Assembly of CdS Quantum Dots onto Hierarchical TiO2 Structure for Quantum Dots Sensitized Solar Cell Applications
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of Hierarchical TiO2 Structure
2.2. Deposition of CdS Quantum Dots (QD)
2.3. Preparation of Electrolyte Solution
2.4. Fabrication of QDSCs
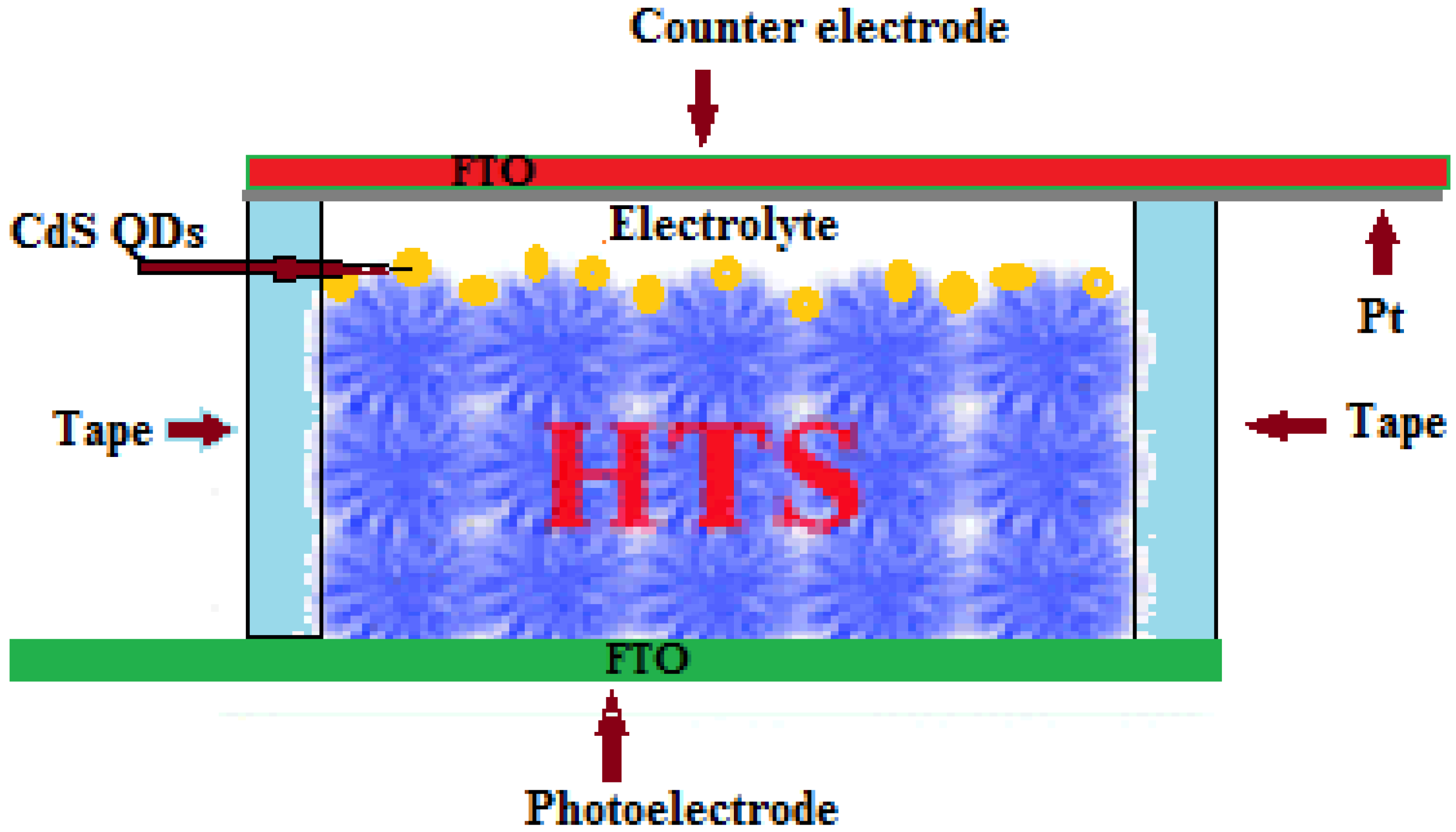
2.5. Characterization
3. Results and Discussion
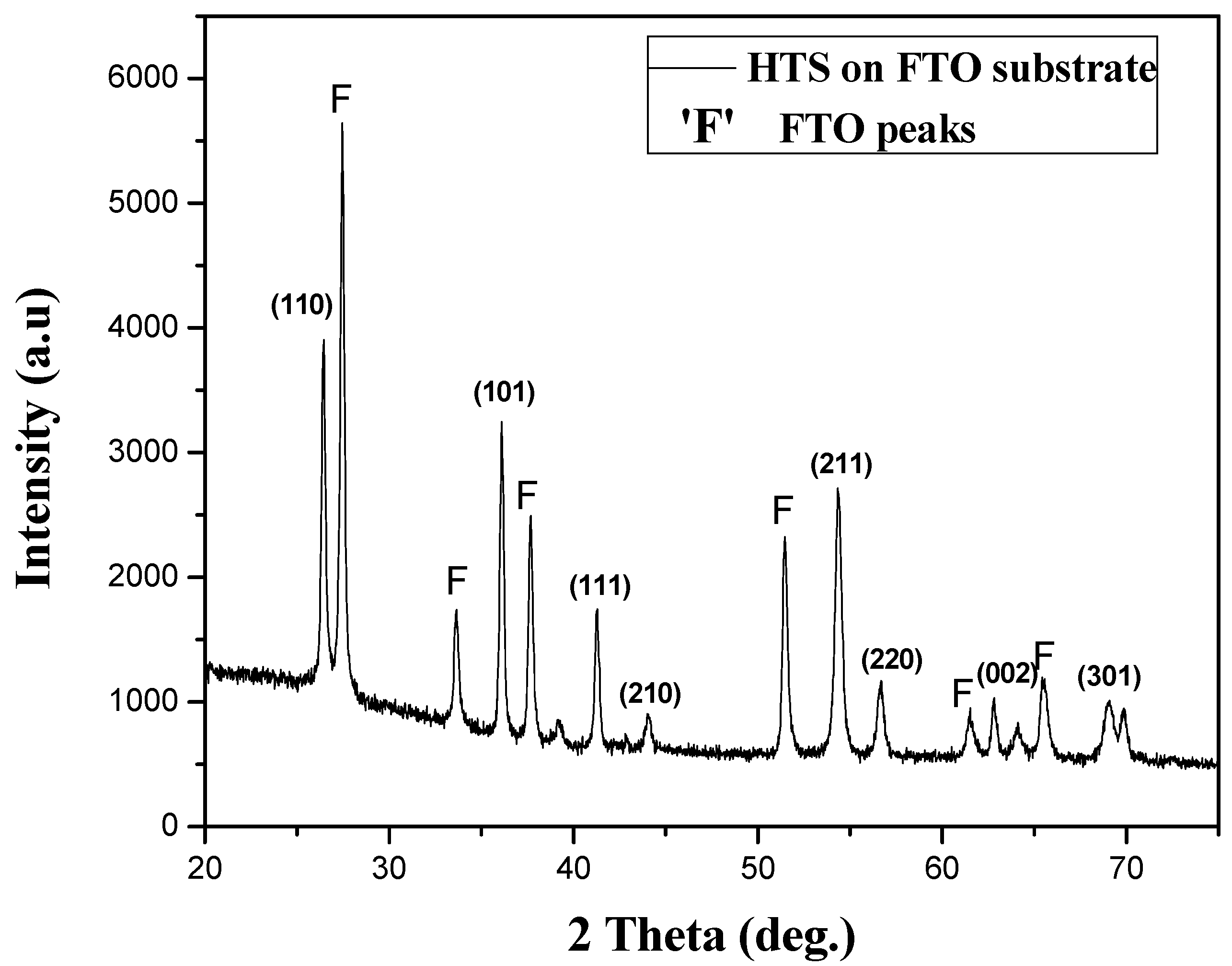
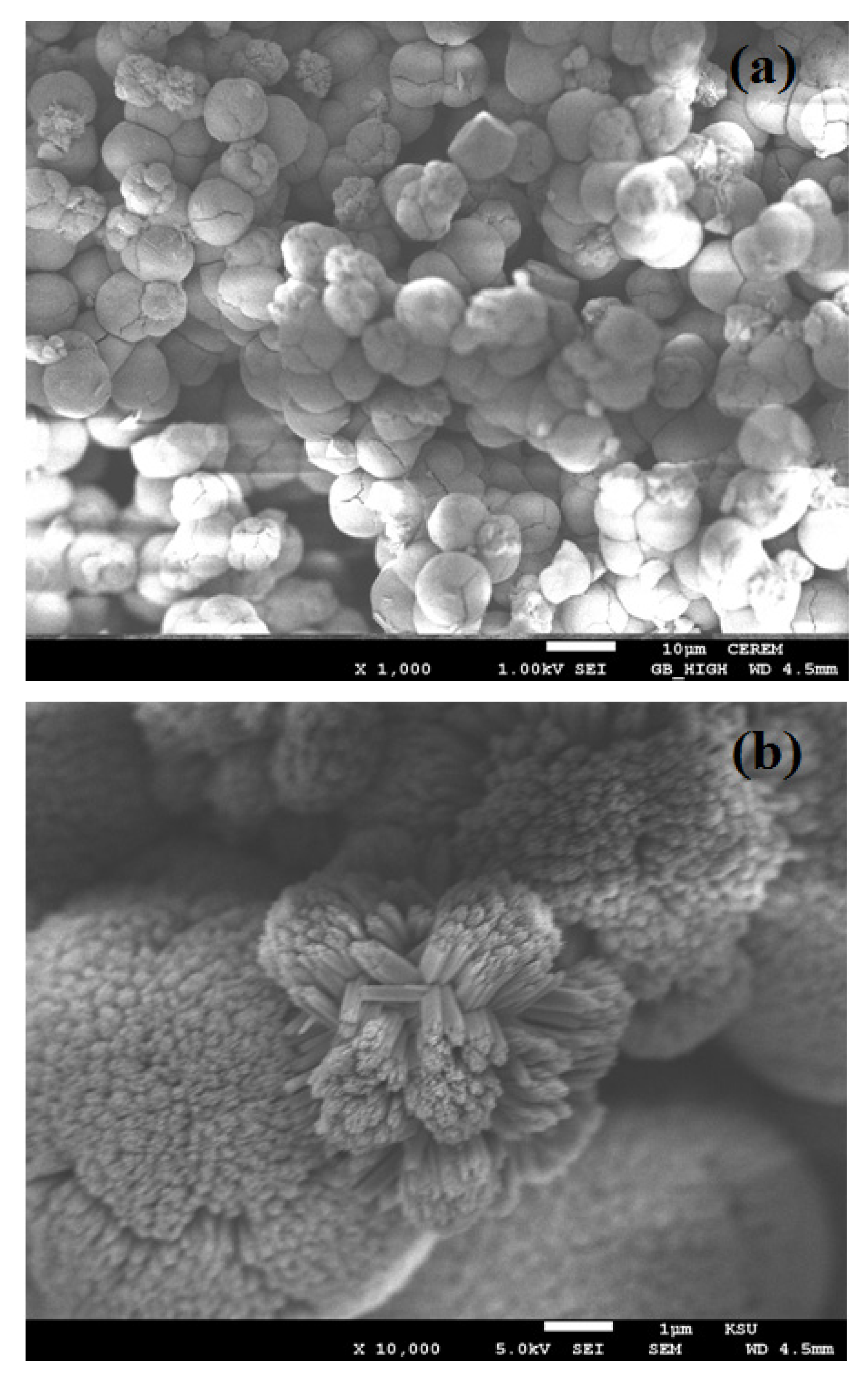
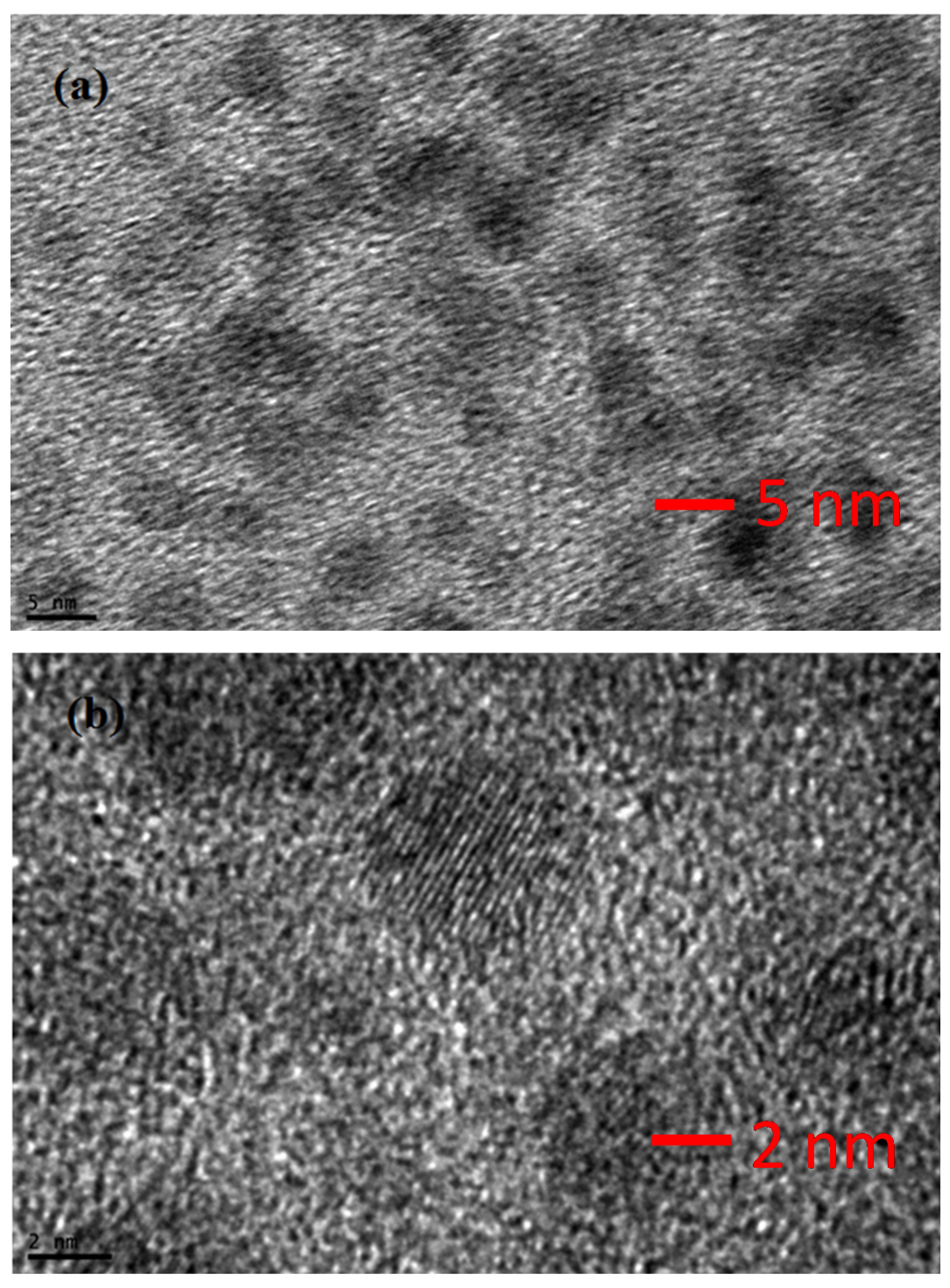
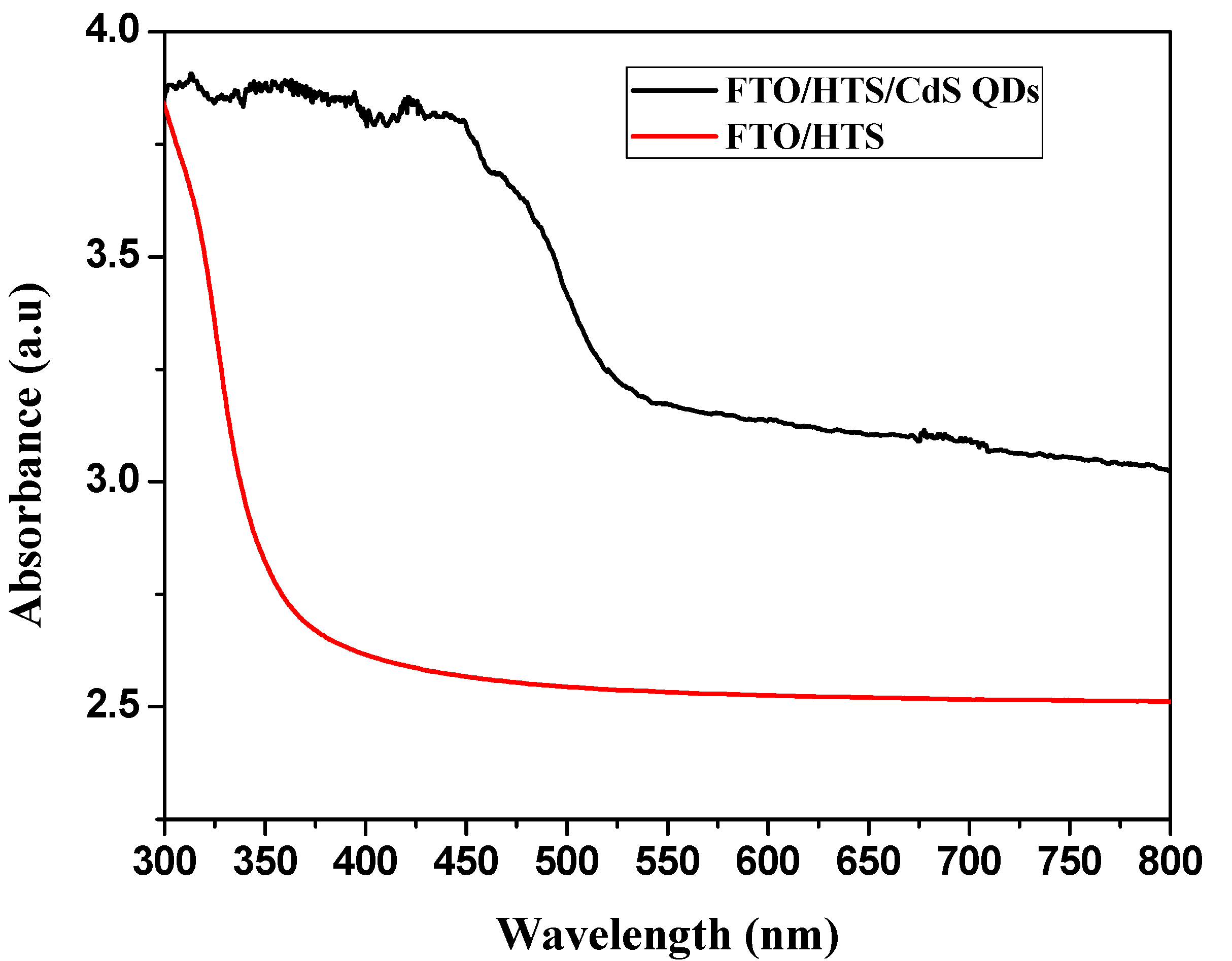
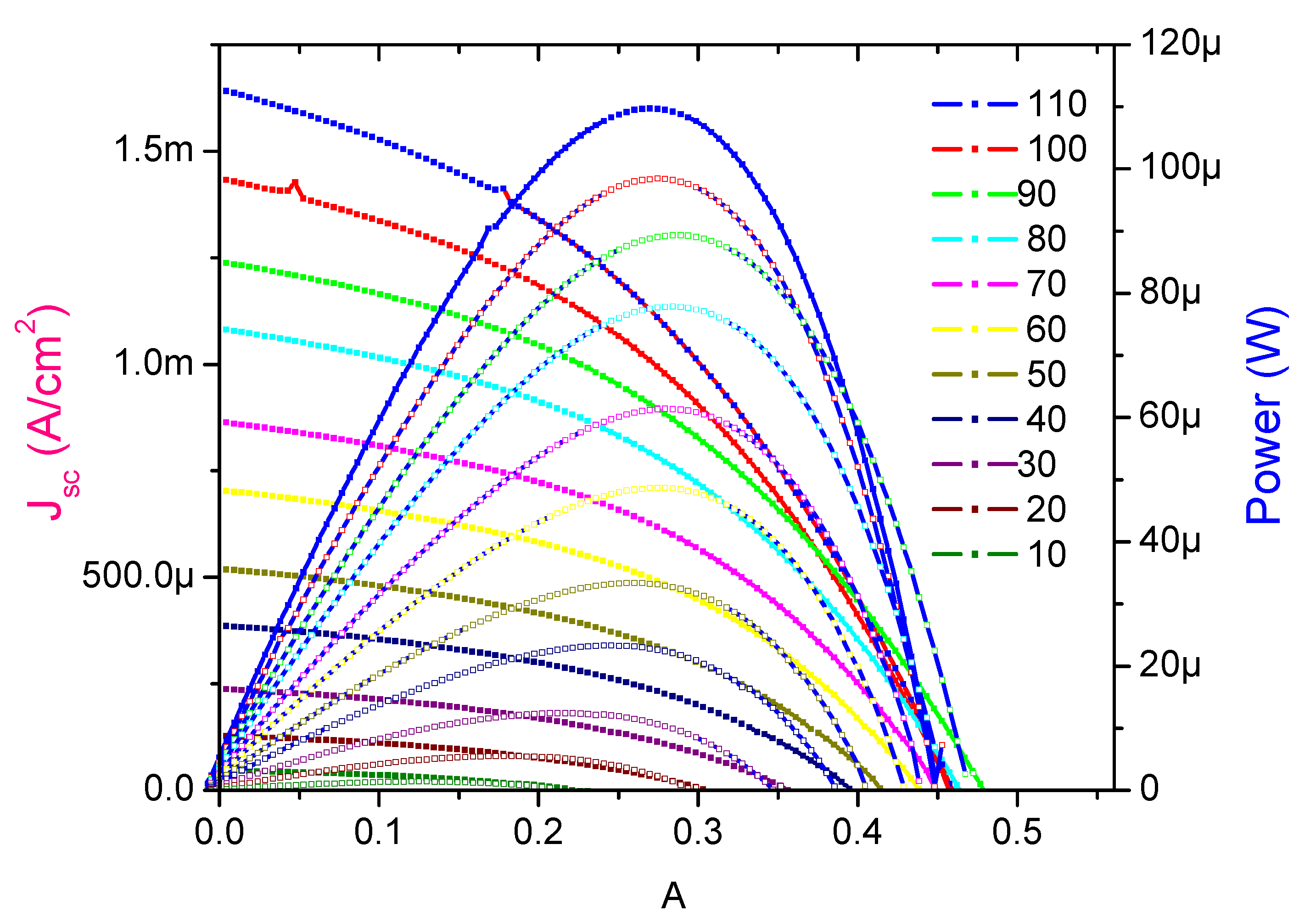

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 335, 737–740. [Google Scholar] [CrossRef]
- Yin, X.; Xue, Z.S.; Liu, B. Electrophoretic deposition of Pt nanoparticles on plastic substrates as counter electrode for flexible dye-sensitized solar cells. J. Power Sources 2011, 196, 2422–2426. [Google Scholar] [CrossRef]
- Song, H.K.; Yoon, J.S.; Won, J.; Kim, H.; Yeom, M.S. New approach to the reduction of recombination in dye-sensitised solar cells via complexation of oxidised species. J. Nanosci. Nanotechnol. 2013, 13, 5136–5141. