Effect of Water Vapor and Surface Morphology on the Low Temperature Response of Metal Oxide Semiconductor Gas Sensors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sensing Layers and Surface Morphology
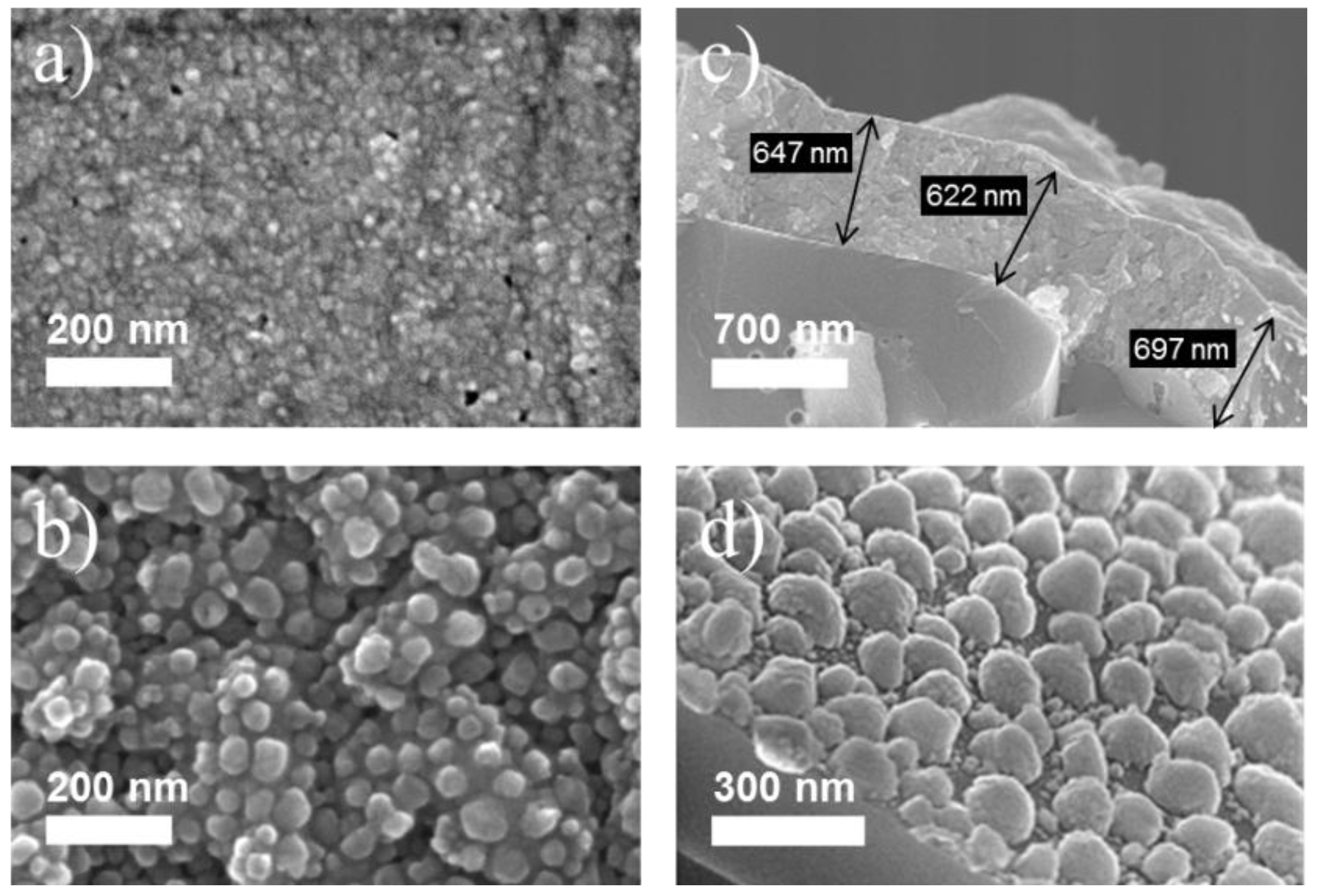
2.2. Gas Sensing Properties
2.2.1. Humidity Response
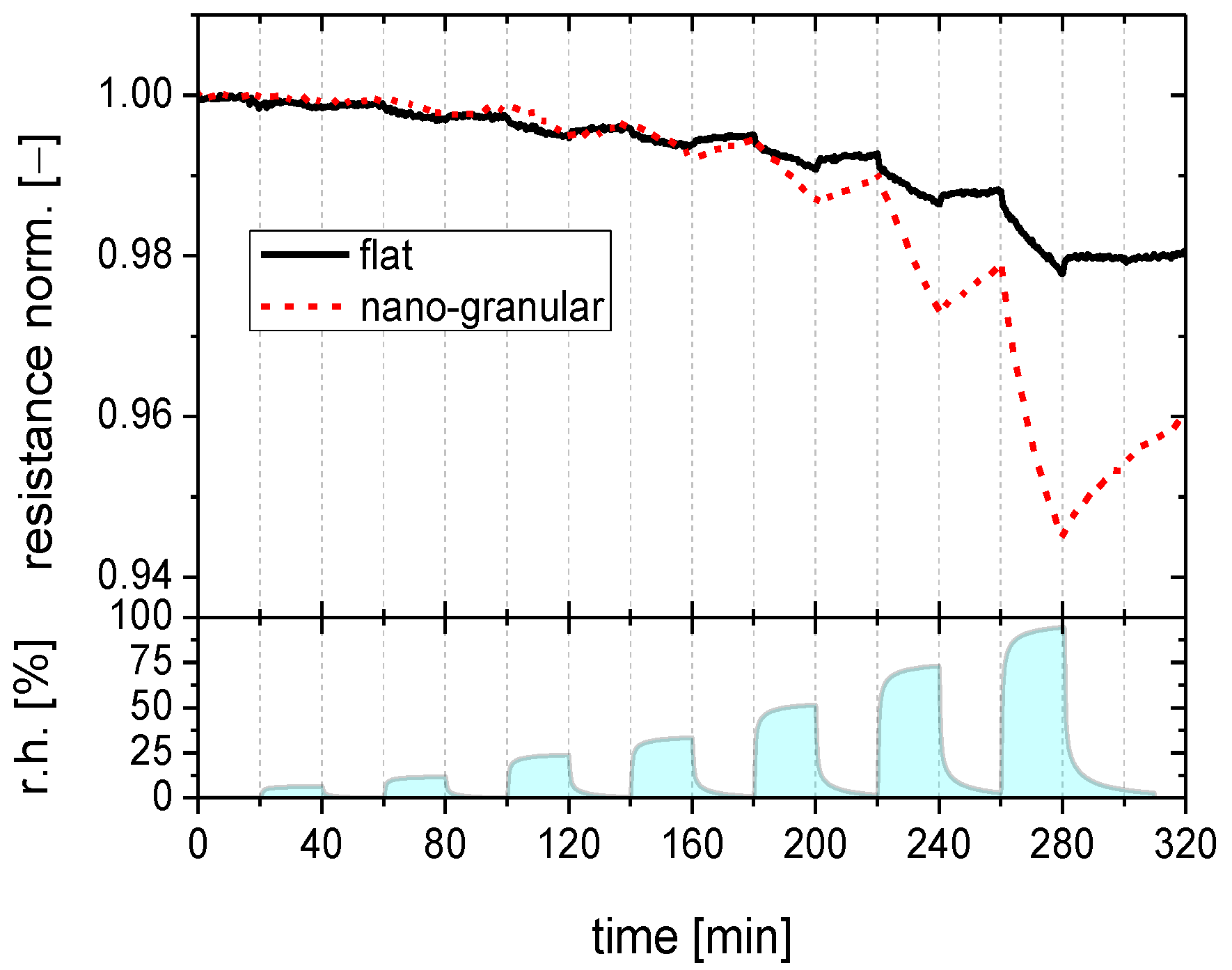
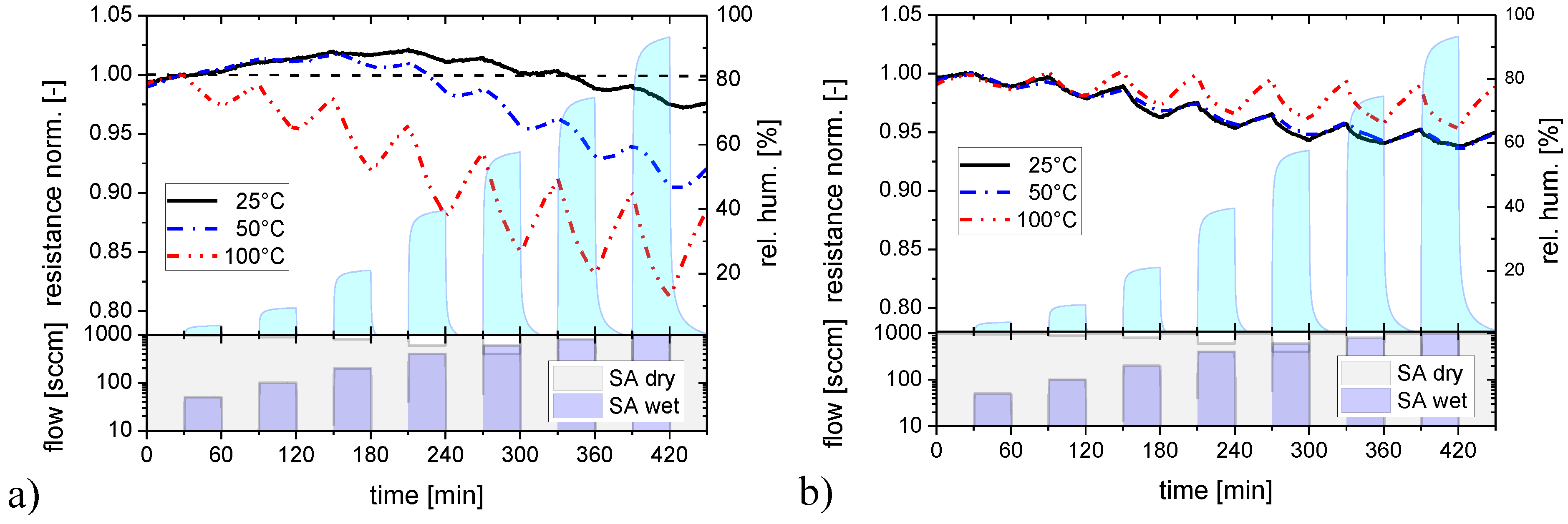
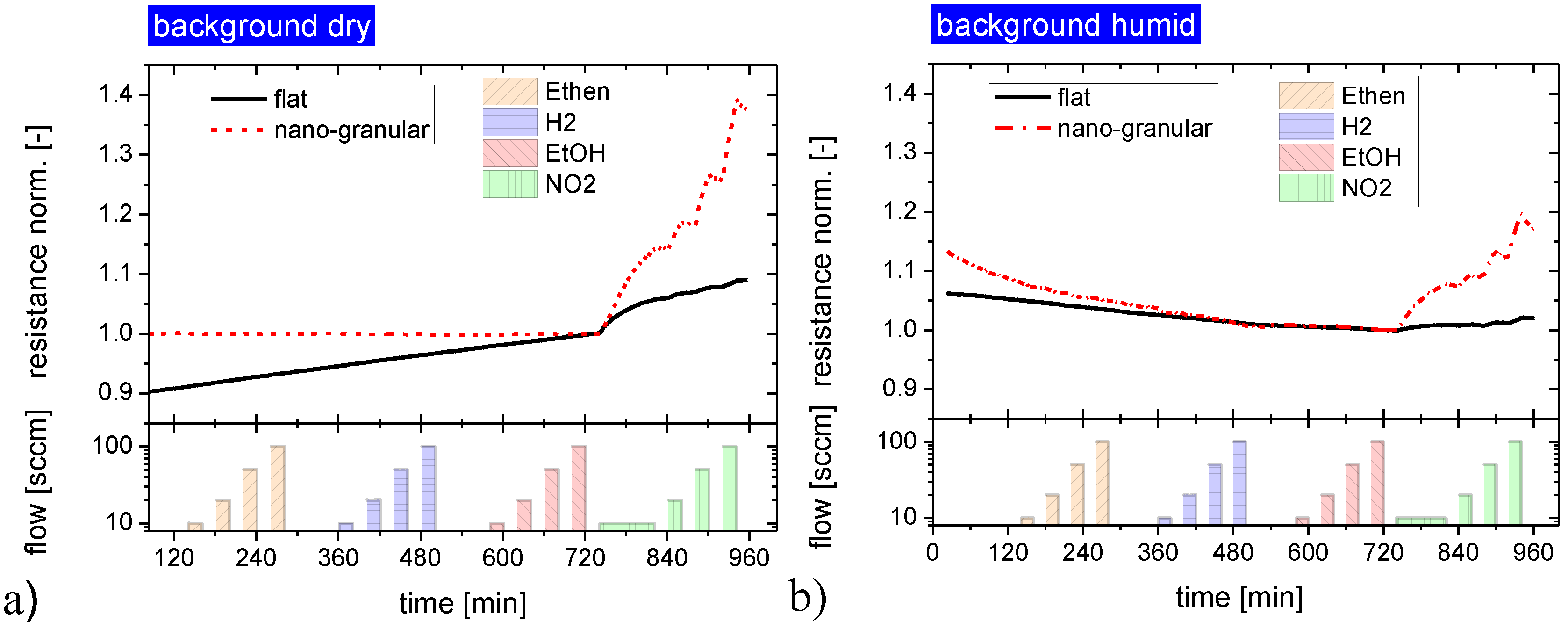
2.2.2. Response to Acid- and Base-Forming Gases
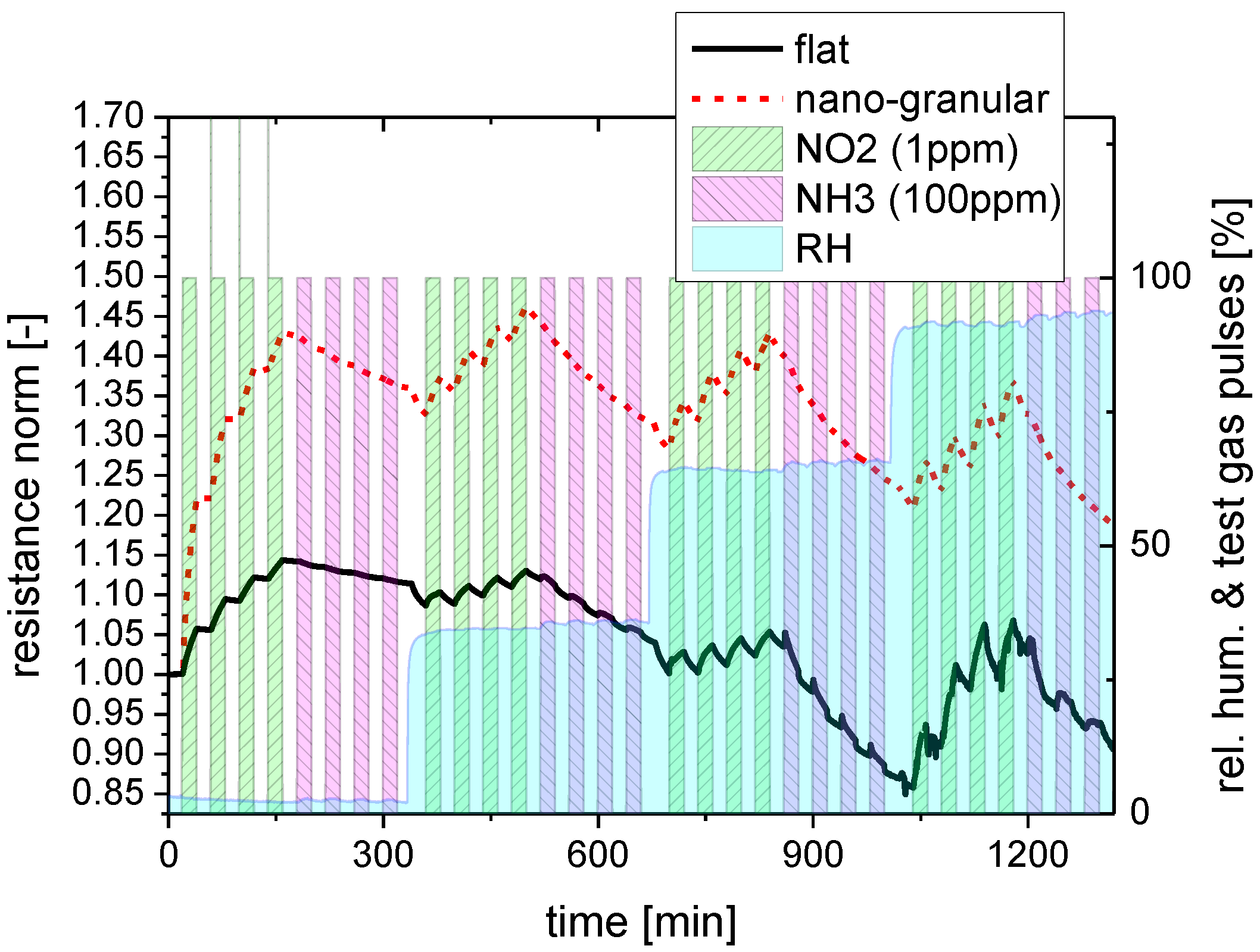
2.2.3. Response to Non-Acid and Non-Base Forming Gases
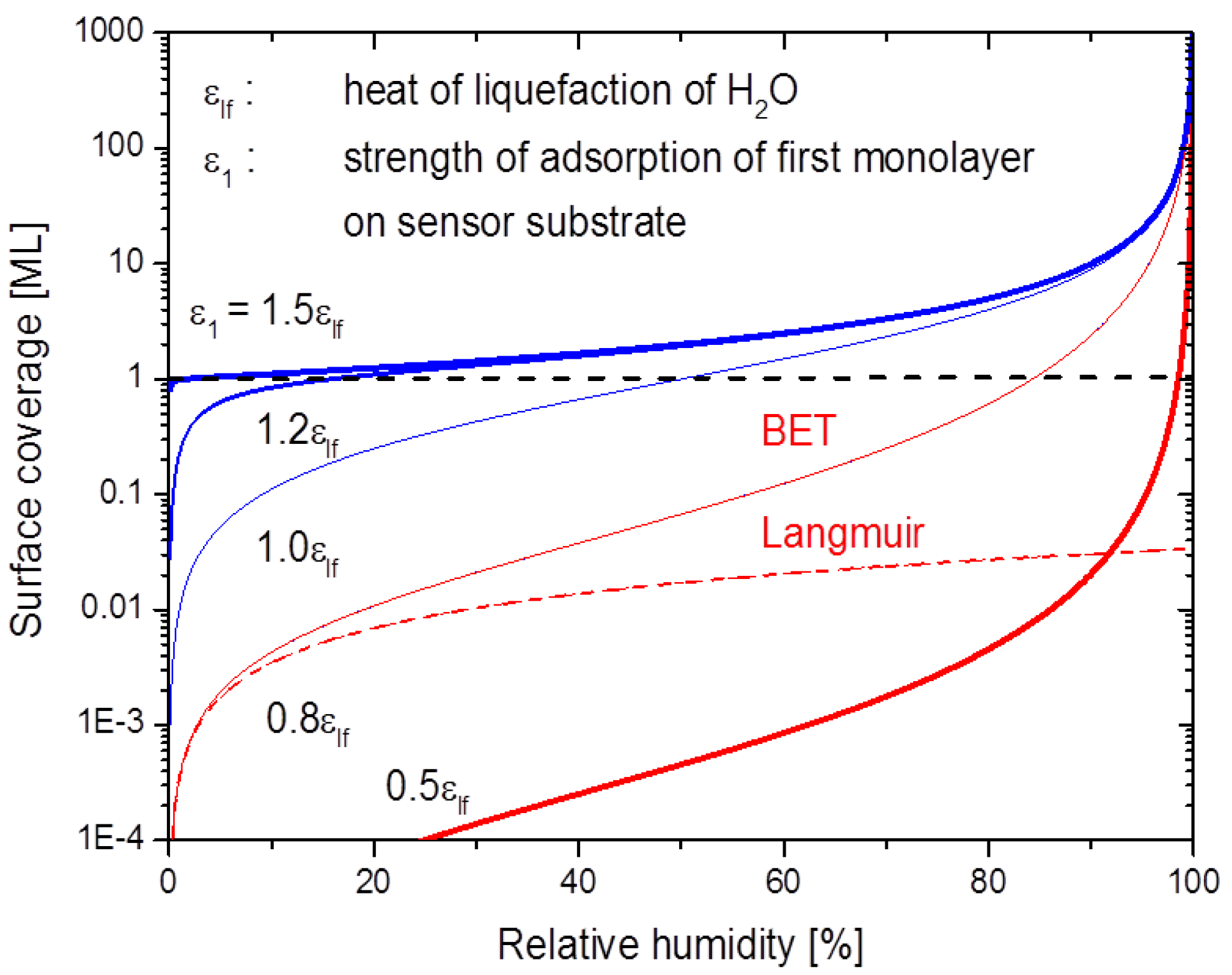
2.3. Formation of BET Adsorbates
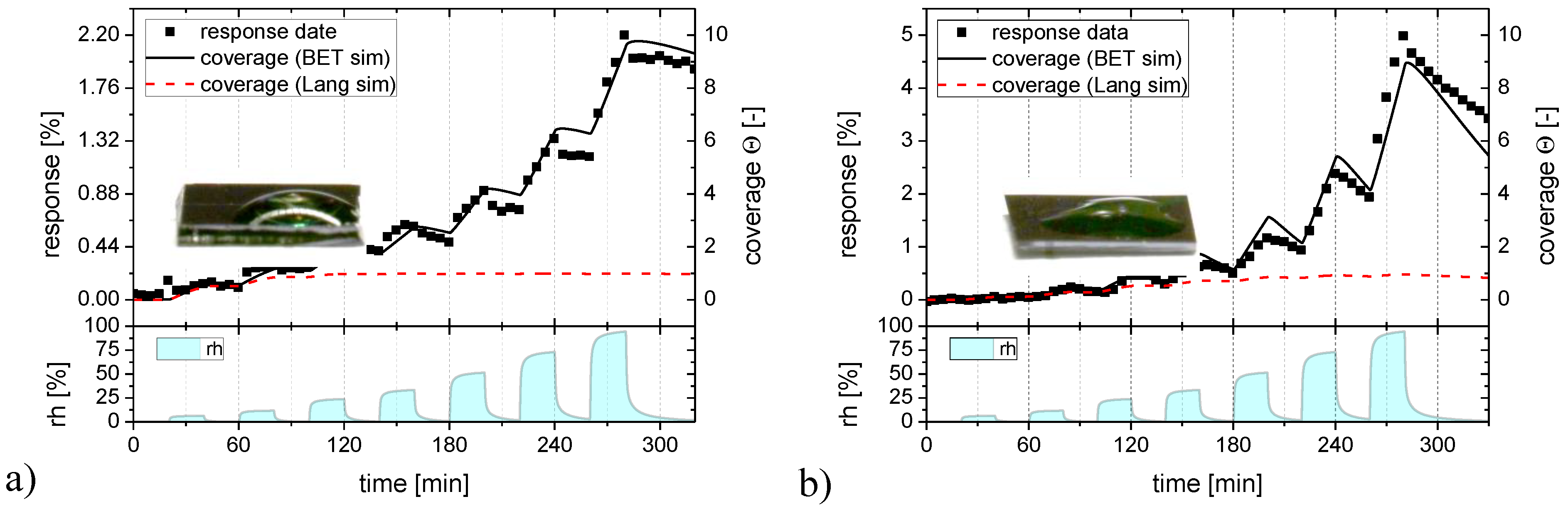
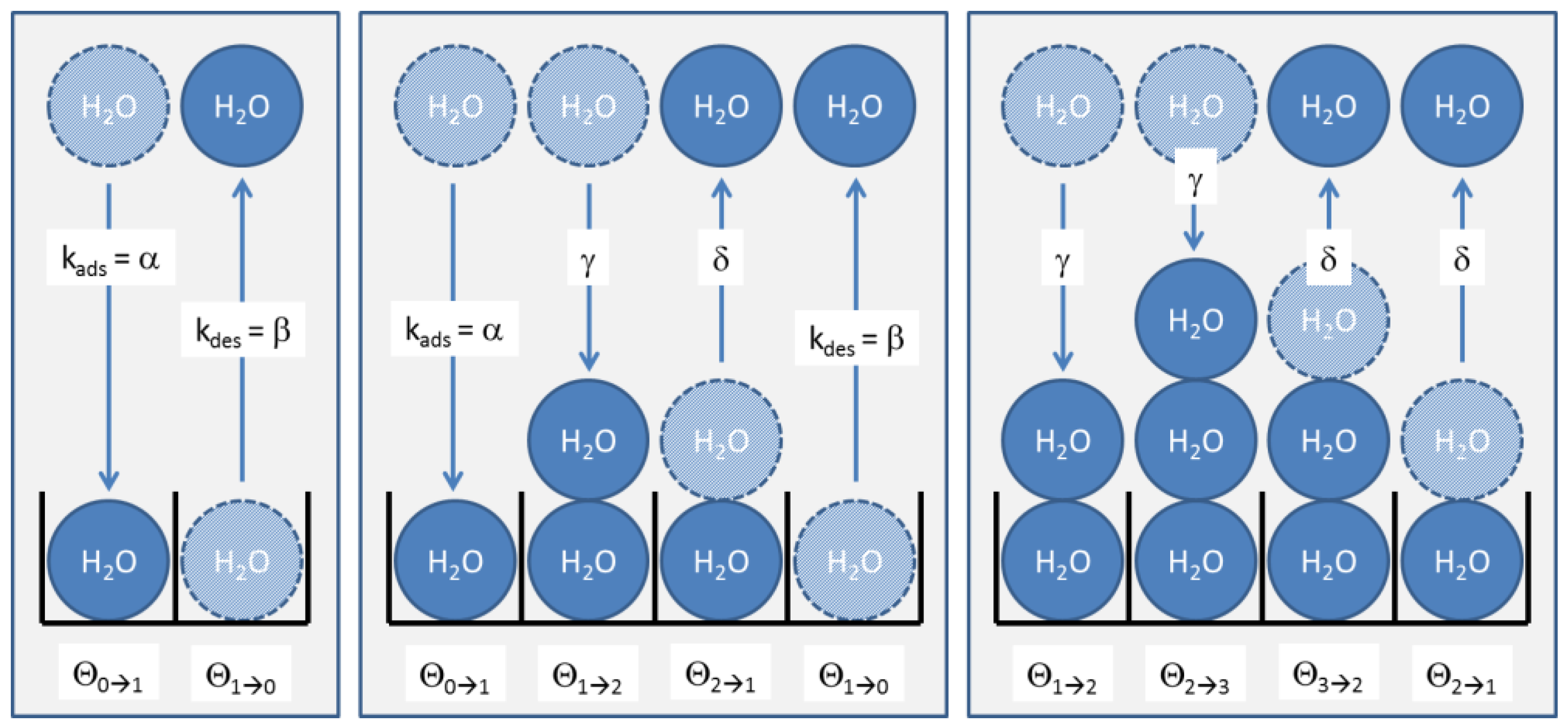
2.4. Adsorption and Desorption Kinetics
| Process | Kinetic Parameter | Flat Surface | Nano-Granular |
|---|---|---|---|
| Langmuir adsorption | kads | 1 × 10−4 | 1 × 10−4 |
| Langmuir desorption | kdes | 0.2 kads | 0.2 kads |
| BET adsorption on substrate | α | 1 × 10−4 | 1 × 10−4 |
| BET desorption from substrate | 0.2 α | 0.35 α | |
| BET adsorption onto pre-adsorbed H2O | γ | 0.35 α | 0.5 α |
| BET desorption from pre-adsorbed H2O | δ | 8.75 α | 15 α |
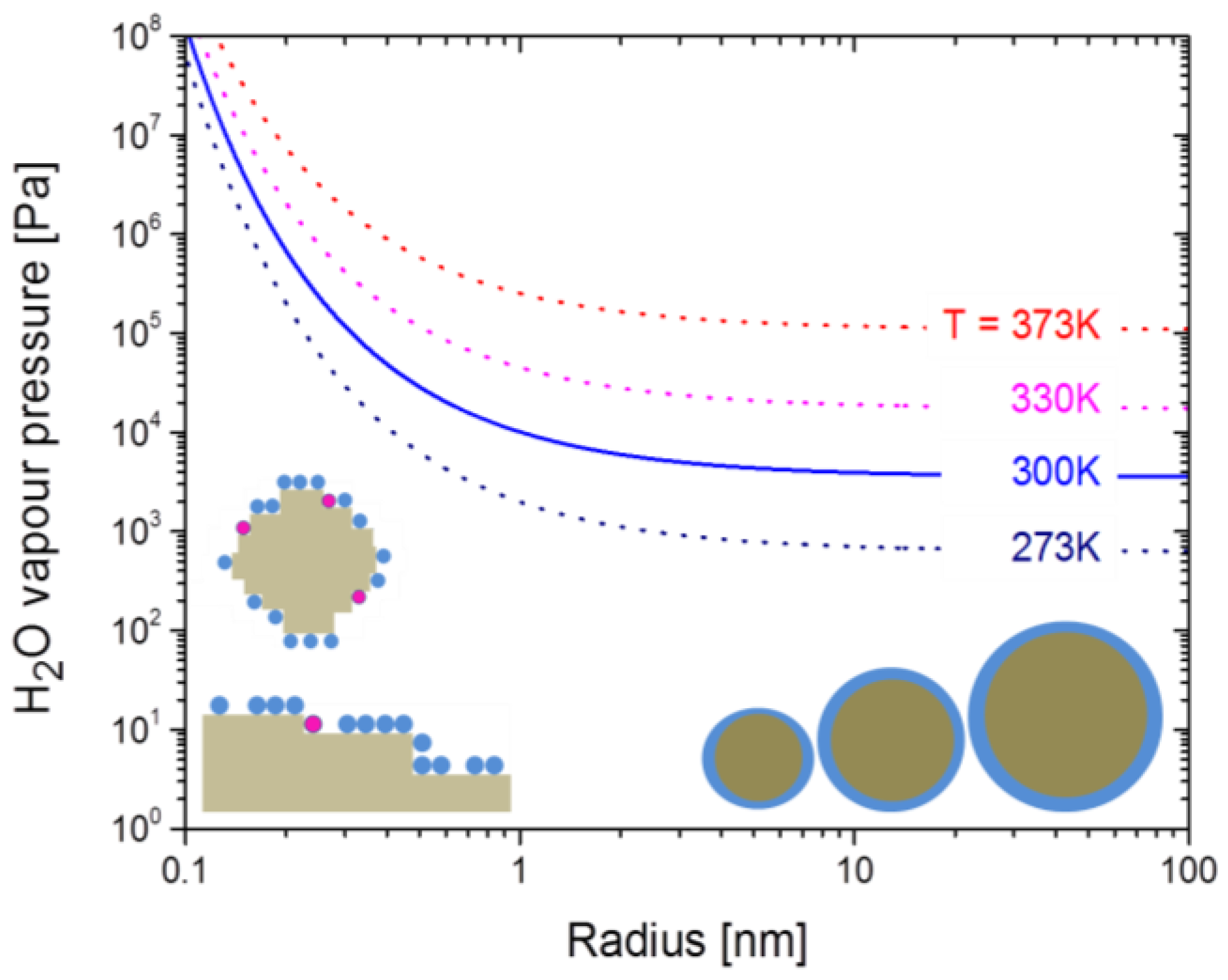
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix: Kinetics of BET Multi-Layer Adsorption
A.1. Langmuir Kinetics
A.2. BET Kinetics
References
- Zhao, J.; Huo, L.-H.; Gao, S.; Zhao, H.; Zhao, J.-G. Alcohols and acetone sensing properties of SnO2 thin films deposited by dip-coating. Sens. Actuators B Chem. 2006, 115, 460–464. [Google Scholar] [CrossRef]
