Preclinical in vivo Performance of Novel Biodegradable, Electrospun Poly(lactic acid) and Poly(lactic-co-glycolic acid) Nanocomposites: A Review
Abstract
:1. Introduction
2. Material Characteristics and Degradation Behavior

3. Biocompatibility in Preclinical Studies
3.1. Aim of the Review on Biocompatibility
3.2. Search Strategy
3.3. Review Process
3.4. Results
3.4.1. Description of Materials
3.4.2. Description of Experimental Methods
| Author | Scaffold Components | Scaffold Architecture | Fiber Diameter |
|---|---|---|---|
| Adegani et al. [33] | PLGA 15% (wt/wt) solution dissolved in DMF/THF coating with willmite nanoparticles | porous structure | 300 ± 500 nm; willmite coating did not affect fiber diameter |
| Dinarvand et al. [34] | PLLA dissolved in chloroform with a 4% (w/v) concentration coating with HA, BG, TCP; HA + BG | nanofibrous structure with homogeneous distribution of bioceramics along the surface of PLLA | 822 ± 97 nm |
| Jaiswal et al. [35] | PLLA with molecular weight 300,000 Da blend with G (3:1) composited with HA | no information | no information |
| Jiang et al. [36] | PLGA (85:15) 10% with molecular weight of 80,000 Da dissolved in a mixture of chloroform + DMF (1:1) mixed with HA (20:1) mixed with HA + SIM (20:1:1) | scaffolds with smooth and nanofibrous morphology | PLGA: 550 ± 50 nm PLGA + HA: 240 ± 30 nm PLGA + HA + SIM: 270 ± 30 nm |
| Lee et al. [38] | PLLA (5.7–8.2 dL/g viscosity; Resomer L 214 S) dissolved in HFIP (2 wt % for random, 2.5 wt % for aligned fibers) coating with polydopamine | scaffolds with random and aligned fiber orientation | 1 μm in both structures |
| Ko et al. [37] | PLLA (3.3–4.3 dL/g viscosity; Resomer L 210 S) dissolved in trifluorethanol mixed with DBP (1.0:0.2) | nanofibrous scaffold with randomly oriented fibers with a homogeneous distribution | 300–700 nm |
| Schneider et al. [9] | PLGA (Resomer) (85:15) with a molecular weight of 380,300 g/mol and 181,900 g/mol blend with TCP nanoparticles (40 wt %) | fibers exhibiting a porous structure, TCP-containing fibers revealed an increased roughness | 5–10 μm |
| Schofer et al. [30] | PLLA (Resomer) 4% (w/w) dissolved in DCM incorporation of BMP-2 | three-dimensional non-woven network of nanofibers, fibers showed a porous structure | 775 ± 294 nm |
| Shim et al. [31] | PLLA (intrinsic viscosity 0.63 dL/g, molecular weight: 250,000 g/mol); 8% PLLA dissolved in DCM/HFIP or in DCM/DMF or in DCM/acetone with volume ratios (90:10) 3% PLLA in DCM/HFIP (90:10) | PLLA mixture below 2% w/v resulted in beaded fibers, for concentrations > 4%, the fibers fused at the contact points | 400 nm–7 μm |
| Yanagida et al. [32] | PLLA (Lactel: intrinsic viscosity: 0.9–1.2 dL/g) dissolved in DMC at 15 wt % mixed or coated, or mixed and coated with HA nanocrystals | PLLA/HA nanocomposite fibers, where HA nanocrystals were mixed into the PLLA matrix as well as coated onto the PLLA surface had submicron-sized dimples on their surfaces | PLLA fibers: 6.1 ± 1.9 μm PLLA/HA mixed: 7.6 ± 1.9 μm |
| Author | Animal Model | Defect Size (Diameter) and Wound Treatment | Time of Evaluation | Methods of Evaluation | Area of Regenerated Bone | Histological Results |