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.X.; Xu, C.; Wang, X.; Wang, S.H.; Pan, C.F.; Lin, C.J.; Wang, Z.L. Rectangular bunched rutile TiO2 nanorod arrays grown on carbon fiber for dye-sensitized solar cells. J. Am. Chem. Soc. 2012, 134, 4437–4441. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.M.; Sun, Q.; Li, Y.; Sui, L.N.; Dong, L.F. Effects of calcination treatment on the morphology and crystallinity, and photoelectric properties of all-solid-state dye-sensitized solar cells assembled by TiO2 nanorod arrays. Phys. Chem. Chem. Phys. 2013, 15, 18716–18720. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Zhou, Z.J.; Li, M.; Zhou, W.H.; Yuan, S.J.; Yao, R.Y.; Zhao, Y.; Wu, S.X. Wurtzite copper-zinc-tin sulfide as a superior counter electrode material for dye-sensitized solar cells. Nanoscale Res. Lett. 2013, 8. [Google Scholar] [CrossRef]
- Grätzel, M. Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 2005, 44, 6841–6851. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Chu, Z.Z.; Wang, F.Z.; Zhang, C.; Chen, L.; Chen, Y.; Tang, Y.W.; Zou, D.C. Wire-shaped flexible dye-sensitized solar cells. Adv. Mater. 2008, 20, 592–595. [Google Scholar] [CrossRef]
