Template-Free Synthesis of Monoclinic BiVO4 with Porous Structure and Its High Photocatalytic Activity
Abstract
:1. Introduction
2. Experimental
2.1. Experimental Materials
2.2. Synthesis
2.3. Characterization
2.4. Evaluation of Photocatalytic Activity
3. Results and Discussion
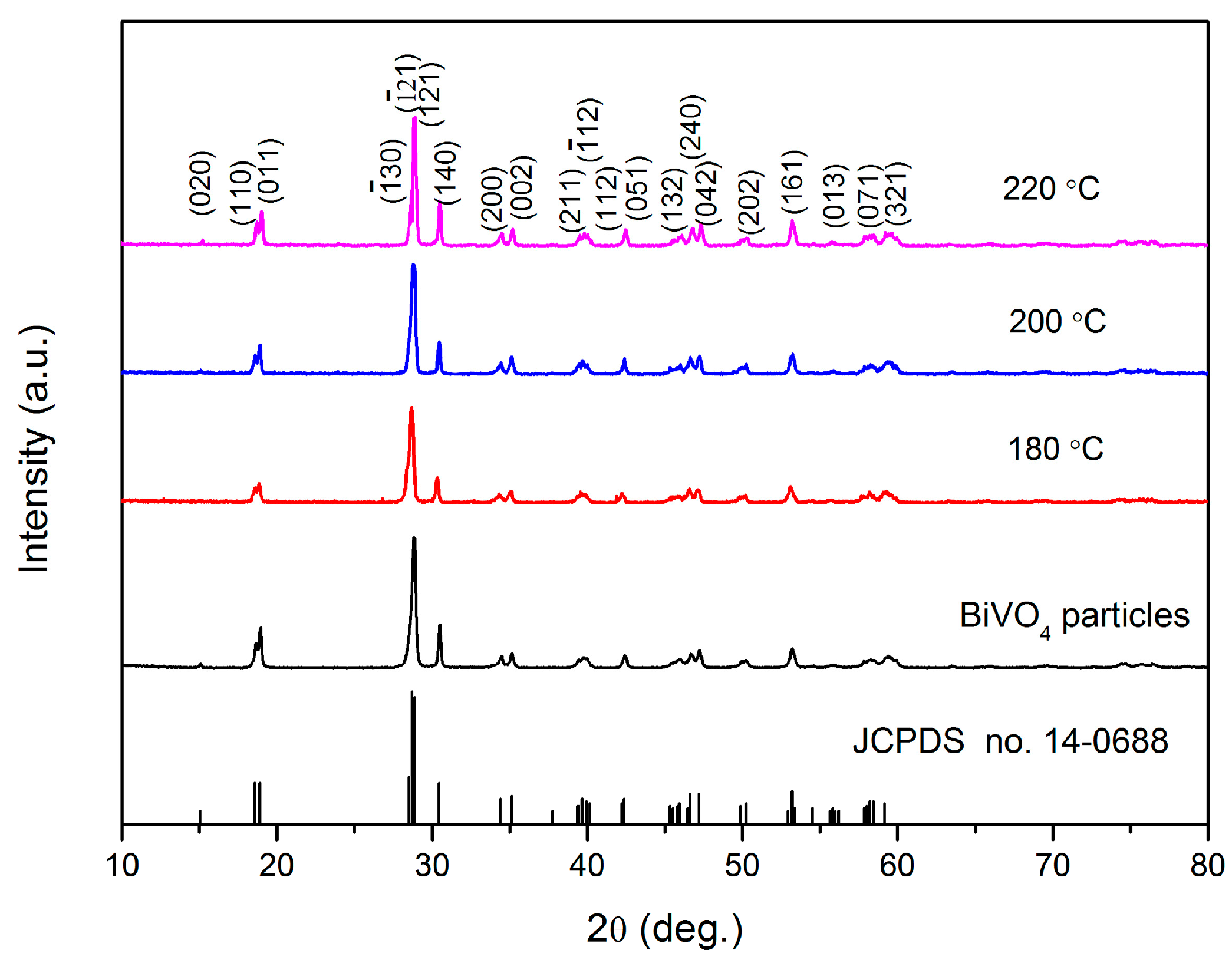
3.1. XRD Analysis
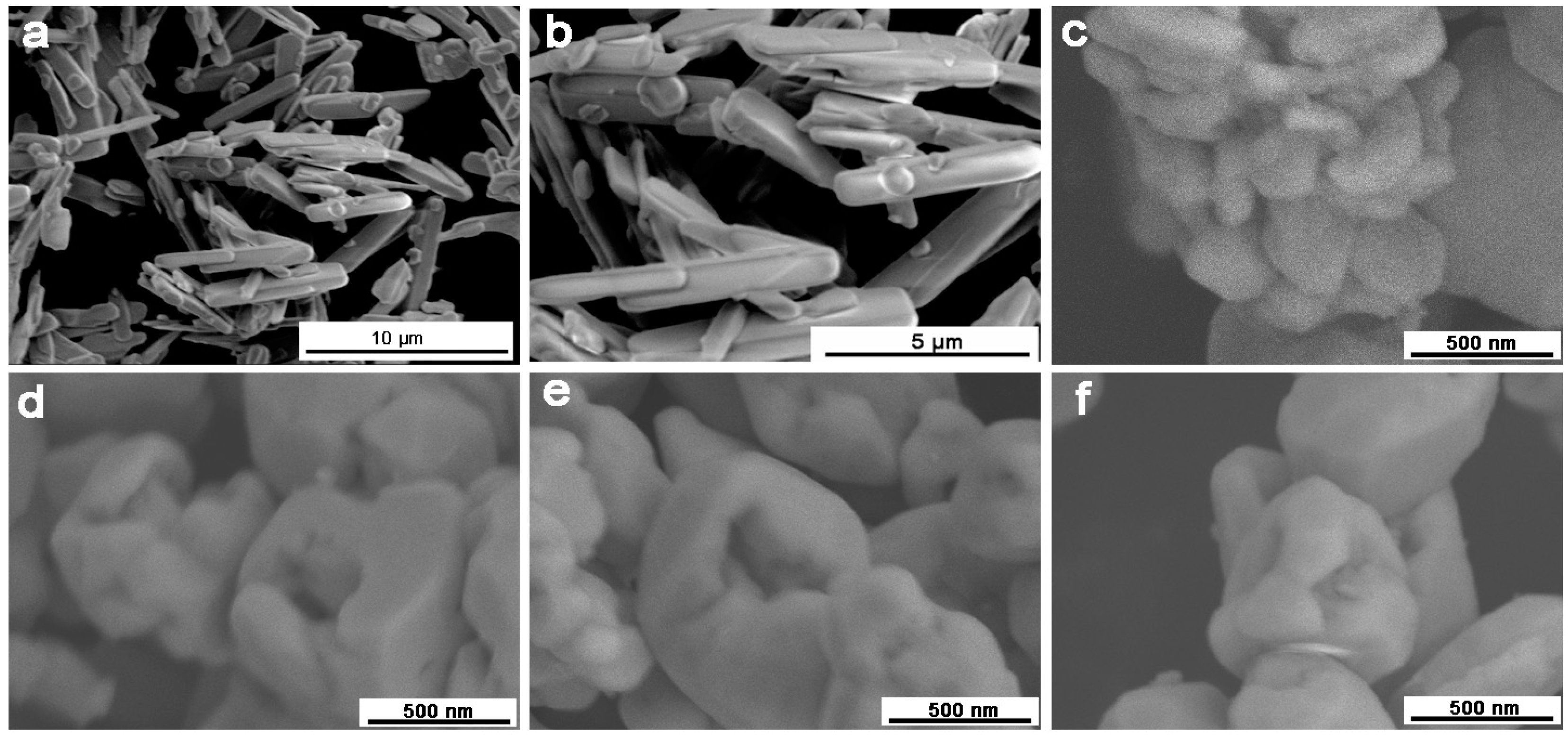
3.2. SEM Analysis
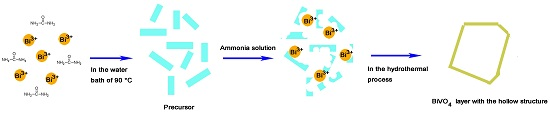
3.3. Formation Mechanism
3.4. Nitrogen Adsorption–Desorption
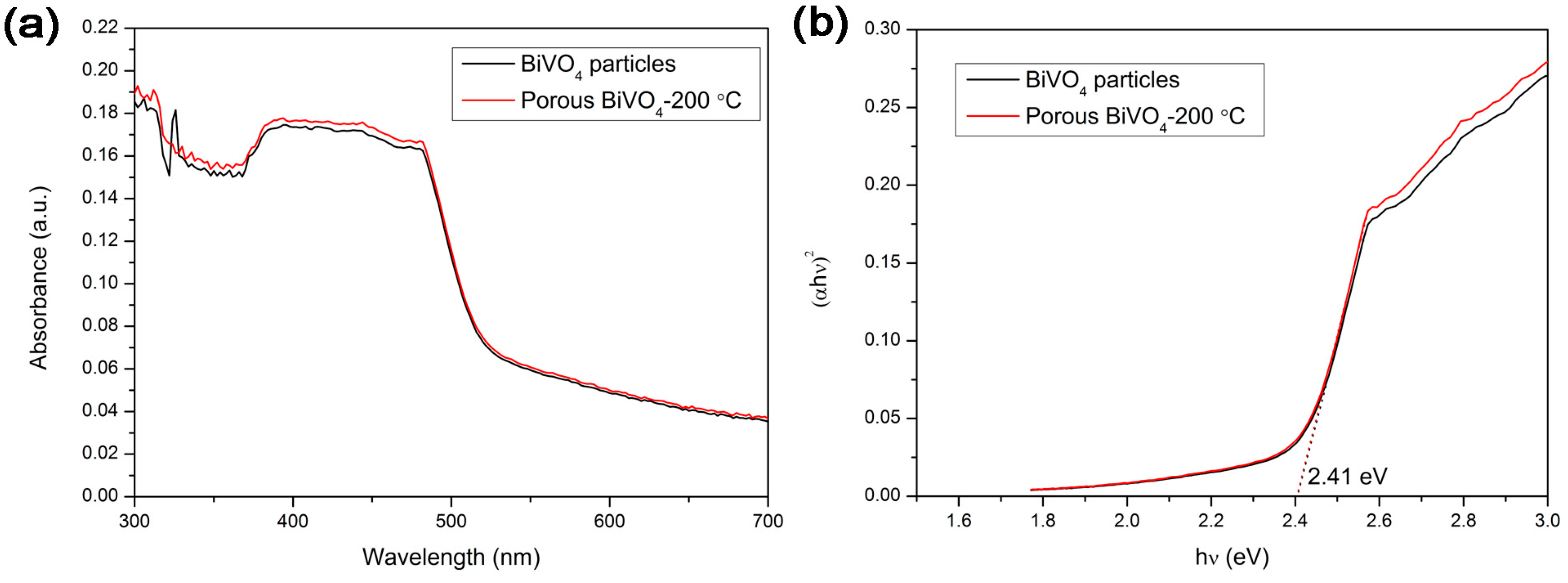
3.5. UV-Vis Diffuse Reflectance Spectroscopy
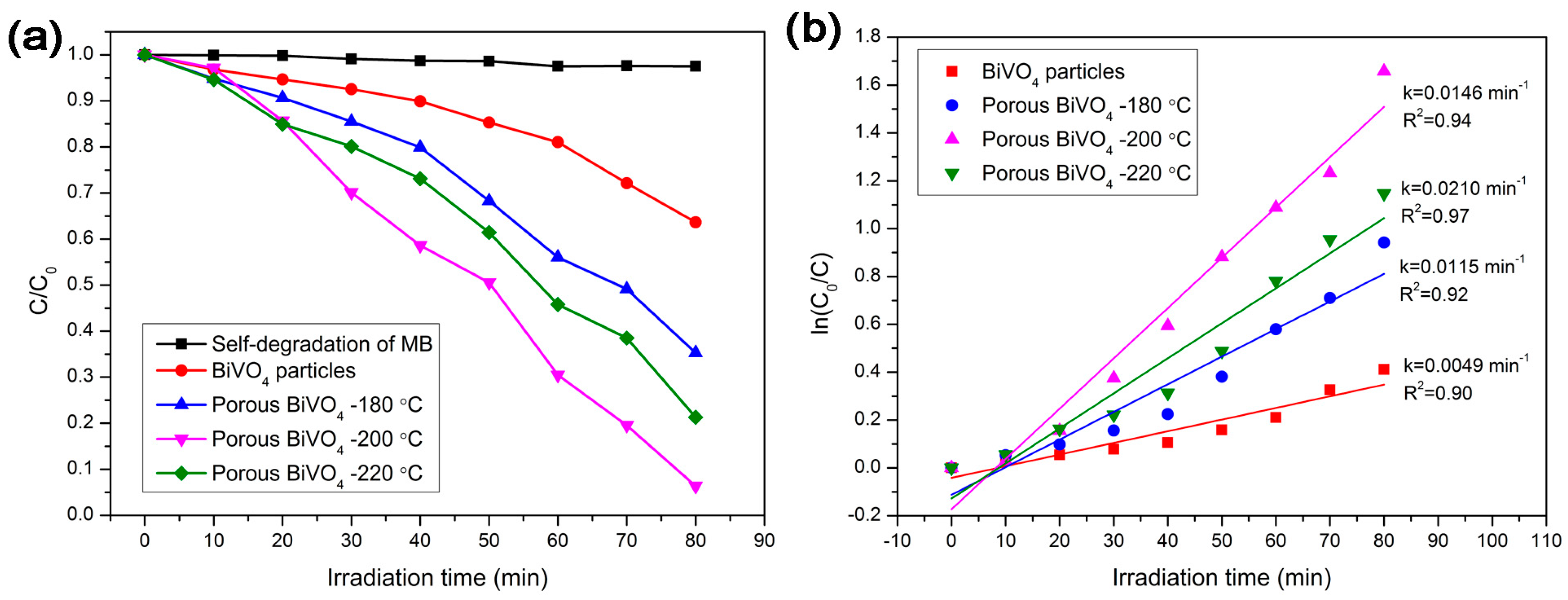
3.6. Photocatalytic Activity and Electrochemical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C 2012, 23, 169–189. [Google Scholar] [CrossRef]
- Li, R.; Zhang, F.; Wang, D.; Yang, J.; Li, M.; Zhu, J.; Zhou, X.; Han, H.; Li, C. Spatial separation of photogenerated electrons and holes among {010} and {110} crystal facets of BiVO4. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zhou, P.; Yu, J. Recent advances in visible light Bi-based photocatalysts. Chin. J. Catal. 2014, 35, 989–1007. [Google Scholar]
- Qu, Z.; Liu, P.; Yang, X.; Wang, F.; Zhang, W.; Fei, C. Microstructure and Characteristic of BiVO4 Prepared under Different pH Values: Photocatalytic Efficiency and Antibacterial Activity. Materials 2016, 9. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.S. BiVO4-Based Heterostructured Photocatalysts for Solar Water Splitting: A Review. Energy Environ. Focus 2014, 3, 339–353. [Google Scholar]
