Dispersion Process and Effect of Oleic Acid on Properties of Cellulose Sulfate- Oleic Acid Composite Film
Abstract
:1. Introduction
2. Results and Discussion
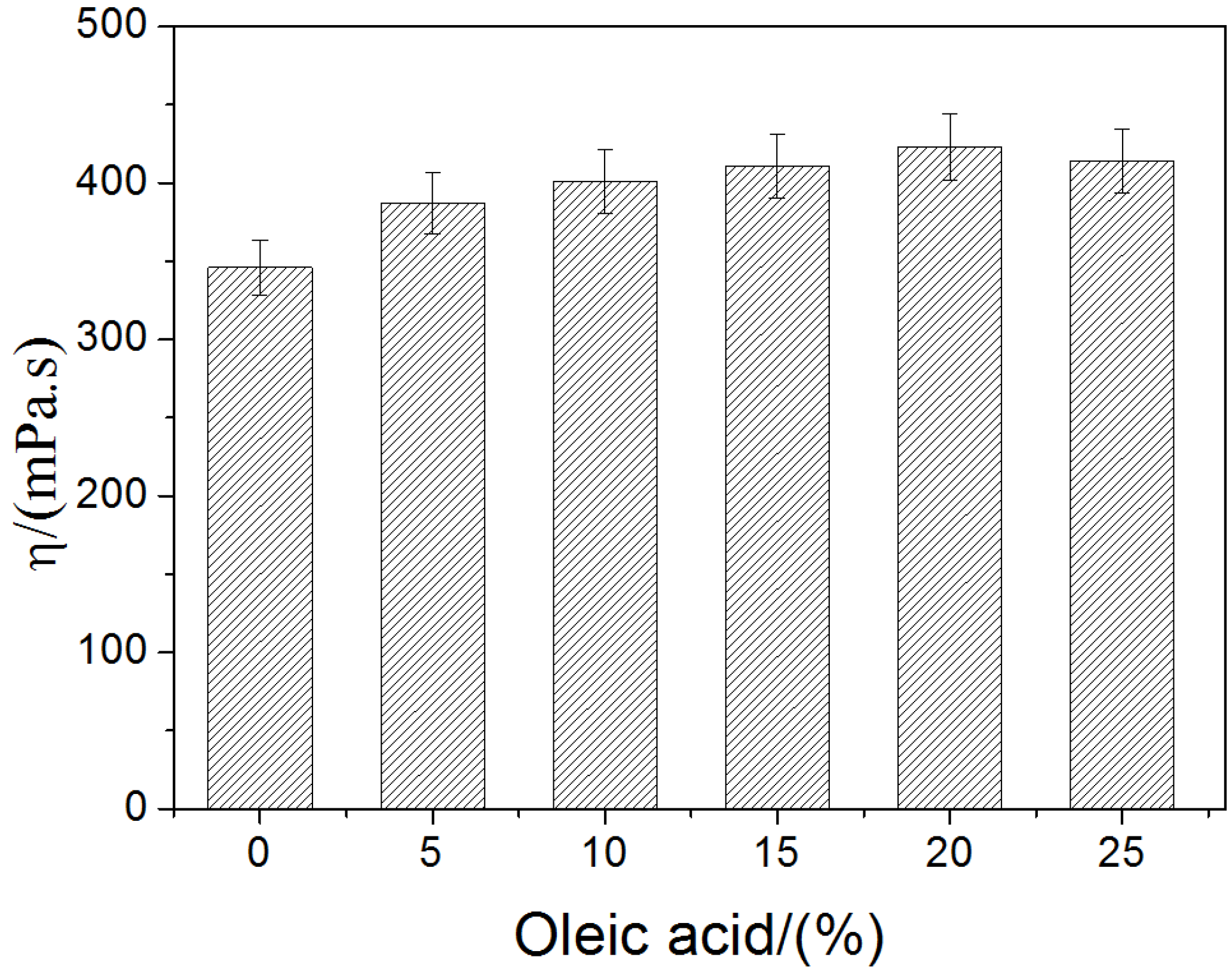
2.1. Rheological Behavior of the Film-Forming Emulsions

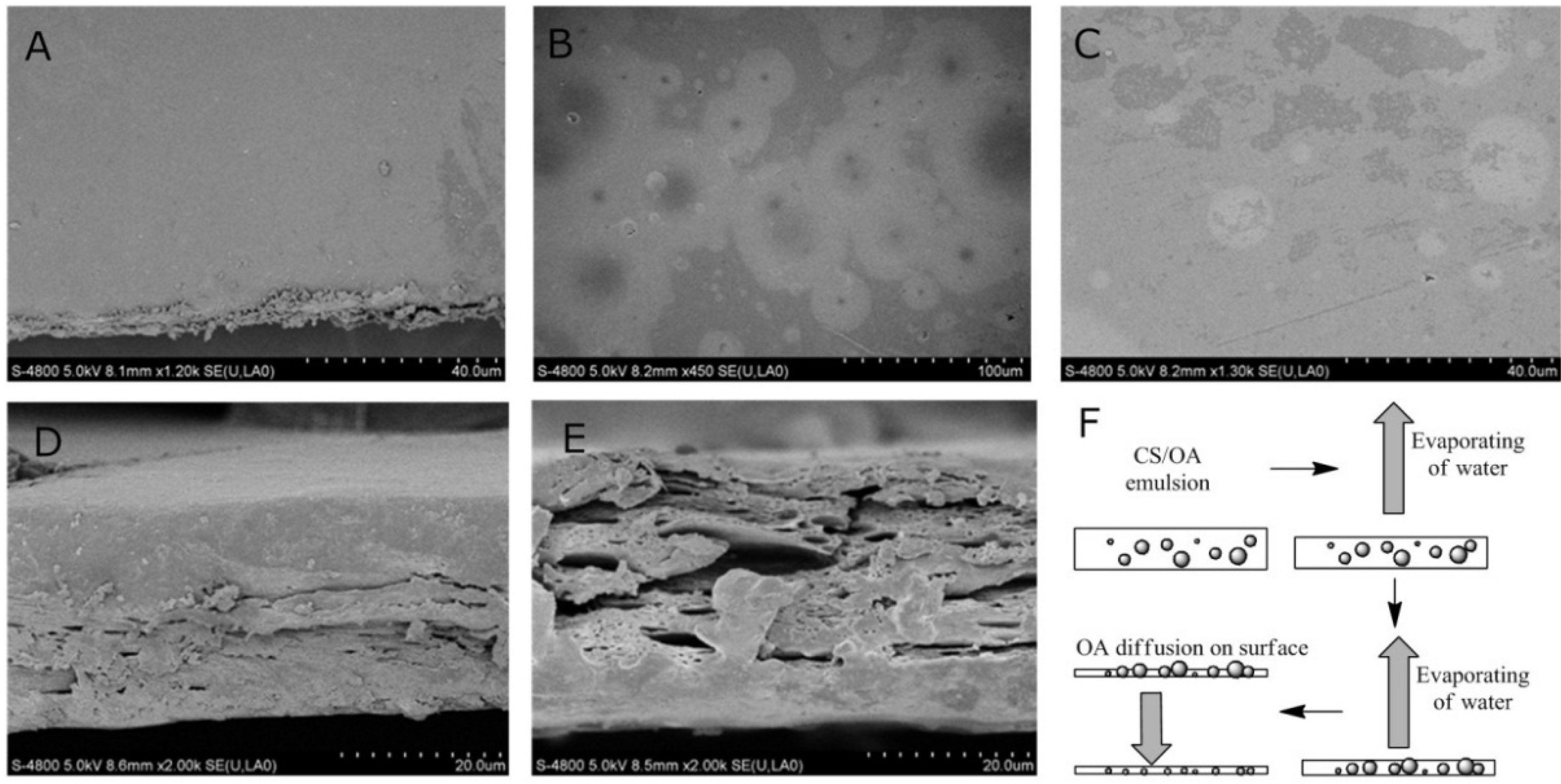
2.2. Microstructure of CS-OA Films
2.3. Surface Water Contact Angle of CS-OA Films

2.4. The Effect of Oleic Acid Content on Properties of CS-OA Films
| Sample | δ (μm) | Inte | Fold | S (h) | OP (%) | T (%) | TS (MPa) | ε (%) | WVP × 10−11 (gm−1s−1Pa−1) |
|---|---|---|---|---|---|---|---|---|---|
| 2g CS + 0.3g Gly | 25.00 ± 0.16 a | good | good | 0.02 ± 0.01 a | 0.2 ± 0.1% a | 89.3 ± 3.2 a | 14.5 ± 1.5 a | 27.9 ± 0.8 a | 3.92 ± 0.23 a |
| 2g CS + 0.3g Gly + 0.1g OA | 25.02 ± 0.10 a | good | good | 1.50 ± 0.10 b | 0.2 ± 0.2% a | 85.0 ± 5.6 a | 28.2 ± 2.1 b | 18.6 ± 0.6 b | 3.49 ± 0.21 a |
| 2g CS + 0.3g Gly + 0.2g OA | 25.85 ± 0.18 b | good | good | 2.33 ± 0.08 c | 1.9 ± 0.2% b | 76.9 ± 2.8 b | 37.1 ± 1.7 c | 13.9 ± 0.3 a | 2.41 ± 0.16 b |
| 2g CS + 0.3g Gly + 0.3g OA | 29.04 ± 0.12 c | good | good | 4.45 ± 0.12 d | 9.0 ± 0.3% c | 73.5 ± 4.8 b | 43.5 ± 1.3 d | 9.2 ± 0.5 d | 1.91 ± 0.12 bc |
| 2g CS + 0.3g Gly + 0.4g OA | 36.60 ± 0.26 d | good | good | 5.13 ± 0.50 de | 14.6 ± 0.5% d | 67.4 ± 2.3 c | 36.8 ± 1.5 e | 7.2 ± 0.3 e | 1.57 ± 0.21 c |
| 2g CS + 0.3g Gly + 0.5g OA | 55.20 ± 0.32 e | good | good | 5.55 ± 0.36 e | 25.1 ± 0.8% e | 68.2 ± 3.1 c | 34.3 ± 2.2 e | 6.4 ± 0.5 e | 1.52 ± 0.14 c |
2.5. The Interactions among Components of Edible Film
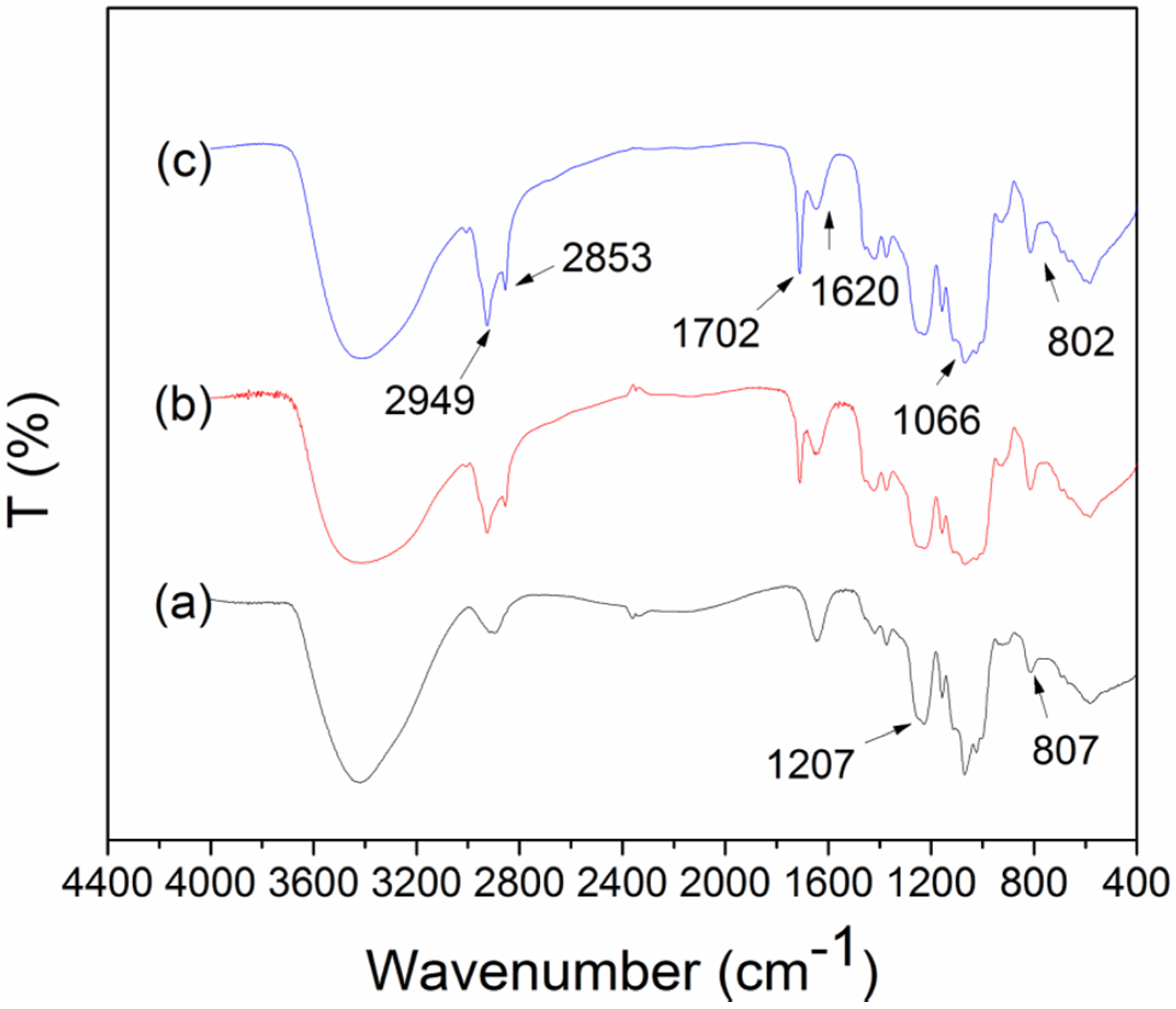
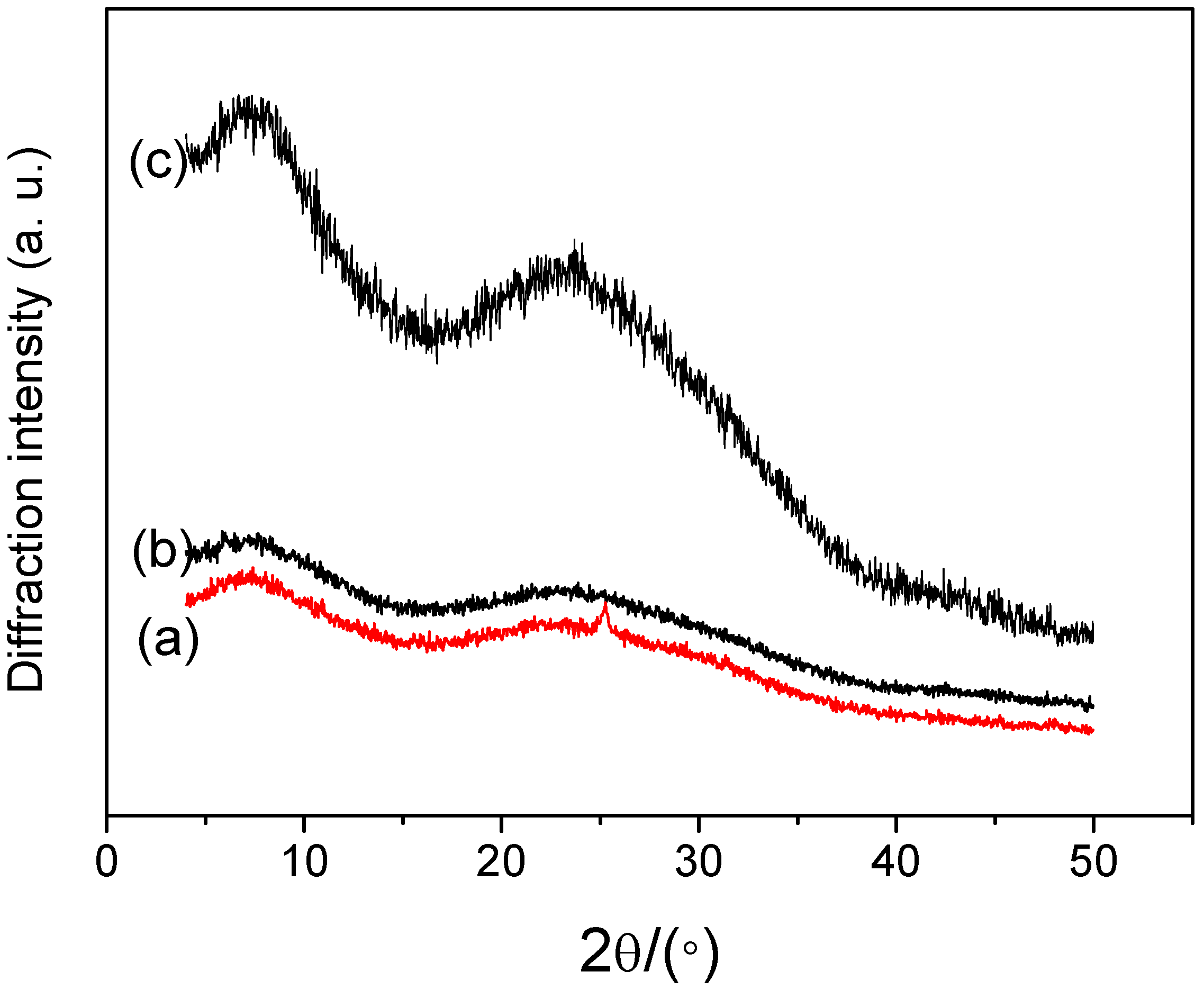
3. Experimental Section
3.1. Materials
3.2. Preparation of Cellulose Sulfate Films
3.3. Rheological Behavior of the Film Forming Emulsions
