Fabrication of CH3NH3PbI3/PVP Composite Fibers via Electrospinning and Deposition
Abstract
:1. Introduction
2. Results and Discussion
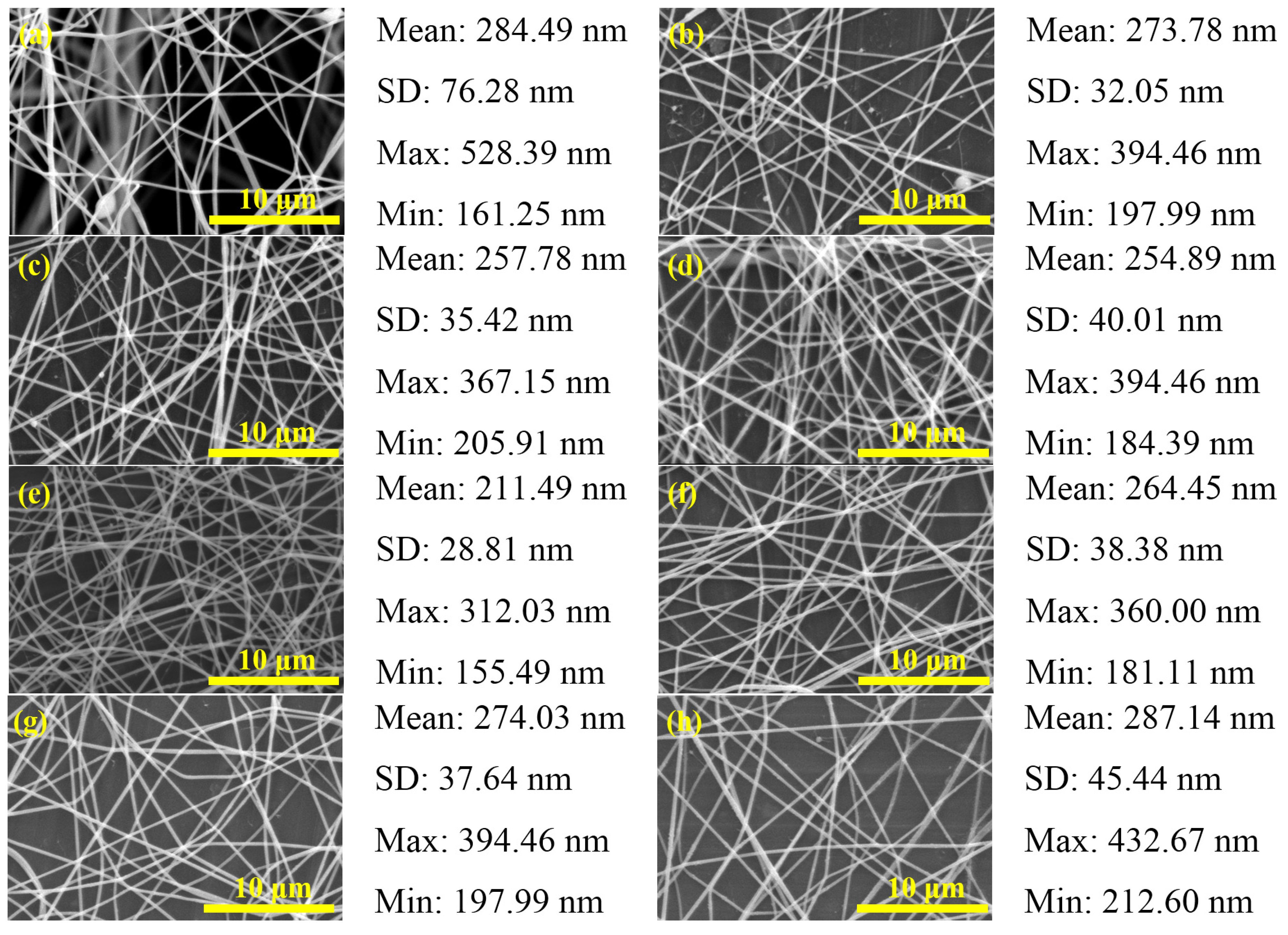
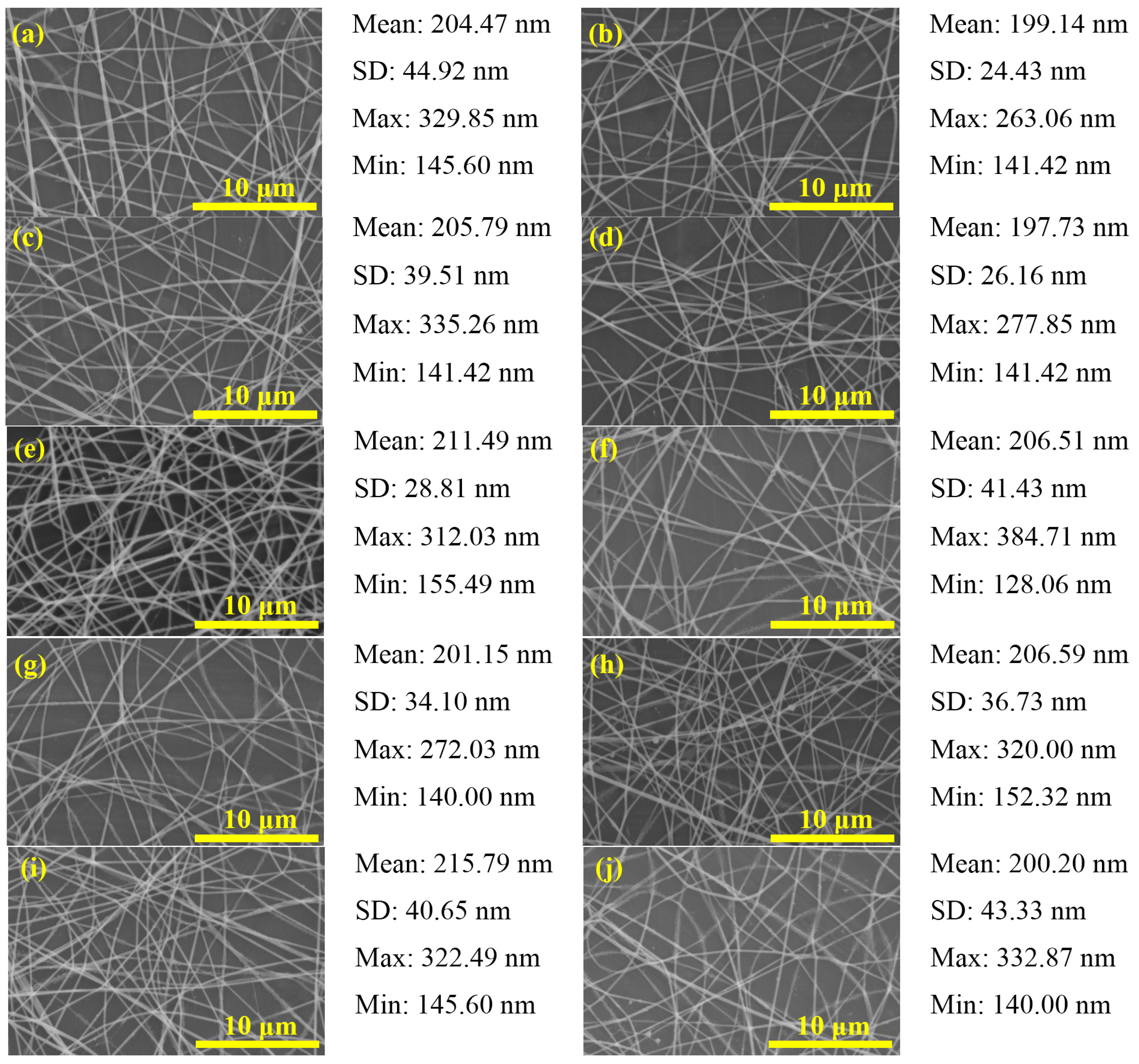
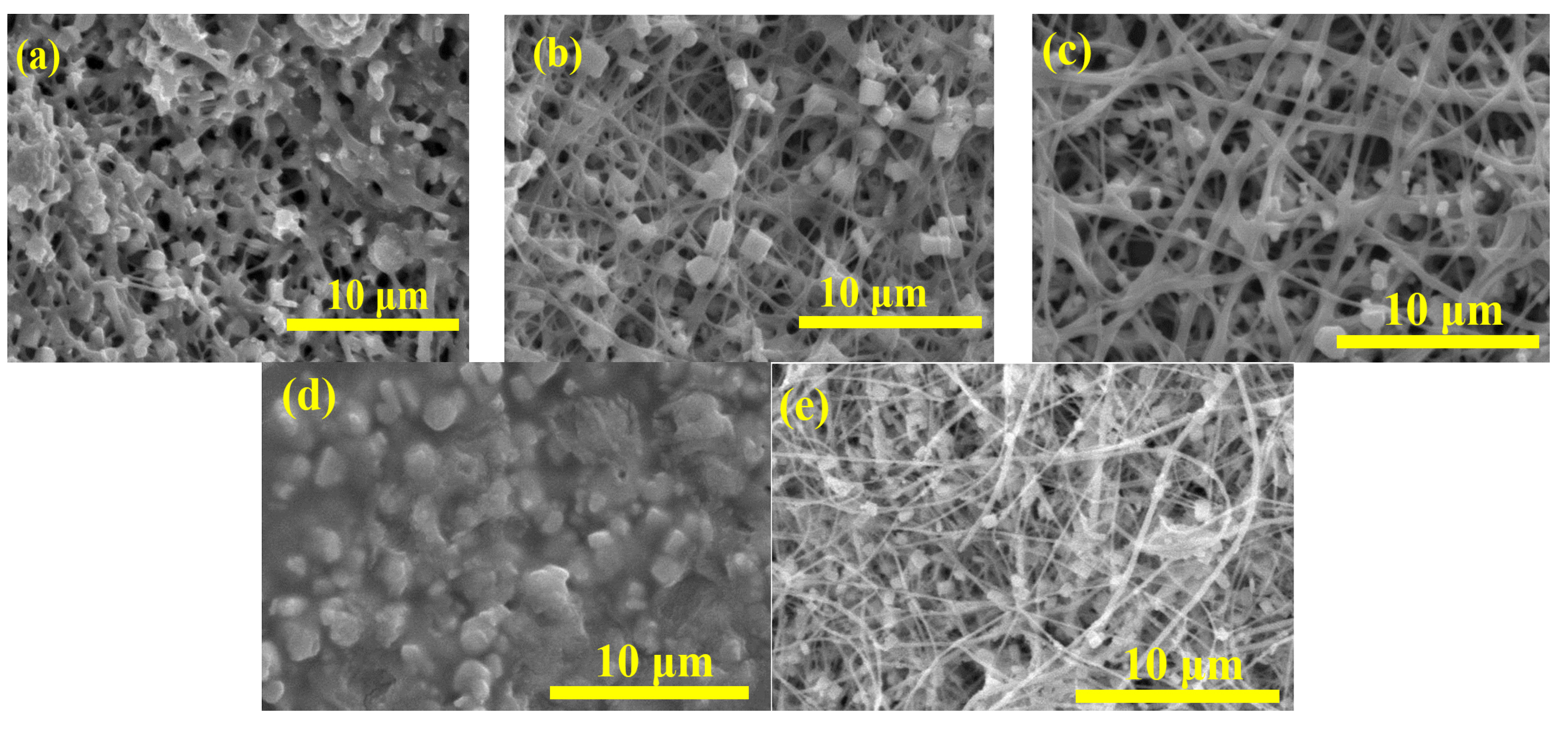
2.1. PbI2/PVP Composite Fibers
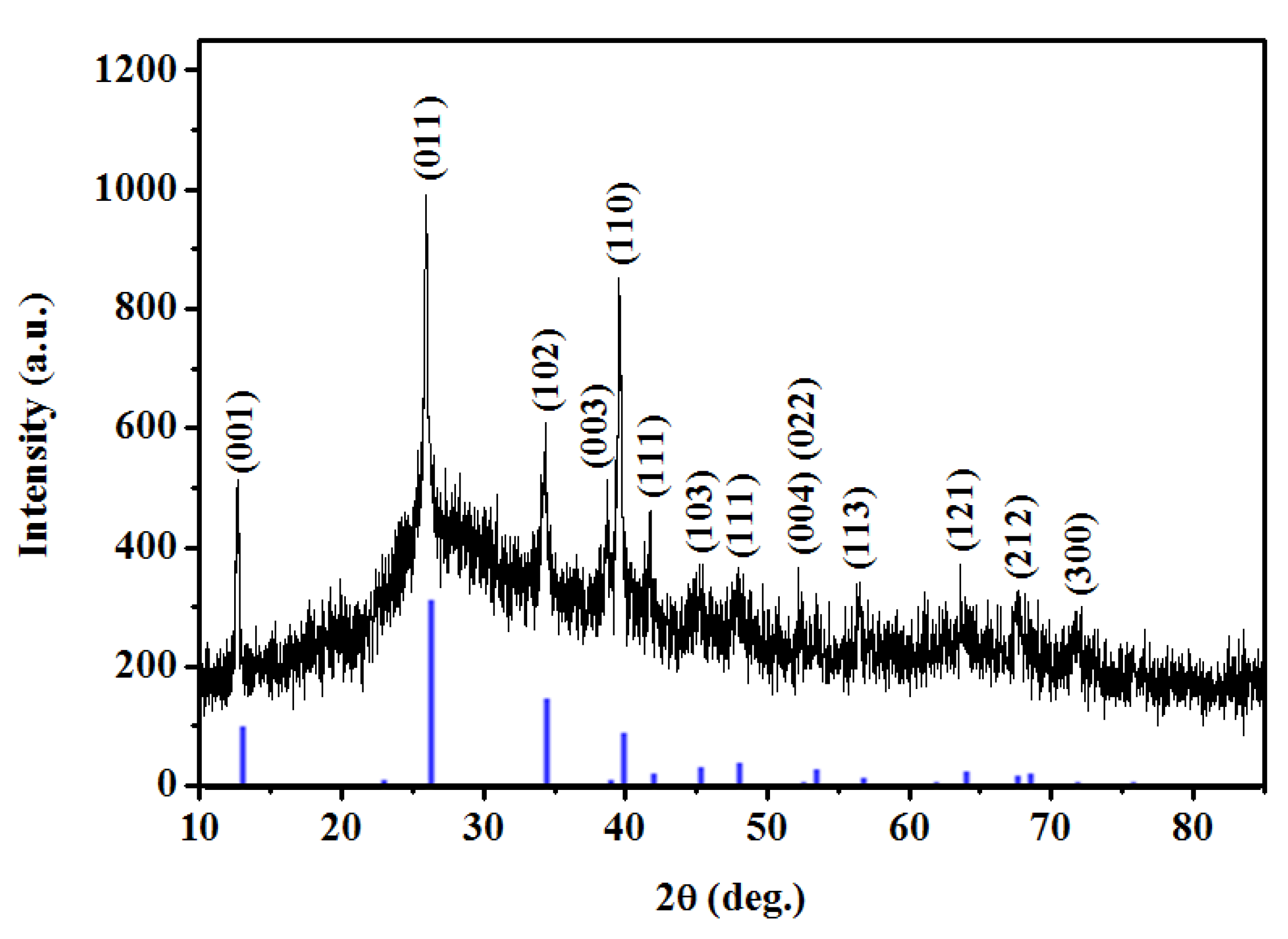
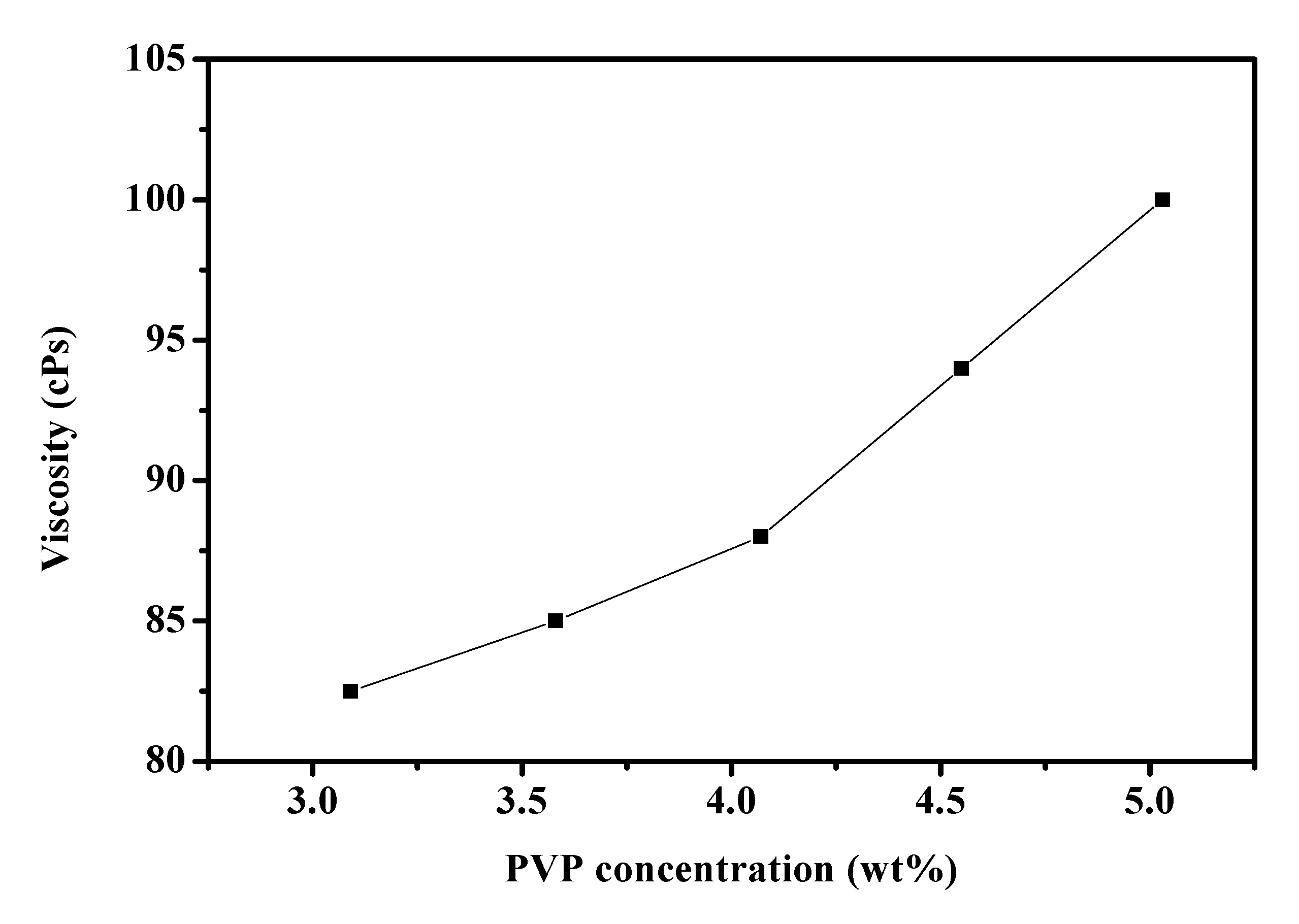

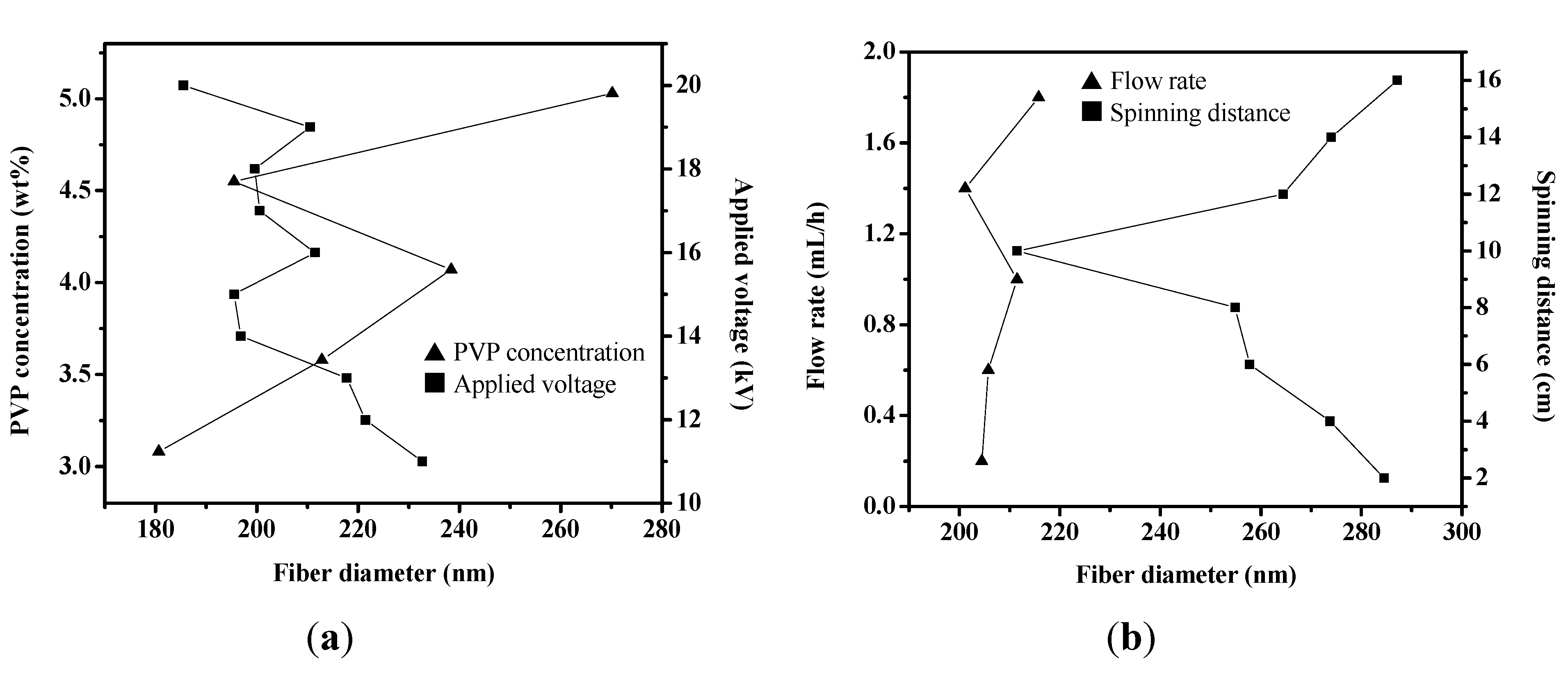
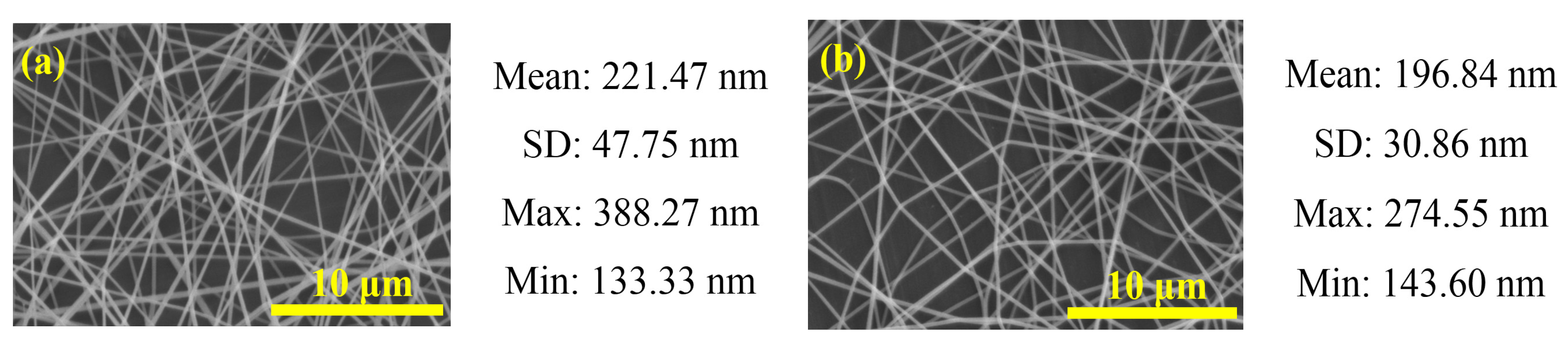
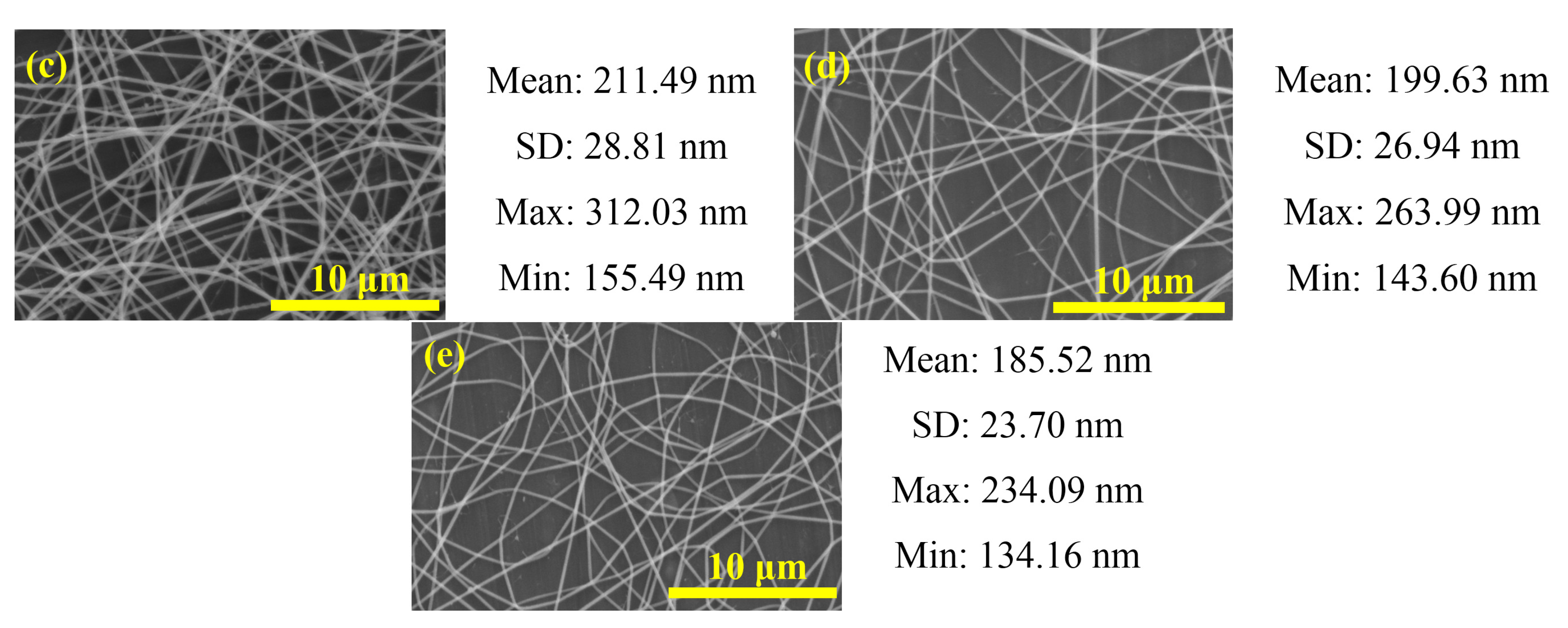
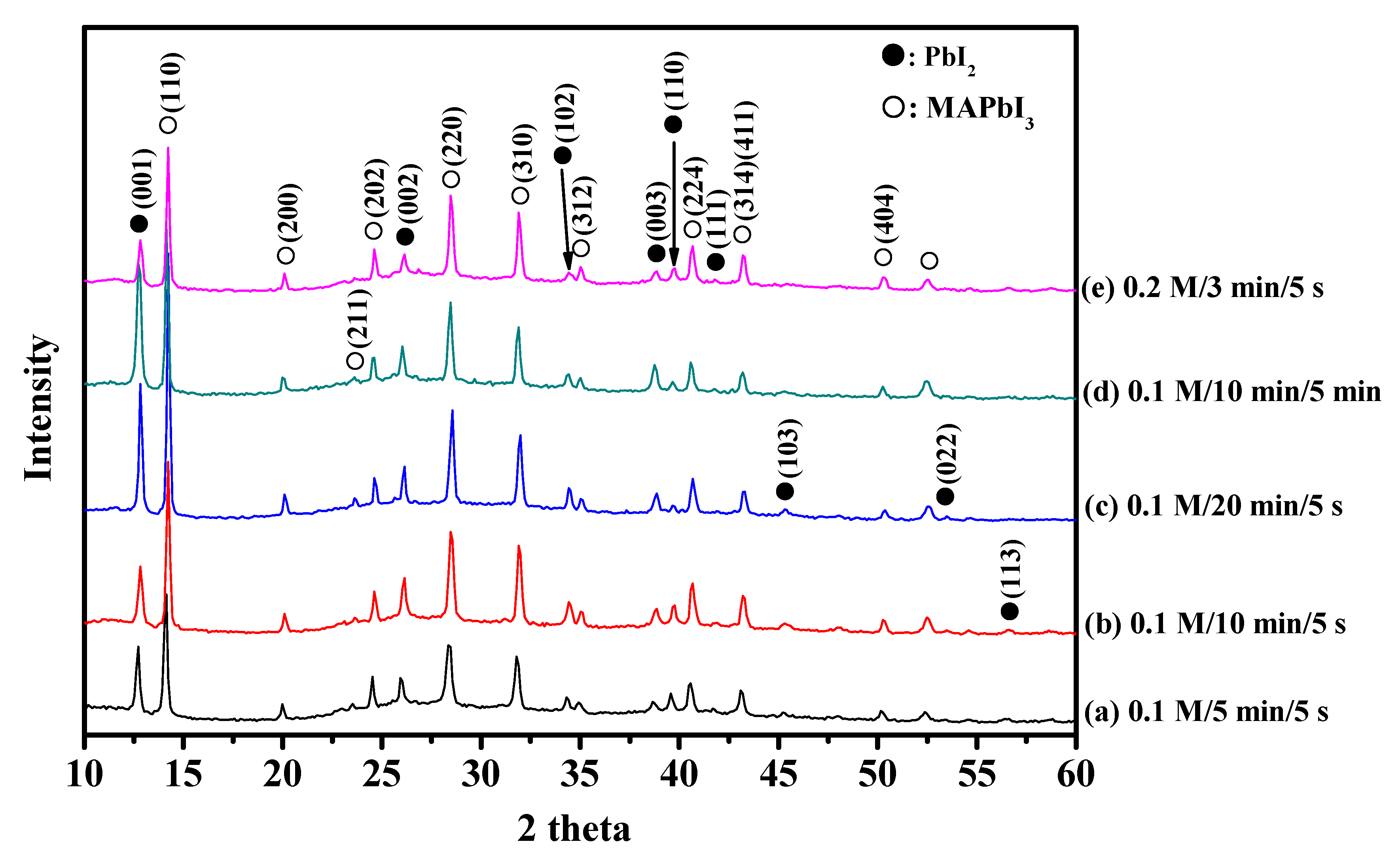
2.2. MAPbI3/PVP Composite Fibers
3. Experimental Section
3.1. Materials
3.2. Synthesis of PbI2/PVP Composite Fibers
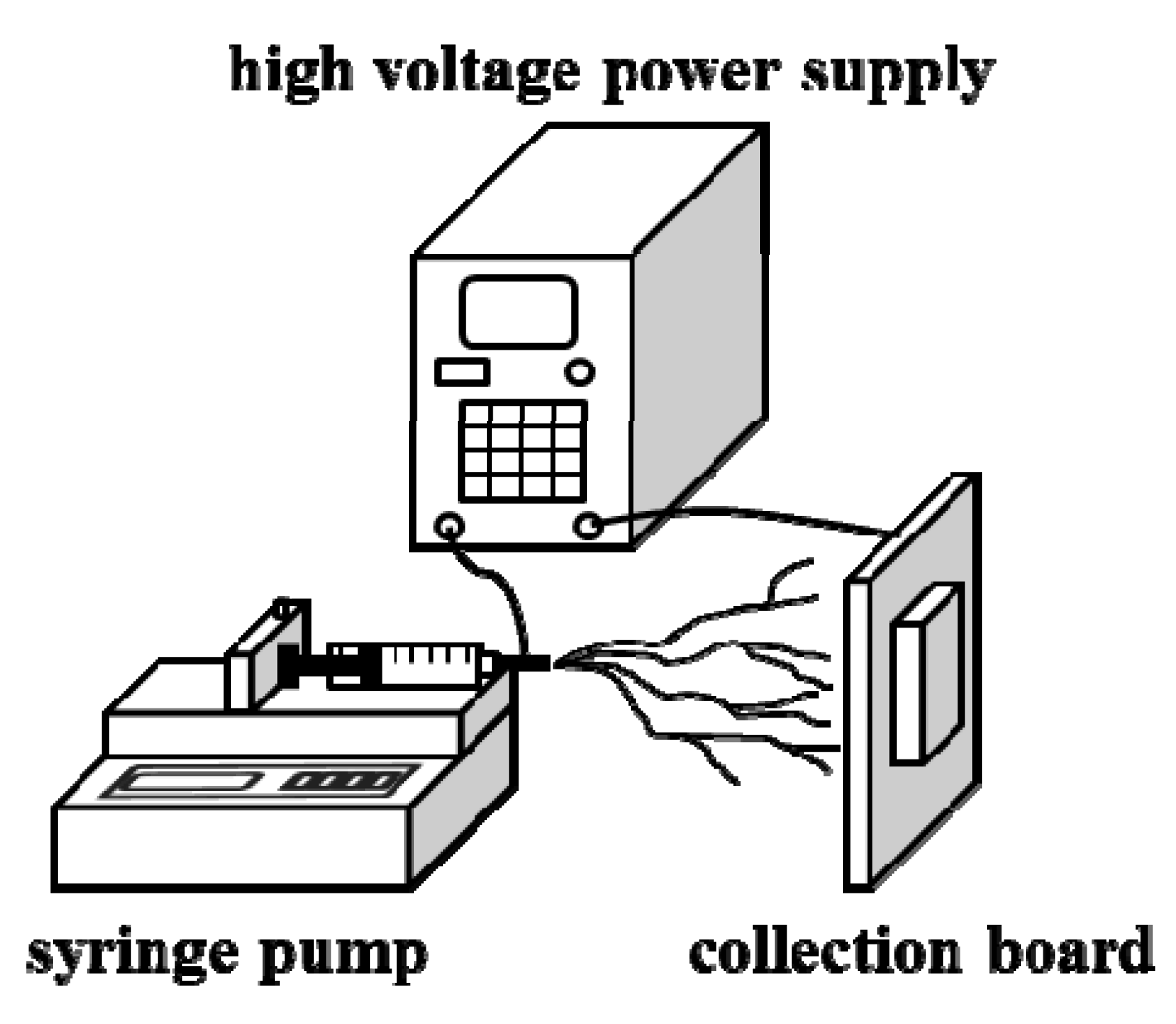
3.3. Synthesis of CH3NH3PbI3/PVP Composite Fibers
