Surface Morphology and Tooth Adhesion of a Novel Nanostructured Dental Restorative Composite
Abstract
:1. Introduction
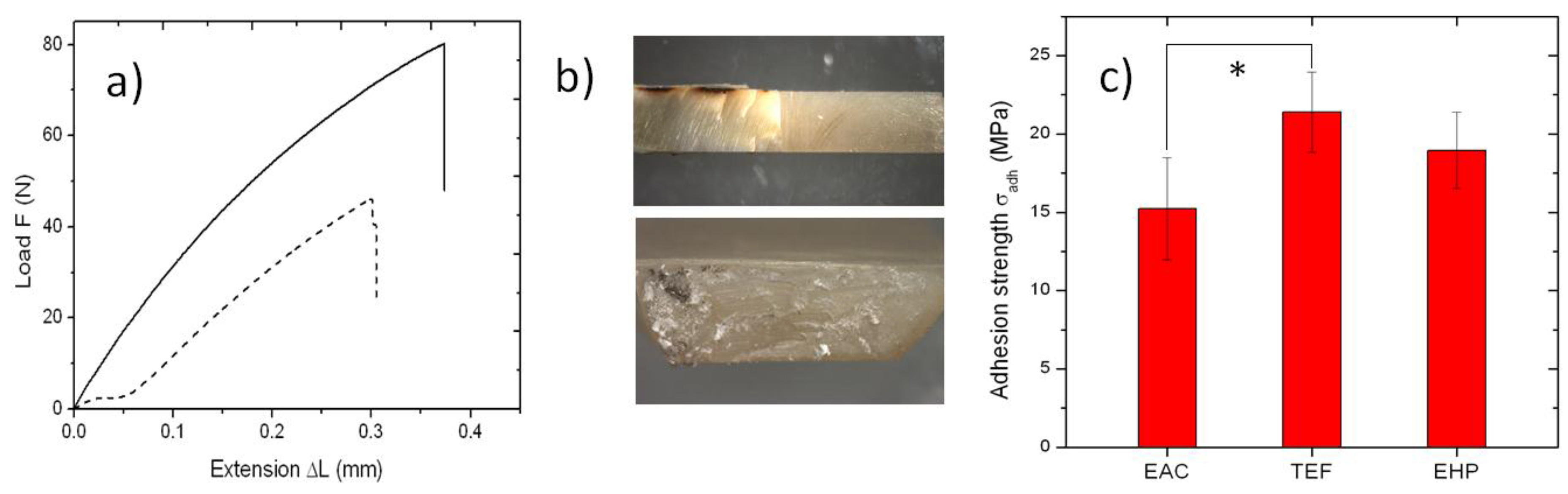
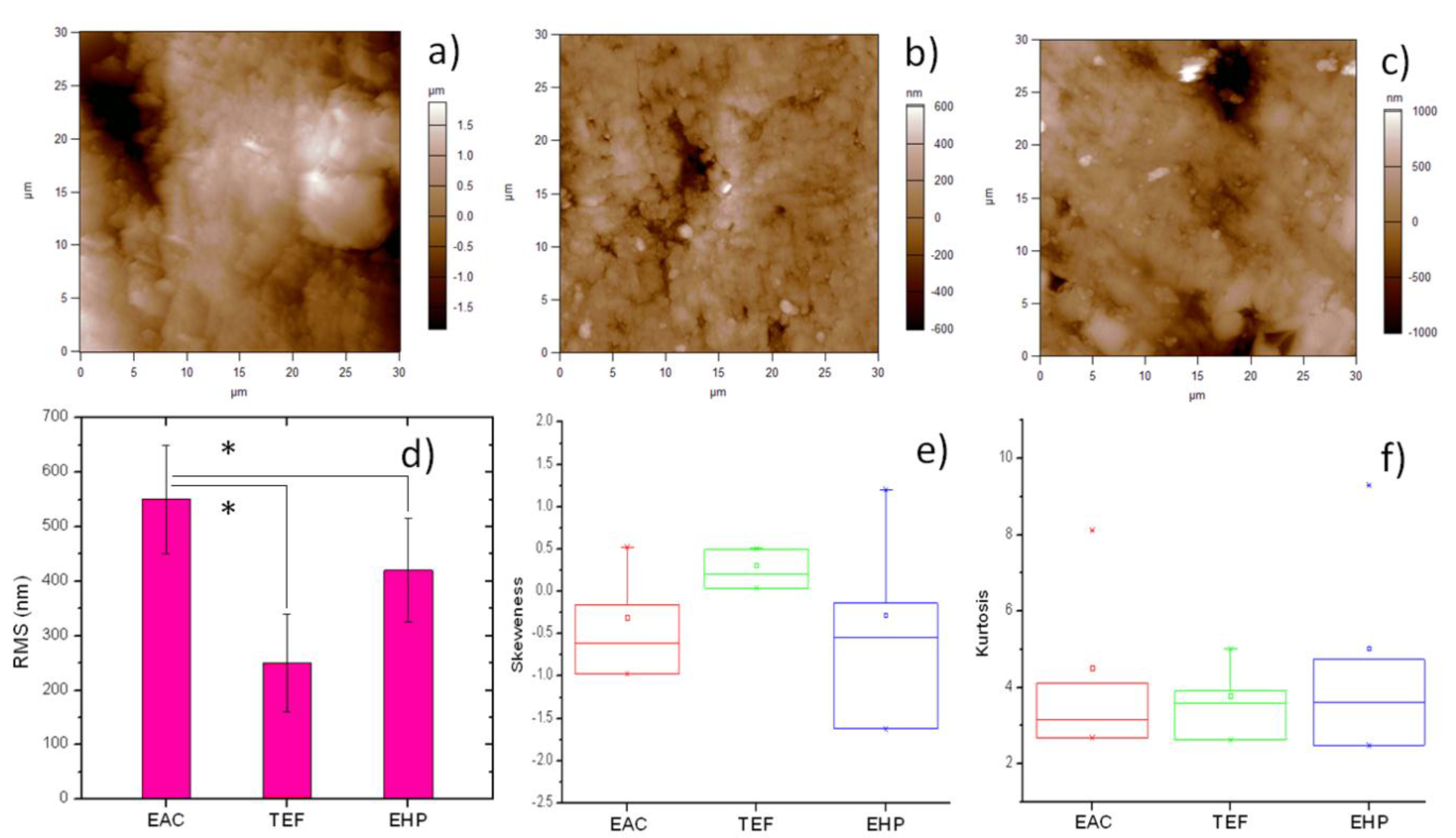
2. Results and Discussion
3. Materials and Methods
3.1. APA Fillers
3.2. Resin Composites
3.3. Sample Preparation
3.3.1. Tensile Tests
3.3.2. AFM Characterization
3.3.3. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| APA | anodic porous alumina |
| EAC | experimental APA-based composite |
| TEF | Tetric EVO Flow |
| EHP | Enamel HRi Plus |
| AFM | atomic force microscope |
| RMS | root mean square |
| DC | direct current |
| rpm | rounds per minute |
References
- Darvell, B.W. Materials Science for Dentistry, 9th ed.; Woodhead Publishing: New Delhi, India, 2009. [Google Scholar]
- Jandt, K.D.; Sigusch, B.W. Future perspectives of resin-based dental materials. Dent. Mater. 2009, 25, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Resin composite--state of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Janus, J.; Fauxpoint, G.; Arntz, Y.; Pelletier, H.; Etienne, O. Surface roughness and morphology of three nanocomposites after two different polishing treatments by a multitechnique approach. Dent. Mater. 2010, 26, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Musanje, L.; Darvell, B. Aspects of water sorption from the air, water and artificial saliva in resin composite restorative materials. Dent. Mater. 2003, 19, 414–422. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and / or surface topography on biofilm development. Clin. Oral Implantol. Res. 2006, 17, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Miyazaki, M.; Takamizawa, T. Influence of polishing duration on surface roughness of resin composites. J. Oral Sci. 2005, 47, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Cross, S.E.; Hsueh, C.; Wali, R.P.; Stieg, A.Z.; Gimzewski, J.K. Nanocharacterization in dentistry. Int. J. Mol. Sci. 2010, 11, 2523–2545. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V.; Bertassoni, L.E. Nanotechnology in Dental Sciences: Moving towards a Finer Way of Doing Dentistry. Materials (Basel) 2010, 3, 1674–1691. [Google Scholar] [CrossRef]
- Salerno, M.; Patra, N.; Cingolani, R. Use of Ionic Liquid in Fabrication, Characterization, and Processing of Anodic Porous Alumina. Nanoscale Res. Lett. 2009, 4, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Thorat, S.B.; Diaspro, A.; Salerno, M. In vitro investigation of coupling-agent-free dental restorative composite based on nano-porous alumina fillers. J. Dent. 2014, 42, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Goracci, C.; Sadek, F.T.; Monticelli, F.; Cardoso, P.E.C.; Ferrari, M. Influence of substrate, shape, and thickness on microtensile specimens’ structural integrity and their measured bond strengths. Dent. Mater. 2004, 20, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Hamouda Current perspectives of nanoparticles in medical and dental biomaterials. J. Biomed. Res. 2012, 26, 143–151.
