Solvent-Free Esterification of Carboxylic Acids Using Supported Iron Oxide Nanoparticles as an Efficient and Recoverable Catalyst
Abstract
:1. Introduction
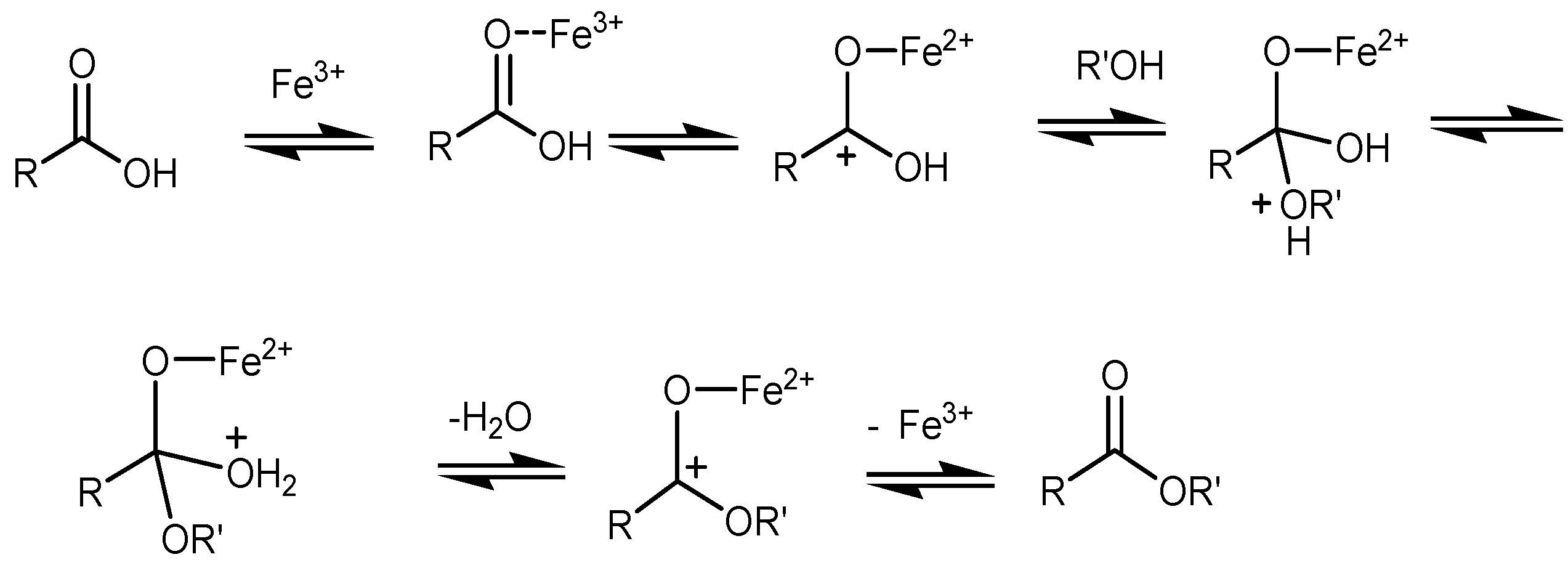
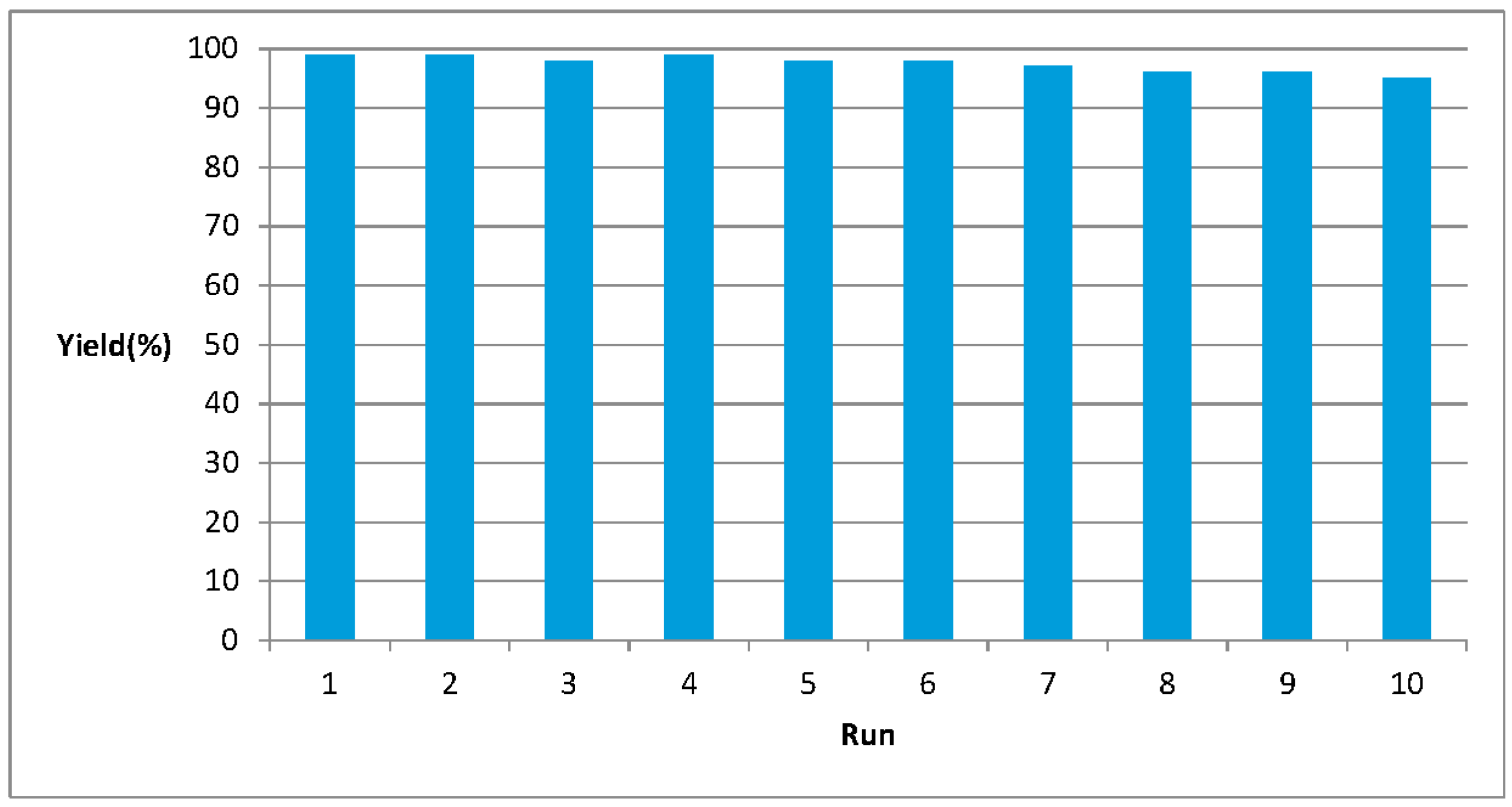
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Preparation of Aminopropyl-Functionalized SBA-15 Materials (SBA-15-NH2)
3.3. Preparation of Supported Iron Oxide Nanoparticles (FeNP@SBA-15)
3.4. General Reaction Procedure
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Othmer, K. Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed.; John Wiley & Sons: New York, NY, USA, 1994; p. 797. [Google Scholar]
- Fischer, E.; Speier, A. Darstellung der Ester. Ber. Dtsch. Chem. Ges. 1895, 28, 3252–3258. [Google Scholar] [CrossRef]
- Aoyama, T.; Shioiri, T. New methods and reagents in organic synthesis. 8. Trimethylsilyldiazomethane. A new, stable, and safe reagent for the classical Arndt-Eistert synthesis. Tetrahedron Lett. 1980, 21, 4461–4462. [Google Scholar] [CrossRef]
- Crimmins, M.T.; DeLoach, J.A. Intramolecular photocycloadditions-cyclobutane fragmentation: Total synthesis of (±)-pentalenene, (±)-pentalenic acid, and (±)-deoxypentalenic acid. J. Am. Chem. Soc. 1986, 108, 800–806. [Google Scholar] [CrossRef]
- Widmer, U. A convenient preparation of t-butyl esters. Synthesis 1983, 1983, 135–136. [Google Scholar] [CrossRef]