3.4. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schadler, L.S. Polymer-Based and Polymer-Filled Nanocomposites. In Nanocomposite Science and Technology; Ajayan, P.M., Schadler, L.S., Braun, P.V., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; pp. 77–153. [Google Scholar]
- Li, Z.; Wang, C. One-Dimensional Nanostructures; Springer: Berlin, Germany; Heidelberg, Germany, 2013. [Google Scholar]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Z.; Hong, Y.; Zhao, Y.; Qiu, S.; Wang, C.; Wei, Y. Influence of solvents on the formation of ultrathin uniform poly(vinyl pyrrolidone) nanofibers with electrospinning. J. Polym. Sci. Pol. Phys. 2004, 42, 3721–3726. [Google Scholar] [CrossRef]
- Schlüter, I.C.; Schlüter, M. Electronic structure and optical properties of PbI2. Phys. Rev. B 1974, 9, 1652–1663. [Google Scholar] [CrossRef]
- Zhu, G.; Hojamberdiev, M.; Liua, P.; Peng, J.; Zhou, J.; Bian, X.; Huang, X. The effects of synthesis parameters on the formation of PbI2 particles under DTAB-assisted hydrothermal process. Mater. Chem. Phys. 2011, 131, 64–71. [Google Scholar] [CrossRef]
- Artemyev, M.V.; Rakovich, Y.P.; Yablonski, G.P. Effect of dc electric field on photoluminescence from quantum-confined PbI2 nanocrystals. J. Cryst. Growth 1997, 171, 447–452. [Google Scholar] [CrossRef]
- Chang, Y.C.; James, R.B. Phonon dispersion and polar-optical scattering in 2H PbI2. Phys. Rev. B 1997, 55, 8219–8225. [Google Scholar] [CrossRef]
- Schlesinger, T.E.; James, R.B.; Schieber, M.; Toney, J.; Scyoc, J.M.V.; Salary, L.; Hermon, H.; Lund, J.; Burger, A.; Chen, K.T.; et al. Characterization of lead iodide for nuclear spectrometers. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Dect. Assoc. Equip. 1996, 380, 193–197. [Google Scholar] [CrossRef]
