Lamellae Evolution of Stereocomplex-Type Poly(Lactic Acid)/Organically-Modified Layered Zinc Phenylphosphonate Nanocomposites Induced by Isothermal Crystallization
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication of the SC-PLA/m-PPZn Nanocomposites
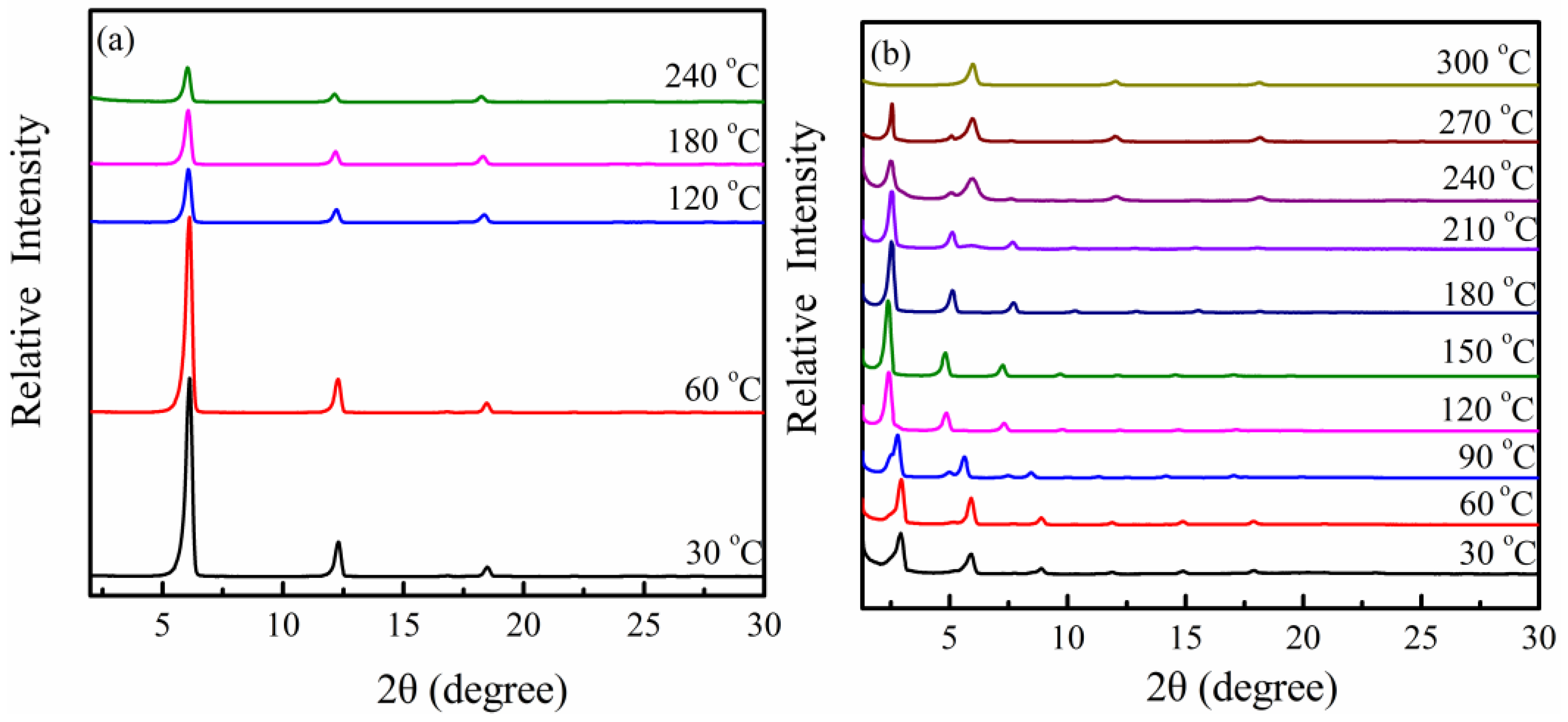
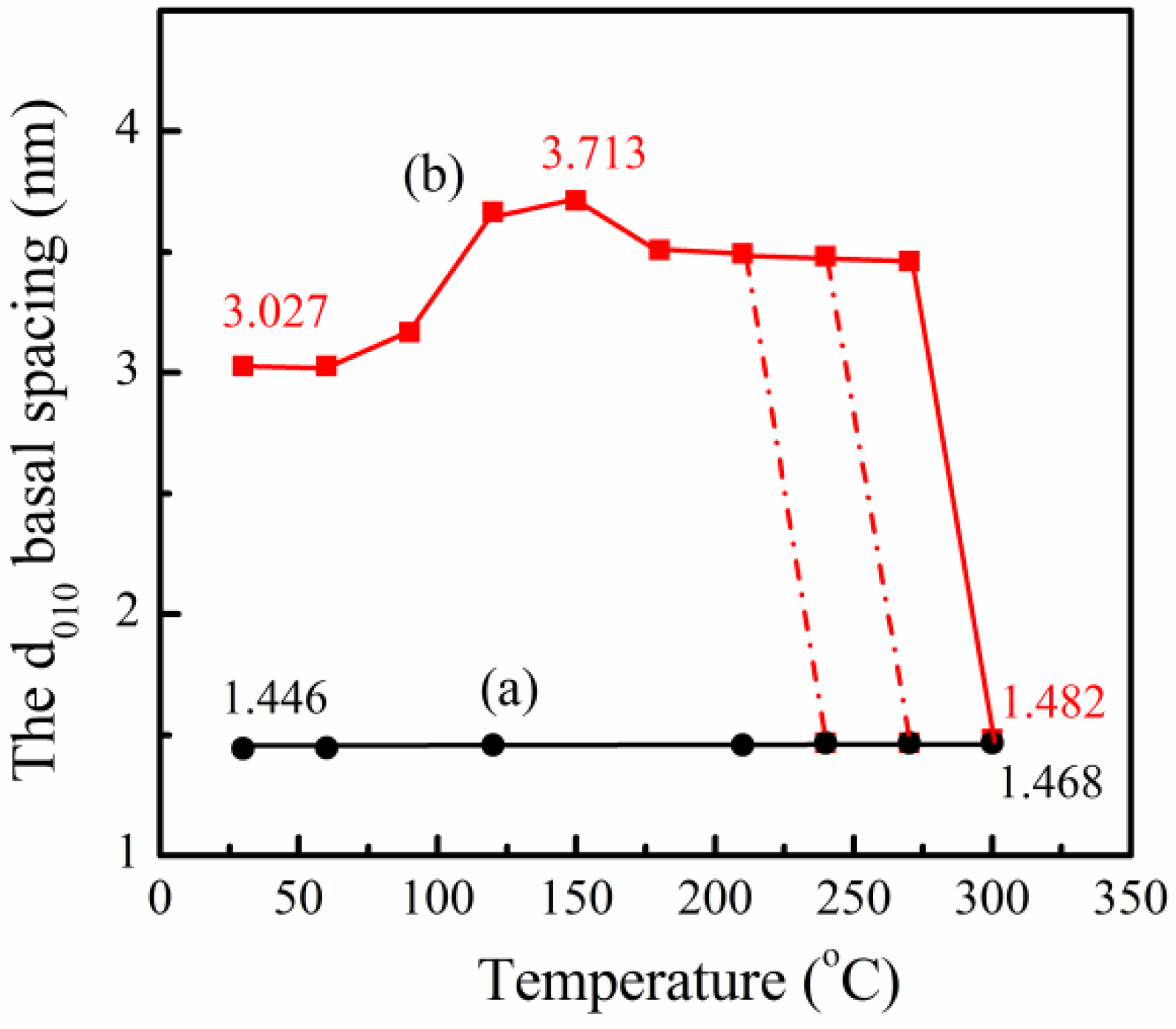
2.3. Wide-Angle X-Ray Diffraction
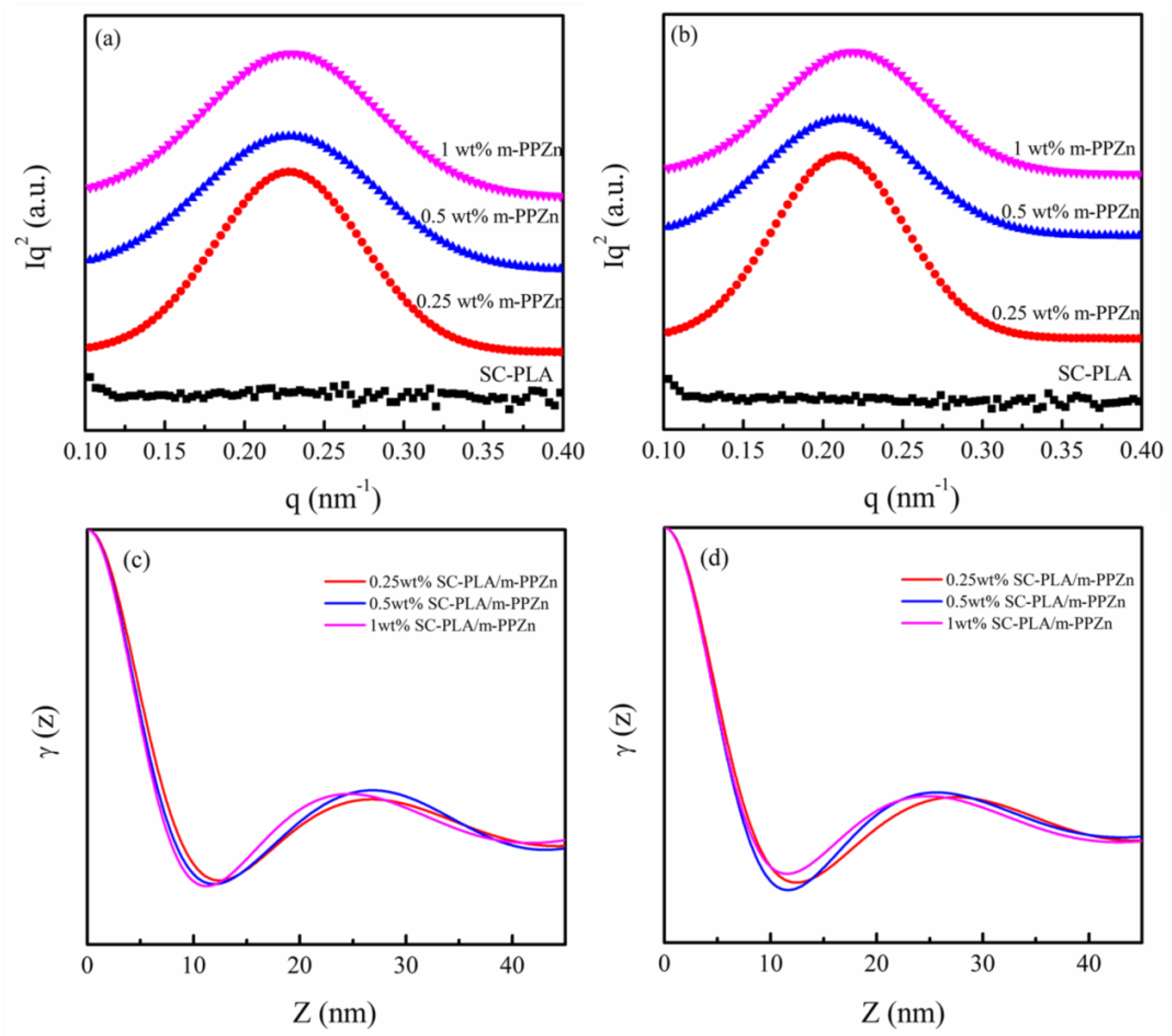
2.4. Small-Angle X-Ray Scattering
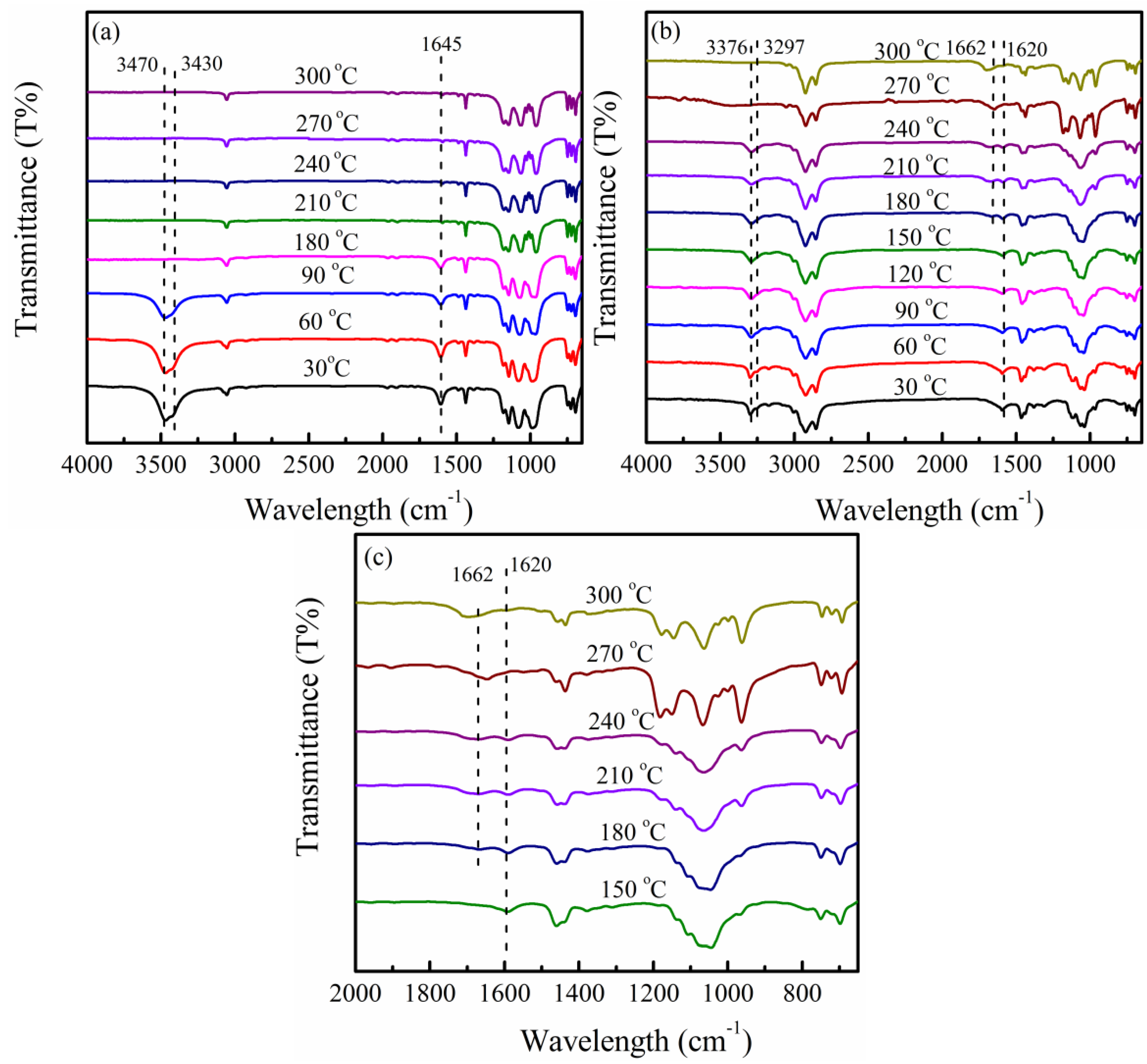
2.5. Fourier Transform Infrared Spectroscopy
2.6. Transmission Electron Microscopy
2.7. Polarized Optical Microscopy
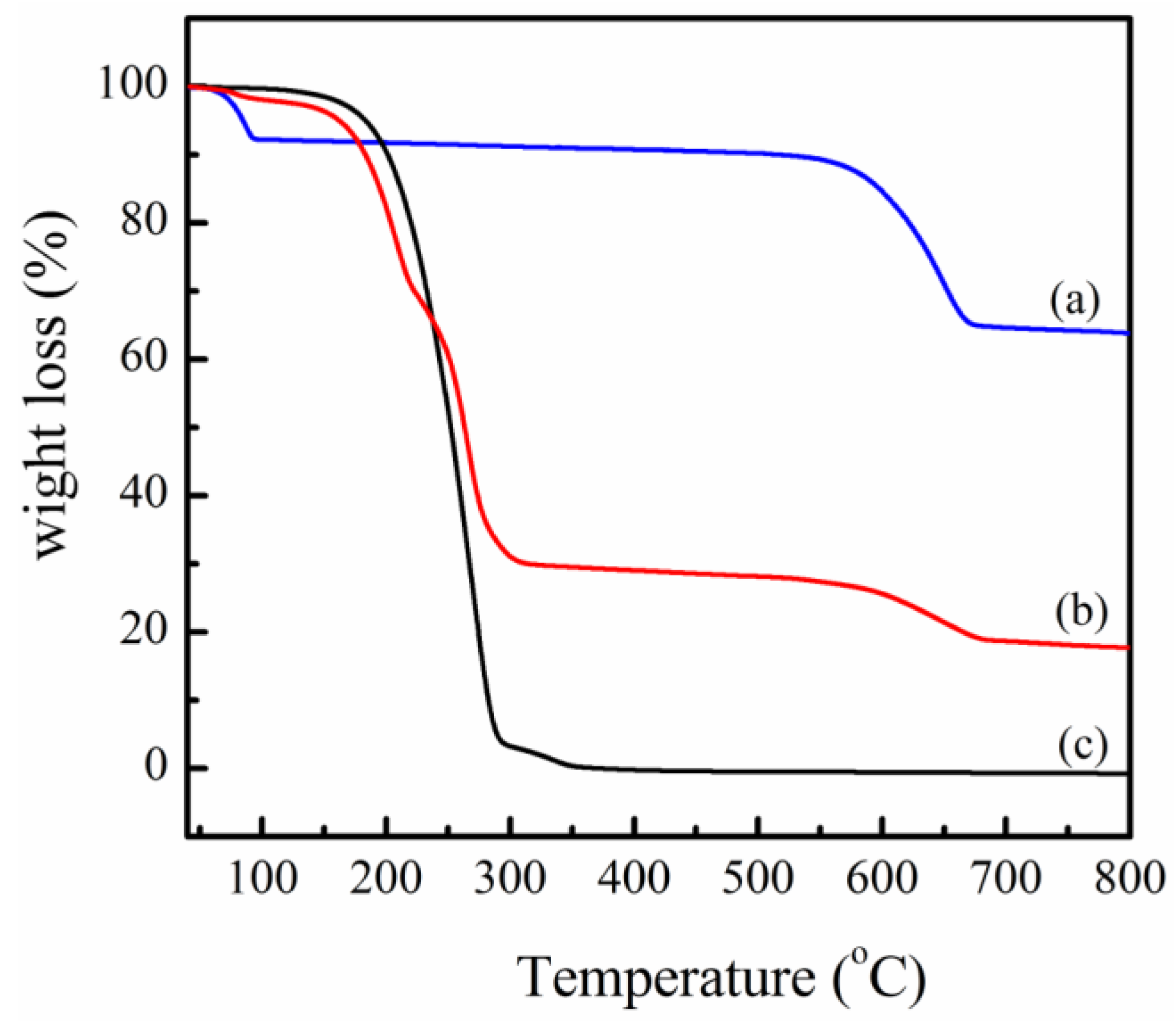
2.8. Thermogravimetric Analysis
3. Results and Discussion
3.1. PPZn and m-PPZn Characterization
3.2. In Situ Study of the Intercalation Behavior and the Thermal Properties of PPZn and m-PPZn
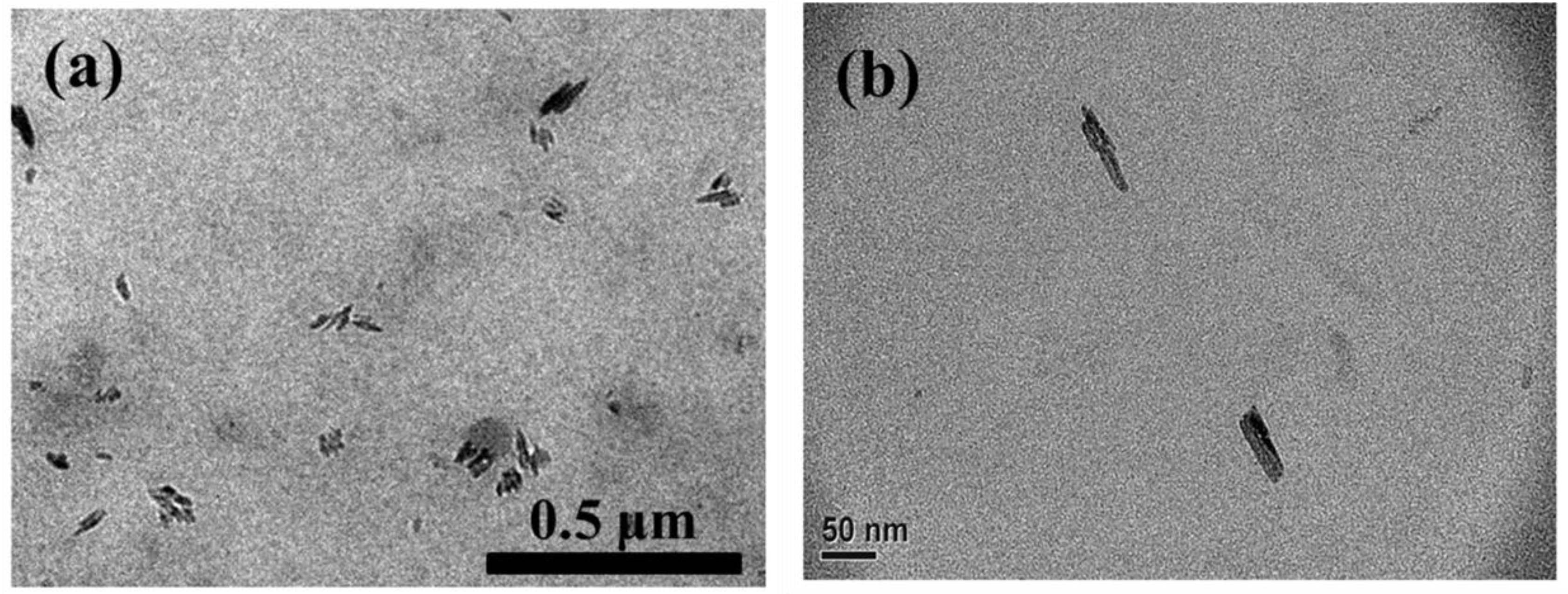
3.3. Morphology of SC-PLA and SC-PLA/m-PPZn Nanocomposites
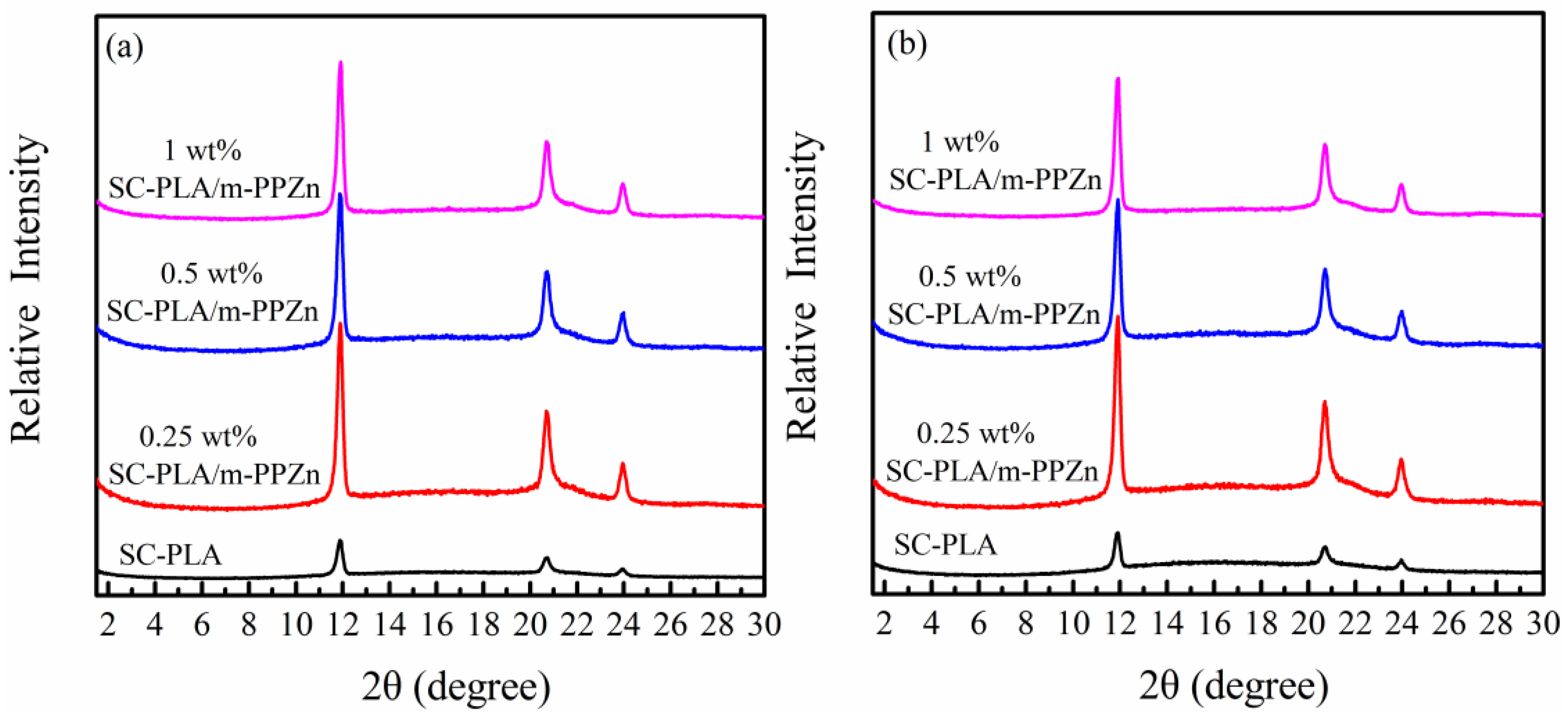
3.4. Microstructure of SC-PLA and SC-PLA/m-PPZn Nanocomposites
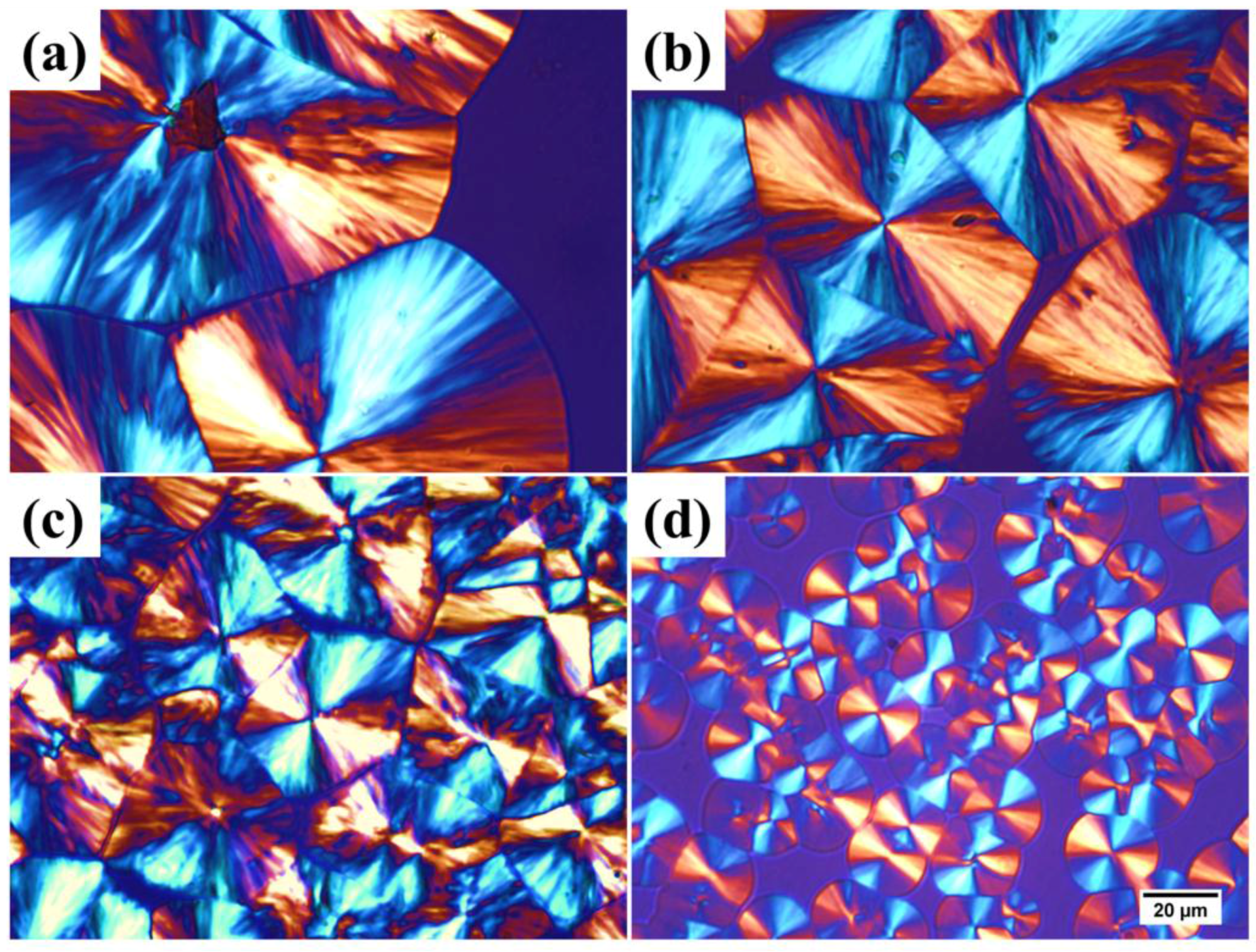
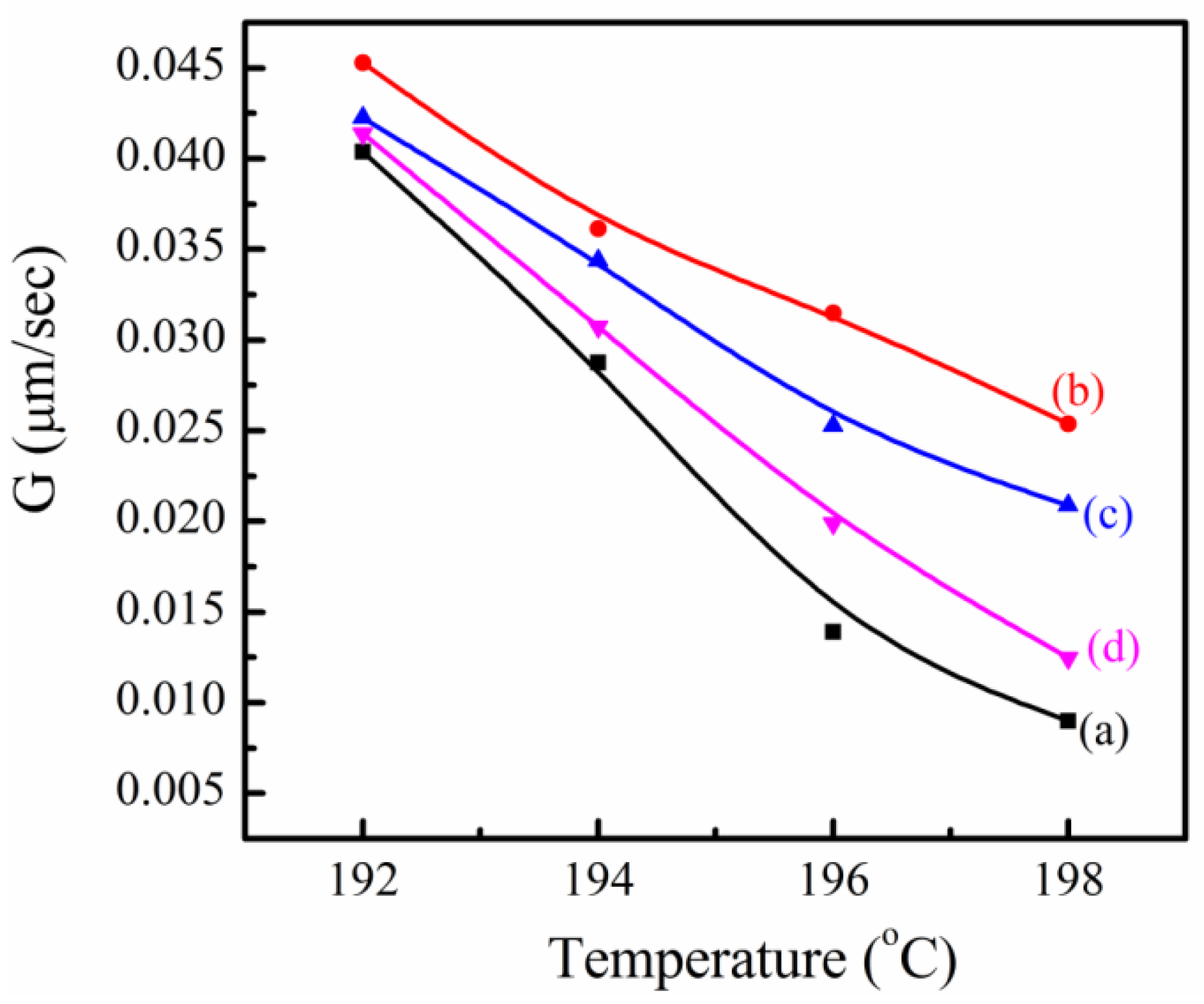
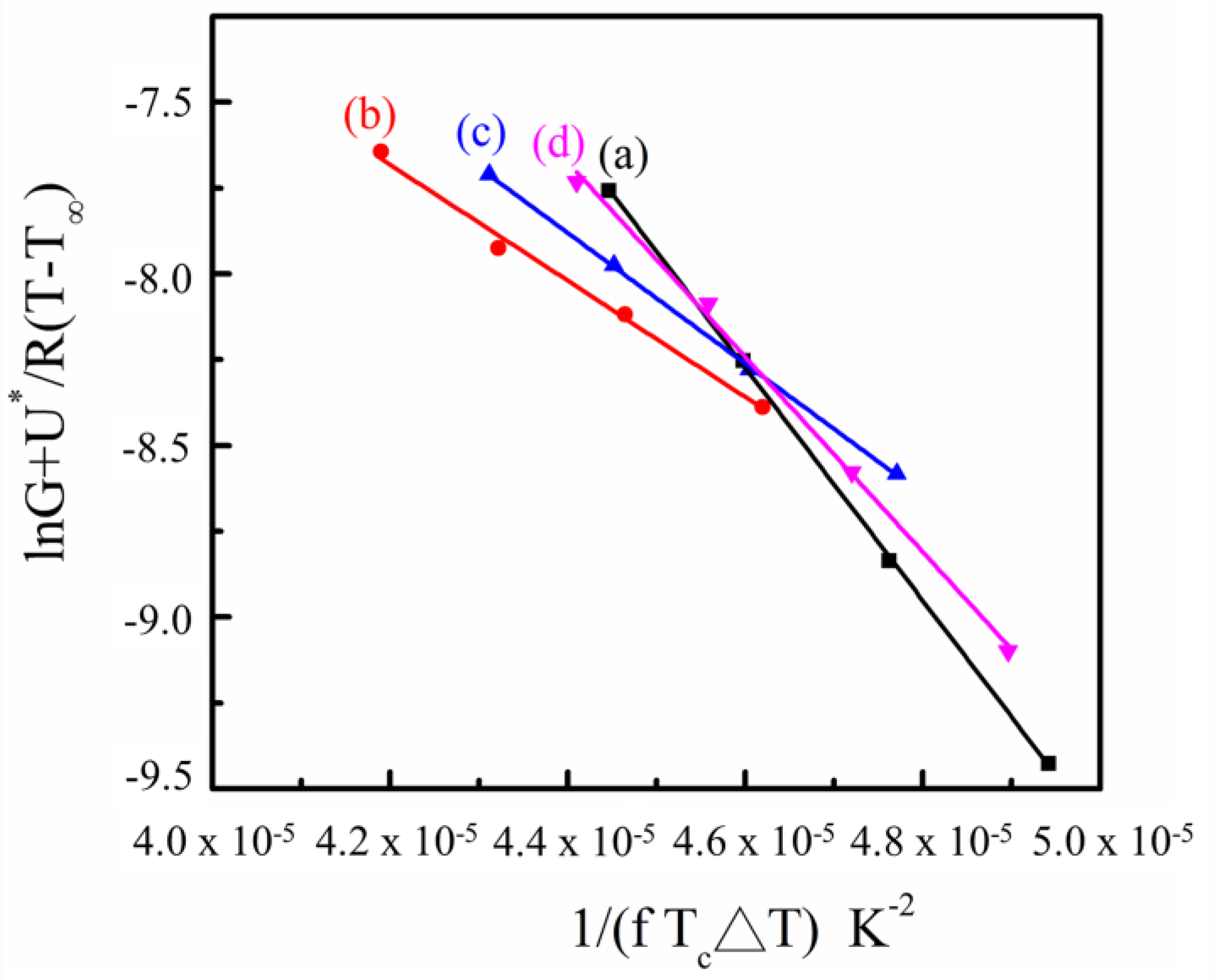
3.5. Crystallization Behavior of SC-PLA and SC-PLA/m-PPZn Nanocomposites
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 1987, 20, 904–906. [Google Scholar]
- Tsuji, H.; Ikada, Y.; Hyon, S.H.; Kimura, Y.; Kitao, T. Stereocomplex formation between enantiomeric poly(lactic acid). VIII. Complex fibers spun from mixed solution of poly(d-lactic acid) and poly(l-lactic acid). J. Appl. Polym. Sci. 1994, 51, 337–344. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(lactide) Stereocomplexes: Formation, structure, properties, degradation, and applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.A.; Wu, T.M. Crystallization kinetics of poly(1,4-butylene adipate) with stereocomplexed poly(lactic acid) serving as a nucleation agent. Ind. Eng. Chem. Res. 2014, 53, 16689–16695. [Google Scholar] [CrossRef]
