Fabrication of Pt/Ti/TiO2 Photoelectrodes by RF-Magnetron Sputtering for Separate Hydrogen and Oxygen Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Photoelectrodes
2.2. Characterization of the Photoelectrodes
2.3. IPCE Measurements
2.4. Separate H2 and O2 Photocatalytic Production Tests
3. Results and Discussion
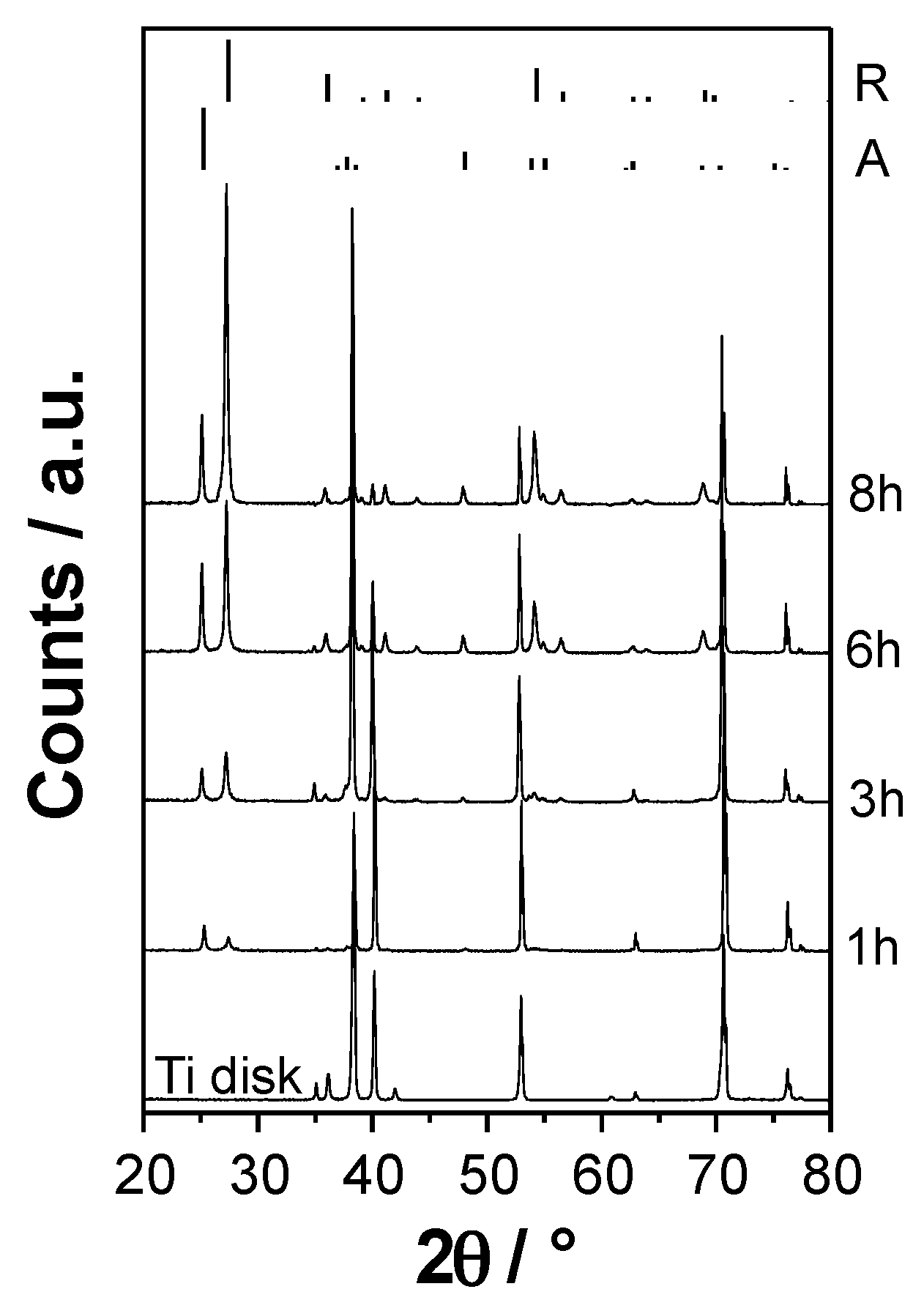
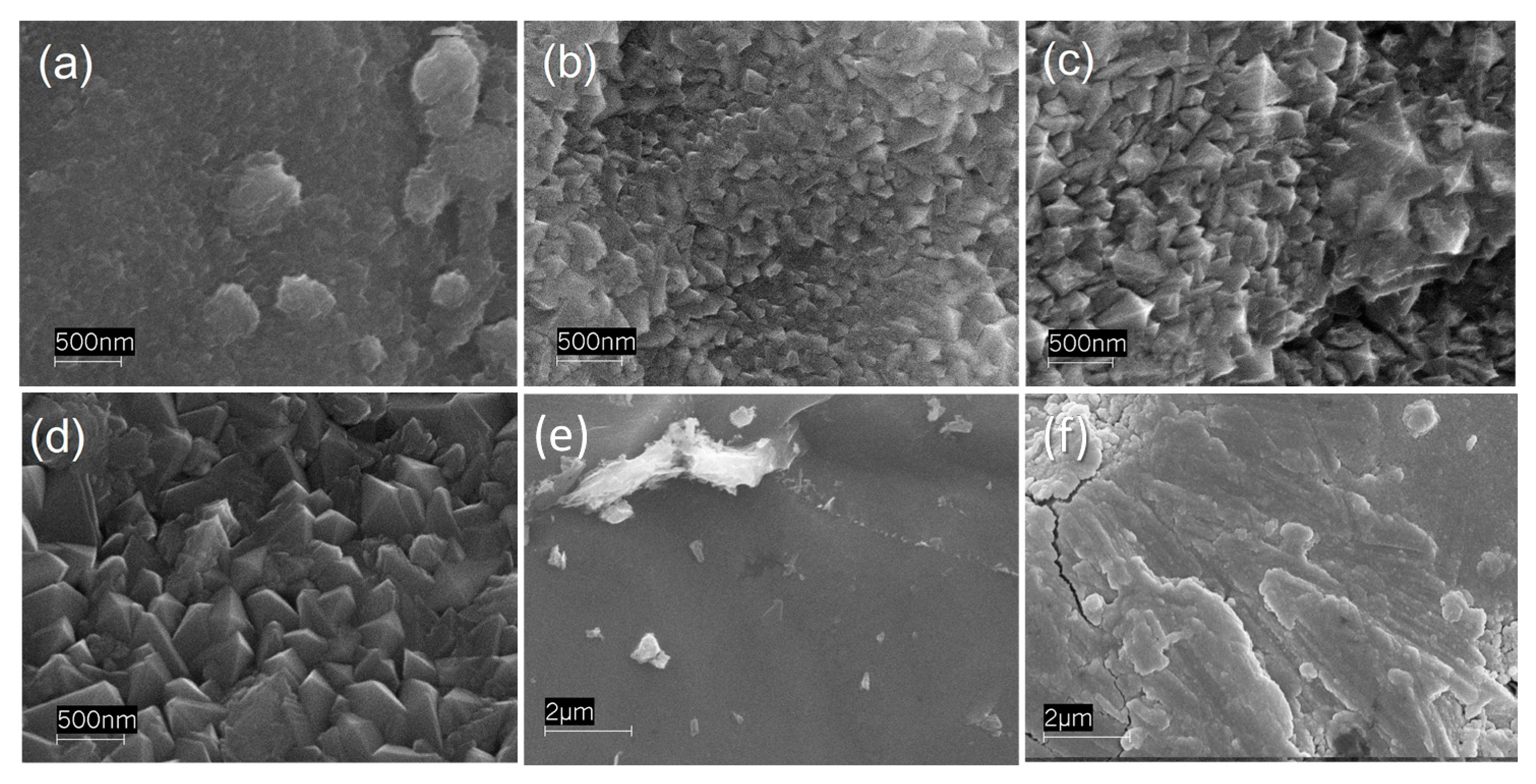
3.1. XRD and SEM Investigation
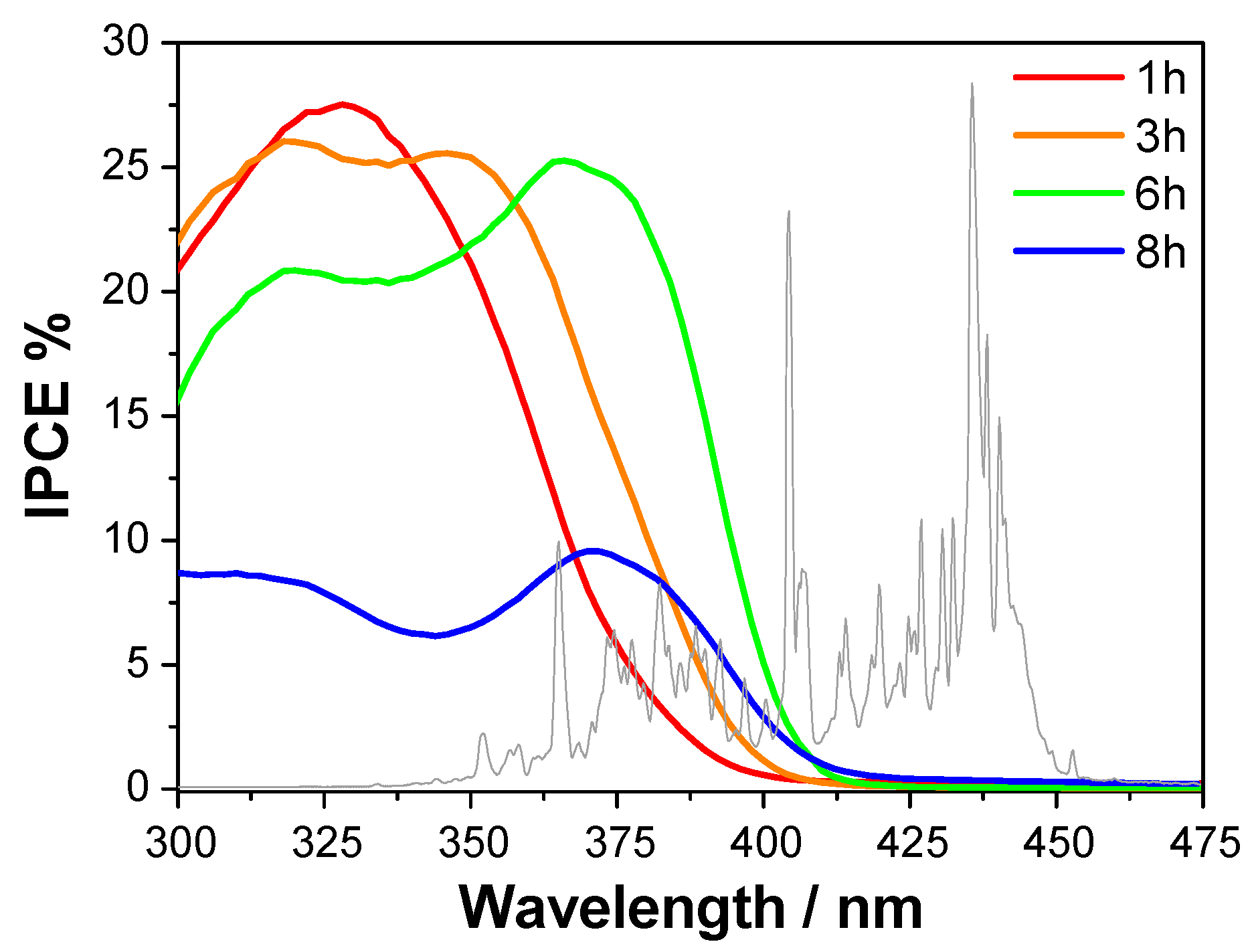
3.2. UV-vis-NIR Diffuse Reflectance and IPCE Measurements
3.3. Photocatalytic Water Splitting Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| RF | Radio frequency |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscopy |
| DRS | Diffuse reflectance spectroscopy |
| IPCE | Incident photon to current efficiency |
References
- Li, J.; Wu, N. Semiconductor-based photocatalysts and photoelectrochemical cells for solar fuel generation: A review. Catal. Sci. Technol. 2015, 5, 1360–1384. [Google Scholar] [CrossRef]
- Lianos, P. Production of electricity and hydrogen by photocatalytic degradation of organic wastes in a photoelectrochemical cell. The concept of the Photofuelcell: A review of a re-emerging research field. J. Hazard. Mater. 