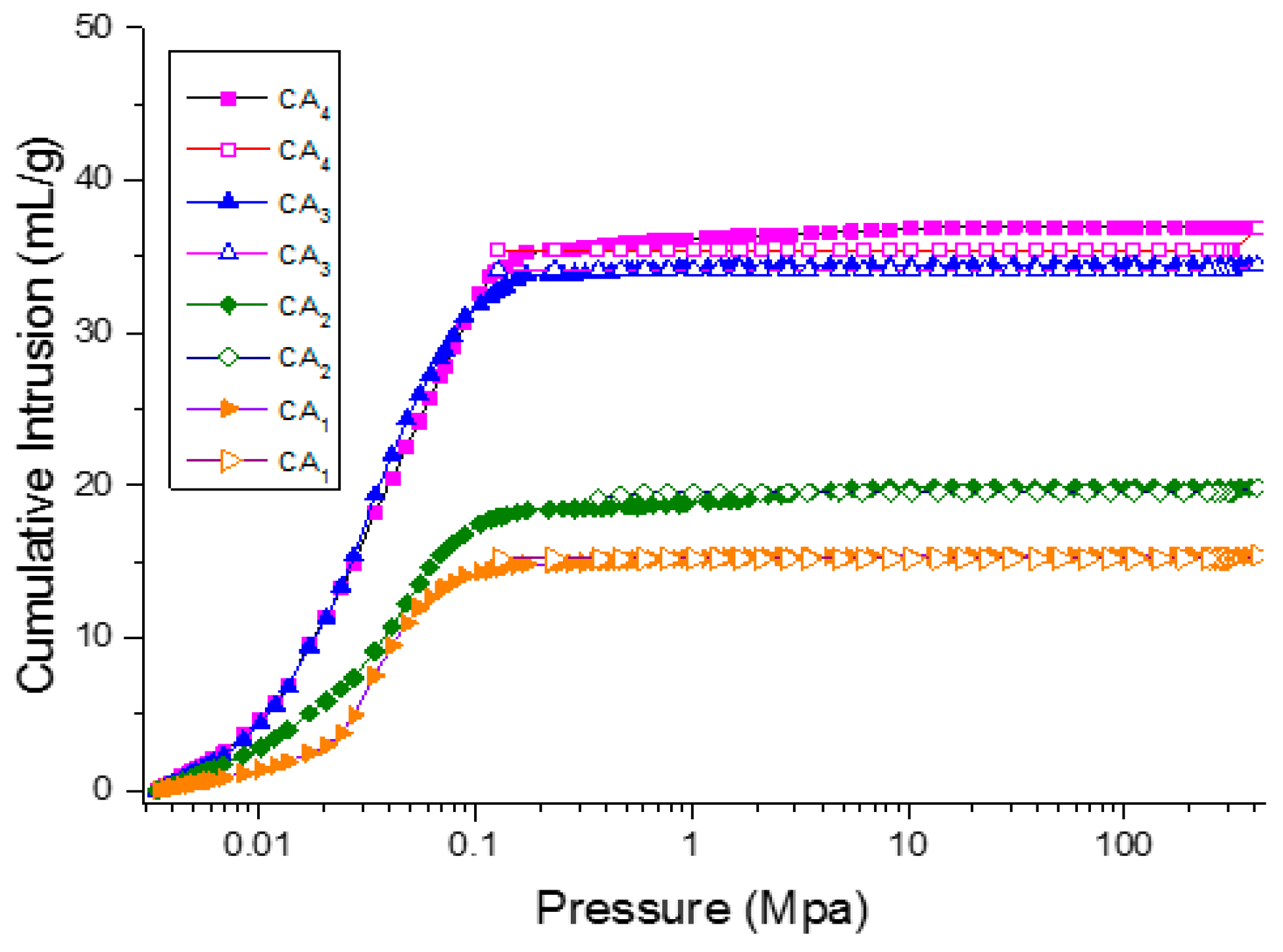
2.4. Surface Area, Pore Diameter Distribution and Hydrophobicity
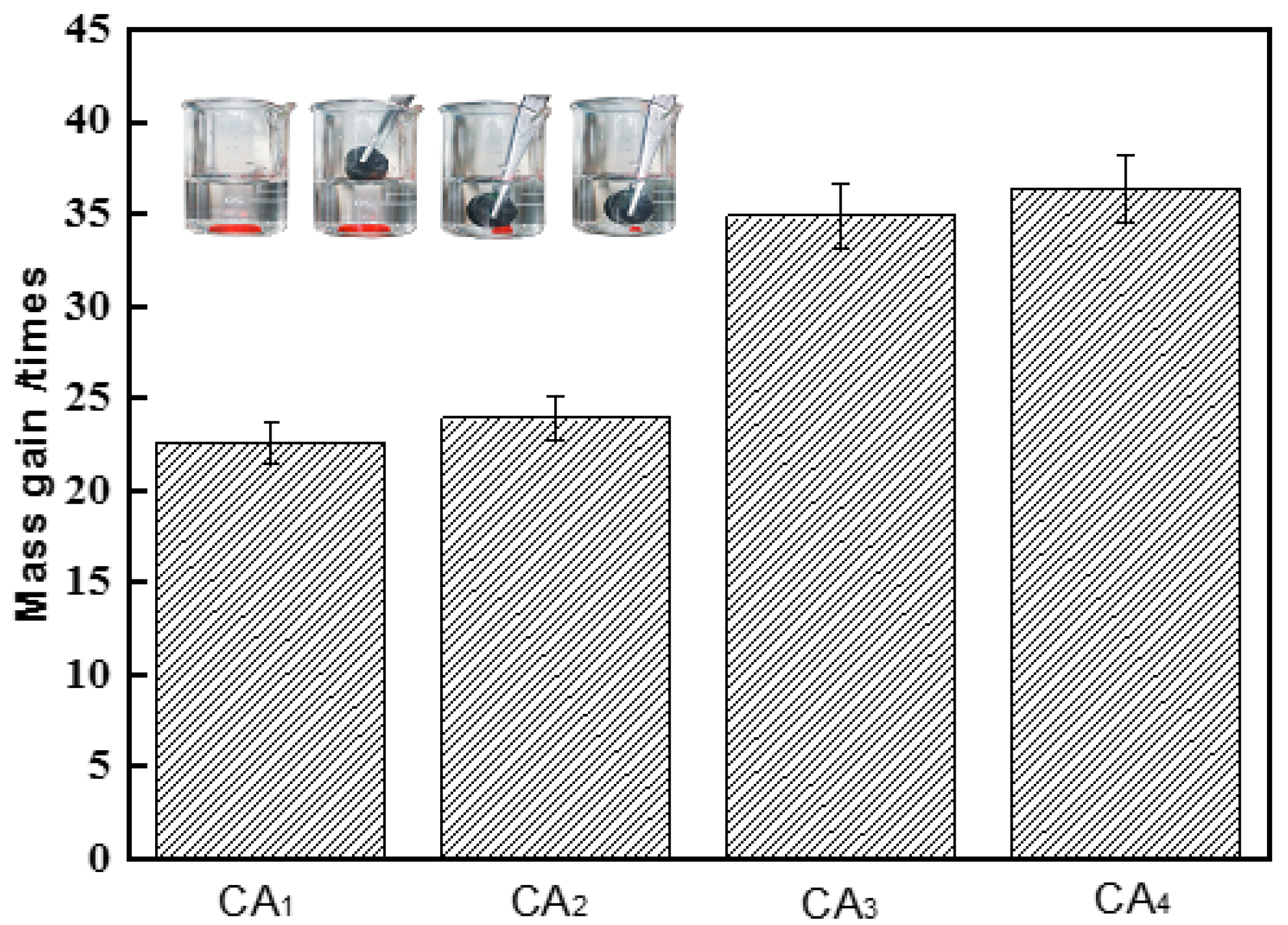
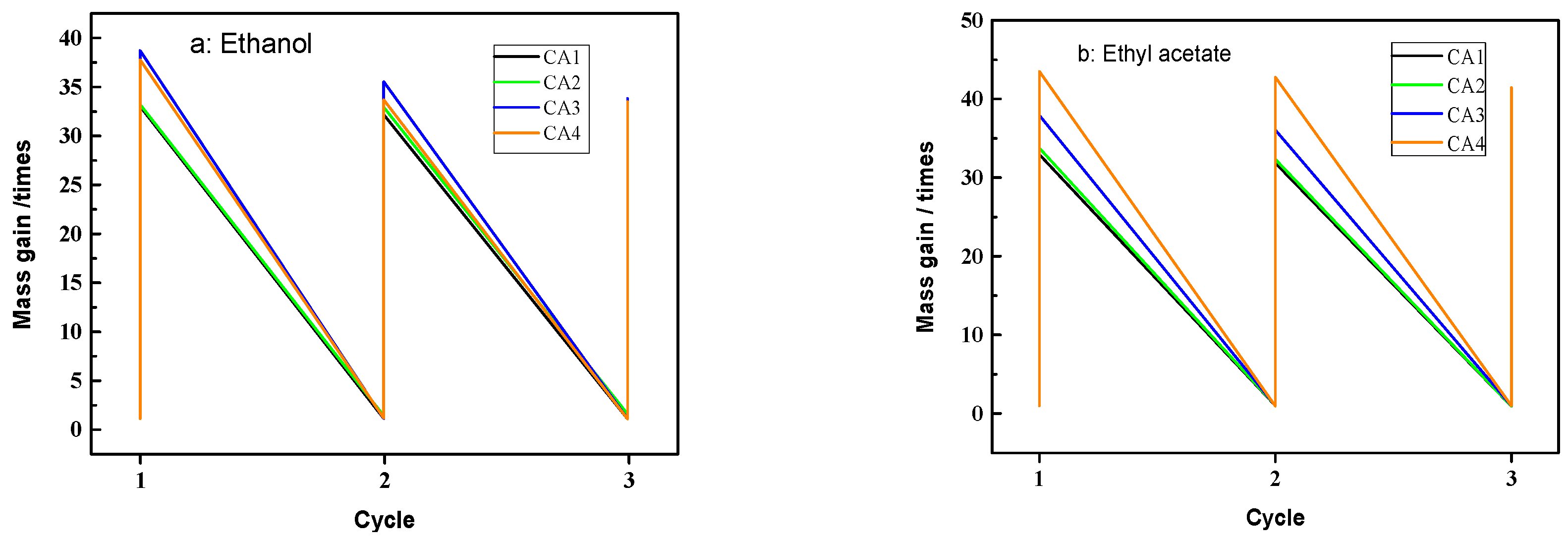
2.5. Absorption Capacities for Oil and Organic Solvents
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Carbon Aerogels
3.2.2. Characterizations
3.2.3. Adsorption of Oils and Organic Solvents and Reusability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bi, H.C.; Xie, X.; Yin, K.B.; Zhou, Y.L.; Wan, S.; He, L.B.; Xu, F.; Banhart, F.; Sun, L.T.; Ruoff, R.S. Spongy graphene as a highly efficient and recyclable sorbent for oils and organic solvents. Adv. Funct. Mater. 2012, 22, 4421–4425. [Google Scholar] [CrossRef]
- Bi, H.C.; Yin, Z.Y.; Cao, X.H.; Xie, X.; Tan, C.L.; Huang, X.; Chen, B.; Chen, F.T.; Yang, Q.L.; Bu, X.Y.; et al. Carbon fiber aerogel made from raw cotton: A novel, efficient and recyclable sorbent for oils and organic solvents. Adv. Mater. 2013, 25, 5916–5921. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.C.; Zeng, Z.P.; Lin, Z.Q.; Gan, Q.M.; Xiang, R.; Zhu, Y.; Cao, A.Y.; Tang, Z.K. Magnetic and highly recyclable macroporous carbon nanotubes for spilled oil sorption and separation. ACS Appl. Mater. Inter. 2013, 5, 5845–5850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Li, Z.; Liu, K.S.; Jiang, L. Bioinspired multifunctional foam with self-cleaning and oil/water separation. Adv. Funct. Mater. 2013, 23, 2881–2886. [Google Scholar] [CrossRef]