- Tokunaga, S.; Kato, H.; Kudo, A. Selective preparation of monoclinic and tetragonal BiVO4 with scheelite structure and their photocatalytic properties. Chem. Mater. 2001, 13, 4624–4628. [Google Scholar] [CrossRef]
- Zhang, X.; Ai, Z.; Jia, F.; Zhang, L.; Fan, X.; Zou, Z. Selective synthesis and visible-light photocatalytic activities of BiVO4 with different crystalline phases. Mater. Chem. Phys. 2007, 103, 162–167. [Google Scholar] [CrossRef]
- Yin, W.; Wang, W.; Zhou, L.; Sun, S.; Zhang, L. CTAB-assisted synthesis of monoclinic BiVO4 photocatalyst and its highly efficient degradation of organic dye under visible-light irradiation. J. Hazard. Mater. 2010, 173, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Omori, K.; Kato, H. A novel aqueous process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Ge, L. Novel visible-light-driven Pt/BiVO4 photocatalyst for efficient degradation of methyl orange. J. Mol. Catal. A Chem. 2008, 282, 62–66. [Google Scholar] [CrossRef]
- Jiang, H.; Meng, X.; Dai, H.; Deng, J.; Liu, Y.; Zhang, L.; Zhao, Z.; Zhang, R. High-performance porous spherical or octapod-like single-crystalline BiVO4 photocatalysts for the removal of phenol and methylene blue under visible-light illumination. J. Hazard. Mater. 