Experimental results from reference [
11] are used to verify the proposed model. Papadakis [
11] studied property developments of high-calcium fly ash blended mortars. A rapid-setting Portland cement was used (400 m
2/kg Blaine’s fineness). Prior to use, high-calcium fly ash was pulverized to meet the cement mean particle size. The physical properties and chemical compositions of cement and FH are shown in
Table 2. The total CaO content in FH is 23%, the free CaO content in FH is 5%, and the glass content in FH is 50%. The mixture proportions are given in
Table 3. In the control specimen, the water-cement ratio (
W/
C) was 0.5 and the aggregate-cement ratio (
A/
C) was 3. Two different cases were studied: FH replaces either aggregate or cement. When FH replaces aggregate, three contents of FH were selected: 10%, 20%, and 30% additions to the control cement weight for specimens FHA, 10%, FHA, 20%, and FHA, 30%, respectively. When FH replaces the cement, the same FH contents were selected: 10%, 20%, and 30% replacements of the control cement weight for specimens FHC, 10%, FHC, 20%, and FHC, 30%, respectively.
The developments of strength, porosity, bound water, and calcium hydroxide were measured [
11]. The specimens for the strength measurements were prisms of 40 × 40 × 160 mm, cured under lime-saturated water at 20 °C and tested after 3, 14, 28, 49, 91, 182, and 364 days. FH-cement pastes were also prepared, representing the paste matrix of the mortar specimens, and analyzed for CH content, chemically bound water content, and porosity at 3, 7, 14, 28, 49, 112, 182, and 364 days after casting and moist curing [
11]. For each test, the moist-cured paste specimen was stripped, placed in a pre-weighed glass mortar, and pulverized. The mortar with the material was placed in an oven at 105 °C until the weight was constant. These weight indications were used to determine the chemically bound water content and porosity. To determine the CH content, a paste sample of the oven-dried powder was examined by a combination of quantitative X-ray diffraction analysis and TG/DTA (thermogravimetry/differential thermal analysis). The mass loss corresponding to the decomposition of Ca(OH)
2 occurs between 440 °C and 520 °C. The paste properties were reduced to the corresponding mortar specimen volume by multiplying the appropriate paste volume/mortar volume fraction [
11].
3.1. Evaluation of Calcium Hydroxide Contents
In the proposed model, the reaction of free CaO and other phases in FH are simulated separately. Equation (3) is used to model the rapid reaction of free CaO in FH, and Equation (4) is used to model the reaction of phases in FH other than free CaO. Using the chemical analysis of FH shown in
Table 2, we can determine the weight fractions of S, A, C, and
in the FH other than free CaO. Furthermore, the active part of FH other than free CaO, γ
active, which includes an aluminosilicate glass phase, a calcium phase, and a sulfate phase, can be calculated. The weight fractions of FH used for modeling are shown in
Table 4.
Using the experimental results of calcium hydroxide from Portland cement and a predictor-corrector algorithm [
19], the reaction coefficients of Portland cement
Bce,
Cce,
De0, and
kr can be determined. Using the experimental results of calcium hydroxide from cement-FH paste, the FH reaction coefficients
BFH,
CFH,
De0FH, and
krFH can be determined. The values of the reaction coefficients are shown in
Table 5. The fit parameters for a material are not changed from one mix to the other. For concrete with different water to binder ratios or FH replacement ratios, the reaction coefficients of cement and FH do not change.
Table 4.
Weight fractions of FH.
Table 4.
Weight fractions of FH.
| γS | fS, p | γA | fA, p | | fC, p | CaOfree |
|---|
| 0.7 | 0.413 | 0.7 | 0.1711 | 0.0453 | 0.1856 | 0.0518 |
| γactive = γSfS, p(1−CaOfree) + γAfA, p(1−CaOfree) + fC, p(1−CaOfree) + (1−CaOfree) | 0.61 |
Table 5.
Coefficients of the reaction model.
Table 5.
Coefficients of the reaction model.