|---|---|---|---|---|---|---|
| Adegani et al. [33] | rats | 8 mm calvarial critical size defects, precise treatment of the wound is not described | 8 weeks | MSCT histology evaluation by two independent radiologists | PLGA + willmite: 70% PLGA: 35% Empty: 5% | No sign of inflammation |
| Dinarvand et al. [34] | rats | 8 mm calvarial critical size defects, wound was closed with sutures | 8 weeks | MSCT Digital mammo-graphy histology evaluation by two independent radiologists | PLLA-HA-BG: 63% PLLA-TCP: 44% PLLA-HA: 23% PLLA-BG: 20% PLLA: 13% Empty: 12% | No sign of inflammation |
| Jaiswal et al. [35] | rats | 5 mm calvarial critical size defects, pericranium and skin was closed in layers | 6 and 10 weeks | Micro-CT digital X-ray, hematology and serum biochemistry histology evaluation with an image software | 6 weeks: PLLA-G-HA: ≈94% PLLA-HA: ≈64% Empty: 30% PLLA: 26% PLLA-G: 13% 10 weeks: PLLA-G-HA: 98% PLLA-G: 80% PLLA-HA: 76% PLLA: 60% Empty: 34% | No sign of inflammation |
| Jiang et al. [36] | rats | 5 mm calvarial defects, wound was closed with sutures | 4 and 8 weeks | Micro-CT histology evaluation with an image software | 4 weeks: PLGA-HA-SIM: ≈4.2% PLGA-HA: <1% Empty: <1% 8 weeks: PLGA-HA-SIM: ≈10% PLGA-HA: <4% Empty: <2% | - |
| Lee et al. [38] | mice | 4 mm calvarial critical size defects, wound was closed with sutures | 8 weeks | Micro-CT SEM histology precise method of evaluation is not described | PLLA + DA aligned fibers: 28.86 ± 6.5% PLLA + DA random fibers: 10.58 ± 0.9% PLLA aligned fibers: 5.25 ± 3.7% PLLA random fibers: 3.35 ± 1.8% | No sign of inflammation |
| Ko et al. [37] | rats | 8 mm calvarial critical size defects, a polyvinyl membrane was laid over the defects and the wound was closed with sutures | 8 and 12 weeks | Micro-CT nhistology precise method of evaluation is not described | 8 weeks: PLLA: minimal newly formed bone PLLA + DBP: greater extent of newly formed bone than PLLA alone 12 weeks: PLLA: 70% PLLA + DBP: 90% | PLLA: large numbers of inflammatory cells (12 weeks) PLLA + DBP: Minimal inflammatory reactions (12 weeks) |
| Schneider et al. [9] | rabbits | 6 mm calvarial non-critical size, wound was closed with sutures | 4 weeks | Radiography Micro-CT histology evaluation with an image software | PLGA/TCP: 34.9 ± 17% Bio Oss: 30.8 ± 14.3% Empty: 28.4 ± 14.9% PLGA: 25.1 ± 14.6% | No sign of inflammation |
| Schofer et al. [30] | rats | 5 mm calvarial critical size defects, the wound was closed by suturing the overlaying tissue and skin | 4, 8, and 12 weeks | CCT histology evaluation with an image software | 4 weeks: PLLA/BMP-2: 31% BS: 4% PLLA: 3% Empty: 1% 8 weeks: PLLA/BMP-2: 48% BS: 6% LLA: 5% Empty: 3% 12 weeks: PLLA/BMP-2: 48% BS: 26% PLLA: 2% Empty: 9% | No sign of inflammation |
| Shim et al. [31] | rabbits | 8mm calvarial defects Wound was closed with sutures | 2 and 4 weeks | histology | - | 2 weeks: cells (mostly connective tissue and inflammatory cells) penetrated the three-dimensional scaffolds. 4 weeks: new bone formation was observed |
| Yanagida et al. [32] | rats | 3 mm calvarial defects Wound was closed with sutures | 4 weeks | histology | - | PLLA: rarely new bone HAP-mixed/coated PLLA: new bone was more elongated |