2011, 185, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, Y.; Toyao, T.; Takeuchi, M.; Matsuoka, M.; Anpo, M. Recent advances in visible-light-responsive photocatalysts for hydrogen production and solar energy conversion—From semiconducting TiO2 to MOF/PCP photocatalysts. Phys. Chem. Chem. Phys. 2013, 15, 13243–13253. [Google Scholar] [CrossRef] [PubMed]
- Selli, E.; Chiarello, G.L.; Quartarone, E.; Mustarelli, P.; Rossetti, I.; Forni, L. A photocatalytic water splitting device for separate hydrogen and oxygen evolution. Chem. Commun. 2007, 47, 5022–5024. [Google Scholar]
- Chiarello, G.L.; Zuliani, A.; Ceresoli, D.; Martinazzo, R.; Selli, E. Exploiting the photonic crystal properties of TiO2 nanotube arrays to enhance photocatalytic hydrogen production. ACS Catal. 2016, 6, 1345–1353. [Google Scholar] [CrossRef]
- Chen, Z.; Jaramillo, T.F.; Deutsch, T.G.; Kleiman-Shwarsctein, A.; Forman, A.J.; Gaillard, N.; Garland, R.; Takanabe, K.; Heske, C.; Sunkara, M.; et al. Accelerating materials development for photoelectrochemical hydrogen production: Standards for methods, definitions, and reporting protocols. J. Mater. Res. 2010, 25, 3–16. [Google Scholar] [CrossRef]
- Molinari, R.; Marino, T.; Argurio, P. Photocatalytic membrane reactors for hydrogen production from water. Int. J. Hydrog. Energy 2014, 39, 7247–7261. [Google Scholar] [CrossRef]