| Bce (cm/h) | Cce (cm/h) | kr (cm/h) | De0 (cm2/h) |
|---|
| 1 × 10−8 | 0.81 | 2.33 × 10−6 | 3.79 × 10−10 |
| BFH (cm/h) | CFH (cm/h) | krFH (cm/h) | De0FH (cm2/h) |
| 2.22 × 10−11 | 0.08 | 5.45 × 10−7 | 2 × 10−8 |
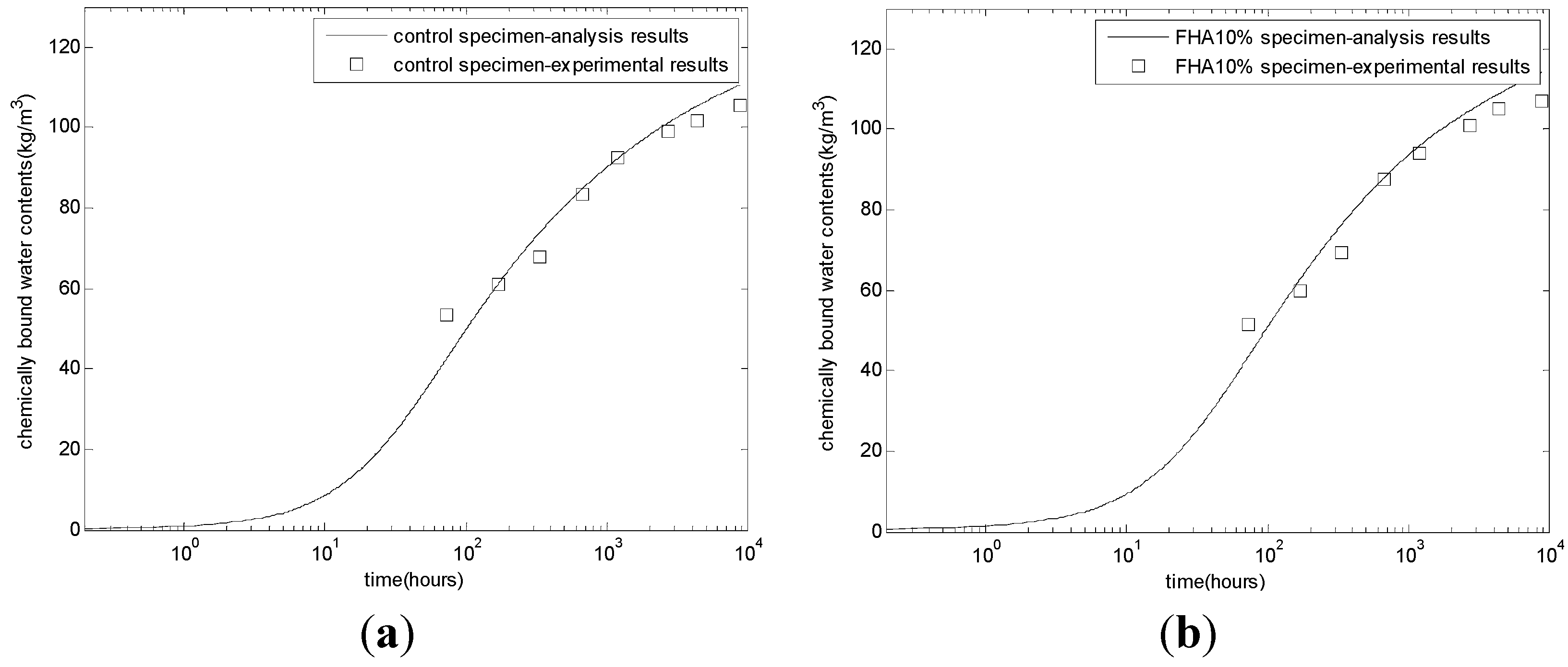
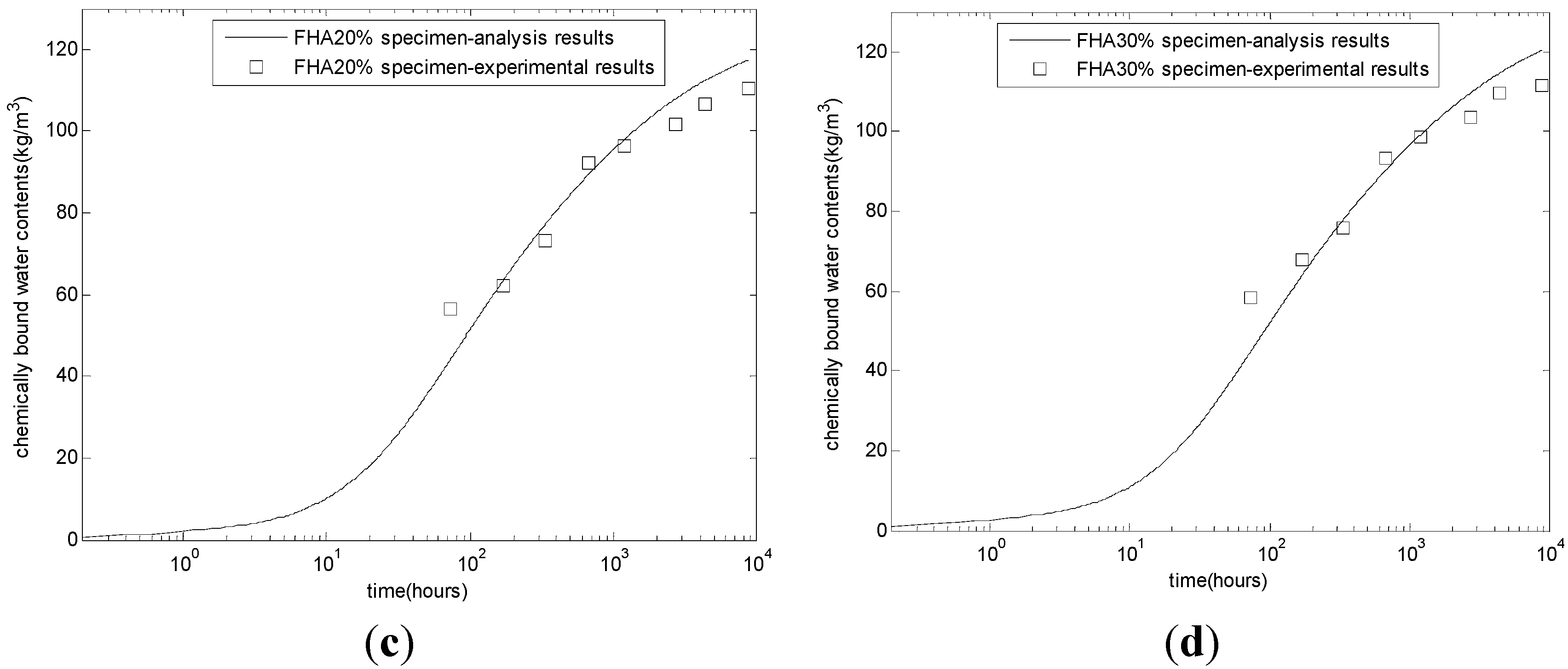
After calibrating the coefficients of the reaction model, multiple checks, such as the evolution of chemically bound water, porosity, CSH contents, and compressive strength, were performed to verify the proposed model. The procedure of the proposed model is similar to that of Tomosawa
et al. [
15,
19]. In Tomosawa’s model, the heat evolution of Portland cement was adopted to calibrate the coefficients of the hydration model. Furthermore, the evolution of the mechanical properties in early-age concrete was described using functions of the degree of hydration [
15,
19].