- White, E.H.; Baum, A.A.; Eitel, D.E. 1-Methyl-3-p-tolyltriazene and its use in the esterification of acids. In Organic Syntheses, Coll. Vol. 5; John Wiley & Sons: New York, NY, USA, 1973; p. 797. [Google Scholar]
- Calo, F.; Richardson, J.; Barrett, A.G.M. Total synthesis of Citrafungin A. J. Org. Chem. 2008, 73, 9692–9697. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Chiu, J.-Y.; Lin, H.-M. Kinetics of catalytic esterification of propionic acid and n-butanol over amberlyst 35. Ind. Eng. Chem. Res. 2002, 41, 2882–2887. [Google Scholar] [CrossRef]
- Altıokka, M.R.; Çıtak, A. Kinetics study of esterification of acetic acid with isobutanol in the presence of amberlite catalyst. Appl. Catal. A Gen. 2003, 239, 141–148. [Google Scholar] [CrossRef]
- Izci, A.; Bodur, F. Liquid-phase esterification of acetic acid with isobutanol catalyzed by ion-exchange resins. React. Funct. Polym. 2007, 67, 1458–1464. [Google Scholar] [CrossRef]
- Hoek, I.; Nijhuis, T.A.; Stankiewicz, A.I.; Moulijn, J.A. Kinetics of solid acid catalysed etherification of symmetrical primary alcohols: Zeolite BEA catalysed etherification of 1-octanol. Appl. Catal. A Gen. 2004, 266, 109–116. [Google Scholar] [CrossRef]
- Kirumakki, S.R.; Nagaraju, N.; Narayanan, S. A comparative esterification of benzyl alcohol with acetic acid over zeolites Hβ, HY and HZSM5. Appl. Catal. A Gen. 2004, 273, 1–9. [Google Scholar] [CrossRef]