- Pessoa, R.S.; Fraga, M.A.; Santos, L.V.; Massi, M.; Maciel, H.S. Nanostructured thin films based on TiO2 and/or SiC for use in photoelectrochemical cells: A review of the material characteristics, synthesis and recent applications. Mater. Sci. Semicond. Process. 2014, 29, 56–68. [Google Scholar] [CrossRef]
- Liao, C.-H.; Huang, C.-W.; Wu, J.C.S. Novel dual-layer photoelectrode prepared by RF magnetron sputtering for photocatalytic water splitting. Int. J. Hydrog. Energy 2012, 37, 11632–11639. [Google Scholar] [CrossRef]
- Tealdi, C.; Quartarone, E.; Galinetto, P.; Grandi, M.S.; Mustarelli, P. Flexible deposition of TiO2 electrodes for photocatalytic applications: Modulation of the crystal phase as a function of the layer thickness. J. Solid State Chem. 2013, 199, 1–6. [Google Scholar] [CrossRef]
- Binetti, E.; El Koura, Z.; Patel, N.; Dashora, A.; Miotello, A. Rapid hydrogenation of amorphous TiO2 to produce efficient H-doped anatase for photocatalytic water splitting. Appl. Catal. A Gen. 2014, 500, 69–73. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Kitano, M.; Iyatani, K.; Horiuchi, Y.; Takeuchi, M.; Matsuoka, M.; Anpo, M. Effect of the sputtering parameters on the physical properties and photocatalytic reactivity of TiO2 thin films prepared by an RF magnetron sputtering deposition method. Res. Chem. Intermed. 2012, 39, 1593–1602. [Google Scholar] [CrossRef]