3.4. Fourier Transform Infrared Spectroscopy (FTIR)
3.5. X-ray Diffraction
3.6. Film Thickness
3.7. Scanning Electron Microscopy
3.8. Mechanical Properties
3.9. Water Vapor Permeability (WVP)
3.10. Contact Angle
3.11. Flexibility
3.12. Integrity
3.13. Oil Permeability
3.14. Water Solubility
3.15. Transparency
3.16. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Debeaufort, F.; Quezada-Gallo, J.A.; Voilley, A. Edible films and coatings: Tomorrow’s packagings: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and biodegradable starch films: A review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Clasen, C.; Kulicke, W.M. Determination of viscoelastic and rheo-optical material functions of water-soluble cellulose derivatives. Progr. Polym. Sci. 2001, 26, 1839–1919. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Jiménez, A.; Talens, P.; Chiralt, A. Properties of starch-hydroxypropyl methylcellulose based films obtained by compression molding. Carbohydr. Polym. 2014, 109, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Slavutsky, A.M.; Bertuzzi, M.A. Water barrier properties of starch films reinforced with cellulose nanocrystals obtained from sugarcane bagasse. Carbohydr. Polym. 2014, 110, 53–61. [Google Scholar] [CrossRef] [PubMed]
- De Moura, M.R.; Lorevice, M.V.; Mattoso, L.H.C.; Zucolotto, V. Highly stable, edible cellulose films incorporating chitosan nanoparticles. J. Food Sci. 2011, 76, S25–S29. [Google Scholar] [CrossRef]
- Malmiri, H.J.; Osman, A.; Tan, C.P.; Rahman, R.A. Effects of edible surface coatings (sodium carboxymethyl cellulose, sodium caseinate and glycerol) on storage quality of berangan banana (musa sapientum cv. Berangan) using response surface methodology. J. Food Process. Preserv. 2012, 36, 252–261. [Google Scholar] [CrossRef]