2012, 217–218, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, D.; Zheng, Y.; Chen, W.; He, Y.; Shao, Y.; Fu, X.; Xiao, G. BiVO4/TiO2 nanocrystalline heterostructure: A wide spectrum responsive photocatalyst towards the highly efficient decomposition of gaseous benzene. Appl. Catal. B 2011, 104, 30–36. [Google Scholar] [CrossRef]
- Kohtani, S.; Koshiko, M.; Kudo, A.; Tokumura, K.; Ishigaki, Y.; Toriba, A.; Hayakawa, K.; Nakagaki, R. Photodegradation of 4-alkylphenols using BiVO4 photocatalyst under irradiation with visible light from a solar simulator. Appl. Catal. B 2003, 46, 573–586. [Google Scholar] [CrossRef]
- Kohtani, S.; Hiro, J.; Yamamoto, N.; Kudo, A.; Tokumura, K.; Nakagaki, R. Adsorptive and photocatalytic properties of Ag-loaded BiVO4 on the degradation of 4-n-alkylphenols under visible light irradiation. Catal. Commun. 2005, 6, 185–189. [Google Scholar] [CrossRef]
- Shang, M.; Wang, W.; Zhou, L.; Sun, S.; Yin, W. Nanosized BiVO4 with high visible-light-induced photocatalytic activity: Ultrasonic-assisted synthesis and protective effect of surfactant. J. Hazard. Mater. 2009, 172, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, W.; Liu, S.; Zhang, L.; Xu, H.; Zhu, W. A sonochemical route to visible-light-driven high-activity BiVO4 photocatalyst. J. Mol. Catal. A Chem. 2006, 252, 120–124. [Google Scholar] [CrossRef]