3.4.3. New Bone Formation at Different Time Points
(1) PLGA
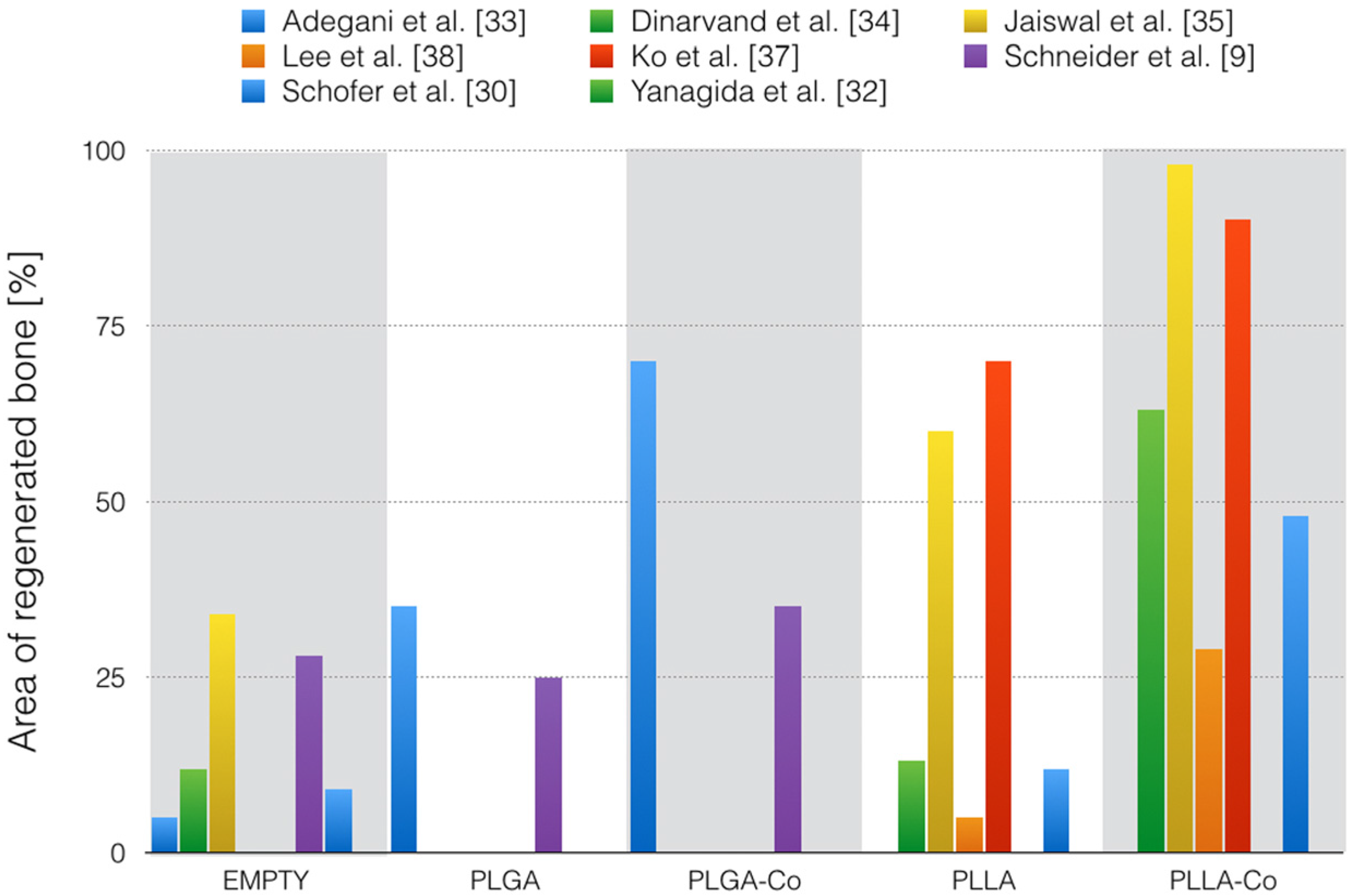
(2) PLLA
3.4.4. Biocompatibility Based on the Descriptive Histological Evaluation
(1) PLGA
(2) PLLA
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, M.L.; Raut, C.P. Modern surgical therapy: Limb salvage and the role of amputation for extremity soft-tissue sarcomas. Surg. Oncol. Clin. N. Am. 2012, 21, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Martou, G.; Antonyshyn, O.M. Advances in surgical approaches to the upper facial skeleton. Curr. Opin. Otolaryngol. Head Neck Surg. 2011, 19, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.A.; Greenwald, M.A.; Grossi, P.A. Transmission of infection with human allografts: Essential considerations in donor screening. Clin. Infect. Dis. 2012, 55, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Lanao, R.P.F.; Jonker, A.M.; Wolke, J.G.C.; Jansen, J.A.; Van Hest, J.C.M.; Leeuwenburgh, S.C.G. Physicochemical properties and applications of poly(lactic-co-glycolic acid) for use in bone regeneration. Tissue Eng. Part B Rev. 2013, 19, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Schneider, O.D.; Loher, S.; Brunner, T.J.; Uebersax, L.; Simonet, M.; Grass, R.N.; Merkle, H.P.; Stark, W.J. Cotton wool-like nanocomposite biomaterials prepared by electrospinning: In vitro bioactivity and osteogenic differentiation of human mesenchymal stem cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84B, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Schneider, O.D.; Mohn, D.; Fuhrer, R.; Klein, K.; Kämpf, K.; Nuss, K.M.R.; Sidler, M.; Zlinszky, K.; von Rechenberg, B.; Stark, W.J. Biocompatibility and bone formation of flexible, cotton wool-like PLGA/calcium phosphate nanocomposites in sheep. Open Orthop. J. 2011, 5, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Schneider, O.D.; Weber, F.; Brunner, T.J.; Loher, S.; Ehrbar, M.; Schmidlin, P.R.; Stark, W.J. In vivo and in vitro evaluation of flexible, cottonwool-like nanocomposites as bone substitute material for complex defects. Acta Biomater. 2009, 5, 1775–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ignatius, A.A.; Claes, L.E. In vitro biocompatibility of bioresorbable polymers: Poly(L,DL-lactide) and poly(L-lactide-co-glycolide). Biomaterials 1996, 17, 831–839. [Google Scholar] [CrossRef]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, K.A.; Agrawal, C.M.; Barber, F.A.; Burkhart, S.S. Orthopaedic applications for PLA-PGA biodegradable polymers. Arthrosc. J. Arthrosc. Relat. Surg. 1998, 14, 726–737. [Google Scholar] [CrossRef]
- Peppas, N.A.; Langer, R. New challenges in biomaterials. Science 1994, 263, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Treiser, M.; Abramson, S.; Langer, R.; Kohn, J. Degradable and resorbable biomaterials. In Biomaterials Science, 3rd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemon, J.E., Eds.; Academic Press: Oxford, UK, 2013; pp. 179–195. [Google Scholar]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- von Burkersroda, F.; Schedl, L.; Göpferich, A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials 2002, 23, 4221–4231. [Google Scholar] [CrossRef]