- Wang, S.; Kuang, X.; Li, B.; Wu, X.; Huang, T. Physical properties and antimicrobial activity of chilled meat pads containing sodium carboxymethyl cellulose. J. Appl. Polym. Sci. 2013, 127, 612–619. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; López, J.; Kenny, J.M. Bionanocomposite films based on plasticized pla–phb/cellulose nanocrystal blends. Carbohydr. Polym. 2015, 121, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Peltzer, M.; Armentano, I.; Torre, L.; Jiménez, A.; Kenny, J.M. Effects of modified cellulose nanocrystals on the barrier and migration properties of pla nano-biocomposites. Carbohydr. Polym. 2012, 90, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Ayranci, E.; Tunc, S. A method for the measurement of the oxygen permeability and the development of edible films to reduce the rate of oxidative reactions in fresh foods. Food Chem. 2003, 80, 423–431. [Google Scholar] [CrossRef]
- Bravin, B.; Peressini, D.; Sensidoni, A. Development and application of polysaccharide-lipid edible coating to extend shelf-life of dry bakery products. J. Food Eng. 2006, 76, 280–290. [Google Scholar] [CrossRef]
- Navarro-Tarazaga, M.L.; Del Rio, M.A.; Krochta, J.M.; Perez-Gago, M.B. Fatty acid effect on hydroxypropyl methylcellulose-beeswax edible film properties and postharvest quality of coated ‘ortanique’ mandarins. J. Agric. Food Chem. 2008, 56, 10689–10696. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Tarazaga, M.L.; Massa, A.; Perez-Gago, M.B. Effect of beeswax content on hydroxypropyl methylcellulose-based edible film properties and postharvest quality of coated plums (cv. Angeleno). Lwt-Food Sci. Technol. 2011, 44, 2328–2334. [Google Scholar] [CrossRef]