- Dholam, R.; Patel, N.; Adami, M.; Miotello, A. Hydrogen production by photocatalytic water-splitting using Cr- or Fe-doped TiO2 composite thin films photocatalyst. Int. J. Hydrog. Energy 2009, 34, 5337–5346. [Google Scholar] [CrossRef]
- Wu, M.; Liu, J.; Jin, J.; Wang, C.; Huang, S.; Deng, Z.; Li, Y.; Su, B.L. Probing significant light absorption enhancement of titania inverse opal films for highly exalted photocatalytic degradation of dye pollutants. Appl. Catal. B Environ. 2014, 150–151, 411–420. [Google Scholar] [CrossRef]
- El Koura, Z.; Patel, N.; Edla, R.; Miotello, A. Multilayer films of indium tin oxide/TiO2 codoped with vanadium and nitrogen for efficient photocatalytic water splitting. Int. J. Nanotechnol. 2014, 11, 1017–1027. [Google Scholar] [CrossRef]
- Rahman, M.; Dang, B.H.Q.; McDonnell, K.; MacElroy, J.M.D.; Dowling, D.P. Effect of doping (C or N) and co-doping (C + N) on the photoactive properties of magnetron sputtered titania coatings for the application of solar water-splitting. J. Nanosci. Nanotechnol. 2012, 12, 4729–4735. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hu, Q.Q.; Huang, J.Q.; Deng, Z.H.; Shi, H.L.; Wu, L.; Liu, Z.G.; Cao, Y.G. Effective water splitting using N-doped TiO2 films: Role of preferred orientation on hydrogen production. Int. J. Hydrog. Energy 2014, 39, 1967–1971. [Google Scholar] [CrossRef]
