Study of Different Sol-Gel Coatings to Enhance the Lifetime of PDMS Devices: Evaluation of Their Biocompatibility
Abstract
:1. Introduction
2. Results
2.1. Fabrication of the Channel
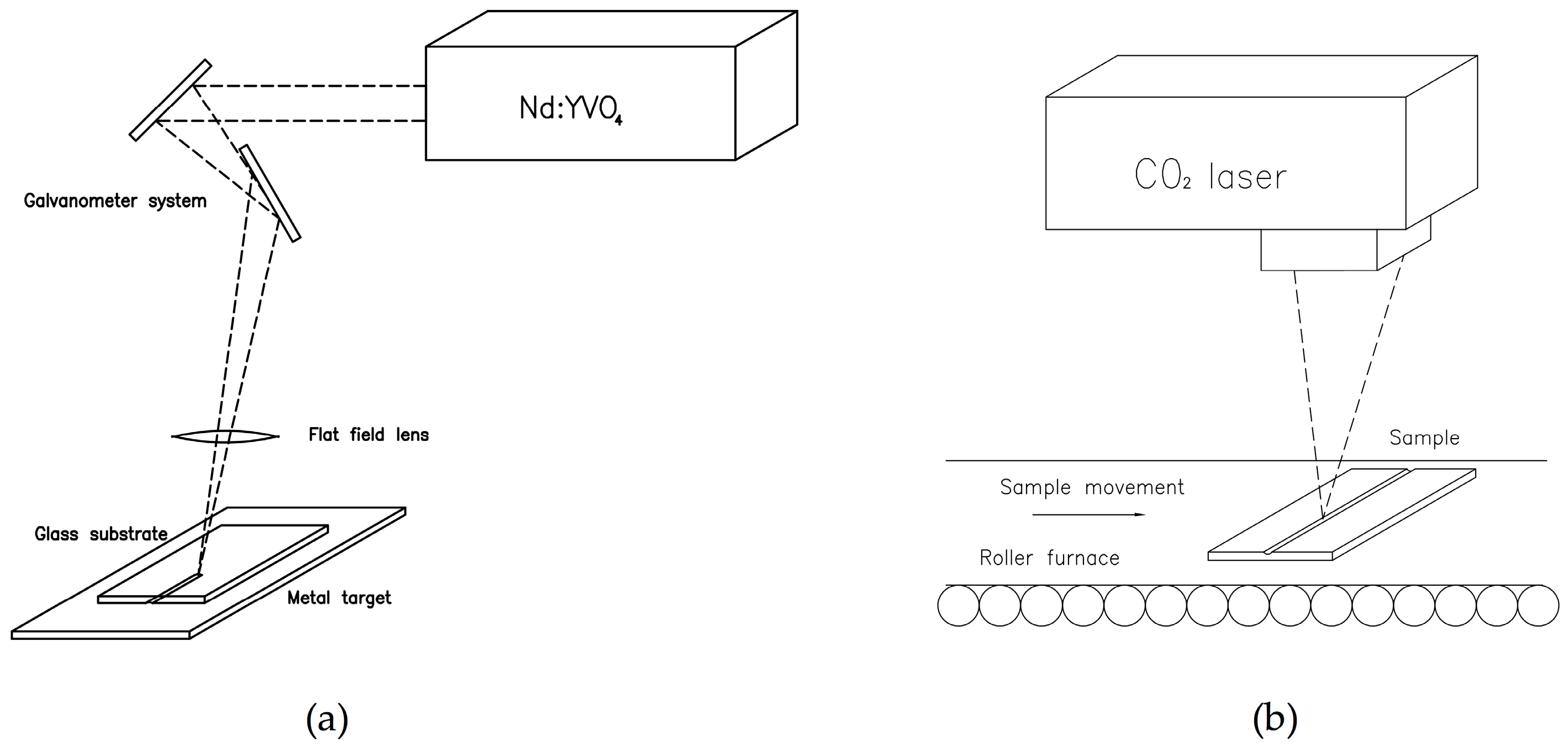
2.1.1. Master Fabrication with Laser Technologies
2.1.2. Master Replica Using Soft Lithography Technique
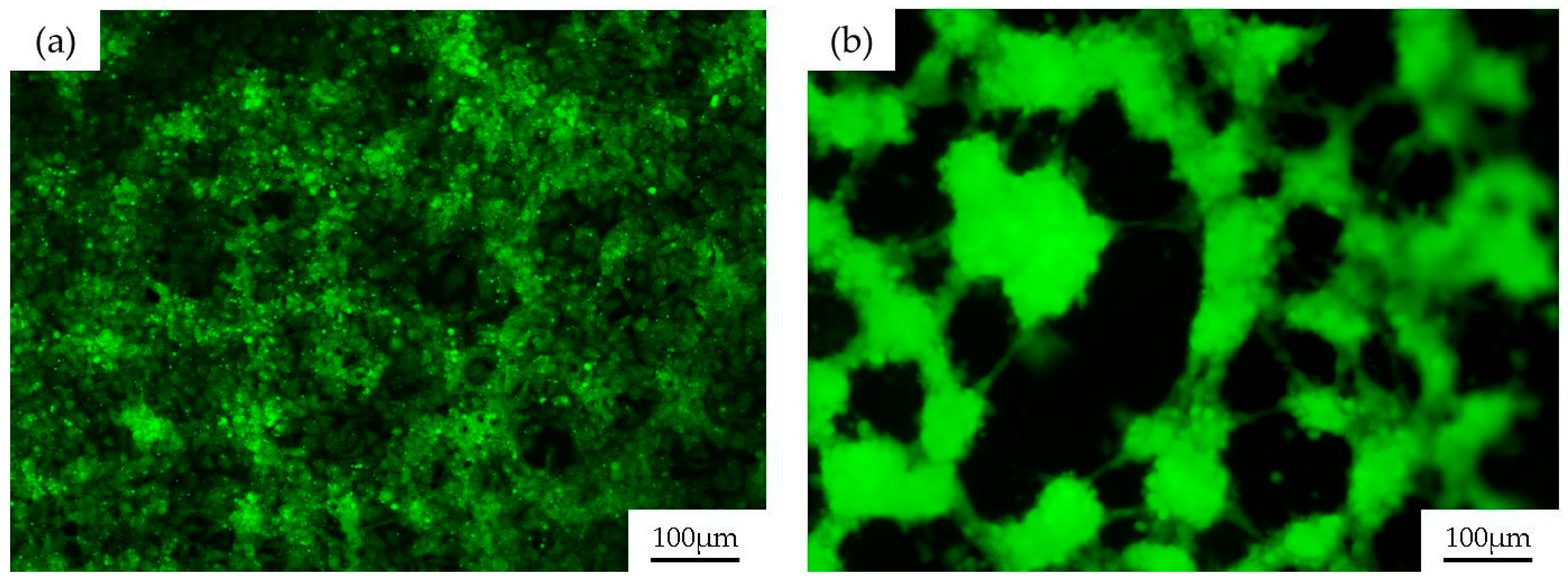
2.2. Endothelial Cell Seeding in PDMS Channels
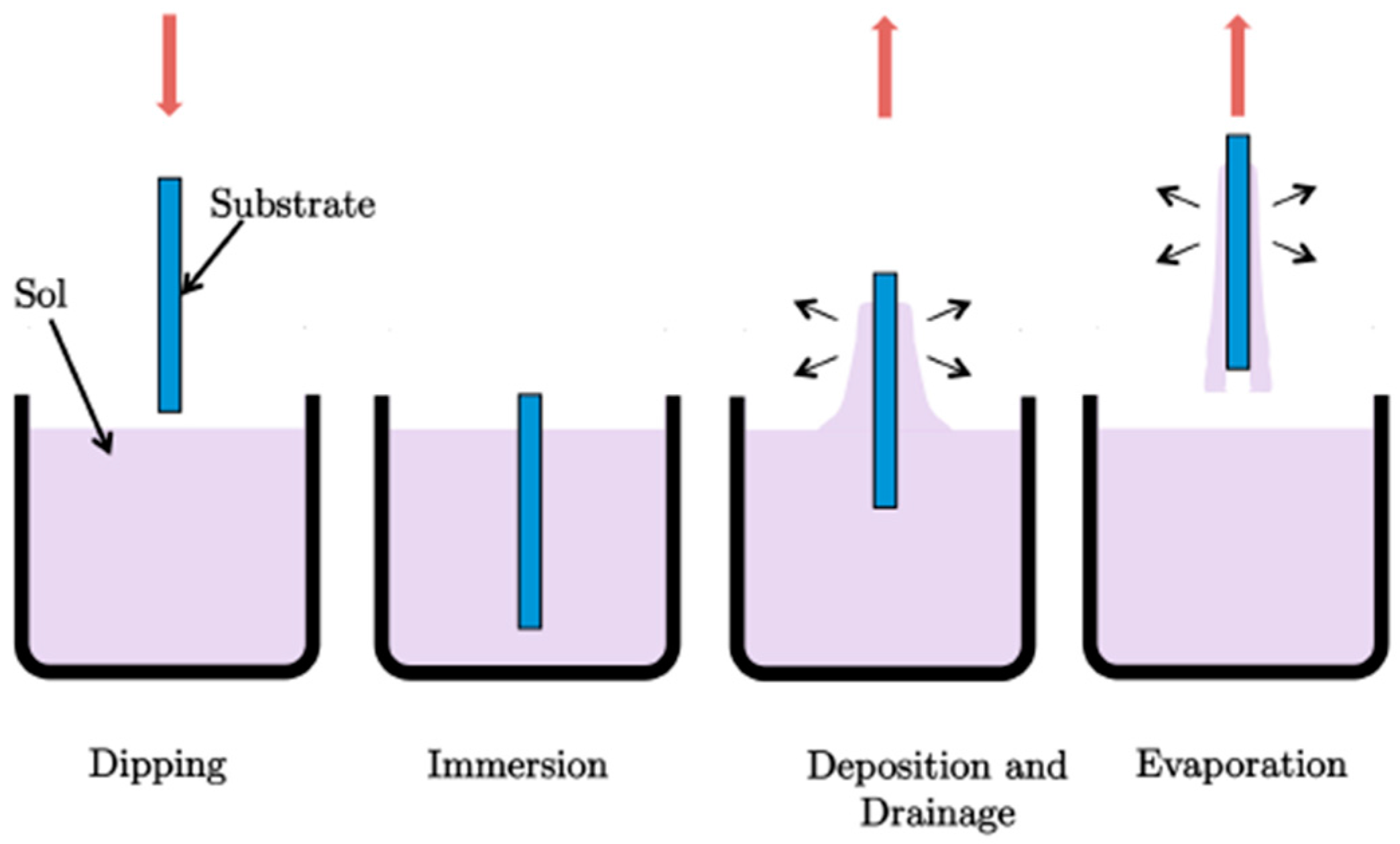
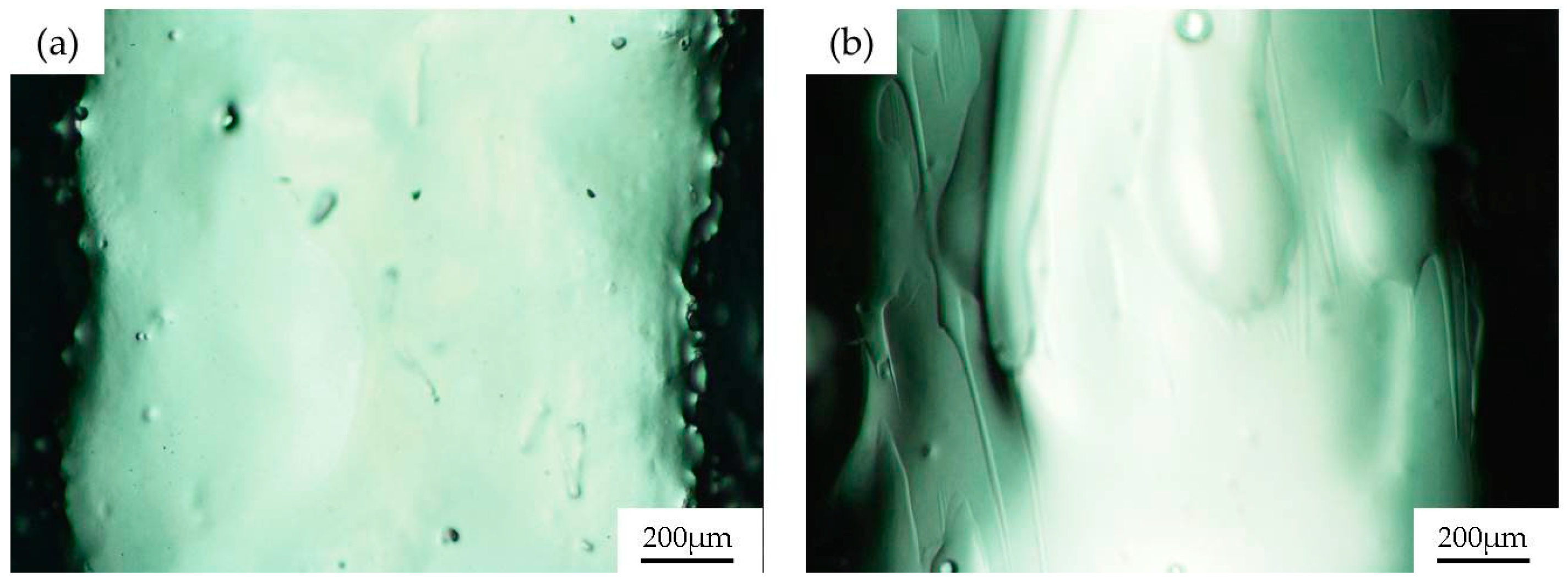
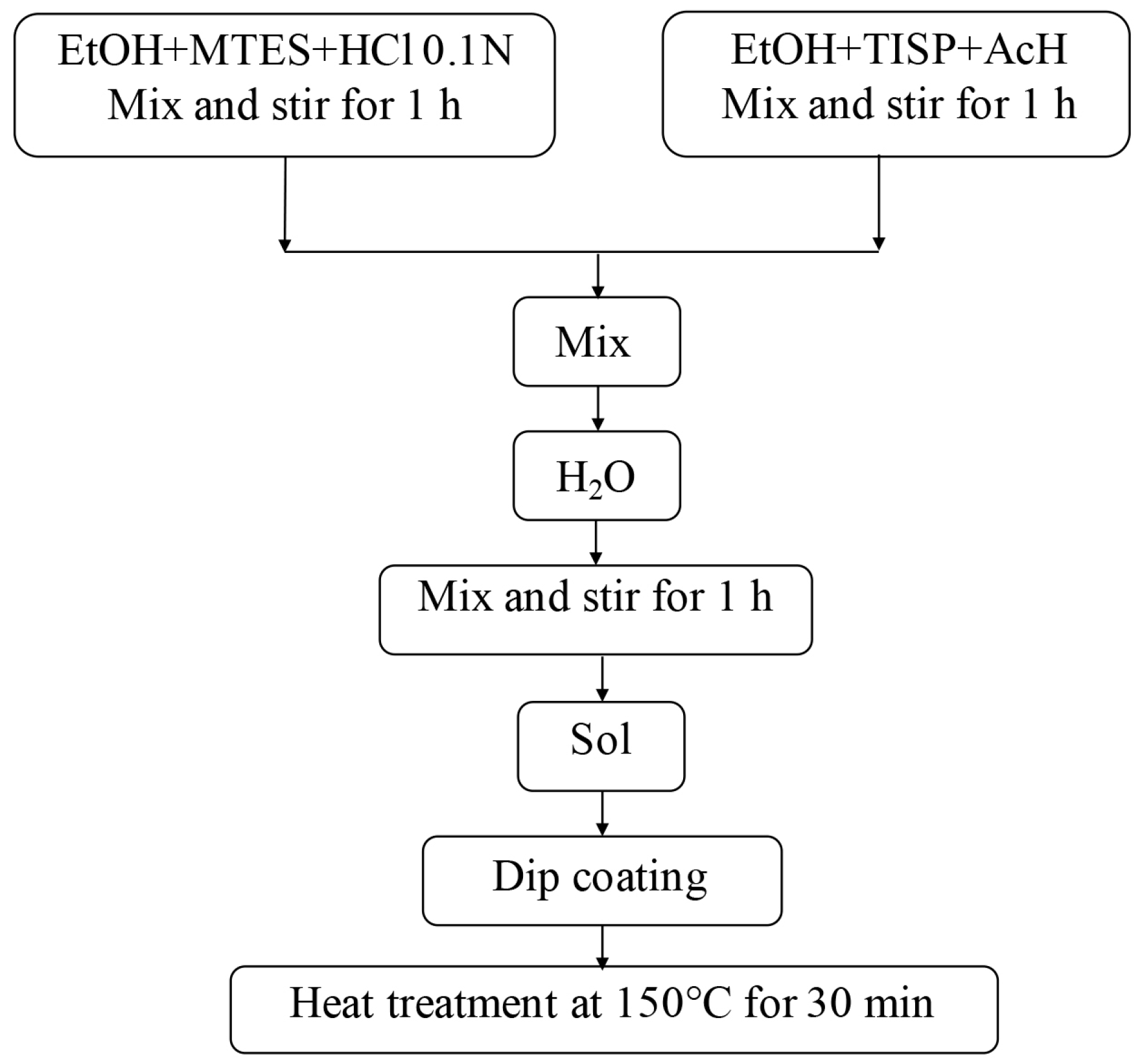
2.3. Sol-Gel Coatings
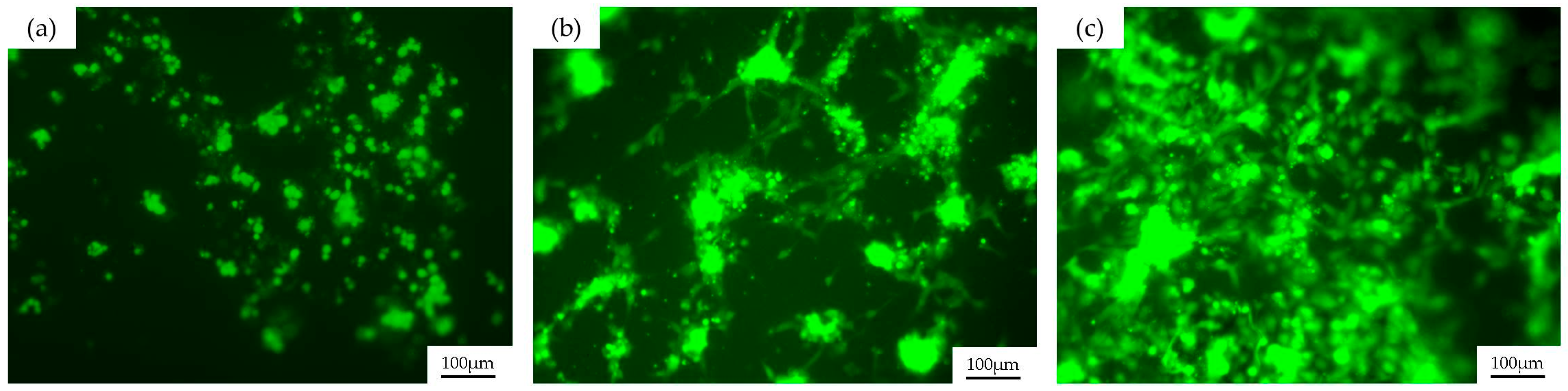
2.4. Biological Validation
3. Discussion
4. Materials and Methods
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ziober, B.L.; Mauk, M.G.; Falls, E.M.; Chen, Z.; Ziober, A.F.; Bau, H.H. Lab-on-a-chip for oral cancer screening and diagnosis. Head Neck 2008, 30, 111–121. [Google Scholar]
- Fiddes, L.K.; Raz, N.; Srigunapalan, S.; Tumarkan, E.; Simmons, C.A.; Wheeler, A.R.; Kumacheva, E. A circular cross-section PDMS microfluidics system for replication of cardiovascular flow conditions. Biomaterials 2010, 31, 3459–3464. [Google Scholar]