- Wang, H.C.; Li, Y.; Yang, M.J. Fast response thin film SnO2 gas sensors operating at room temperature. Sens. Actuators B Chem. 2006, 119, 380–383. [Google Scholar] [CrossRef]
- Anothainart, K.; Burgmair, M.; Karthigeyan, A.; Zimmer, M.; Eisele, I. Light enhanced NO2 gas sensing with tin oxide at room temperature: Conductance and work function measurements. Sens. Actuators B Chem. 2003, 93, 580–584. [Google Scholar] [CrossRef]
- Wei, B.-Y.; Hsu, M.-C.; Su, P.-G.; Lin, H.-M.; Wu, R.-J.; Lai, H.-J. A novel SnO2 gas sensor doped with carbon nanotubes operating at room temperature. Sens. Actuators B Chem. 2004, 101, 81–89. [Google Scholar] [CrossRef]
- Comini, E. Metal oxide nano-crystals for gas sensing. Anal. Chim. Acta 2006, 568, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Suchea, M.; Katsarakis, N.; Christoulakis, S.; Nikolopoulou, S.; Kiriakidis, G. Low temperature indium oxide gas sensors. Sens. Actuators B Chem. 2006, 118, 135–141. [Google Scholar] [CrossRef]
- Afshar, M.; Preiß, E.M.; Sauerwald, T.; Rodner, M.; Feili, D.; Straub, M.; König, K.; Schütze, A.; Seidel, H. Indium-tin-oxide single-nanowire gas sensor fabricated via laser writing and subsequent etching. Sens. Actuators B Chem. 2015, 215, 525–535. [Google Scholar] [CrossRef]
- Comini, E.; Cristalli, A.; Faglia, G.; Sberveglieri, G. Light enhanced gas sensing properties of indium oxide and tin dioxide sensors. Sens. Actuators B Chem. 2000, 65, 260–263. [Google Scholar] [CrossRef]
- Comini, E.; Faglia, G.; Sberveglieri, G. UV light activation of tin oxide thin films for NO2 sensing at low temperatures. Sens. Actuators B Chem. 2001, 78, 73–77. [Google Scholar] [CrossRef]