- Sakakihara, H.; Takakashi, Y.; Tadokoro, H.; Oguni, N.; Tani, H. Structural studies of isotactic poly(tert-butylethylene oxide). Macromolecules 1973, 6, 205–212. [Google Scholar] [CrossRef]
- Bosscher, F.; ten Brinke, G.; Challa, G. Complex formation between enantiomeric polyesters. J. Polym. Sci. Polym. Phys. 1984, 22, 577–587. [Google Scholar]
- Lavallee, C.; Prud’homme, R.E. Stereocomplexation of isotactic polyesters of opposite configurations. Macromolecules 1989, 22, 2438–2446. [Google Scholar] [CrossRef]
- Matsubayshi, H.; Chatani, Y.; Tadokoro, H.; Dumas, P.; Spassky, N.; Gigwalt, P. Crystal structures of optically active and inactive poly(tert-butylethylene sulfide). Macromolecules 1977, 10, 996–1002. [Google Scholar] [CrossRef]
- Bosscher, F.; ten Brinke, G.; Challa, G. Crystal structure of isotactic poly(methyl methacrylate). Macromolecules 1982, 15, 1364–1368. [Google Scholar] [CrossRef]
- Koennecke, K.; Rehage, G. Crystallization and stereo assocation of stereoregular PMMA, 2. Crystallization behavious of the stereocomplex in comparison with that of pure stereoregular PMMA. Makromol. Chem. 1983, 184, 2679–2691. [Google Scholar]
- Okihara, T.; Tsuji, M.; Kawaguchi, A.; Katayama, K.; Tsuji, H.; Hyon, S.H.; Ikada, Y. Crystal structure of stereocomplex of poly(l-lactide) and poly(d-lactide). J. Macromol. Sci. Phys. 1991, B30, 119–140. [Google Scholar] [CrossRef]
- Brizzolara, D.; Cantow, H.J.; Diederichs, K.; Keller, E.; Domb, A.J. Mechanism of the stereocomplex formation between enantiomeric poly(lactide)s. Macromolecules 1996, 29, 191–197. [Google Scholar] [CrossRef]