- Van der Meer, A.D.; Orlova, V.V.; ten Dijke, P.; van den Berg, A.; Mummery, C.L. Three-dimensional co-cultures of human endothelial cells and embryonic stem cell-derived pericytes inside a microfluidic device. Lab Chip 2013, 13, 3562–3568. [Google Scholar]
- Cui, J.; Björnmalm, M.; Liang, K.; Xu, C.; Best, J.P.; Zhang, X.; Caruso, F. Super-Soft Hydrogel Particles with Tunable Elasticity in a Microfluidic Blood Capillary Model. Adv. Mater. 2014, 26, 7295–7299. [Google Scholar]
- Hasan, A.; Paul, A.; Memic, A.; Khademhosseini, A. A multilayered microfluidic blood vessel-like structure. Biomed. Microdevices 2015, 17, 1–13. [Google Scholar]
- Rezvan, A.; Ni, C.W.; Alberts-Grill, N.; Jo, H. Animal, in vitro, and ex vivo models of flow-dependent atherosclerosis: Role of oxidative stress. Antioxid. Redox Sign. 2011, 15, 1433–1448. [Google Scholar]
- Hongbin, Y.; Guangya, Z.; Siong, C.F.; Shouhua, W.; Feiwen, L. Novel polydimethylsiloxane (PDMS) based microchannel fabrication method for lab-on-a-chip application. Sens. Actuators B 2009, 137, 754–761. [Google Scholar]
- Xu, B.B.; Zhang, Y.L.; Xia, H.; Dong, W.F.; Ding, H.; Sun, H.B. Fabrication and multifunction integration of microfluidic chips by femtosecond laser direct writing. Lab Chip 2013, 13, 1677–1690. [Google Scholar]