- Yang, T.-Y.; Lin, H.-M.; Wei, B.-Y.; Wu, C.-Y.; Lin, C.-K. UV enhancement of the gas sensing properties of nano-TiO2. Rev. Adv. Mater. Sci. 2003, 4, 48–54. [Google Scholar]
- Mishra, S.; Ghanshyam, C.; Ram, N.; Bajpai, R.P.; Bedi, R.K. Detection mechanism of metal oxide gas sensor under UV radiation. Sens. Actuators B Chem. 2004, 97, 387–390. [Google Scholar] [CrossRef]
- Fu, T. Sensing properties and mechanism of gas sensor for H2S and NO2 based on [Cu5(bipyO2)6Cl8]Cl2. Sens. Actuators B Chem. 2007, 123, 1113–1119. [Google Scholar] [CrossRef]
- Helwig, A.; Müller, G.; Eickhoff, M.; Sberveglieri, G. Dissociative Gas Sensing at Metal Oxide Surfaces. IEEE Sens. J. 2007, 7, 1675–1679. [Google Scholar] [CrossRef]
- Schalwig, J.; Müller, G.; Ambacher, O.; Stutzmann, M. Group-III-nitride based gas sensing devices. Phys. Status Solidi 2001, 185, 39–45. [Google Scholar] [CrossRef]
- Schalwig, J.; Müller, G.; Eickhoff, M.; Ambacher, O.; Stutzmann, M. Gas sensitive GaN/AlGaN-heterostructures. Sens. Actuators B Chem. 2002, 87, 425–430. [Google Scholar] [CrossRef]
- Stutzmann, M.; Steinhoff, G.; Eickhoff, M.; Ambacher, O.; Nebel, C.E.; Schalwig, J.; Neuberger, R.; Müller, G. GaN-based heterostructures for sensor applications. Diam. Relat. Mater. 2002, 11, 886–891. [Google Scholar] [CrossRef]
- Offermans, P.; Vitushinsky, R.; Fabrication, A.D. NO2 detection with AlGaN/GaN 2DEG channels for air quality monitoring. IEEE Sens. J. 2013, 13, 2823–2827. [Google Scholar] [CrossRef]
- Vitushinsky, R.; Crego-Calama, M.; Brongersma, S.H.; Offermans, P. Enhanced detection of NO2 with recessed AlGaN/GaN open gate structures. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]