- Zhang, H.M.; Liu, J.B.; Wang, H.; Zhang, W.X.; Yan, H. Rapid microwave-assisted synthesis of phase controlled BiVO4 nanocrystals and research on photocatalytic properties under visible light irradiation. J. Nanopart. Res. 2008, 10, 767–774. [Google Scholar] [CrossRef]
- Eda, S.-I.; Fujishima, M.; Tada, H. Low temperature-synthesis of BiVO4 nanorods using polyethylene glycol as a soft template and the visible-light-activity for copper acetylacetonate decomposition. Appl. Catal. B 2012, 125, 288–293. [Google Scholar] [CrossRef]
- Ren, L.; Jin, L.; Wang, J.B.; Yang, F.; Qiu, M.Q.; Yu, Y. Template-free synthesis of BiVO4 nanostructures: I. Nanotubes with hexagonal cross sections by oriented attachment and their photocatalytic property for water splitting under visible light. Nanotechnology 2009, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, D.; Jiao, X. Monoclinic structured BiVO4 nanosheets: Hydrothermal preparation, formation mechanism, and coloristic and photocatalytic properties. J. Phys. Chem. B 2006, 110, 2668–2673. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Wang, W.; Ren, J.; Sun, S.; Zhang, L. A novel BiVO4 hierarchical nanostructure: Controllable synthesis, growth mechanism, and application in photocatalysis. CrystEngComm 2010, 12, 1754–1758. [Google Scholar] [CrossRef]
- Zhang, X.; Du, L.; Wang, H.; Dong, X.; Zhang, X.; Ma, C.; Ma, H. Highly ordered mesoporous BiVO4: Controllable ordering degree and super photocatalytic ability under visible light. Microporous Mesoporous Mater. 2013, 173, 175–180. [Google Scholar] [CrossRef]