- Ardizzone, S.; Bianchi, C.L.; Ragaini, V.; Vercelli, B. SO4-ZrO2 catalysts for the esterification of benzoic acid to methylbenzoate. Catal. Lett. 1999, 62, 59–65. [Google Scholar] [CrossRef]
- Khire, S.; Bhagwat, P.V.; Fernandes, M.; Gangundi, P.B.; Vadalia, H. Esterification of lower aliphatic alcohols with acetic acid in presence of different acid catalysts. Indian J. Chem. Technol. 2012, 19, 342–350. [Google Scholar]
- Sepúlveda, J.H.; Yori, J.C.; Vera, C.R. Repeated use of supported H3PW12O40 catalysts in the liquid phase esterification of acetic acid with butanol. Appl. Catal. A Gen. 2005, 288, 18–24. [Google Scholar] [CrossRef]
- Zhang, F.M.; Wang, J.; Yuan, C.S.; Ren, X.Q. Catalytic performances of heteropoly compounds supported on dealuminated ultra-stable Y zeolite for liquid-phase esterification. Sci. China Ser. B 2006, 49, 140–147. [Google Scholar] [CrossRef]
- Parida, K.M.; Mallick, S. Silicotungstic acid supported zirconia: An effective catalyst for esterification reaction. J. Mol. Catal. A Chem. 2007, 275, 77–83. [Google Scholar] [CrossRef]
- Bhorodwaj, S.K.; Pathak, M.G.; Dutta, D.K. Esterification of acetic acid with n-butanol using heteropoly acid supported modified clay catalyst. Catal. Lett. 2009, 133, 185–191. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Khosousi, T.; Hydarzadeh, S. Highly selective esterification of tert-butanol by acetic acid anhydride over alumina-supported InCl3, GaCl3, FeCl3, ZnCl2, CuCl2, NiCl2, CoCl2 and MnCl2 catalysts. J. Mol. Catal. A Chem. 2005, 235, 150–153. [Google Scholar] [CrossRef]