- Pearton, S.J.; Kang, B.S.; Kim, S.; Ren, F.; Gila, B.P.; Abernathy, C.R.; Lin, J.; Chu, S.N.G. GaN-based diodes and transistors for chemical, gas, biological and pressure sensing. J. Phys. Condens. Matter 2004, 16, R961–R994. [Google Scholar] [CrossRef]
- Paul, S.; Helwig, A.; Müller, G.; Furtmayr, F.; Teubert, J.; Eickhoff, M.; Sumit, P. Opto-chemical sensor system for the detection of H2 and hydrocarbons based on InGaN/GaN nanowires. Sens. Actuators B Chem. 2012, 173, 120–126. [Google Scholar] [CrossRef]
- Teubert, J.; Paul, S.; Helwig, A.; Müller, G.; Eickhoff, M. Group III-Nitride Chemical Nanosensors with Optical Readout. In Gas Sensing Fundamentals; Kohl, C.-D., Wagner, T., Eds.; Springer Series on Chemical Sensors and Biosensors; Springer: Berlin, Germany; Heidelberg, Germany, 2014; Volume 15, pp. 311–338. [Google Scholar]
- Maier, K.; Helwig, A.; Müller, G.; Becker, P.; Hille, P.; Schörmann, J.; Teubert, J.; Eickhoff, M. Detection of oxidising gases using an optochemical sensor system based on GaN/InGaN nanowires. Sens. Actuators B Chem. 2014, 197, 87–94. [Google Scholar] [CrossRef]
- Weidemann, O.; Hermann, M.; Steinhoff, G.; Wingbrant, H.; Lloyd Spetz, A.; Stutzmann, M.; Eickhoff, M. Influence of surface oxides on hydrogen-sensitive Pd:GaN Schottky diodes. Appl. Phys. Lett. 2003, 83. [Google Scholar] [CrossRef]
- Steinhoff, G.; Hermann, M.; Schaff, W.J.; Eastman, L.F.; Stutzmann, M.; Eickhoff, M. pH response of GaN surfaces and its application for pH-sensitive field-effect transistors. Appl. Phys. Lett. 2003, 83. [Google Scholar] [CrossRef]