- Ge, M.; Liu, L.; Chen, W.; Zhou, Z. Sunlight-driven degradation of Rhodamine B by peanut-shaped porous BiVO4 nanostructures in the H2O2-containing system. CrystEngComm 2012, 14, 1038–1044. [Google Scholar] [CrossRef]
- Liu, W.; Cao, L.; Su, G.; Liu, H.; Wang, X.; Zhang, L. Ultrasound assisted synthesis of monoclinic structured spindle BiVO4 particles with hollow structure and its photocatalytic property. Ultrason. Sonochem. 2010, 17, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, D.; Yu, J.C. Ordered Mesoporous BiVO4 through Nanocasting: A Superior Visible Light-Driven Photocatalyst. Chem. Mater. 2008, 20, 3983–3992. [Google Scholar] [CrossRef]
- Yao, M.; Liu, M.; Gan, L.; Zhao, F.; Fan, X.; Zhu, D.; Xu, Z.; Hao, Z.; Chen, L. Monoclinic mesoporous BiVO4: Synthesis and visible-light-driven photocatalytic property. Colloids Surf. A Physicochem. Eng. Aspects 2013, 433, 132–138. [Google Scholar] [CrossRef]
- Yin, W.; Wang, W.; Shang, M.; Zhou, L.; Sun, S.; Wang, L. BiVO4 Hollow Nanospheres: Anchoring Synthesis, Growth Mechanism, and Their Application in Photocatalysis. Eur. J. Inorg. Chem. 2009, 2009, 4379–4384. [Google Scholar] [CrossRef]
- Jiang, H.; Dai, H.; Meng, X.; Ji, K.; Zhang, L.; Deng, J. Porous olive-like BiVO4: Alcoho-hydrothermal preparation and excellent visible-light-driven photocatalytic performance for the degradation of phenol. Appl. Catal. B 2011, 105, 326–334. [Google Scholar] [CrossRef]