- MacLeod, C.S.; Harvey, A.P.; Lee, A.F.; Wilson, K. Evaluation of the activity and stability of alkali-doped metal oxide catalysts for application to an intensified method of biodiesel production. Chem. Eng. J. 2008, 135, 63–70. [Google Scholar] [CrossRef]
- Shumaker, J.L.; Crofcheck, C.; Tackett, S.A.; Santillan-Jimenez, E.; Morgan, T.; Ji, Y.; Crocker, M.; Toops, T.J. Biodiesel synthesis using calcined layered double hydroxide catalysts. Appl. Catal. B Environ. 2008, 82, 120–130. [Google Scholar] [CrossRef]
- Cantrell, D.G.; Gillie, L.J.; Lee, A.F.; Wilson, K. Structure-reactivity correlations in MgAl hydrotalcite catalysts for biodiesel synthesis. Appl. Catal. A Gen. 2005, 287, 183–190. [Google Scholar] [CrossRef]
- Noureddini, H.; Gao, X.; Philkana, R.S. Immobilized Pseudomonas cepacia lipase for biodiesel fuel production from soybean oil. Bioresour. Technol. 2005, 96, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Iso, M.; Chen, B.; Eguchi, M.; Kudo, T.; Shrestha, S. Production of biodiesel fuel from triglycerides and alcohol using immobilized lipase. J. Mol. Catal. B Enzym. 2001, 16, 53–58. [Google Scholar] [CrossRef]
- Rajabi, F.; Karimi, N.; Saidi, M.R.; Primo, A.; Varma, R.S.; Luque, R. Unprecedented selective oxidation of styrene derivatives using a supported iron oxide nanocatalyst in aqueous medium. Adv. Synth. Catal. 2012, 354, 1707–1711. [Google Scholar] [CrossRef]