- George, M.A.; Azoulay, M.; Jayatirtha, H.N.; Biao, Y.; Burger, A.; Collins, W.E.; Silberman, E. Atomic force microscopy of lead iodide crystal surfaces. J. Cryst. Growth 1994, 137, 299–303. [Google Scholar] [CrossRef]
- Sandroff, C.J.; Hwang, D.M.; Chung, W.M. Carrier confinement and special crystallite dimensions in layered semiconductor colloids. Phys. Rev. B 1986, 33, 5953–5955. [Google Scholar] [CrossRef]
- Sengupta, A.; Jiang, B.; Mandal, K.C.; Zhang, J.Z. Ultrafast electronic relaxation dynamics in PbI2 semiconductor colloidal nanoparticles: A femtosecond transient absorption study. J. Phys. Chem. B 1999, 103, 3128–3137. [Google Scholar] [CrossRef]
- Mu, R.; Tung, Y.S.; Ueda, A.; Henderson, D.O. Chemical and size characterization of layered lead iodide quantum dots via optical spectroscopy and atomic force microscopy. J. Phys. Chem. 1996, 100, 19927–19932. [Google Scholar] [CrossRef]
- Gao, P.; Grätze, M.; Nazeeruddin, M.K. Organohalide lead perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 2448–2463. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, K. Optical bleaching of perovskite (CH3NH3)PbI3 through room-temperature phase transformation induced by ammonia. Chem. Commun. 2014, 50, 1605–1607. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, D.; Ma, Y.; Lu, Z.; Chen, Z.; Wang, S.; Xiao, L.; Gong, Q. Morphology control of the perovskite films for efficient solar cells. Dalton Trans. 2015, 44, 10582–10593. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, S.; Chen, X.; Zheng, Y.C.; Hou, Y.; Li, Y.H.; Zeng, H.D.; Yang, H.G. Direct insight into crystallization and stability of hybrid perovskite CH3NH3PbI3 via solvothermal synthesis. J. Mater. Chem. A 2015, 3, 15854–15857. [Google Scholar] [CrossRef]