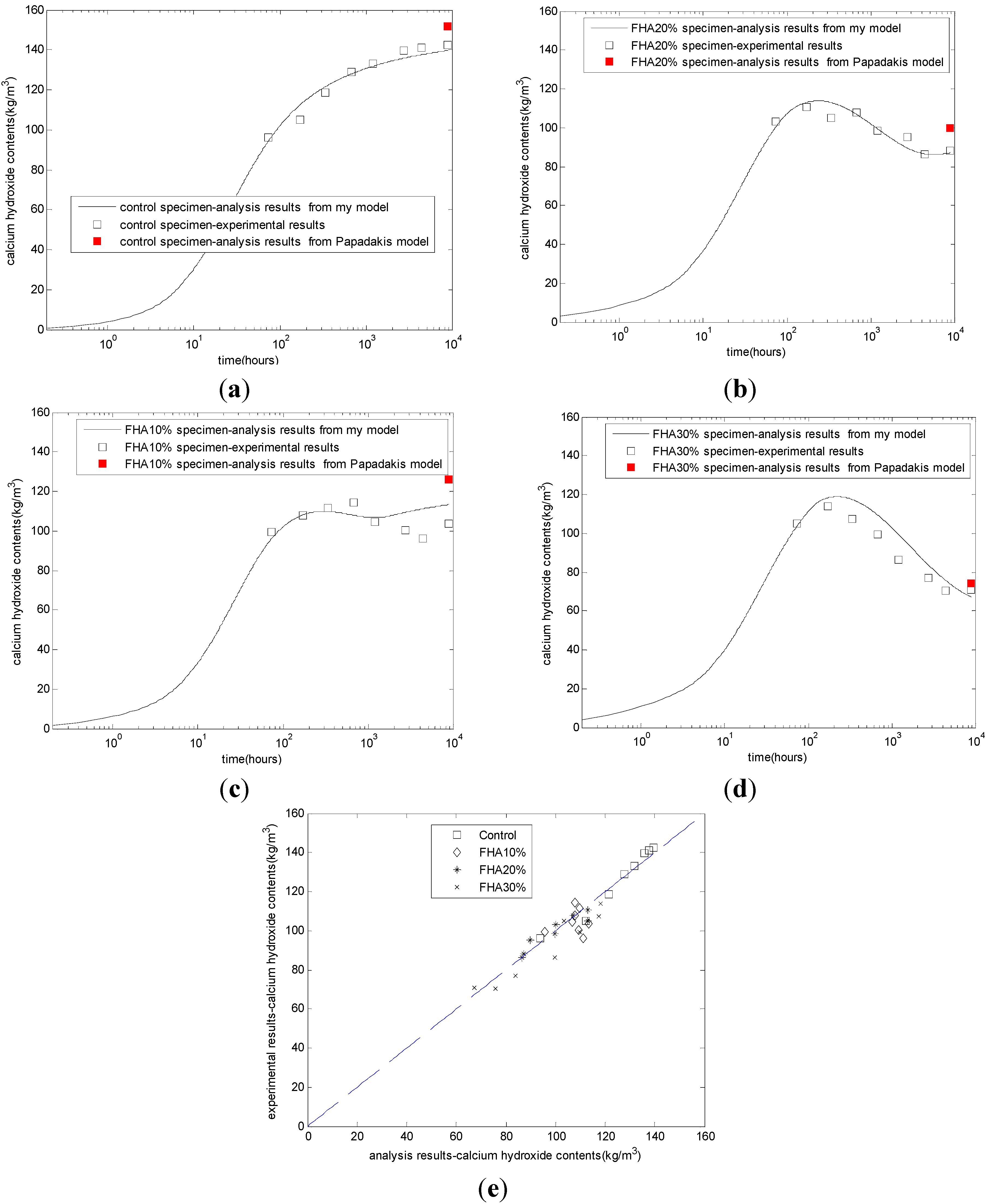
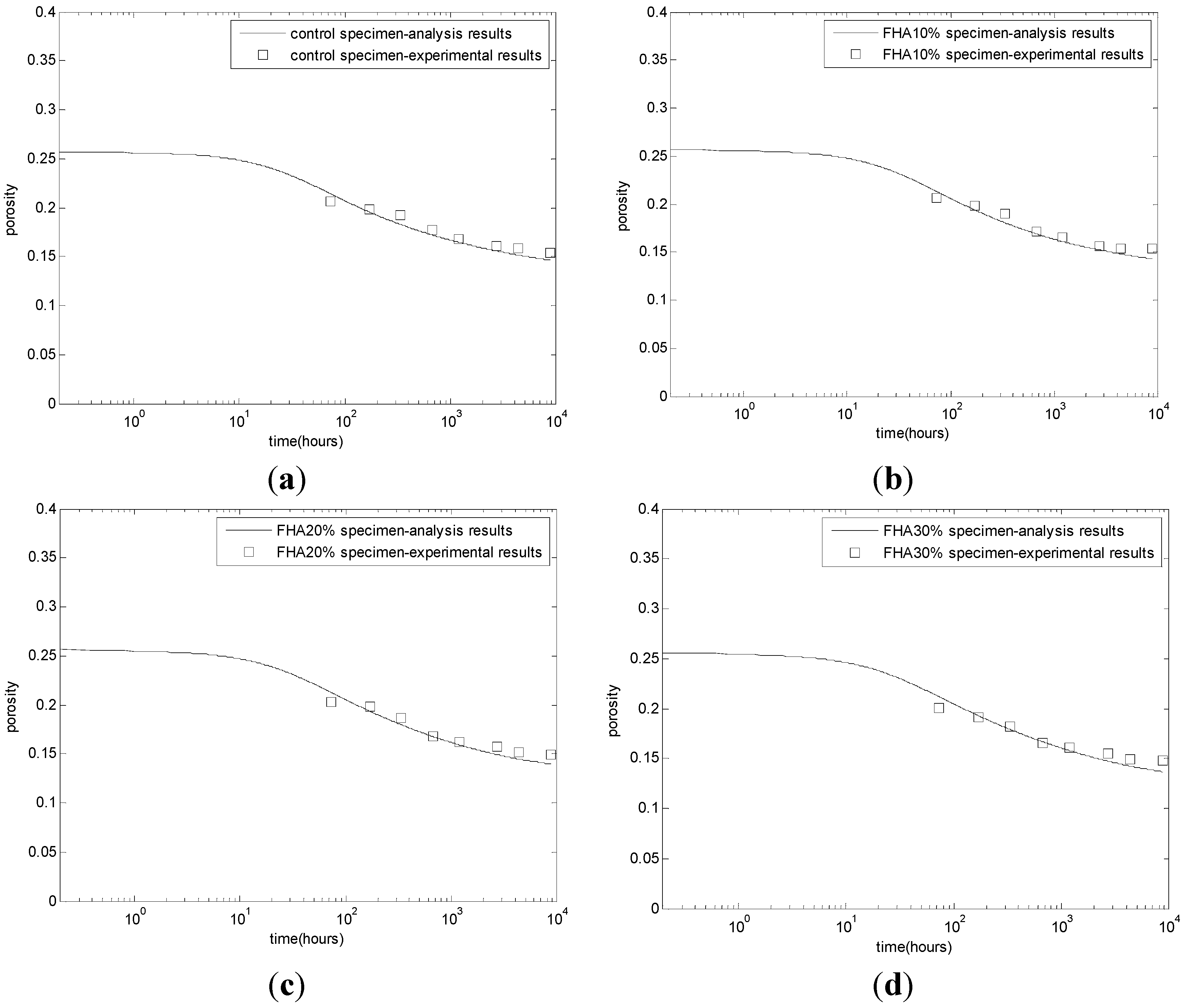
The evaluation results of the calcium hydroxide contents are shown in
Figure 2. The CH content of the control specimen increases with time until a steady state is attained (
Figure 2a). The CH content of FHA specimens presents a rather complicated picture due to the simultaneous CH production from the reaction of cement and the free CaO and CH consumption from other phases of the FH. In the first week, due to the rapid free CaO hydration, the CH contents are higher in the fly ash specimens (
Figure 2b–d) than that of the control specimen (
Figure 2a), These CH contents pass through a maximum due to higher CH production but decrease afterward as the FH-CH reaction proceeds at higher rates. At a late age, with the FH replacing ratio increasing from 10% to 30%, the CH contents decrease correspondingly. For concrete with 10% FH (
Figure 2c) and 20% FH (
Figure 2b), at the age of one year, the calcium hydroxide will slightly increase. This result may be due to the depletion of the active part of FH. Because the proposed model has modeled the rapid production of calcium hydroxide from the free CaO reaction in FH, the production of calcium hydroxide from cement hydration, and the consumption of calcium hydroxide from other phase reactions in FH, the proposed model can describe the complicated evolution process of cement-FH blends.