- Lee, M.R.; Eckert, R.D.; Forberich, K.; Dennler, G.; Brabec, C.J.; Gaudiana, R.A. Solar power wires based on organic photovoltaic materials. Science 2009, 324, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.K.; Hsu, W.D.; Wu, T.C.; Meen, T.H.; Chong, W.J. Effect of compressed TiO2 nanoparticle thin film thickness on the performance of dye-sensitized solar cells. Nanoscale Res. Lett. 2013, 8. [Google Scholar] [CrossRef]
- Wang, D.; Hou, S.C.; Wu, H.W.; Zhang, C.; Chu, Z.Z.; Zou, D.C. Fiber-shaped all-solid state dye sensitized solar cell with remarkably enhanced performance via substrate surface engineering and TiO2 film modification. J. Mater. Chem. 2011, 21, 6383–6388. [Google Scholar] [CrossRef]
- Gorer, S.; Hodes, G. Quantum size effects in the study of chemical solution deposition mechanisms of semiconductor films. J. Phys. Chem. 1994, 98, 5338–5346. [Google Scholar] [CrossRef]
- Moreels, I.; Lambert, K.; de-Muynck, D.; Vanhaecke, F.; Poelman, D.; Martins, J.C.; Allan, G.; Hens, Z. Composition and size-dependent extinction coefficient of colloidal PbSe quantum dots. Chem. Mater. 2007, 19, 6101–6106. [Google Scholar] [CrossRef]