- Eickhoff, M.; Neuberger, R.; Steinhoff, G.; Ambacher, O.; Müller, G.; Stutzmann, M. Wetting Behaviour of GaN Surfaces with Ga- or N-Face Polarity. Phys. Status Solidi 2001, 228, 519–522. [Google Scholar] [CrossRef]
- Tan, O.Z.; Tsai, K.H.; Wu, M.C.H.; Kuo, J.L. Structural and dynamic properties of water on the GaN polar surface. J. Phys. Chem. C 2011, 115, 22444–22450. [Google Scholar] [CrossRef]
- Bermudez, V.M.; Long, J.P. Chemisorption of H2O on GaN(0001). Surf. Sci. 2000, 450, 98–105. [Google Scholar] [CrossRef]
- Coan, M.R.; León-Plata, P.; Seminario, J.M. Ab Initio Analysis of the Interactions of GaN Clusters with Oxygen and Water. J. Phys. Chem. C 2012, 116, 12079–12092. [Google Scholar] [CrossRef]
- Helwig, A.; Müller, G.; Garrido, J.A.; Eickhoff, M. Gas sensing properties of hydrogen-terminated diamond. Sens. Actuators B Chem. 2008, 133, 156–165. [Google Scholar] [CrossRef]
- Beer, S.; Helwig, A.; Müller, G.; Garrido, J.; Stutzmann, M. Water adsorbate mediated accumulation gas sensing at hydrogenated diamond surfaces. Sens. Actuators B Chem. 2013, 181, 894–903. [Google Scholar] [CrossRef]
- Krstev, I.; Helwig, A.; Müller, G.; Garrido, J.; Stutzmann, M. Detection of random vapour concentrations using an integrating diamond gas sensor. Sens. Actuators B Chem. 2014, 195, 603–608. [Google Scholar] [CrossRef]
- Groß, A.; Beulertz, G.; Marr, I.; Kubinski, D.J.; Visser, J.H.; Moos, R. Dual mode NOx sensor: Measuring both the accumulated amount and instantaneous level at low concentrations. Sensors. (Basel) 2012, 12, 2831–2850. [Google Scholar] [CrossRef] [PubMed]
- Marr, I.; Groß, A.; Moos, R. Overview on conductometric solid-state gas dosimeters. J. Sens. Sens. Syst. 2014, 3, 29–46. [Google Scholar] [CrossRef]