- Soazo, M.; Rubiolo, A.C.; Verdini, R.A. Effect of drying temperature and beeswax content on moisture isotherms of whey protein emulsion film. Procedia Food Sci. 2011, 1, 210–215. [Google Scholar] [CrossRef]
- Contreras-Oliva, A.; Rojas-Argudo, C.; Perez-Gago, M.B. Effect of solid content and composition of hydroxypropyl methylcellulose-lipid edible coatings on physico-chemical and nutritional quality of ‘oronules’ mandarins. J. Sci. Food Agric. 2012, 92, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, B.; Zhao, J.; Chen, H.W. Improved process for the production of cellulose sulfate using sulfuric acid/ethanol solution. Carbohydr. Polym. 2013, 95, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Nogues, C.; Horan, J.; Ryan, G.; Kassem, M.; O’Brien, T. Encapsulation of human mesenchymal stem cells in sodium cellulose sulfate-based microcapsules requires immortalization. Cytotherapy 2014, 16, S94. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Yan, X.Q.; Zhang, H.M.; Lin, D.Q.; Yao, S.J.; Jiang, L. Determination of apparent drug permeability coefficients through chitosan-sodium cellulose sulfate polyelectrolyte complex films. Acta Phys.-Chim. Sin. 2014, 30, 365–370. [Google Scholar]
- Chen, G.; Liu, B.; Zhang, B. Characterization of composite hydrocolloid film based on sodium cellulose sulfate and cassava starch. J. Food Eng. 2014, 125, 105–111. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, B.; Zhao, J.; Chen, H.W. Development and characterization of food packaging film from cellulose sulfate. Food Hydrocoll. 2014, 35, 476–483. [Google Scholar] [CrossRef]
- Chillo, S.; Flores, S.; Mastromatteo, M.; Conte, A.; Gerschenson, L.; Del Nobile, M.A. Influence of glycerol and chitosan on tapioca starch-based edible film properties. J. Food Eng. 2008, 88, 159–168. [Google Scholar] [CrossRef]