- Cartier, L.; Okihara, T.; Lotz, B. Triangular Polymer Single Crystals: stereocomplexes, twins, and frustrated structures. Macromolecules 1997, 30, 6313–6322. [Google Scholar] [CrossRef]
- Xiong, Z.; Liu, G.; Zhang, X.; Wen, T.; Vos, S.; Joziasse, C.; Wang, D. Temperature dependence of crystalline transition of highly-oriented poly(l-lactide)/poly(d-lactide) blend: In-situ synchrotron X-ray scattering study. Polymer 2013, 54, 964–971. [Google Scholar] [CrossRef]
- Pan, P.; Han, L.; Bao, J.; Xie, Q.; Shan, G.; Bao, Y. Competitive stereocomplexation, homocrystallization, and polymorphic crystalline transition in poly(l-lactic acid)/poly(d-lactic acid) racemic blends: Molecular weight effects. J. Phys. Chem. B 2015, 119, 6462–6470. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Shan, G.; Bao, Y.; Pan, P. Exclusive Stereocomplex Crystallization of linear and multiarm star-shaped high-molecular-weight stereo diblock poly(lactic acid)s. J. Phys. Chem. B 2015, 119, 14270–14279. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Xiang, S.; Bian, X.; Sun, J.; Li, G.; Chen, X. Remarkable melting behavior of PLA stereocomplex in linear PLLA/PDLA blends. Ind. Eng. Chem. Res. 2015, 54, 2246–2253. [Google Scholar] [CrossRef]
- Shin, E.J.; Jones, A.E.; Waymouth, R.M. Stereocomplexation in cyclic and linear polylactide blends. Macromolecules 2012, 45, 595–598. [Google Scholar] [CrossRef]
- Fujita, M.; Sawayanagi, T.; Abe, H.; Tanaka, T.; Iwata, T.; Ito, K.; Fujisawa, T.; Maeda, M. Stereocomplex formation through reorganization of poly(l-lactic acid) and poly(d-lactic acid) crystals. Macromolecules 2008, 41, 2852–2858. [Google Scholar] [CrossRef]
- Chiang, M.F.; Chen, E.C.; Wu, T.M. Preparation, mechanical properties and thermal stability of poly(l-lactide)/γ-polyglutamate-modified layered double hydroxide nanocomposites. Polym. Degrad. Stab. 2012, 97, 995–1001. [Google Scholar] [CrossRef]