- Fakhouri, H.; Pulpytel, J.; Smith, W.; Zolfaghari, A.; Mortaheb, H.R.; Meshkini, F.; Jafari, R.; Sutter, E.; Arefi-Khonsari, F. Control of the visible and UV light water splitting and photocatalysis of nitrogen doped TiO2 thin films deposited by reactive magnetron sputtering. Appl. Catal. B Environ. 2014, 144, 12–21. [Google Scholar] [CrossRef]
- Kitano, M.; Iyatani, K.; Tsujimaru, K.; Matsuoka, M.; Takeuchi, M.; Ueshima, M.; Thomas, J.M.; Anpo, M. The effect of chemical etching by HF solution on the photocatalytic activity of visible light-responsive TiO2 thin films for solar water splitting. Top. Catal. 2008, 49, 24–31. [Google Scholar] [CrossRef]
- Matsuoka, M.; Kitano, M.; Fukumoto, S.; Iyatani, K.; Takeuchi, M.; Anpo, M. The effect of the hydrothermal treatment with aqueous NaOH solution on the photocatalytic and photoelectrochemical properties of visible light-responsive TiO2 thin films. Catal. Today 2008, 132, 159–164. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Bish, D.L.; Howard, S.A. Quantitative phase analysis using the Rietveld method. J. Appl. Crystallogr. 1988, 21, 86–91. [Google Scholar] [CrossRef]
- Altomare, A.; Burla, M.C.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Rizzi, R. Quanto: A Rietveld program for quantitative phase analysis of polycrystalline mixtures. J. Appl. Crystallogr. 2001, 34, 392–397. [Google Scholar] [CrossRef]
- Lazzeri, M.; Vittadini, A.; Selloni, A. Structure and energetics of stoichiometric TiO2 anatase surfaces. Phys. Rev. B 2001, 63. [Google Scholar] [CrossRef]