- Wu, X.S.; Wang, N. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers. Part II: Biodegradation. J. Biomater. Sci. Polym. Ed. 2001, 12, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Grizzi, I.; Garreau, H.; Li, S.; Vert, M. Hydrolytic degradation of devices based on poly(DL-lactic acid) size-dependence. Biomaterials 1995, 16, 305–311. [Google Scholar] [CrossRef]
- Park, T.G. Degradation of poly(lactic-co-glycolic acid) microspheres: Effect of copolymer composition. Biomaterials 1995, 16, 1123–1130. [Google Scholar] [CrossRef]
- Bergsma, J.E.; De Bruijn, W.C.; Rozema, F.R.; Bos, R.R.M.; Boering, G. Late degradation tissue response to poly(l-lactide) bone plates and screws. Biomaterials 1995, 16, 25–31. [Google Scholar] [CrossRef]
- Weir, N.A.; Buchanan, F.J.; Orr, J.F.; Dickson, G.R. Degradation of poly-L-lactide: Part 1: In vitro and in vivo physiological temperature degradation. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2004, 218, 307–319. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Vert, M.; Li, S.M.; Spenlehauer, G.; Guerin, P. Bioresorbability and biocompatibility of aliphatic polyesters. J. Mater. Sci. Mater. Med. 1992, 3, 432–446. [Google Scholar] [CrossRef]
- Ara, M.; Watanabe, M.; Imai, Y. Effect of blending calcium compounds on hydrolytic degradation of poly(DL-lactic acid-co-glycolic acid). Biomaterials 2002, 23, 2479–2483. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibres. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Hild, N.; Schneider, O.D.; Mohn, D.; Luechinger, N.A.; Koehler, F.M.; Hofmann, S.; Vetsch, J.R.; Thimm, B.W.; Mueller, R.; Stark, W.J. Two-layer membranes of calcium phosphate/collagen/PLGA nanofibres: In vitro biomineralisation and osteogenic differentiation of human mesenchymal stem cells. Nanoscale 2011, 3, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Schofer, M.D.; Roessler, P.P.; Schaefer, J.; Theisen, C.; Schlimme, S.; Heverhagen, J.T.; Voelker, M.; Dersch, R.; Agarwal, S.; Fuchs-Winkelmann, S.; Paletta, J.R.J. Electrospun PLLA nanofiber scaffolds and their use in combination with BMP-2 for reconstruction of bone defects. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, I.K.; Jung, M.R.; Kim, K.H.; Seol, Y.J.; Park, Y.J.; Park, W.H.; Lee, S.J. Novel three-dimensional scaffolds of poly((L)-lactic acid) microfibers using electrospinning and mechanical expansion: Fabrication and bone regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95B, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, H.; Okada, M.; Masuda, M.; Narama, I.; Nakano, S.; Kitao, S.; Takakuda, K.; Furuzono, T. Preparation and in vitro/in vivo evaluations of dimpled poly(l-lactic acid) fibers mixed/coated with hydroxyapatite nanocrystals. J. Artif. Organs 2011, 14, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Adegani, F.J.; Langroudi, L.; Ardeshirylajimi, A.; Dinarvand, P.; Dodel, M.; Doostmohammadi, A.; Rahimian, A.; Zohrabi, P.; Seyedjafari, E.; Soleimani, M. Coating of electrospun poly(lactic-co-glycolic acid) nanofibers with willemite bioceramic: Improvement of bone reconstruction in rat model. Cell Biol. Int. 2014, 38, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Dinarvand, P.; Seyedjafari, E.; Shafiee, A.; Jandaghi, A.B.; Doostmohammadi, A.; Fathi, M.H.; Farhadian, S.; Soleimani, M. New approach to bone tissue engineering: Simultaneous application of hydroxyapatite and bioactive glass coated on a poly(L-lactic acid) scaffold. ACS Appl. Mater. Interfaces 2011, 3, 4518–4524. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.K.; Dhumal, R.V.; Ghosh, S.; Chaudhari, P.; Nemani, H.; Soni, V.P.; Vanage, G.R.; Bellare, J.R. Bone healing evaluation of nanofibrous composite scaffolds in rat calvarial defects: A comparative study. J. Biomed. Nanotechnol. 2013, 9, 2073–2085. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Sun, H.; Yuan, A.; Zhang, K.; Li, D.; Li, C.; Shi, C.; Li, X.; Gao, K.; Zheng, C.; Yang, B.; Sun, H. Enhancement of osteoinduction by continual simvastatin release from poly(lactic-co-glycolic acid)-hydroxyapatite-simvastatin nano-fibrous scaffold. J. Biomed. Nanotechnol. 2013, 9, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.K.; Jeong, S.I.; Rim, N.G.; Lee, Y.M.; Shin, H.; Lee, B.K. In vitro osteogenic differentiation of human mesenchymal stem cells and in vivo bone formation in composite nanofiber meshes. Tissue Eng. Part A 2008, 14, 2105–2119. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, Y.J.; Cho, H.J.; Shin, H. Guidance of in vitro migration of human mesenchymal stem cells and in vivo guided bone regeneration using aligned electrospun fibers. Tissue Eng. Part A 2014, 20, 2031–2042. [Google Scholar] [CrossRef] [PubMed]
- Lanao, R.P.F.; Leeuwenburgh, S.C.G.; Wolke, J.G.C.; Jansen, J.A. In vitro degradation rate of apatitic calcium phosphate cement with incorporated PLGA microspheres. Acta Biomater. 2011, 7, 3459–3468. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Kawazoe, N.; Tateishi, T.; Chen, G. In vitro evaluation of biodegradation of poly(lactic-co-glycolic acid) sponges. Biomaterials 2008, 29, 3438–3443. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, S.H.; Santos, A.R.; Zavaglia, C.A.C.; Duek, E.A.R. Porous and dense poly(L-lactic acid) and poly(D,L-lactic acid co-glycolic acid) scaffolds: In vitro degradation in culture medium and osteoblasts culture. J. Mater. Sci. Mater. Med. 2004, 15, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Ramakrishna, S. Nano-featured scaffolds for tissue engineering: A review of spinning methodologies. Tissue Eng. 2006, 12, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.Y.; Park, J.C.; Um, Y.J.; Jung, U.W.; Kim, C.S.; Cho, K.S.; Choi, S.H. Spontaneous healing capacity of rabbit cranial defects of various sizes. J. Periodontal Implant Sci. 2010, 40, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.E.; Holtorf, H.L.; Reis, R.L.; Mikos, A.G. Influence of the porosity of starch-based fiber mesh scaffolds on the proliferation and osteogenic differentiation of bone marrow stromal cells cultured in a flow perfusion bioreactor. Tissue Eng. 2006, 12, 801–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, Y.; Yaszemski, M.J.; Mikos, A.G.; Laurencin, C.T. Tissue engineering of bone: Material and matrix considerations. J. Bone Jt. Surg. 2008, 90, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X.; Choi, J.W. Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Eng. 2001, 7, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, J.; Low, S.; Choon, A.T.; Ramakrishna, S. Interaction of cells and nanofiber scaffolds in tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84B, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Mitchell, G.; Messina, A.; Price, L.; Thompson, E.; Penington, A.; Morrison, W.; O’Connor, A.; Stevens, G.; Cooper-White, J. The influence of architecture on degradation and tissue ingrowth into three-dimensional poly(lactic-co-glycolic acid) scaffolds in vitro and in vivo. Biomaterials 2006, 27, 2854–2864. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.K.; Morris, F.; Shah, S.A.; Tuan, R.S. Surface composition of orthopaedic implant metals regulates cell attachment, spreading, and cytoskeletal organization of primary human osteoblasts in vitro. Clin. Orthop. Relat. Res. 1994, 305, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.C.; Shaw, S.Y.; Lin, H.R.; Lee, T.M.; Yang, C.Y. Bone tissue engineering evaluation based on rat calvaria stromal cells cultured on modified PLGA scaffolds. Biomaterials 2006, 27, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Saito, E.; Liao, E.E.; Hu, W.W.; Krebsbach, P.H.; Hollister, S.J. Effects of designed PLLA and 50:50 PLGA scaffold architectures on bone formation in vivo. J. Tissue Eng. Regen. Med. 2013, 7, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cells Mater. 2007, 13, 1–10. [Google Scholar]
- Uludag, H.; D’Augusta, D.; Golden, J.; Li, J.; Timony, G.; Riedel, R.; Wozney, J.M. Implantation of recombinant human bone morphogenetic proteins with biomaterial carriers: A correlation between protein pharmacokinetics and osteoinduction in the rat ectopic model. J. Biomed. Mater. Res. 2000, 50, 227–238. [Google Scholar] [CrossRef]
- Fu, Y.C.; Nie, H.; Ho, M.L.; Wang, C.K.; Wang, C.H. Optimized bone regeneration based on sustained release from three-dimensional fibrous PLGA/HAp composite scaffolds loaded with BMP-2. Biotechnol. Bioeng. 2008, 99, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-R.; Thuy Ba Linh, N.; Min, Y.-K.; Lee, B.-T. In vitro and in vivo studies of BMP-2-loaded PCL-gelatin-BCP electrospun scaffolds. Tissue Eng. Part A 2014, 20, 3279–3289. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, S.S.; Kim, Y.H.; Kim, S.H.; Kim, B.S.; Kim, S.; Choi, C.Y.; Kim, S.H. A poly(lactic acid)/calcium metaphosphate composite for bone tissue engineering. Biomaterials 2005, 26, 6314–6322. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Lee, H.H.; Knowles, J.C. Electrospinning biomedical nanocomposite fibers of hydroxyapaite/poly(lactic acid) for bone regeneration. J. Biomed. Mater. Res. Part A 2006, 79, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.; Zuo, Y.; Li, J.; Ma, S.; Cheng, L. Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials 2007, 28, 3338–3348. [Google Scholar] [CrossRef] [PubMed]