- Stjernström, M.; Roeraade, J. Method for fabrication of microfluidic systems in glass. J. Micromech. Microeng. 1998, 8, 33. [Google Scholar] [CrossRef]
- Carlen, E.T.; Bomer, J.G.; van Nieuwkasteele, J.W.; van den Berg, A. Silicon and Glass Micromachining. In Lab-on-a-Chip Technology for Biomedical and Biological Applications. Volume 1: Fabrication and Microfluidics; Herold, K.E., Rasooly, A., Eds.; Caister Academic Press: Norfolk, UK, 2009; pp. 83–114. [Google Scholar]
- Tsao, C.W.; DeVoe, D.L. Bonding of thermoplastic polymer microfluidics. Microfluid. Nanofluid. 2009, 6, 1–16. [Google Scholar]
- Wu, H.; Odom, T.W.; Chiu, D.T.; Whitesides, G.M. Fabrication of complex three-dimensional microchannel systems in PDMS. J. Am. Chem. Soc. 2003, 125, 554–559. [Google Scholar]
- Lee, S.H. Microtechnology to Fabricate Lab-on-a-Chip for Biology Applications. In Lab-on-a-Chip Technology for Biomedical and Biological Applications. Volume 1: Fabrication and Microfluidics; Herold, K.E., Rasooly, A., Eds.; Caister Academic Press: Norfolk, UK, 2009; pp. 125–138. [Google Scholar]
- Waddell, E.A.; Locascio, L.E.; Kramer, G.W. UV laser micromachining of polymers for microfluidic applications. J. Assoc. Lab. Autom. 2002, 7, 78–82. [Google Scholar]