- Rajabi, F.; Arancon, R.A.D.; Luque, R. Oxidative esterification of alcohols and aldehydes using supported iron oxide nanoparticle catalysts. Catal. Commun. 2015, 59, 101–103. [Google Scholar] [CrossRef]
- Rajabi, F.; Naserian, S.; Primo, A.; Luque, R. Efficient and highly selective aqueous oxidation of sulfides to sulfoxides at room temperature catalysed by supported iron oxide nanoparticles on SBA-15. Adv. Synth. Catal. 2011, 353, 2060–2066. [Google Scholar] [CrossRef]
- Rajabi, F.; Raessi, M.; Arancon, R.A.D.; Saidi, M.R.; Luque, R. Supported cobalt oxide nanoparticles as efficient catalyst in esterification and amidation reactions. Catal. Commun. 2015, 59, 122–126. [Google Scholar] [CrossRef]
- Cai, Y.Q.; Yu, G.Q.; Liu, C.D.; Xu, Y.Y.; Wang, W. Imidazolium ionic liquid-supported sulfonic acids: Efficient and recyclable catalysts for esterification of benzoic acid. Chin. Chem. Lett. 2012, 23, 1–4. [Google Scholar] [CrossRef]
- Zhou, X.S.; Liu, J.B.; Luo, W.F.; Zhang, Y.W.; Song, H. Novel Brønsted-acidic ionic liquids based on benzothiazoliumcations as catalysts for esterification reactions. J. Serb. Chem. Soc. 2011, 76, 1607–1615. [Google Scholar] [CrossRef]
- Aavula, S.K.; Chikkulapalli, A.; Hanumanthappa, N.; Jyothi, I.; Vinod Kumar, C.H.; Manjunatha, S.G. Palladium on carbon-bromobenzene mediated esterification and transesterification. Tetrahedron Lett. 2013, 54, 5690–5694. [Google Scholar] [CrossRef]