Figure 2e shows the comparison between the experimental results and the analysis results for different mixing proportions. The correlation coefficient between the experimental results and the analysis results is 0.95.
In addition,
Figure 2 shows the analysis results of calcium hydroxide from Papadakis’ model [
11]. Papadakis assumed that at the age of 365 days, the reaction rate of cement and FH is very slow. The age of 365 days can be approximately regarded as the steady state age of FH blended concrete. As shown in
Figure 2, Papadakis’ model can calculate the final CH content. However, because Papadakis’ model does not consider the kinetic reaction process of FH, this model cannot calculate the evolution of CH in cement-FH blends. The calculation result of Papadakis’ model is a point, not a curve. Conversely, the calculation result from our kinetic hydration model is a curve.
Figure 2.
Development of calcium hydroxide contents. (a) Control concrete; (b) FHA, 20% concrete: FH replaces aggregate by 20% weight of cement; (c) FHA, 10% concrete: FH replaces aggregate by 10% weight of cement; (d) FHA, 30% concrete: FH replaces aggregate by 30% weight of cement; (e) comparison between analysis results and experimental results.
Figure 2.
Development of calcium hydroxide contents. (a) Control concrete; (b) FHA, 20% concrete: FH replaces aggregate by 20% weight of cement; (c) FHA, 10% concrete: FH replaces aggregate by 10% weight of cement; (d) FHA, 30% concrete: FH replaces aggregate by 30% weight of cement; (e) comparison between analysis results and experimental results.