- Landstrass, M.I.; Ravi, K.V. Hydrogen passivation of electrically active defects in diamond. Appl. Phys. Lett. 1989, 55. [Google Scholar] [CrossRef]
- Maki, T.; Shikama, S.; Komori, M.; Sakaguchi, Y.; Sakuta, K.; Kobayashi, T. Hydrogenating Effect of Single-Crystal Diamond Surface. Jpn. J. Appl. Phys. 1992, 31, L1446–L1449. [Google Scholar] [CrossRef]
- Hokazono, A.; Kawarada, H. Enhancement/depletion surface channel field effect transistors of diamond and their logic circuits. Jpn. J. Appl. Phys. 1997, 36, 7133–7139. [Google Scholar] [CrossRef]
- Hayashi, K.; Yamanaka, S.; Okushi, H.; Kajimura, K. Study of the effect of hydrogen on transport properties in chemical vapor deposited diamond films by Hall measurements. Appl. Phys. Lett. 1996, 68, 376–378. [Google Scholar] [CrossRef]
- Chakrapani, V.; Angus, J.C.; Anderson, A.B.; Wolter, S.D.; Stoner, B.R.; Sumanasekera, G.U. Charge transfer equilibria between diamond and an aqueous oxygen electrochemical redox couple. Science 2007, 318, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Kromka, A.; Davydova, M.; Rezek, B.; Vanecek, M.; Stuchlik, M.; Exnar, P.; Kalbac, M. Gas sensing properties of nanocrystalline diamond films. Diam. Relat. Mater. 2010, 19, 196–200. [Google Scholar] [CrossRef]
- Davydova, M.; Stuchlik, M.; Rezek, B.; Kromka, A. Temperature enhanced gas sensing properties of diamond films. Vacuum 2012, 86, 599–602. [Google Scholar] [CrossRef]
- Maier, F.; Riedel, M.; Mantel, B.; Ristein, J.; Ley, L. Origin of surface conductivity in diamond. Phys. Rev. Lett. 2000, 85, 3472–3475. [Google Scholar] [CrossRef] [PubMed]