- Shaller, R.D.; Klimov, V.I. High efficiency carrier multiplication in PbSe nanocrystals: Implications for solar energy conversion. Phys. Rev. Lett. 2004, 92. [Google Scholar] [CrossRef]
- Michele, P.; Sven, R.; Adam, G.; David, A.K.; Hannah, N.B.; Paolo, M.S.; Daniela, N.; Rodrigo, M.; Assaf, Y.A.; Arie, Z.; et al. TiO2/Cu2O all-oxide heterojunction solar cells produced by spray pyrolysis. Sol. Energy Mater. Sol. Cells 2015, 132, 549–556. [Google Scholar] [CrossRef]
- Hu, L.H. Microstructure design of nanoporous TiO2 photoelectrodes for dye-sensitized solar cell modules. J. Phys. Chem. B 2007, 111, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Lana, V.T.; Monllor, S.D.; Gomez, R. An electrochemical study on the nature of trap states in nanocrystalline rutile thin films. J. Phys. Chem. C 2007, 111, 9936–9942. [Google Scholar] [CrossRef]
- Qu, J.; Li, G.R.; Gao, X.P. One-dimensional hierarchical titania for fast reaction kinetics of photoanode materials of dye-sensitized solar cells. Energy Environ. Sci. 2010, 3, 2003–2009. [Google Scholar] [CrossRef]