- Li, Y.; Han, C.; Zhang, X.; Xu, K.; Bian, J.; Dong, L. Poly(l-lactide)/Poly(d-lactide)/clay nanocomposites: Enhanced dispersion, crystallization, mechanical properties, and hydrolytic degradation. Polym. Eng. Sci. 2014, 54, 914–924. [Google Scholar] [CrossRef]
- Pan, P.; Zhu, B.; Dong, T.; Inoue, Y. Poly(l-lactide)/layered double hydroxides nanocomposites: Preparation and crystallization behavior. J. Polym. Sci. Part B: Polym. Phys. 2008, 46, 2222–2233. [Google Scholar] [CrossRef]
- Chen, H.M.; Chen, J.; Shao, L.N.; Yang, J.H.; Huang, T.; Zhang, N.; Wang, Y. Comparative study of poly(l-lactide) nanocomposites with organic montmorillonite and carbon nanotubes. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 183–196. [Google Scholar] [CrossRef]
- Salehabadi, A.; Bakar, M.A.; Bakar, N.H.H.A. Effect of organo-modified nanoclay on the thermal and bulk structural properties of poly(3-hydroxybutyrate)-epoxidized natural rubber blends: Formation of Multi-Components Biobased Nanohybrids. Materials 2014, 7, 4508–4523. [Google Scholar] [CrossRef]
- Cortés, P.; Fraga, I.; Calventus, Y.; Román, F.; Hutchinson, J.M.; Ferrando, F. A New Epoxy-Based Layered Silicate Nanocomposite Using a Hyperbranched Polymer: Study of the Curing Reaction and Nanostructure Development. Materials 2014, 7, 1830–1849. [Google Scholar] [CrossRef]
- Wang, S.; Han, G.; Bian, J.; Han, L.; Wang, X.; Dong, L. Morphology, crystallization and enzymatic hydrolysis of poly(L-lactide) nucleated using layered metal phosphonates. Polym. Int. 2011, 60, 284–295. [Google Scholar] [CrossRef]