- Helwig, A.; Müller, G.; Sberveglieri, G.; Eickhoff, M. On the low-temperature response of semiconductor gas sensors. J. Sens. 2009, 2009, 1–17. [Google Scholar] [CrossRef]
- Fiz, R.; Hernandez-Ramirez, F.; Fischer, T.; Lopez-Conesa, L.; Estrade, S.; Peiro, F.; Mathur, S. Synthesis, characterization, and humidity detection properties of Nb2O5 nanorods and SnO2/Nb2O5 heterostructures. J. Phys. Chem. C 2013, 117, 10086–10094. [Google Scholar] [CrossRef]
- Maier, K.; Müller, G.; Teubert, J.; Eickhoff, M. H2O adsorption at luminescent InGaN nanowires. Sens. Actuators B Chem. 2015. to be published. [Google Scholar]
- Helwig, A.; Müller, G.; Sberveglieri, G.; Faglia, G. Gas sensing properties of hydrogenated amorphous silicon films. IEEE Sens. J. 2007, 7, 1506–1512. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Henzler, M.; Göpel, W. Oberflächenphysik des Festkörpers, 2nd ed.; Teubner Verlag: Wiesbaden, Germany, 1994. [Google Scholar]
- Lueth, H. Solid Surfaces, Interfaces and Thin Films, 5th ed.; Springer: Berlin, Germany; Heidelberg, Germany, 2010. [Google Scholar]
- Thomson, W.F.R.S. LX. On the equilibrium of vapour at a curved surface of liquid. Philos. Mag. Ser. 4 1871, 42, 448–452. [Google Scholar]
- Moore, W.J. Grundlagen der Physikalischen Chemie; Walter de Gruyter: Berlin, Germany, 1990. [Google Scholar]
- Galvin, K.P. A conceptually simple derivation of the Kelvin equation. Chem. Eng. Sci. 2005, 60, 4659–4660. [Google Scholar] [CrossRef]
- Randeniya, L.K.; Shi, H.; Barnard, A.S.; Fang, J.; Martin, P.J.; Ostrikov, K.K. Harnessing the influence of reactive edges and defects of graphene substrates for achieving complete cycle of room-temperature molecular sensing. Small 2013, 9, 3993–3999. [Google Scholar] [CrossRef] [PubMed]