- Liao, J.Y.; Lei, B.X.; Chen, H.Y.; Kuang, D.B.; Su, C.Y. Oriented hierarchical single crystalline anatase TiO2 nanowire arrays on Ti-foil substrate for efficient flexible dye-sensitized solar cells. Energy Environ. Sci. 2012, 5, 5750–5757. [Google Scholar] [CrossRef]
- Liao, J.Y.; Lei, B.X.; Kuang, D.B.; Su, C.Y. Tri-functional hierarchical TiO2 spheres consisting of anatase nanorods and nanoparticles for high efficiency dye-sensitized solar cells. Energy Environ. Sci. 2011, 4, 4079–4085. [Google Scholar] [CrossRef]
- Ohsaki, Y. Dye-sensitized TiO2 nanotube solar cells: Fabrication and electronic characterization. Phys. Chem. Chem. Phys. 2005, 7, 4157–4163. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Neale, N.R.; Miedaner, A.; Frank, A.J. Enhanced charge-collection efficiencies and light scattering in dye-sensitized solar cells using oriented TiO2 nanotubes arrays. Nano Lett. 2007, 7, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Shankar, K.; Paulose, M.; Grimes, C.A. Tantalum-doped titanium dioxide nanowire arrays for dye-sensitized solar cells with high open-circuit voltage. Angew. Chem. Int. Ed. 2009, 48, 8095–8098. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, B.X.L.; Hua, S.R.; Cheng, Y.S.; Dai, B.K. Hydrothermal fabrication of hierarchically anatase TiO2 nanowire arrays on FTO glass for dye-sensitized solar cells. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Qiang, P.; Zhang, D.; Cai, X.; Tan, S.; Liu, P.; Mai, W. Three-level hierarchical TiO2nanostructure based high efficiency dye-sensitized solar cells. CrysEngComm 2014, 16, 1020–1025. [Google Scholar] [CrossRef]
- Lee, Y.L.; Lo, Y.S. Highly efficient quantum-dot-sensitized solar cell based on co-sensitization of CdS/CdSe. Adv. Funct. Mater. 2009, 19, 604–609. [Google Scholar] [CrossRef]
- Nath, S.S.; Choudhury, M.; Gope, G.; Nath, R.K. PVA embedded ZnO quantum dots for methanol sensing. Nanotrends 2010, 8, 1–4. [Google Scholar]
- Kong, E.H.; Chang, Y.J.; Park, Y.C.; Yoon, Y.H.; Park, H.J.; Jang, H.M. Sea urchin TiO2-nanoparticle hybrid composite photoelectrodes for CdS/CdSe/ZnS quantum-dot-sensitized solar cells. Phys. Chem. Chem. Phys. 2012, 14, 4620–4625. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Chen, C.Y.; Chen, J.G.; Li, J.Y.; Tung, Y.L.; Ho, K.C.; Wu, C.G. Carbazole containing Ru-based photo-sensitizer for dye-sensitized solar cell. J. Chin. Chem. Soc. 2010, 57, 1127–1130. [Google Scholar] [CrossRef]