- Camino, N.A.; Pilosof, A.M.R. Hydroxypropylmethylcellulose at the oil-water interface. Part ii. Submicron-emulsions as affected by ph. Food Hydrocoll. 2011, 25, 1051–1062. [Google Scholar] [CrossRef]
- Mitidieri, F.; Wagner, J. Coalescence of o/w emulsiones stabilized by whey and isolate soybean proteins. Influence of thermal denaturation, salt adittion and competitive interfacial adsorption. Food Res. Int. 2002, 35, 547–557. [Google Scholar] [CrossRef]
- Fabra, M.J.; Hambleton, A.; Talens, P.; Debeaufort, F.; Chiralt, A.; Voilley, A. Influence of interactions on water and aroma permeabilities of ι-carrageenan-oleic acid-beeswax films used for flavour encapsulation. Carbohydr. Polym. 2009, 76, 325–332. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A.; Yarmand, M.S. Characterization of edible emulsified films with low affinity to water based on kefiran and oleic acid. Int. J. Biol. Macromol. 2011, 49, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Limpisophon, K.; Tanaka, M.; Osako, K. Characterisation of gelatin-fatty acid emulsion films based on blue shark (prionace glauca) skin gelatin. Food Chem. 2010, 122, 1095–1101. [Google Scholar] [CrossRef]
- Hong, S.D.; Ha, M.Y.; Balachandar, S. Static and dynamic contact angles of water droplet on a solid surface using molecular dynamics simulation. J. Colloid Interface Sci. 2009, 339, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, Y.; Ghanbarzadeh, B.; Sedaghat, N. Physical properties of edible emulsified films based on pistachio globulin protein and fatty acids. J. Food Eng. 2010, 100, 102–108. [Google Scholar] [CrossRef]
- Bertan, L.C.; Tanada-Palmu, P.S.; Siani, A.C.; Grosso, C.R.F. Effect of fatty acids and ‘brazilian elemi’ on composite films based on gelatin. Food Hydrocoll. 2005, 19, 73–82. [Google Scholar] [CrossRef]