- Pan, P.; Liang, Z.; Cao, A.; Inoue, Y. Layered Metal Phosphonate Reinforced Poly(l-lactide) Composites with a Highly Enhanced Crystallization Rate. ACS Appl. Mat. Interfaces 2009, 1, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, T.; Katayama, H.; Itoh, T. Crystallization behavior of poly(vinylidene fluoride) composites containing zinc phenylphosphonate. Polym. Eng. Sci. 2013, 53, 843–848. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Y.; He, D.; Xu, Y.; Li, Q.; Shen, C. Nucleation effect of layered metal phosphonate on crystallization of isotactic polypropylene. Polym. Test. 2014, 34, 131–139. [Google Scholar] [CrossRef]
- Yu, F.; Pan, P.; Nakamura, N.; Inoue, Y. Nucleation Effect of Layered Metal Phosphonate on Crystallization of Bacterial Poly[(3-hydroxybutyrate)-co-(3-hydroxyhexanoate)]. Macromol. Mater. Eng. 2011, 296, 103–112. [Google Scholar] [CrossRef]
- Wu, N.; Wang, H. Effect of zinc phenylphosphonate on the crystallization behavior of poly(l-lactide). J. Appl. Polym. Sci. 2013, 130, 2744–2752. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Chen, Q.; Xi, Z.; Wang, C.; Chen, X.; Feng, X.; Liang, R.; Yang, J. Modulated crystallization behavior, polymorphic crystalline structure and enzymatic degradation of poly(butylene adipate): Effects of layered metal phosphonate. Eur. Polym. J. 2015, 72, 222–237. [Google Scholar] [CrossRef]
- Poojary, D.M.; Clearfield, A. Coordinative Intercalation of Alkylamines into Layered Zinc Phenylphosphonate. Crystal Structures from X-ray Powder Diffraction Data. J. Am. Chem. Soc. 1995, 117, 11278–11284. [Google Scholar] [CrossRef]
- Zhang, Y.; Scott, K.J.; Clearfield, A. Intercalation of alkylamines into dehydrated and hydrated zinc phenyiphosphonates. J. Mater. Chem. 1995, 5, 315–318. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Liz-Marzán, L.M. Oleylamine in Nanoparticle Synthesis. Chem. Mater. 2013, 25, 1465–1476. [Google Scholar] [CrossRef]