- Rosenblut, M.L.; Lewis, N.S. Ideal behavior of the open circuit voltage of semiconductor liquid junction. J. Phys. Chem. 1989, 93, 3735–3740. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Lindström, H.; Södergren, S.; Lindquist, S.E. Photoelectrochemical studies of colloidal TiO2 films: The effect of oxygen studied by photocurrent transients. J. Electroanal. Chem. 1995, 381, 39–46. [Google Scholar] [CrossRef]
- Sheng, X.; Zhao, Y.; Zhai, J.; Jiang, L.; Zhu, D. Electro-hydrodynamic fabrication of ZnO-based dye sensitized solar cells. Appl. Phys. A 2007, 87, 715–719. [Google Scholar] [CrossRef]
- Sogabe, T.; Shoji, Y.; Ohba, M.; Yoshida, K.; Tamaki, R.; Hong, H.F.; Wu, C.H.; Kuo, C.T.; Tomic, S.; Okada, Y.; et al. Intermediate band dynamic of quantum dots solar cell in concentrated photovoltaic modules. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Breitenstein, O.; Langenkamp, M.; Rakotoniaina, J.P.; Zettner, J. The imaging of shunts in solar cells by infrared lock-in thermography. In Proceedings of the 17th European Photovoltaic Solar Energy Conference, Munich, Germany, 22–26 October 2001; pp. 1499–1502.