- Chakraborti, A.K.; Singh, B.; Chankeshwara, S.V.; Patel, A.R. Protic acid immobilized on solid support as an extremely efficient recyclable catalyst system for a direct and atom economical esterification of carboxylic acids with alcohols. J. Org. Chem. 2009, 74, 5967–5974. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, K.S.K.; Chan, J.C.C.; Cheng, S. Direct synthesis and catalytic applications of ordered large pore aminopropyl-functionalized SBA-15 mesoporous materials. J. Phys. Chem. B 2005, 109, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
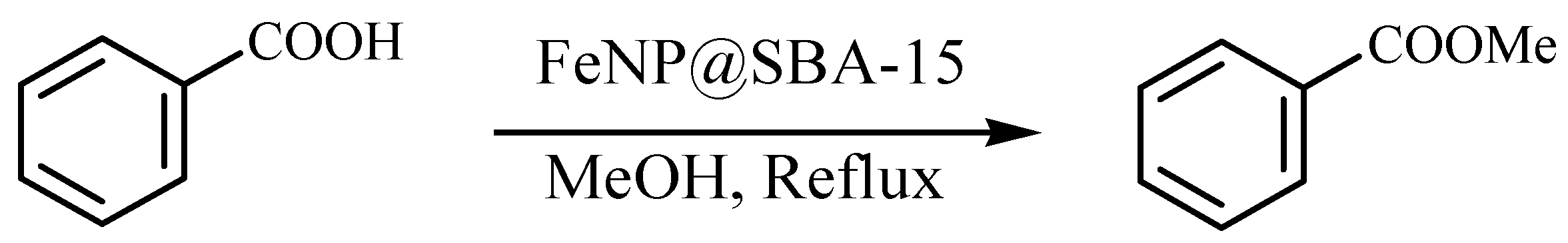
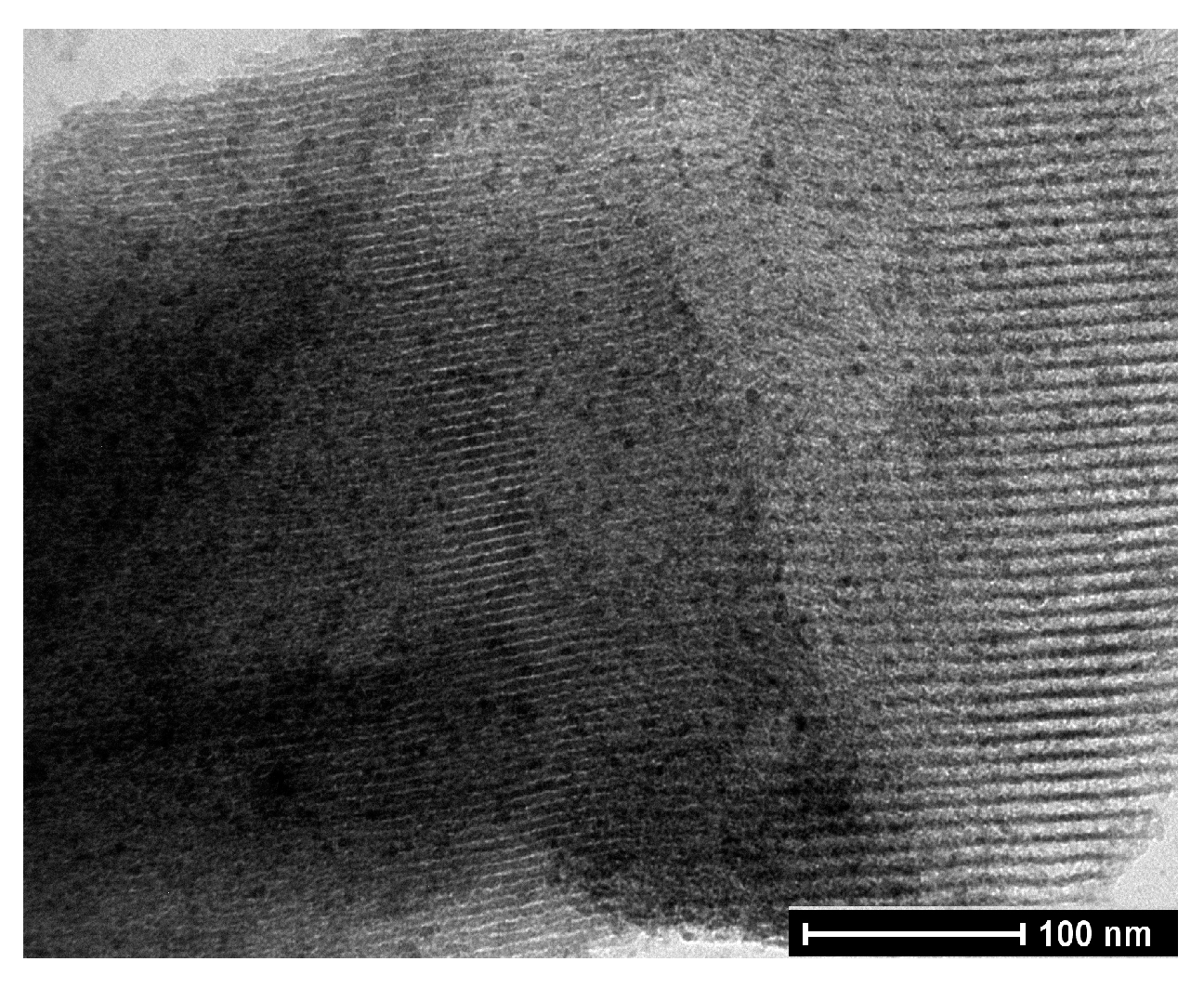
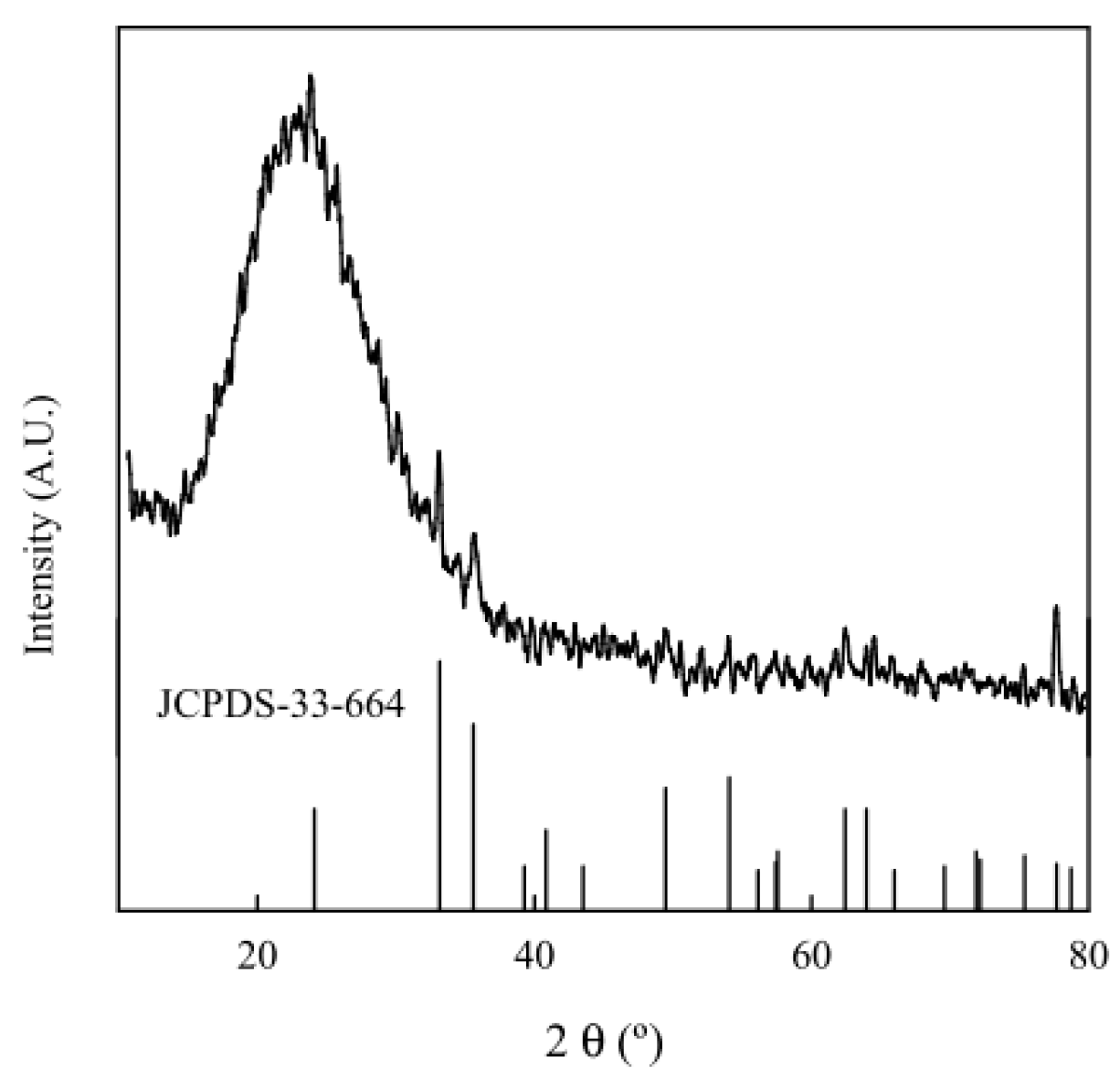
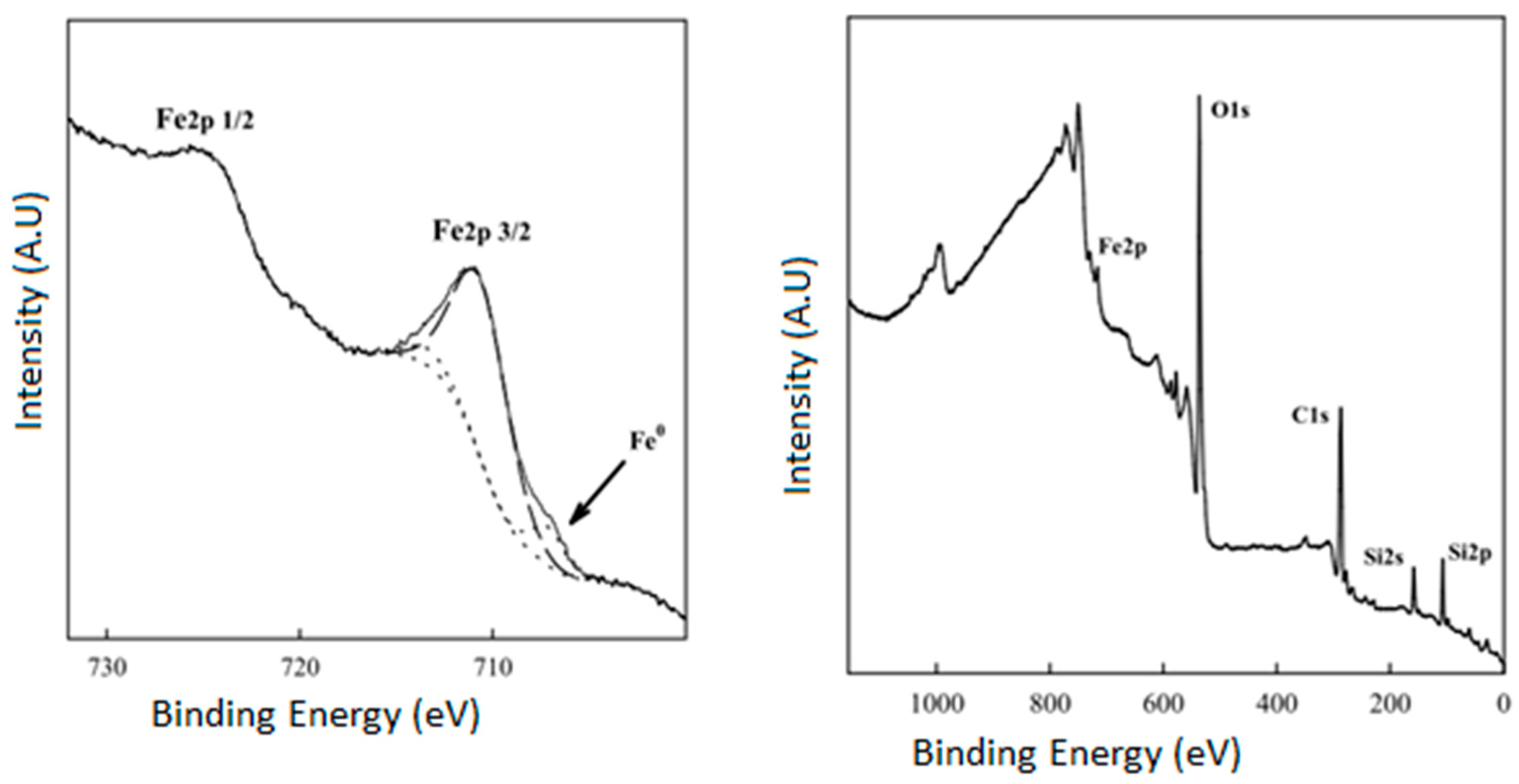
| Entry | FeNP (mol. %) | Time (h) | T (°C) | Yield (%) b |
|---|---|---|---|---|
| 1 | - | 10 | 60 | - |
| 2 | 0.3 | 10 | r.t. | 10 |
| 3 | 0.3 | 10 | 40 | 22 |
| 4 | 0.3 | 10 | reflux | 99 |
| 5 | 0.2 | 10 | reflux | 99 |
| 6 | 0.1 | 10 | reflux | 99 |
| 7 | 0.07 | 10 | reflux | 48 |
| 8 | 0.1 | 6 | reflux | 99 |
| 9 | 0.1 | 4 | reflux | 91 |
| Entry | Carboxylic Acid | Product (Ester) | Alcohol | Yield (%) b |
|---|---|---|---|---|
| 1 | 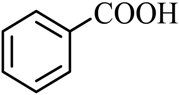 | 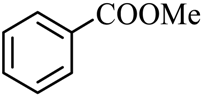 | CH3OH | 99 |