- Chen, N.; Pan, Q.M. Versatile fabrication of ultralight magnetic foams and application for oil-water separation. ACS Nano 2013, 7, 6875–6883. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Tai, N.H.; Lee, S.B.; Kuo, W.S. Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energy Environ. Sci. 2012, 5, 7908–7912. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Li, C.; Liang, H.W.; Chen, J.F.; Yu, S.H. Ultralight, flexible, and fire-resistant carbon nanofiber aerogels from bacterial cellulose. Angew. Chem. Int. Ed. 2013, 52, 2925–2929. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.K.; Oh, J.; Lima, M.; Kozlov, M.E.; Kim, S.J.; Baughman, R.H. Elastomeric conductive composites based on carbon nanotube forests. Adv. Mater. 2010, 22, 2663–2667. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Samad, Y.A.; Polychronopoulou, K.; Alhassan, S.M.; Liao, K. Carbon aerogel from winter melon for highly efficient and recyclable oils and organic solvents absorption. ACS Sustain. Chem. Eng. 2014, 2, 1492–1497. [Google Scholar] [CrossRef]
- Wu, X.L.; Wen, T.; Guo, H.L.; Yang, S.B.; Wang, X.K.; Xu, A.W. Biomass-derived sponge-like carbonaceous hydrogels and aerogels for supercapacitors. ACS Nano 2013, 7, 3589–3597. [Google Scholar] [CrossRef] [PubMed]
- Titirici, M.M.; Thomas, A.; Yu, S.H.; Muller, J.O.; Antonietti, M. A direct synthesis of mesoporous carbons with bicontinuous pore morphology from crude plant material by hydrothermal carbonization. Chem. Mater. 2007, 19, 4205–4212. [Google Scholar] [CrossRef]
- Zia-ur-Rehman, Z.; Islam, M.; Shah, W.H. Effect of microwave and conventional cooking on insoluble dietary fibre components of vegetables. Food Chem. 2003, 80, 237–240. [Google Scholar] [CrossRef]