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.M.; Aslam, M.; Farooq, W.A.; Fatehmulla, A.; Atif, M. Assembly of CdS Quantum Dots onto Hierarchical TiO2 Structure for Quantum Dots Sensitized Solar Cell Applications. Materials 2015, 8, 2376-2386. https://doi.org/10.3390/ma8052376
Ali SM, Aslam M, Farooq WA, Fatehmulla A, Atif M. Assembly of CdS Quantum Dots onto Hierarchical TiO2 Structure for Quantum Dots Sensitized Solar Cell Applications. Materials. 2015; 8(5):2376-2386. https://doi.org/10.3390/ma8052376
Chicago/Turabian StyleAli, Syed Mansoor, Mohamed Aslam, W. A. Farooq, Amanullah Fatehmulla, and M. Atif. 2015. "Assembly of CdS Quantum Dots onto Hierarchical TiO2 Structure for Quantum Dots Sensitized Solar Cell Applications" Materials 8, no. 5: 2376-2386. https://doi.org/10.3390/ma8052376
APA StyleAli, S. M., Aslam, M., Farooq, W. A., Fatehmulla, A., & Atif, M. (2015). Assembly of CdS Quantum Dots onto Hierarchical TiO2 Structure for Quantum Dots Sensitized Solar Cell Applications. Materials, 8(5), 2376-2386. https://doi.org/10.3390/ma8052376