- Anderson, J.R.; Chiu, D.T.; Jackman, R.J.; Cherniavskaya, O.; McDonald, J.C.; Wu, H.; Whitesides, S.H.; Whitesides, G.M. Fabrication of topologically complex three-dimensional microfluidic systems in PDMS by rapid prototyping. Anal. Chem. 2000, 72, 3158–3164. [Google Scholar]
- McDonald, J.C.; Whitesides, G.M. Poly (dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar]
- Bartholomeusz, D.A.; Boutté, R.W.; Andrade, J.D. Xurography: Rapid prototyping of microstructures using a cutting plotter. J. Microelectromech. 2005, 14, 1364–1374. [Google Scholar]
- Li, C.W.; Cheung, C.N.; Yang, J.; Tzang, C.H.; Yang, M. PDMS-based microfluidic device with multi-height structures fabricated by single-step photolithography using printed circuit board as masters. Analyst 2003, 128, 1137–1142. [Google Scholar]
- Etsion, I. State of the art in laser surface texturing. J. Tribol. 2005, 127, 248–253. [Google Scholar]
- Nieto, D.; Flores-Arias, M.T.; O’Connor, G.M.; Gomez-Reino, C. Laser direct-write technique for fabricating microlens arrays on soda-lime glass with a Nd: YVO 4 laser. Appl. Opt. 2010, 49, 4979–4983. [Google Scholar]
- Roman, G.T.; Hlaus, T.; Bass, K.J.; Seelhammer, T.G.; Culbertson, C.T. Sol-gel modified poly (dimethylsiloxane) microfluidic devices with high electroosmotic mobilities and hydrophilic channel wall characteristics. Anal. Chem. 2005, 77, 1414–1422. [Google Scholar]