- Ying, Y.; Tao, F.; Hong, T.; Wang, L. Controlled fabrication of bismuth vanadium oxide hierarchical microtubes with enhanced visible light photocatalytic activity. Mater. Sci. Semicond. Process. 2015, 32, 82–89. [Google Scholar] [CrossRef]
- Ma, Z.Y.; Yao, B.H. Preparation of BiVO4 Hollow Spheres and its Photocatalytic Degradation of Methylene Blue. Chin. J. Chem. Phys. 2013, 26, 341–346. [Google Scholar] [CrossRef]
- Cheng, B.; Wang, W.; Shi, L.; Zhang, J.; Ran, J.; Yu, H. One-Pot Template-Free Hydrothermal Synthesis of Monoclinic BiVO4 Hollow Microspheres and Their Enhanced Visible-Light Photocatalytic Activity. Int. J. Photoenergy 2012, 2012. [Google Scholar] [CrossRef]
- Li, F.; Yang, C.; Li, Q.; Cao, W.; Li, T. The pH-controlled morphology transition of BiVO4 photocatalysts from microparticles to hollow microspheres. Mater. Lett. 2015, 145, 52–55. [Google Scholar] [CrossRef]
- Yin, Y.; Erdonmez, C.K.; Cabot, A.; Hughes, S.; Alivisatos, A.P. Colloidal Synthesis of Hollow Cobalt Sulfide Nanocrystals. Adv. Funct. Mater. 2006, 16, 1389–1399. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Xu, Z.; Zhang, H.; Dong, P.; Guo, L.; Li, F.; Xin, S.; Zeng, W. Preparation and drug-delivery properties of hollow YVO4:Ln3+ and mesoporous YVO4:Ln3+@nSiO2@mSiO2 (Ln = Eu, Yb, Er, and Ho). J. Mater. Chem. B 2013, 1, 330–338. [Google Scholar] [CrossRef]