- Borges, J.; Ribeiro, J.A.; Pereira, E.M.; Carreira, C.A.; Pereira, C.M.; Silva, F. Preparation and characterization of DNA films using oleylamine modified Au surfaces. J. Colloid. Interface. Sci. 2001, 358, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, Y.; Morioka, K.; Tamegai, H.; Yoshie, N. Preparation and some properties of stereocomplex-type poly(lactic acid)/layered silicate nanocomposites. J. Appl. Polym. Sci. 2013, 127, 1615–1622. [Google Scholar] [CrossRef]
- Purnama, P.; Jung, Y.; Kim, S.H. An Advanced class of bio-hybrid materials: Bionanocomposites of inorganic clays and organic stereocomplex polylactides. Macromol. Mater. Eng. 2013, 298, 263–269. [Google Scholar] [CrossRef]
- Re, G.L.; Benali, S.; Habibi, Y.; Raquez, J.M.; Dubois, P. Stereocomplexed PLA nanocomposites: From in situ polymerization to materials properties. Eur. Polym. J. 2014, 54, 138–150. [Google Scholar] [CrossRef]
- Han, L.; Pan, P.; Shan, G.; Bao, Y. Stereocomplex crystallization of high-molecular-weight poly(l-lactic acid)/poly(d-lactic acid) racemic blends promoted by a selective nucleator. Polymer 2015, 63, 144–153. [Google Scholar] [CrossRef]
- Chen, Y.A.; Chen, E.C.; Wu, T.M. Organically modified layered zinc phenylphosphonate reinforced stereocomplex-type poly(lactic acid) nanocomposites with highly enhanced mechanical properties and degradability. J. Mater. Sci. 2015, 50, 7770–7778. [Google Scholar] [CrossRef]
- Bao, R.Y.; Yang, W.; Wei, X.F.; Xie, B.H.; Yang, M.B. Enhanced Formation of Stereocomplex Crystallites of High Molecular Weight Poly(L lactide)/Poly(D-lactide) Blends from Melt by Using Poly(ethylene glycol). ACS Sustainable Chem. Eng. 2014, 2, 2301–2309. [Google Scholar] [CrossRef]