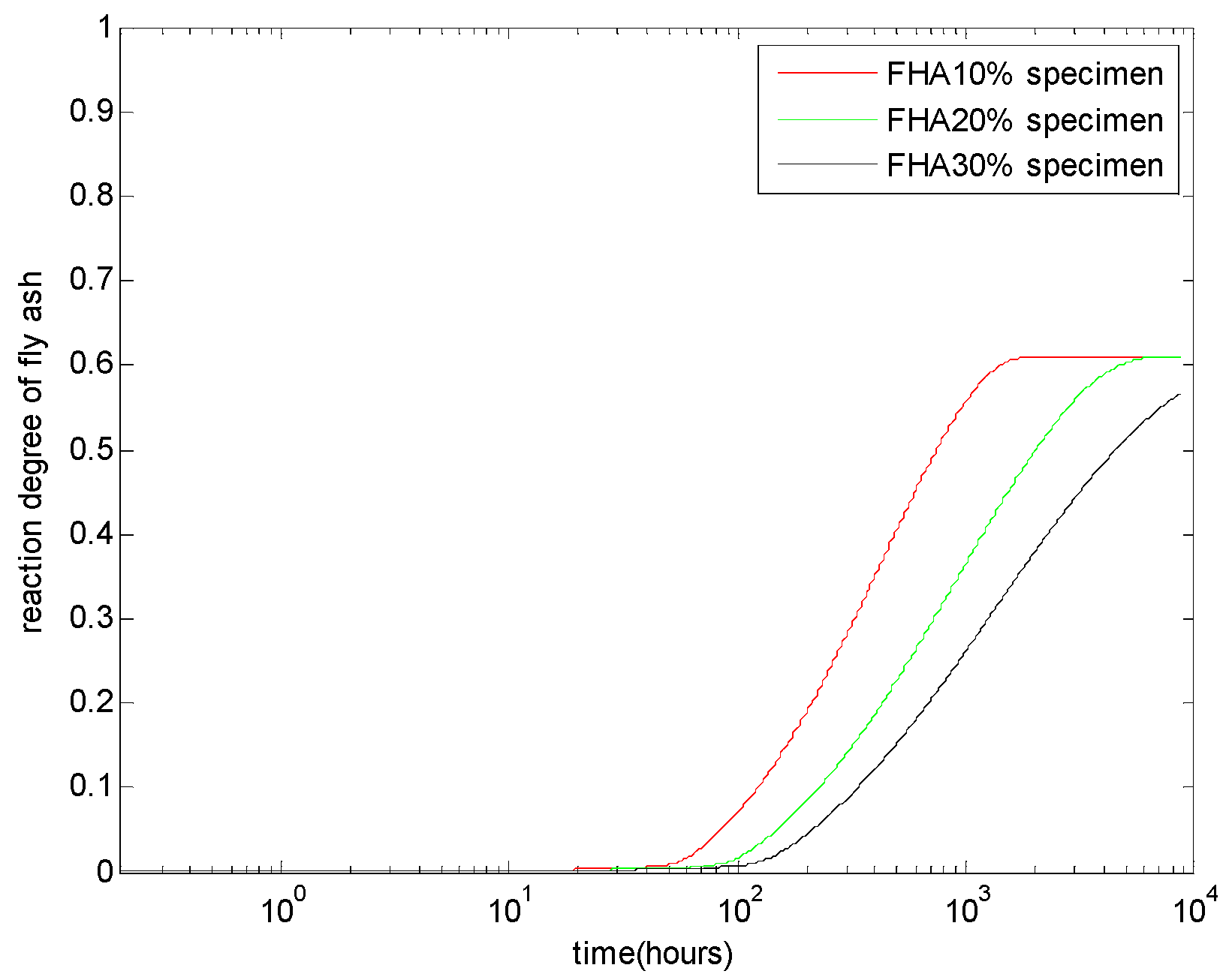
The evaluation of the reaction degree of fly ash α
FH-total is shown in
Figure 3. As shown in this figure, given a certain water to binder ratio, with an increasing replacement level of fly ash, the alkaline-activating effect of the cement would be weaker, so that the reactivity of the fly ash decreases [
20]. Conversely, at a late age, for concrete with 10% and 20% FH, the active part of FH has totally reacted (the ultimate reaction degree equals the weight fraction of the active part γ
active). For concrete with 30% FH, until the age of one year, part of FH is still un-reacted.
Figure 3.
Reaction degree of high-calcium fly ash with different FH contents: FH replaces aggregate by 10%, 20%, and 30% weight of cement and a water to binder ratio of 0.5.
Figure 3.
Reaction degree of high-calcium fly ash with different FH contents: FH replaces aggregate by 10%, 20%, and 30% weight of cement and a water to binder ratio of 0.5.
3.3. Evaluation of Compressive Strength
Papadakis [
11,
12] proposed that the compressive strength of cement-based materials, such as concrete containing low-calcium fly ash, high-calcium fly ash, and slag, can be estimated from the CSH content. Using the proposed hydration model considering both cement hydration and FH reaction (Equation (8d)), we can calculate the CSH contents for mortars with different FH contents, such as the control specimen, FHA, 10% (FH replaces aggregate by 10% weight of cement), FHA, 20% (FH replaces aggregate by 20% weight of cement), FHA30% (FH replaces aggregate by 30% weight of cement), and FHC, 20% (FH replaces cement by 20%).