- Hellmich, W.; Bosch-v. Braunmühl, C.; Müller, G.; Sberveglieri, G.; Berti, M.; Perego, C. The kinetics of formation of gas-sensitive RGTO-SnO2 films. Thin Solid Films 1995, 263, 231–237. [Google Scholar] [CrossRef]
- Sberveglieri, G.; Faglia, G.; Groppelli, S.; Nelli, P. RGTO: A New Technique for Preparing SnO2 Sputtered Thin Film As Gas Sensors. In Proceedings of the TRANSDUCERS’91: 1991 International Conference on Solid-State Sensors and Actuators, San Francisco, CA, USA, 24–27 June 1991.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maier, K.; Helwig, A.; Müller, G.; Hille, P.; Eickhoff, M. Effect of Water Vapor and Surface Morphology on the Low Temperature Response of Metal Oxide Semiconductor Gas Sensors. Materials 2015, 8, 6570-6588. https://doi.org/10.3390/ma8095323
Maier K, Helwig A, Müller G, Hille P, Eickhoff M. Effect of Water Vapor and Surface Morphology on the Low Temperature Response of Metal Oxide Semiconductor Gas Sensors. Materials. 2015; 8(9):6570-6588. https://doi.org/10.3390/ma8095323
Chicago/Turabian StyleMaier, Konrad, Andreas Helwig, Gerhard Müller, Pascal Hille, and Martin Eickhoff. 2015. "Effect of Water Vapor and Surface Morphology on the Low Temperature Response of Metal Oxide Semiconductor Gas Sensors" Materials 8, no. 9: 6570-6588. https://doi.org/10.3390/ma8095323
APA StyleMaier, K., Helwig, A., Müller, G., Hille, P., & Eickhoff, M. (2015). Effect of Water Vapor and Surface Morphology on the Low Temperature Response of Metal Oxide Semiconductor Gas Sensors. Materials, 8(9), 6570-6588. https://doi.org/10.3390/ma8095323