- Li, Y.; Han, C.; Bian, Y.; Dong, Q.; Zhao, H.; Zhang, X.; Xu, M.; Dong, L. Miscibility, thermal properties and polymorphism of stereocomplexation of high-molecular-weightpolylactide/poly(D,L-lactide) blends. Thermochim. Acta. 2014, 580, 53–62. [Google Scholar] [CrossRef]
- Strobl, G.R.; Schneider, M. Direct evaluation of the electron densitycorrelation function of partially crystalline polymers. J. Polym. Sci. Polym. Phys. Ed. 1980, 18, 1343–1359. [Google Scholar] [CrossRef]
- Barbi, V.; Funari, S.S.; Gehrke, R.; Scharnagl, N.; Stribeck, N. SAXS and the gas transport in polyether-block-polyamide copolymer membranes. Macromolecules 2003, 36, 749–758. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Weeks, J.J. Melting process and the equilibrium melting temperature of polychlorotrifluoroethylene. J. Res. Natl. Bur. Stand. Sect. A. 1962, 66, 13–28. [Google Scholar] [CrossRef]
- Hoffman, J.D. Regime III crystallization in melt-crystallized polymers: The variable cluster model of chain folding. Polymer 1983, 24, 3–26. [Google Scholar] [CrossRef]
- Lauritzen, J.I.; Hoffman, J.D. Extension of theory of growth of chain-Folded polymer crystals to large undercoolings. J. Appl. Phys. 1973, 44, 4340–4352. [Google Scholar] [CrossRef]
- Baimark, Y.; Srihanam, P. Influence of chain extender on thermal properties and melt flow index of stereocomplex PLA. Polym. Test. 2015, 45, 52–57. [Google Scholar] [CrossRef]
- Sun, J.; Yu, H.; Zhuang, X.; Chen, X.; Jing, X. Crystallization behavior of asymmetric PLLA/PDLA blends. J. Phys. Chem. B 2011, 115, 2864–2869. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, J.I. Effect of a finite substrate length upon polymer crystal lamellar growth rate. J. Appl. Phys. 1973, 44, 4353–4359. [Google Scholar] [CrossRef]
| Sample | Temperature (°C) | Xc (%) | LP (nm) | lc (nm) | la (nm) |
|---|---|---|---|---|---|