- Yang, L.; Paulson, A.T. Effects of lipids on mechanical and moisture barrier properties of edible gellan film. Food Res. Int. 2000, 33, 571–578. [Google Scholar] [CrossRef]
- Handa, A.; Gennadios, A.; Hanna, M.A.; Weller, C.L.; Kuroda, N. Physical and molecular properties of egg-white lipid films. J. Food Sci. 1999, 64, 860–864. [Google Scholar] [CrossRef]
- Shellhammer, T.H.; Krochta, J.M. Whey protein emulsion film performance as affected by lipid type and amount. J. Food Sci. 1997, 62, 390–394. [Google Scholar] [CrossRef]
- De la Caba, K.; Pena, C.; Ciannamea, E.M.; Stefani, P.M.; Mondragon, I.; Ruseckaite, R.A. Characterization of soybean protein concentrate-stearic acid/palmitic acid blend edible films. J. Appl. Polym. Sci. 2012, 124, 1796–1807. [Google Scholar]
- Nobrega, M.M.; Olivato, J.B.; Muller, C.M.O.; Yamashita, F. Addition of saturated fatty acids to biodegradable films: Effect on the crystallinity and viscoelastic characteristics. J. Polym. Environ. 2013, 21, 166–171. [Google Scholar] [CrossRef]
- Rezvani, E.; Schleining, G.; Sumen, G.; Taherian, A.R. Assessment of physical and mechanical properties of sodium caseinate and stearic acid based film-forming emulsions and edible films. J. Food Eng. 2013, 116, 598–605. [Google Scholar] [CrossRef]
- Fabra, M.J.; Talens, P.; Chiralt, A. Tensile properties and water vapor permeability of sodium caseinate films containing oleic acid-beeswax mixtures. J. Food Eng. 2008, 85, 393–400. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, J.; Wang, X.-X.; Zhang, N.; Sun, X.-X.; Ma, Z.-S. The effects of ultrasonic/microwave assisted treatment on the water vapor barrier properties of soybean protein isolate-based oleic acid/stearic acid blend edible films. Food Hydrocoll. 2014, 35, 51–58. [Google Scholar] [CrossRef]