- Greg, S.; Sing, K. Adsorption, Surface Area and Porosity; Academic Press: London, UK, 1982. [Google Scholar]
- Bavykin, D.; Parmon, V.; Lapkin, A.; Walsh, F. The effect of hydrothermal conditions on the mesoporous structure of TiO2 nanotubes. J. Mater. Chem. 2004, 14, 3370–3377. [Google Scholar] [CrossRef]
- Butler, M.A. Photoelectrolysis and physical properties of the semiconducting electrode WO2. J. Appl. Phys. 1977, 48, 1914–1920. [Google Scholar] [CrossRef]
- Sun, S.; Wang, W.; Zhou, L.; Xu, H. Efficient Methylene Blue Removal over Hydrothermally Synthesized Starlike BiVO4. Ind. Eng. Chem. Res. 2009, 48, 1735–1739. [Google Scholar] [CrossRef]
- Chen, L.; Yin, S.-F.; Huang, R.; Zhang, Q.; Luo, S.-L.; Au, C.-T. Hollow peanut-like m-BiVO4: Facile synthesis and solar-light-induced photocatalytic property. CrystEngComm 2012, 14, 4217–4222. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic Materials: Possibilities and Challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hao, H.; Zhu, Z. Construction of g-C3N4-WO3-Bi2WO6 double Z-scheme system with enhanced photoelectrochemical performance. Mater. Lett. 2016, 168, 180–183. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Wang, Y.; Yao, W.; Zhu, Y. Enhanced oxidation ability of g-C3N4 photocatalyst via C60 modification. Appl. Catal. B 2014, 152–153, 262–270. [Google Scholar] [CrossRef]