- Hile, D.D.; Doherty, S.A.; Trantolo, D.J. Prediction of resorption rates for composite polylactide/hydroxylapatite internal fixation devices based on initial degradation profiles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 71B, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Meijer, G.J.; de Bruijn, J.D.; Koole, R.; van Blitterswijk, C.A. Cell-based bone tissue engineering. PLoS Med. 2007, 4. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.C.; de Castro Lima, C.E.V.; Frederico, L.; de Castro, R.P.D.S.; Anbinder, A.L. The influence of local administration of simvastatin in calvarial bone healing in rats. J. Cranio-Maxillofac. Surg. 2011, 39, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Inoda, H.; Yamamoto, G.; Hattori, T. rh-BMP2-induced ectopic bone for grafting critical size defects: A preliminary histological evaluation in rat calvariae. Int. J. Oral Maxillofac. Surg. 2007, 36, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Soh, B.W.; Fu, Y.C.; Wang, C.H. Three-dimensional fibrous PLGA/HAp composite scaffold for BMP-2 delivery. Biotechnol. Bioeng. 2008, 99, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.S.; Young, S.; Tabata, Y.; Jansen, J.A.; Wong, M.E.K.; Mikos, A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 2008, 43, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Rim, N.G.; Kim, S.J.; Shin, Y.M.; Jun, I.; Lim, D.W.; Park, J.H.; Shin, H. Mussel-inspired surface modification of poly(L-lactide) electrospun fibers for modulation of osteogenic differentiation of human mesenchymal stem cells. Colloid Surf. B Biointerfaces 2012, 91, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Tanigo, T.; Takaoka, R.; Tabata, Y. Sustained release of water-insoluble simvastatin from biodegradable hydrogel augments bone regeneration. J. Control. Release 2010, 143, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Teraoka, F.; Fujimoto, S.; Hamada, Y.; Kibayashi, H.; Takahashi, J. Improvement of cell adhesion on poly(L-lactide) by atmospheric plasma treatment. J. Biomed. Mater. Res. Part A 2006, 77, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Chang, J. pH-compensation effect of bioactive inorganic fillers on the degradation of PLGA. Compos. Sci. Technol. 2005, 65, 2226–2232. [Google Scholar] [CrossRef]
- Manninen, M.J.; Päivärinta, U.; Pätiälä, H.; Rokkanen, P.; Taurio, R.; Tamminmäki, M.; Törmälä, P. Shear strength of cancellous bone after osteotomy fixed with absorbable self-reinforced polyglycolic acid and poly-L-lactic acid rods. J. Mater. Sci. Mater. Med. 1992, 3, 245–251. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holderegger, C.; Schmidlin, P.R.; Weber, F.E.; Mohn, D. Preclinical in vivo Performance of Novel Biodegradable, Electrospun Poly(lactic acid) and Poly(lactic-co-glycolic acid) Nanocomposites: A Review. Materials 2015, 8, 4912-4931. https://doi.org/10.3390/ma8084912
Holderegger C, Schmidlin PR, Weber FE, Mohn D. Preclinical in vivo Performance of Novel Biodegradable, Electrospun Poly(lactic acid) and Poly(lactic-co-glycolic acid) Nanocomposites: A Review. Materials. 2015; 8(8):4912-4931. https://doi.org/10.3390/ma8084912
Chicago/Turabian StyleHolderegger, Claudia, Patrick R. Schmidlin, Franz E. Weber, and Dirk Mohn. 2015. "Preclinical in vivo Performance of Novel Biodegradable, Electrospun Poly(lactic acid) and Poly(lactic-co-glycolic acid) Nanocomposites: A Review" Materials 8, no. 8: 4912-4931. https://doi.org/10.3390/ma8084912