- Abate, A.R.; Lee, D.; Do, T.; Holtze, C.; Weitz, D.A. Glass coating for PDMS microfluidic channels by sol-gel methods. Lab Chip 2008, 8, 516–518. [Google Scholar]
- Hench, L.L.; West, J.K. The Sol-Gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar]
- Rey-García, F.; Gómez-Reino, C.; Flores-Arias, M.T.; De La Fuente, G.F.; Durán, A.; Castro, Y. Sol-gel coatings: An alternative route for producing planar optical waveguides. Thin Solid Films 2011, 519, 7982–7986. [Google Scholar]
- Gómez-Varela, A.I.; Castro, Y.; Durán, A.; De Beule, P.A.A.; Flores-Arias, M.T.; Bao-Varela, C. Synthesis and characterization of erbium-doped SiO2-TiO2 thin films prepared by sol-gel and dip-coating techniques onto commercial glass substrates as a route for obtaining active gradient-index materials. Thin Solid Films 2015, 583, 115–121. [Google Scholar]
- Roman, G.T.; Culbertson, C.T. Surface engineering of poly (dimethylsiloxane) microfluidic devices using transition metal sol-gel chemistry. Langmuir 2006, 22, 4445–4451. [Google Scholar] [PubMed]
- Gomez-Sjoberg, R.; Leyrat, A.A.; Houseman, B.T.; Shokat, K.; Quake, S.R. Biocompatibility and Reduced Drug Absorption of Sol− Gel-Treated Poly (dimethyl siloxane) for Microfluidic Cell Culture Applications. Anal. Chem. 2010, 82, 8954–8960. [Google Scholar]
- Castelo, A.; Nieto, D.; Bao, C.; Flores-Arias, M.T.; Pérez, M.V.; Gómez-Reino, C.; López-Gascón, C.; De La Fuente, G.F. Laser backwriting process on glass via ablation of metal targets. Opt. Commun. 2007, 273, 193–199. [Google Scholar]
- Estepa, L.C.; De la Fuente, G.F. Continuos Furnace with Coupled Laser for the Surface Treatment of Materials. U.S. Patent 20090230105 A1, 7 March 2006. [Google Scholar]
- Ross, A.M.; Jiang, Z.; Bastmeyer, M.; Lahann, J. Physical aspects of cell culture substrates: Topography, roughness, and elasticity. Small 2012, 8, 336–355. [Google Scholar]
- Rodiño-Janeiro, B.K.; González-Peteiro, M.; Ucieda-Somoza, R.; González-Juanatey, J.R.; Álvarez, E. Glycated albumin, a precursor of advanced glycation end-products, up-regulates NADPH oxidase and enhances oxidative stress in human endothelial cells: Molecular correlate of diabetic vasculopathy. Diabetes-Metab. Res. Rev. 2010, 26, 550–558. [Google Scholar]


| Composition | Molar Ratio |
|---|---|
| MTES/TISP | 70/30 |
| H2O/Alcoxides | 1.5 |
| Alkoxides/AcH | 1 |
| Composition | Molar Ratio |
|---|---|