- Lv, Y.H.; Pan, C.S.; Ma, X.G.; Zong, R.L.; Bai, X.J.; Zhu, Y.F. Production of visible activity and UV performance enhancement of ZnO photocatalyst via vacuum deoxidation. Appl. Catal. B 2013, 138, 26–32. [Google Scholar] [CrossRef]

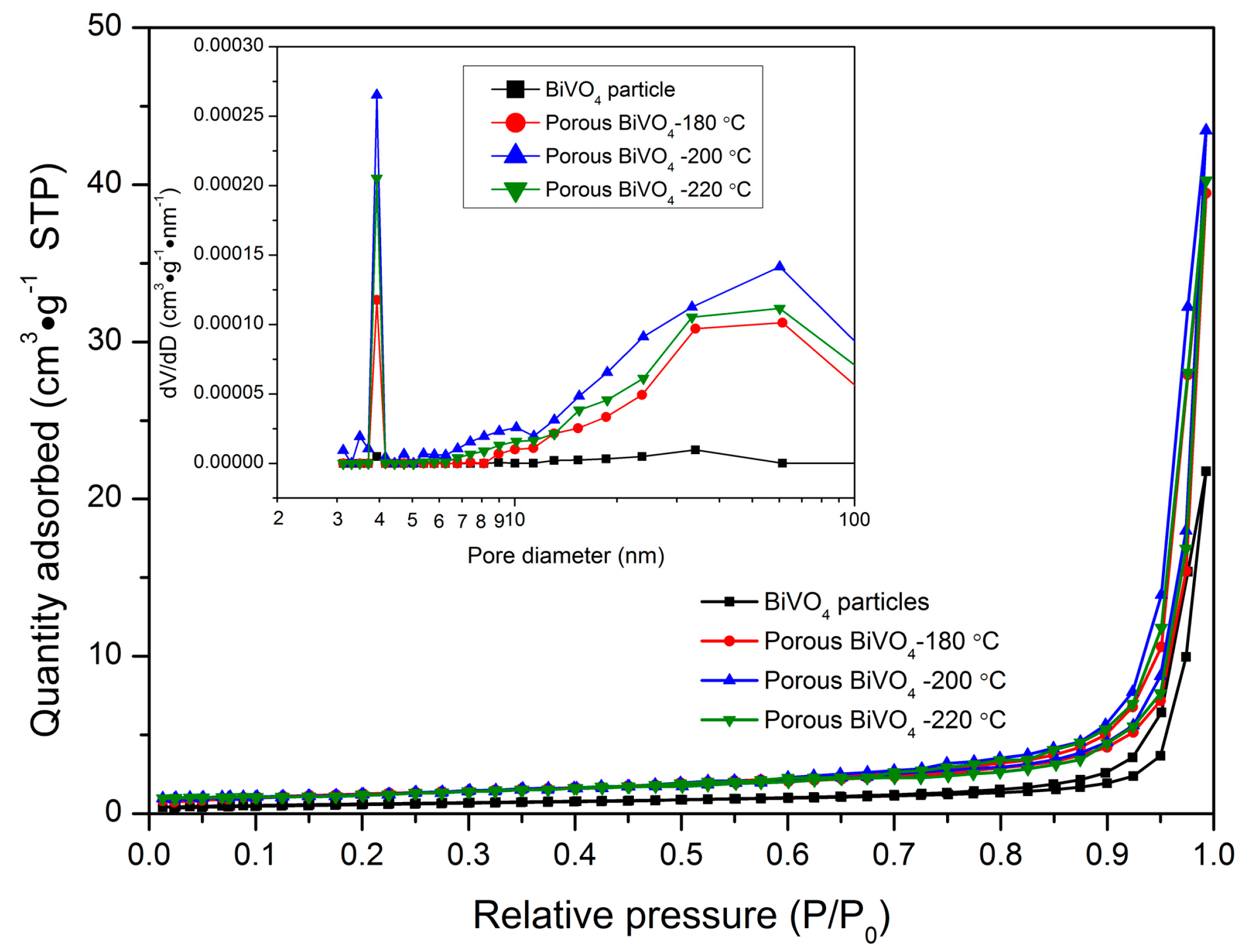

| Samples | BiVO4 Particles | Porous BiVO4-180 °C | Porous BiVO4-200 °C | Porous BiVO4-220 °C |
|---|---|---|---|---|
| BET specific surface area (m2/g) 1 | 1.85 | 4.42 | 5.69 | 4.83 |
| BJH pore volume (cm3/g) 2 | 0.023 | 0.055 | 0.072 | 0.061 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, P.; Xi, X.; Zhang, X.; Hou, G.; Guan, R. Template-Free Synthesis of Monoclinic BiVO4 with Porous Structure and Its High Photocatalytic Activity. Materials 2016, 9, 685. https://doi.org/10.3390/ma9080685
Dong P, Xi X, Zhang X, Hou G, Guan R. Template-Free Synthesis of Monoclinic BiVO4 with Porous Structure and Its High Photocatalytic Activity. Materials. 2016; 9(8):685. https://doi.org/10.3390/ma9080685
Chicago/Turabian StyleDong, Pengyu, Xinguo Xi, Xinjiang Zhang, Guihua Hou, and Rongfeng Guan. 2016. "Template-Free Synthesis of Monoclinic BiVO4 with Porous Structure and Its High Photocatalytic Activity" Materials 9, no. 8: 685. https://doi.org/10.3390/ma9080685
APA StyleDong, P., Xi, X., Zhang, X., Hou, G., & Guan, R. (2016). Template-Free Synthesis of Monoclinic BiVO4 with Porous Structure and Its High Photocatalytic Activity. Materials, 9(8), 685. https://doi.org/10.3390/ma9080685