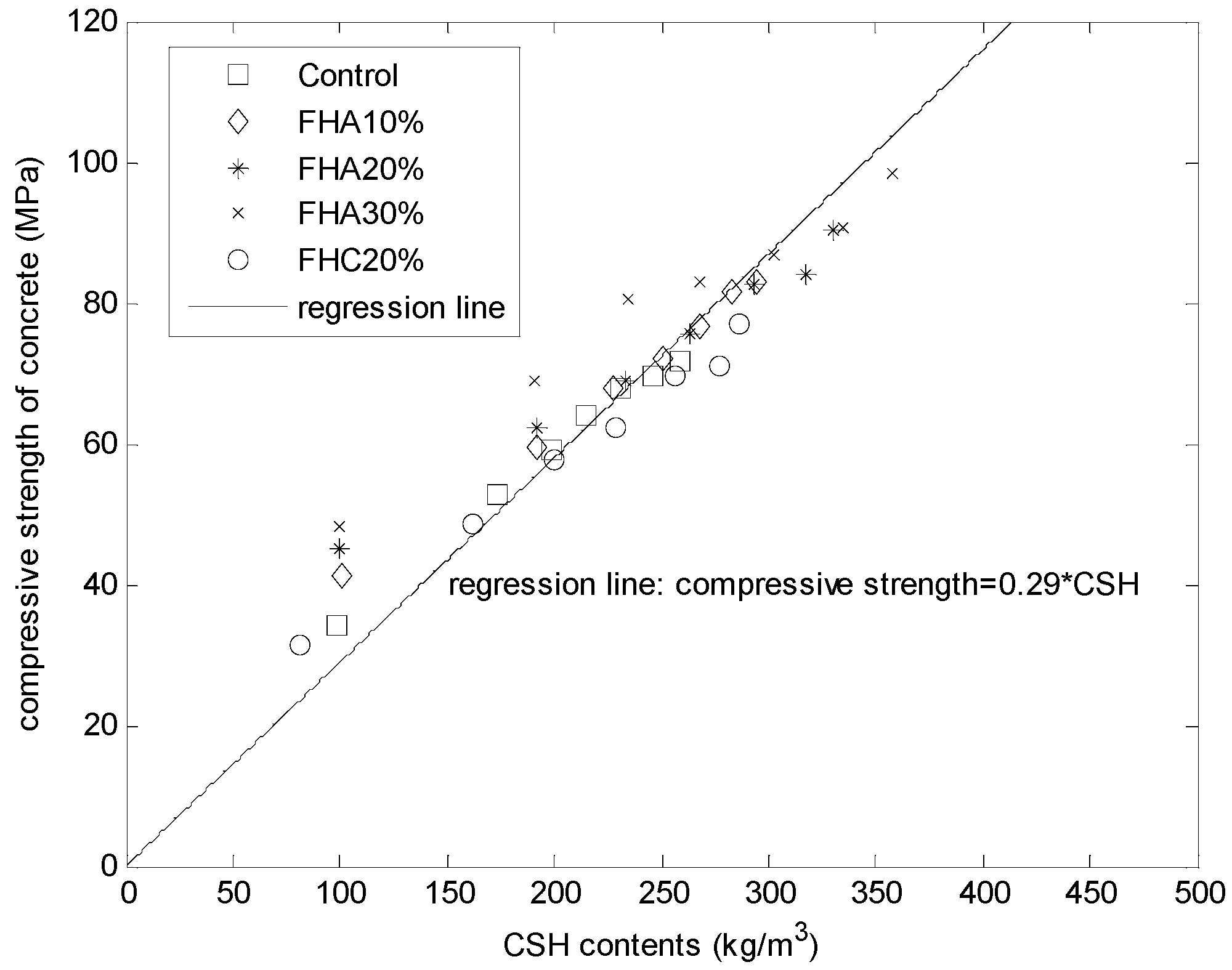
Figure 6 presents the compressive strength of the FH blended mortars as a function of the calculated CSH contents. As shown in
Figure 6, for concrete with different FH contents (control, FHA, 10%, FHA, 20%, FHA, 30%, and FHC, 20%) and at different ages (3, 14, 28, 49, 91, 182, and 364 days), a single linear relationship exists between the compressive strengths and the calculated CSH amounts,
i.e., the compressive strength = 0.29 × CSH.
Figure 6.
Compressive strength of mortars as a function of calculated (CSH) content.
Figure 6.
Compressive strength of mortars as a function of calculated (CSH) content.
Using the regressed linear relationship between the compressive strength and the CSH content, we can evaluate the development of the compressive strength of FH-cement blends.
Figure 7 shows the comparison between the experiment results and the simulation results of the compressive strength. For mortar containing FH-replacing aggregate (FHA mortars, as shown in
Figure 7b–d), the compressive strength of FHA specimens is higher than that of the control specimen (
Figure 7a) since the early ages. However, for mortar containing FH-replacing cement (FHC mortar, as shown in
Figure 7e), at early ages, the compressive strength of the FHC specimen is lower than that of the control specimen (
Figure 7a), and at late ages, due to the evolution of the FH reaction, the compressive strength of the FHC specimen surpasses that of the control specimen. Conversely, in the early age of three days, the simulation value is slightly lower than the experimental value. This result may be due to the omission of some factors, such as the contribution of ettringite from the FH reaction [
11] and the nucleation effects of FH [
11]. For FHA, 30% concrete (
Figure 7d), due to the abundant ettringite produced from the FH reaction, at the early ages of three and 14 days, the analysis results show more deviations from the experimental results than for the other mixing proportions.
Figure 7.
Evaluation of the compressive strength of FH concrete. (a) Control concrete; (b) FHA, 10% concrete: FH replaces aggregate by 10% weight of cement; (c) FHA, 20% concrete: FH replaces aggregate by 20% weight of cement; (d) FHA, 30% concrete: FH replaces aggregate by 30% weight of cement; (e) FHC, 20% concrete: FH replaces cement by 20%.
Figure 7.
Evaluation of the compressive strength of FH concrete. (a) Control concrete; (b) FHA, 10% concrete: FH replaces aggregate by 10% weight of cement; (c) FHA, 20% concrete: FH replaces aggregate by 20% weight of cement; (d) FHA, 30% concrete: FH replaces aggregate by 30% weight of cement; (e) FHC, 20% concrete: FH replaces cement by 20%.