- Martins, S.; Fernandes, J.B. A simple method to prepare high surface area activated carbon from carboxyl methyl cellulose by low temperature physical activation. J. Therm. Anal. Calorim. 2013, 112, 1007–1011. [Google Scholar] [CrossRef]
- American Society for Testing and Materials (ASTM). Standard test method for tensile properties of thin plastic sheeting. In Standard D882. Annual Book of American Standard Testing Methods; ASTM: Philadelphia, PA, USA, 2001. [Google Scholar]
- American Society for Testing and Materials (ASTM). Standard test methods for water vapour transmission of materials. In Standards Designations: E96–95. In Annual Book of ASTM Standards; ASTM: Philadelphia, PA, USA, 1995; pp. 406–413. [Google Scholar]
- Hu, G.; Chen, J.; Gao, J. Preparation and characteristics of oxidized potato starch films. Carbohydr. Polym. 2009, 76, 291–298. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Zhang, B.; Zhao, J. Dispersion Process and Effect of Oleic Acid on Properties of Cellulose Sulfate- Oleic Acid Composite Film. Materials 2015, 8, 2346-2360. https://doi.org/10.3390/ma8052346
Chen G, Zhang B, Zhao J. Dispersion Process and Effect of Oleic Acid on Properties of Cellulose Sulfate- Oleic Acid Composite Film. Materials. 2015; 8(5):2346-2360. https://doi.org/10.3390/ma8052346
Chicago/Turabian StyleChen, Guo, Bin Zhang, and Jun Zhao. 2015. "Dispersion Process and Effect of Oleic Acid on Properties of Cellulose Sulfate- Oleic Acid Composite Film" Materials 8, no. 5: 2346-2360. https://doi.org/10.3390/ma8052346
APA StyleChen, G., Zhang, B., & Zhao, J. (2015). Dispersion Process and Effect of Oleic Acid on Properties of Cellulose Sulfate- Oleic Acid Composite Film. Materials, 8(5), 2346-2360. https://doi.org/10.3390/ma8052346