- Barnard, A.S.; Curtiss, L.A. Prediction of TiO2 nanoparticle phase and shape transitions controlled by surface chemistry. Nano Lett. 2005, 5, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, M.; Vanderbilt, D.; King-Smith, R.D. First-principles calculations of the energetics of stoichiometric TiO2 surfaces. Phys. Rev. B 1994, 49, 16721–16727. [Google Scholar] [CrossRef]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Preparation and characterization of quantum-size titanium dioxide. J. Phys. Chem. 1988, 92, 5196–5201. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Di Paola, A.; Palmisano, L.; Selli, E. Effect of titanium dioxide crystalline structure on the photocatalytic production of hydrogen. Photochem. Photobiol. Sci. 2011, 10, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Ekimov, A.I.; Efros, A.L.; Onushchenko, A.A. Quantum size effect in semiconductor microcrystals. Solid State Commun. 1985, 56, 921–924. [Google Scholar] [CrossRef]

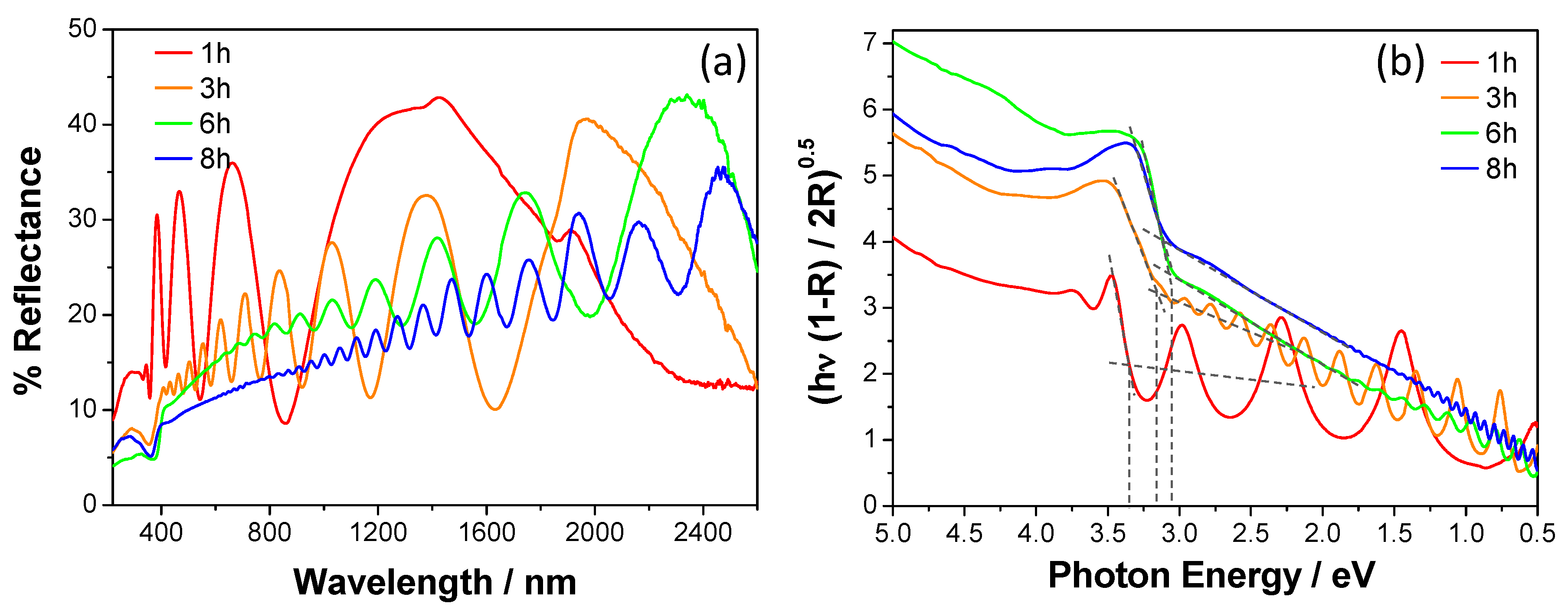
| Deposition Time (h) | Film Thickness (nm) 1 | Anatase (wt.%) | Rutile (wt.%) | Band Gap (eV) |
|---|---|---|---|---|
| 1 | 320 | 54 | 46 | 3.30 |
| 3 | 1066 | 31 | 69 | 3.18 |
| 6 | 1823 | 30 | 70 | 3.05 |
| 8 | 3030 | 28 | 72 | 3.05 |
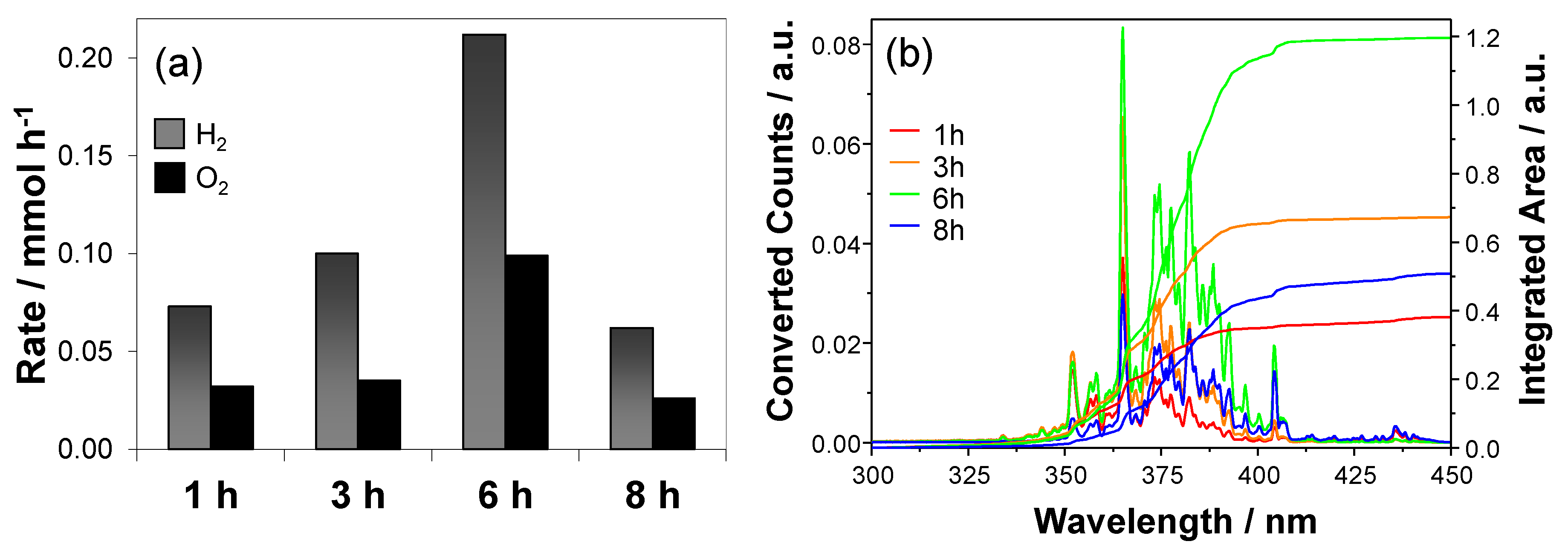