In addition, the free CaO in FH also affects the compressive strength developments of hardening cement-FH blends. Tsimas and Moutsatsou-Tsima [
7] investigated the influence of free CaO contents on the compressive strength development. When the free CaO content increases from 1.8% to 3.5%, the compressive strength will increase correspondingly. This result is mainly due to the stimulus of the free CaO on the initial FH reaction [
7]. However, when the free CaO content further increases from 3.5% to 6.8%, the soundness of the concrete will be impaired and the compressive strength will decrease. Therefore, the free CaO presents double-edged effects on the strength development. For the model proposed in this paper, further investigations should be performed regarding FH with various chemical compositions and free CaO contents.
In the concrete industry, considering the economic and environmental effects, high-calcium fly ash is generally used as a mineral admixture to replace cement. Using the hydration model, we can calculate the CSH contents. Furthermore, using the regressed linear relationship between the compressive strengths and CSH amounts, we can calculate the compressive strength development of FH concrete.
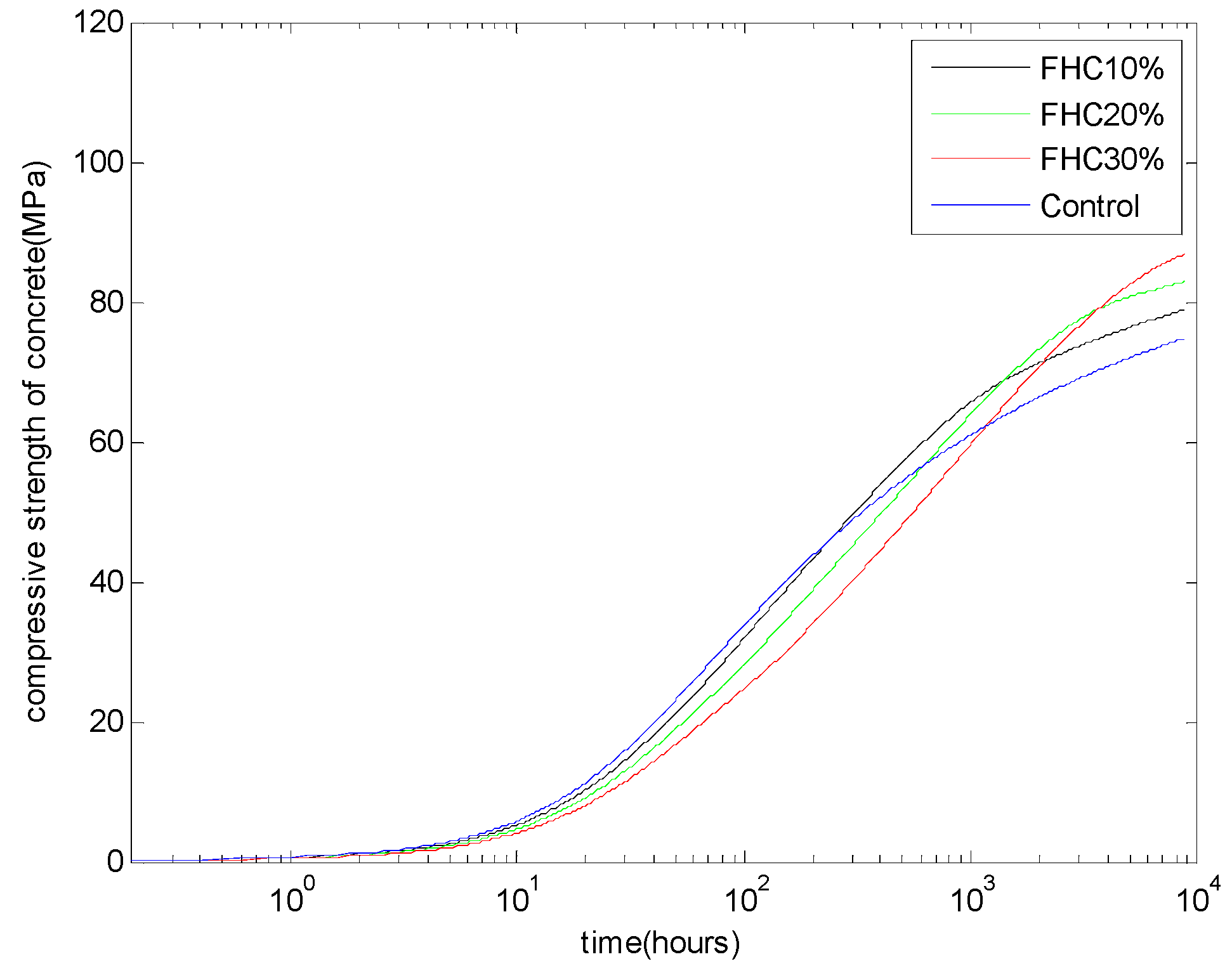
Figure 8 shows the analysis results of the compressive strength of FH blended concrete with a water to binder ratio of 0.5 and different FH contents: FH replacing cement by 10%, 20%, and 30%. The proposed model can reproduce the compressive strength crossover phenomenon between the control Portland cement concrete and the FH blended concrete. With increasing FH replacement ratios, the reactivity of FH will decrease (as shown in
Figure 3) and the age corresponding to the crossover of the compressive strength will be postponed.
Figure 8.
Compressive strength development of FH concrete: water to binder ratio of 0.5 and FH replaces cement by 10%, 20%, and 30%.
Figure 8.
Compressive strength development of FH concrete: water to binder ratio of 0.5 and FH replaces cement by 10%, 20%, and 30%.