| MTES/TISP | 80/20 |
| H2O/Alcoxides | 1.5 |
| Alkoxides/AcH | 1 |
| Composition | Molar Ratio |
|---|---|
| MTES/TEOS | 60/40 |
| H2O/Alcoxides | 1.8 |
| Alkoxides/AcH | 4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aymerich, M.; Gómez-Varela, A.I.; Álvarez, E.; Flores-Arias, M.T. Study of Different Sol-Gel Coatings to Enhance the Lifetime of PDMS Devices: Evaluation of Their Biocompatibility. Materials 2016, 9, 728. https://doi.org/10.3390/ma9090728
Aymerich M, Gómez-Varela AI, Álvarez E, Flores-Arias MT. Study of Different Sol-Gel Coatings to Enhance the Lifetime of PDMS Devices: Evaluation of Their Biocompatibility. Materials. 2016; 9(9):728. https://doi.org/10.3390/ma9090728
Chicago/Turabian StyleAymerich, María, Ana I. Gómez-Varela, Ezequiel Álvarez, and María T. Flores-Arias. 2016. "Study of Different Sol-Gel Coatings to Enhance the Lifetime of PDMS Devices: Evaluation of Their Biocompatibility" Materials 9, no. 9: 728. https://doi.org/10.3390/ma9090728
APA StyleAymerich, M., Gómez-Varela, A. I., Álvarez, E., & Flores-Arias, M. T. (2016). Study of Different Sol-Gel Coatings to Enhance the Lifetime of PDMS Devices: Evaluation of Their Biocompatibility. Materials, 9(9), 728. https://doi.org/10.3390/ma9090728