- Jung, M.; Sehr, K.; Klimek, J. Surface texture of four nanofilled and one hybrid composite after finishing. Oper. Dent. 2007, 32, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-H. Update on dental nanocomposites. J. Dent. Res. 2010, 89, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Vanini, L.; Mangani, F.; Klimovskaia, O. Conservative Restoration in Anterior Teeth; ACME Promoden: Viterbo, Italy, 2012. [Google Scholar]
- Salerno, M.; Patra, N.; Diaspro, A. Atomic force microscopy nanoindentation of a dental restorative midifill composite. Dent. Mater. 2012, 28, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Derchi, G.; Thorat, S.; Ceseracciu, L.; Ruffilli, R.; Barone, A.C. Surface morphology and mechanical properties of new-generation flowable resin composites for dental restoration. Dent. Mater. 2011, 27, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, L.; Derchi, G.; Frustaci, A.; Bruno, O.; Covani, U.; Barone, A.; De Santis, D.; Chiappelli, F. Surface roughness of commercial composites after different polishing protocols: an analysis with atomic force microscopy. Open Dent. J. 2010, 4, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Cresti, S.; Itri, A.; Rebaudi, A.; Diaspro, A.; Salerno, M. Microstructure of Titanium-Cement-Lithium Disilicate Interface in CAD-CAM Dental Implant Crowns: A Three-Dimensional Profilometric Analysis. Clin. Implant Dent. Relat. Res. 2015, 17, e97–e106. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Giacomelli, L.; Derchi, G.; Patra, N.; Diaspro, A. Atomic force microscopy in vitro study of surface roughness and fractal character of a dental restoration composite after air-polishing. Biomed. Eng. Online 2010, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Stępniowski, W.J.; Nowak-stępniowska, A.; Michalska-domańska, M.; Norek, M.; Czujko, T.; Bojar, Z. Fabrication and geometric characterization of highly-ordered hexagonally arranged arrays of nanoporous anodic alumina. Polish J. Chem. Technol. 2013, 16, 63–69. [Google Scholar] [CrossRef]
- Salerno, M.; Patra, N.; Losso, R.; Cingolani, R. Increased growth rate of anodic porous alumina by use of ionic liquid as electrolyte additive. Mater. Lett. 2009, 63, 1826–1829. [Google Scholar] [CrossRef]
- Das, G.; Patra, N.; Gopalakrishnan, A.; Zaccaria, R.P.; Toma, A.; Thorat, S.; Di Fabrizio, E.; Diaspro, A.; Salerno, M. Fabrication of large-area ordered and reproducible nanostructures for SERS biosensor application. Analyst 2012, 137, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Toccafondi, C.; Thorat, S.; La Rocca, R.; Scarpellini, A.; Salerno, M.; Dante, S.; Das, G. Multifunctional substrates of thin porous alumina for cell biosensors. J. Mater. Sci. Mater. Med. 2014, 25, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- ISO. ISO 25178-2:ISO ISO 25178-2:2012, Geometrical product specifications (GPS)—Surface texture: Areal—Part 2: Terms, definitions and surface texture parameters. Available online: http://www.iso.org/iso/iso_catalogue/catalogue_tc/catalogue_detail.htm?csnumber=42785 (accessed on 2 March 2016).
| Ingredient | Manufacturer | Lot # | Amount (wt %) |
|---|---|---|---|
| Bis‑GMA | Sigma-Aldrich (Italy) | MKBD8328 | 34.653 |
| TEGDMA | Sigma-Aldrich (Italy) | BCBC5367V | 14.851 |
| DMAEMA | Sigma-Aldrich (Italy) | 1437599V | 0.248 |
| CQ | Sigma-Aldrich (Italy) | S12442 | 0.248 |
| APA filler | home made | – | 50.000 |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salerno, M.; Loria, P.; Matarazzo, G.; Tomè, F.; Diaspro, A.; Eggenhöffner, R. Surface Morphology and Tooth Adhesion of a Novel Nanostructured Dental Restorative Composite. Materials 2016, 9, 203. https://doi.org/10.3390/ma9030203
Salerno M, Loria P, Matarazzo G, Tomè F, Diaspro A, Eggenhöffner R. Surface Morphology and Tooth Adhesion of a Novel Nanostructured Dental Restorative Composite. Materials. 2016; 9(3):203. https://doi.org/10.3390/ma9030203
Chicago/Turabian StyleSalerno, Marco, Patrizia Loria, Giunio Matarazzo, Francesco Tomè, Alberto Diaspro, and Roberto Eggenhöffner. 2016. "Surface Morphology and Tooth Adhesion of a Novel Nanostructured Dental Restorative Composite" Materials 9, no. 3: 203. https://doi.org/10.3390/ma9030203
APA StyleSalerno, M., Loria, P., Matarazzo, G., Tomè, F., Diaspro, A., & Eggenhöffner, R. (2016). Surface Morphology and Tooth Adhesion of a Novel Nanostructured Dental Restorative Composite. Materials, 9(3), 203. https://doi.org/10.3390/ma9030203