| 2 |  | 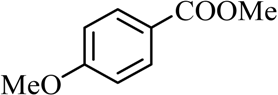 | CH3OH | 98 |
| 3 |  | 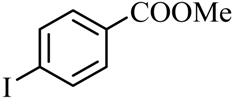 | CH3OH | 97 |
| 4 | 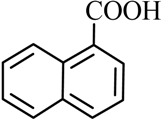 | 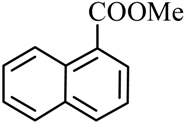 | CH3OH | 96 |
| 5 | 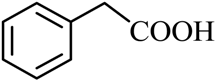 |  | CH3OH | 90 |
| 6 |  |  | CH3OH | 94 |
| 7 | 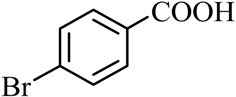 | 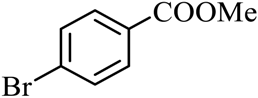 | CH3OH | 95 |
| 8 | 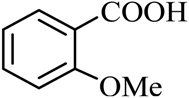 | 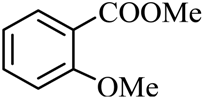 | CH3OH | 88 |
| 9 |  | 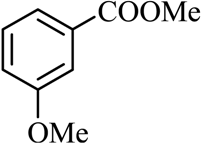 | CH3OH | 90 |
| 10 |  |  | CH3OH | 93 |
| 11 |  | 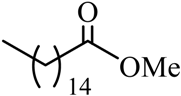 | CH3OH | 95 |
| 12 | 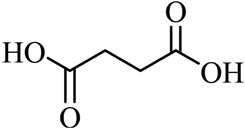 | 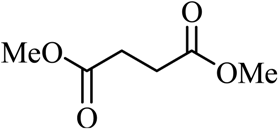 | CH3OH | 90 |
| 13 | 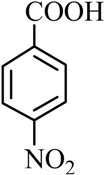 | 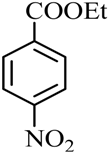 | CH3CH2OH | 90 |
| 14 |  | 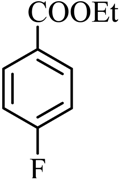 | CH3CH2OH | 92 |
| 15 | 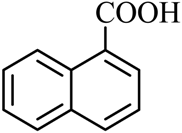 |  | CH3CH2OH | 94 |
| 16 | 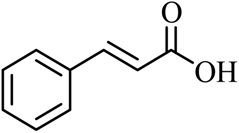 | 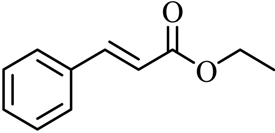 | CH3CH2OH | 95 |
| 17 | 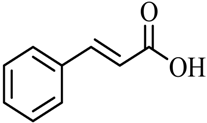 | 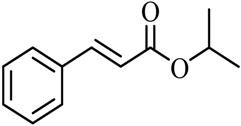 | (CH3)2CHOH | 95 |
| 18 | 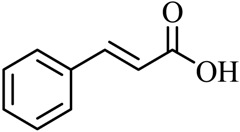 |  | CH3OH | 98 |
| Entry | Catalyst | Mol (%) | T (°C) | Acid/Methanol Molar Ratio | Time (h) | Yield(%) a | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | FeNP@SBA-15 | 0.1 | reflux | 1:2 | 6 | 99 | b |