| SC-PLA | 192 | 11.59 | – | – | – |
| 196 | 10.96 | – | – | – | |
| 0.25 wt% SC-PLA/m-PPZn | 192 | 35.32 | 25.41 | 8.78 | 16.63 |
| 196 | 35.21 | 27.03 | 9.06 | 17.79 | |
| 0.5 wt% SC-PLA/m-PPZn | 192 | 34.89 | 25.20 | 8.49 | 16.71 |
| 196 | 34.42 | 26.95 | 8.74 | 18.21 | |
| 1 wt% | 192 | 33.41 | 24.87 | 8.08 | 16.79 |
| SC-PLA/m-PPZn | 196 | 32.13 | 26.72 | 8.49 | 18.42 |
| sample | (K) | Kg (K2) | σσe (erg2/cm4) |
|---|---|---|---|
| SC-PLA | 516.15 | 3.51 × 105 | 222.13 |
| 0.25 wt% SC-PLA/m-PPZn | 519.43 | 1.66 × 105 | 104.67 |
| 0.5 wt% SC-PLA/m-PPZn | 517.84 | 1.94 × 105 | 122.31 |
| 1 wt% SC-PLA/m-PPZn | 516.59 | 2.84 × 105 | 179.53 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-A.; Chen, E.-C.; Wu, T.-M. Lamellae Evolution of Stereocomplex-Type Poly(Lactic Acid)/Organically-Modified Layered Zinc Phenylphosphonate Nanocomposites Induced by Isothermal Crystallization. Materials 2016, 9, 159. https://doi.org/10.3390/ma9030159
Chen Y-A, Chen E-C, Wu T-M. Lamellae Evolution of Stereocomplex-Type Poly(Lactic Acid)/Organically-Modified Layered Zinc Phenylphosphonate Nanocomposites Induced by Isothermal Crystallization. Materials. 2016; 9(3):159. https://doi.org/10.3390/ma9030159
Chicago/Turabian StyleChen, Yi-An, Erh-Chiang Chen, and Tzong-Ming Wu. 2016. "Lamellae Evolution of Stereocomplex-Type Poly(Lactic Acid)/Organically-Modified Layered Zinc Phenylphosphonate Nanocomposites Induced by Isothermal Crystallization" Materials 9, no. 3: 159. https://doi.org/10.3390/ma9030159
APA StyleChen, Y.-A., Chen, E.-C., & Wu, T.-M. (2016). Lamellae Evolution of Stereocomplex-Type Poly(Lactic Acid)/Organically-Modified Layered Zinc Phenylphosphonate Nanocomposites Induced by Isothermal Crystallization. Materials, 9(3), 159. https://doi.org/10.3390/ma9030159