- Ji, L.; Medford, A.J.; Zhang, X. Electrospun polyacrylonitrile/zinc chloride composite nanofibers and their response to hydrogen sulfide. Polymer 2009, 50, 605–612. [Google Scholar] [CrossRef]
- Chen, M.; Qu, H.; Zhu, J.; Luo, Z.; Khasanov, A.; Kucknoor, A.S.; Haldolaarachchige, N.; Young, D.P.; Wei, S.; Guo, Z. Magnetic electrospun fluorescent polyvinylpyrrolidone nanocomposite fibers. Polymer 2012, 53, 4501–4511. [Google Scholar] [CrossRef]
- Tan, S.H.; Inai, R.; Kotaki, M.; Ramakrishna, S. Systematic parameter study for ultra-fine fiber fabrication via electrospinning process. Polymer 2005, 46, 6128–6134. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Fabrication of titania nanofibers by electrospinning. Nano Lett. 2003, 3, 555–560. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, H.Y.; La, Y.M.; Lee, D.R.; Sung, N. Influence of a mixing solvent with tetrahydrofuran and N,N-dimethylformamide on electrospun poly(vinyl chloride) nonwoven mats. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2259–2268. [Google Scholar] [CrossRef]
- Megelski, S.; Stephens, J.S.; Chase, D.B.; Rabolt, J.F. Micro- and nanostructured surface morphology on electrospun polymer fibers. Macromolecules 2002, 35, 8456–8466. [Google Scholar] [CrossRef]
- Sun, S.; Salim, T.; Mathews, N.; Duchamp, M.; Boothroyd, C.; Xing, G.; Sum, T.C.; Lam, Y.M. The origin of high efficiency in low-temperature solution-processable bilayer organometal halide hybrid solar cells. Energy Environ. Sci. 2014, 7, 399–407. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, L.-M.; Tai, T.-Y.; Chen, Y.-Y.; Lin, P.-Y.; Fu, Y.-S. Fabrication of CH3NH3PbI3/PVP Composite Fibers via Electrospinning and Deposition. Materials 2015, 8, 5467-5478. https://doi.org/10.3390/ma8085256
Chao L-M, Tai T-Y, Chen Y-Y, Lin P-Y, Fu Y-S. Fabrication of CH3NH3PbI3/PVP Composite Fibers via Electrospinning and Deposition. Materials. 2015; 8(8):5467-5478. https://doi.org/10.3390/ma8085256
Chicago/Turabian StyleChao, Li-Min, Ting-Yu Tai, Yueh-Ying Chen, Pei-Ying Lin, and Yaw-Shyan Fu. 2015. "Fabrication of CH3NH3PbI3/PVP Composite Fibers via Electrospinning and Deposition" Materials 8, no. 8: 5467-5478. https://doi.org/10.3390/ma8085256
APA StyleChao, L.-M., Tai, T.-Y., Chen, Y.-Y., Lin, P.-Y., & Fu, Y.-S. (2015). Fabrication of CH3NH3PbI3/PVP Composite Fibers via Electrospinning and Deposition. Materials, 8(8), 5467-5478. https://doi.org/10.3390/ma8085256