| Deposition Time (h) | rH2 (μmol·h−1) | rO2 (μmol·h−1) | rH2/rO2 | φexp | φeff |
|---|---|---|---|---|---|
| 1 | 72.94 ± 0.06 | 31.62 ± 0.03 | 2.3 | 2.3 | 2.5 |
| 3 | 100.15 ± 0.09 | 40.3 ± 0.1 | 2.5 | 4.0 | 3.5 |
| 6 | 212.30 ± 0.03 | 98.4 ± 0.1 | 2.2 | 7.1 | 7.3 |
| 8 | 52.35 ± 0.08 | 21.81 ± 0.05 | 2.4 | 3.0 | 2.2 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiarello, G.L.; Tealdi, C.; Mustarelli, P.; Selli, E. Fabrication of Pt/Ti/TiO2 Photoelectrodes by RF-Magnetron Sputtering for Separate Hydrogen and Oxygen Production. Materials 2016, 9, 279. https://doi.org/10.3390/ma9040279
Chiarello GL, Tealdi C, Mustarelli P, Selli E. Fabrication of Pt/Ti/TiO2 Photoelectrodes by RF-Magnetron Sputtering for Separate Hydrogen and Oxygen Production. Materials. 2016; 9(4):279. https://doi.org/10.3390/ma9040279
Chicago/Turabian StyleChiarello, Gian Luca, Cristina Tealdi, Piercarlo Mustarelli, and Elena Selli. 2016. "Fabrication of Pt/Ti/TiO2 Photoelectrodes by RF-Magnetron Sputtering for Separate Hydrogen and Oxygen Production" Materials 9, no. 4: 279. https://doi.org/10.3390/ma9040279
APA StyleChiarello, G. L., Tealdi, C., Mustarelli, P., & Selli, E. (2016). Fabrication of Pt/Ti/TiO2 Photoelectrodes by RF-Magnetron Sputtering for Separate Hydrogen and Oxygen Production. Materials, 9(4), 279. https://doi.org/10.3390/ma9040279