- Yuan, T.Q.; Xu, F.; He, J.; Sun, R.C. Structural and physico-chemical characterization of hemicelluloses from ultrasound-assisted extractions of partially delignified fast-growing poplar wood through organic solvent and alkaline solutions. Biotechnol. Adv. 2010, 28, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Meng, L.Y.; Xu, F.; Sun, R.C. Pretreatment of partially delignified hybrid poplar for biofuels production: Characterization of organosolv hemicelluloses. Ind. Crop. Prod. 2011, 33, 310–316. [Google Scholar] [CrossRef]
- El Ouaqoudi, F.Z.; El Fels, L.; Winterton, P.; Lemee, L.; Ambles, A.; Hafidi, M. Study of humic acids during composting of ligno-cellulose waste by Infra-red spectroscopic and thermogravimetric/thermal differential analysis. Compos. Sci. Util. 2014, 22, 188–198. [Google Scholar] [CrossRef]
- Volzone, C.; Zagorodny, N. Mercury intrusion porosimetry (MIP) study of archaeological pottery from Hualfin Valley, Catamarca, Argentina. Appl. Clay Sci. 2014, 91, 12–15. [Google Scholar] [CrossRef]
- Sakthivel, T.; Reid, D.L.; Goldstein, I.; Hench, L.; Seal, S. Hydrophobic high surface area zeolites derived from fly ash for oil spill remediation. Environ. Sci. Technol. 2013, 47, 5843–5850. [Google Scholar] [CrossRef] [PubMed]
- Radetic, M.; Ilic, V.; Radojevic, D.; Miladinovic, R.; Jocic, D.; Jovancic, P. Efficiency of recycled wool-based nonwoven material for the removal of oils from water. Chemosphere 2008, 70, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Drelich, J. Guidelines to measurements of reproducible contact angles using a sessile-drop technique. Surf. Innov. 2013, 1, 248–254. [Google Scholar] [CrossRef]