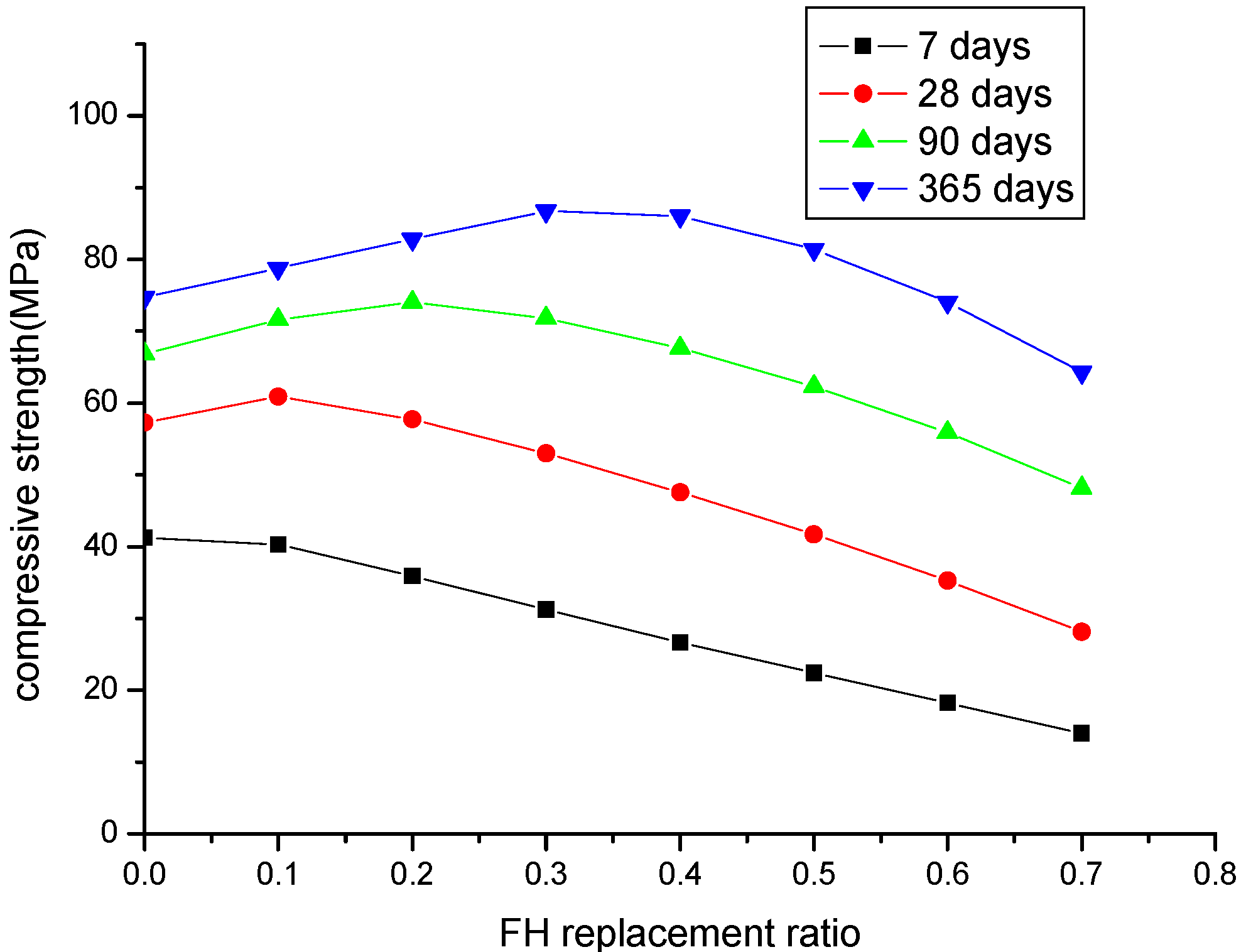
Figure 9 shows the compressive strength development of FH blended concrete with different FH contents (FH replaces cement by 10% to 70%) at different ages of seven days, 28 days, 90 days, and 365 days. At an early age of seven days, compared to the control concrete, the compressive strength of FH concrete almost linearly decreases with increasing FH content. With increasing curing age, due to the development of the FH reaction, the compressive strength of FH concrete will surpass that of the control concrete. However, for concrete incorporating larger FH contents (more than 50% cement is replaced by FH), due to the reduction of FH reactivity, at the age of one year, the compressive strength of FH concrete is still lower than that of the control concrete. Therefore, 50% can be regarded as the maximum replacement ratio of FH. Over this maximum replacement ratio, the compressive strength of FH blended concrete will be lower than that of the control concrete. Conversely, at the age of one year, when the FH replacement ratio is higher than 30%, the compressive strength of concrete will decrease. Therefore, 30% FH can be regarded as an optimum FH content for concrete incorporating FH as a cement-replacing mineral admixture. The optimum FH content can be explained as follows: according to Equation (8d), the CSH content produced from the FH reaction, 2.85γ
SfS,pα
FHP, relates to the FH content
P and the reaction degree of FH α
FH. With increasing FH content
P, the reactivity of FH will decrease (as shown in
Figure 3), and therefore, there is a peak value for the product of FH content
P and the reaction degree of FH α
FH. The FH content corresponding to the peak value can be regarded as the optimum replacement of FH.
Figure 9.
Parameter analysis of the compressive strength of concrete: a water to binder ratio of 0.5 and FH replaces cement by 10% to 70%.
Figure 9.
Parameter analysis of the compressive strength of concrete: a water to binder ratio of 0.5 and FH replaces cement by 10% to 70%.