| 2 | CoNP@SBA-15 | 0.5 | reflux | 1:2 | 12 | 98 | [28] |
| 3 | [C3SO3Hmim]HSO4 | 0.3 | 95 | 1:3 | 2 | 98 | [29] |
| 4 | Ionic liquids based on benzothiazoliumcations | 5 | 120 | 1:4 | 8 | 97.9 | [30] |
| 5 | Pd/C | 0.5 gr | 60 | 1:excess amount | 4 | 90 | [31] |
| 6 | HClO4-SiO2 | 1 | 100 | 1:1 | 3 | 96 | [32] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajabi, F.; Abdollahi, M.; Luque, R. Solvent-Free Esterification of Carboxylic Acids Using Supported Iron Oxide Nanoparticles as an Efficient and Recoverable Catalyst. Materials 2016, 9, 557. https://doi.org/10.3390/ma9070557
Rajabi F, Abdollahi M, Luque R. Solvent-Free Esterification of Carboxylic Acids Using Supported Iron Oxide Nanoparticles as an Efficient and Recoverable Catalyst. Materials. 2016; 9(7):557. https://doi.org/10.3390/ma9070557
Chicago/Turabian StyleRajabi, Fatemeh, Mohammad Abdollahi, and Rafael Luque. 2016. "Solvent-Free Esterification of Carboxylic Acids Using Supported Iron Oxide Nanoparticles as an Efficient and Recoverable Catalyst" Materials 9, no. 7: 557. https://doi.org/10.3390/ma9070557
APA StyleRajabi, F., Abdollahi, M., & Luque, R. (2016). Solvent-Free Esterification of Carboxylic Acids Using Supported Iron Oxide Nanoparticles as an Efficient and Recoverable Catalyst. Materials, 9(7), 557. https://doi.org/10.3390/ma9070557