| Samples | Nitrogen Adsorption | Mercury Intrusion Porosimetry | |||
|---|---|---|---|---|---|
| Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Pore Diameter (nm) | Average Pore Diameter (nm) | Total Porosity (%) | |
| CA1 | 43 | 0.07 | 6.72 | 5968 | 88 |
| CA2 | 48 | 0.08 | 8.72 | 6185 | 89 |
| CA3 | 239 | 0.14 | 3.65 | 10,751 | 92 |
| CA4 | 249 | 0.16 | 2.55 | 16,192 | 94 |
| Hydrothermal Treatment | Carbonization | |
|---|---|---|
| Pyrolyzing Procedures (1) c | Pyrolyzing Procedures (2) d | |
| EH1 a | CA1 | CA2 |
| EH2 b | CA3 | CA4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, A.; Xu, F.; Zhang, X. Fabrication of Biomass-Derived Carbon Aerogels with High Adsorption of Oils and Organic Solvents: Effect of Hydrothermal and Post-Pyrolysis Processes. Materials 2016, 9, 758. https://doi.org/10.3390/ma9090758
Yin A, Xu F, Zhang X. Fabrication of Biomass-Derived Carbon Aerogels with High Adsorption of Oils and Organic Solvents: Effect of Hydrothermal and Post-Pyrolysis Processes. Materials. 2016; 9(9):758. https://doi.org/10.3390/ma9090758
Chicago/Turabian StyleYin, Aishu, Feng Xu, and Xueming Zhang. 2016. "Fabrication of Biomass-Derived Carbon Aerogels with High Adsorption of Oils and Organic Solvents: Effect of Hydrothermal and Post-Pyrolysis Processes" Materials 9, no. 9: 758. https://doi.org/10.3390/ma9090758
APA StyleYin, A., Xu, F., & Zhang, X. (2016). Fabrication of Biomass-Derived Carbon Aerogels with High Adsorption of Oils and Organic Solvents: Effect of Hydrothermal and Post-Pyrolysis Processes. Materials, 9(9), 758. https://doi.org/10.3390/ma9090758







