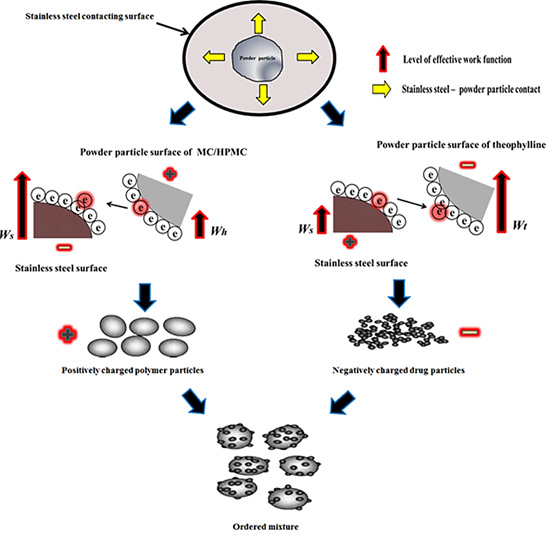
Tribo-electrification and Powder Adhesion Studies in the Development of Polymeric Hydrophilic Drug Matrices
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterisation of Powders

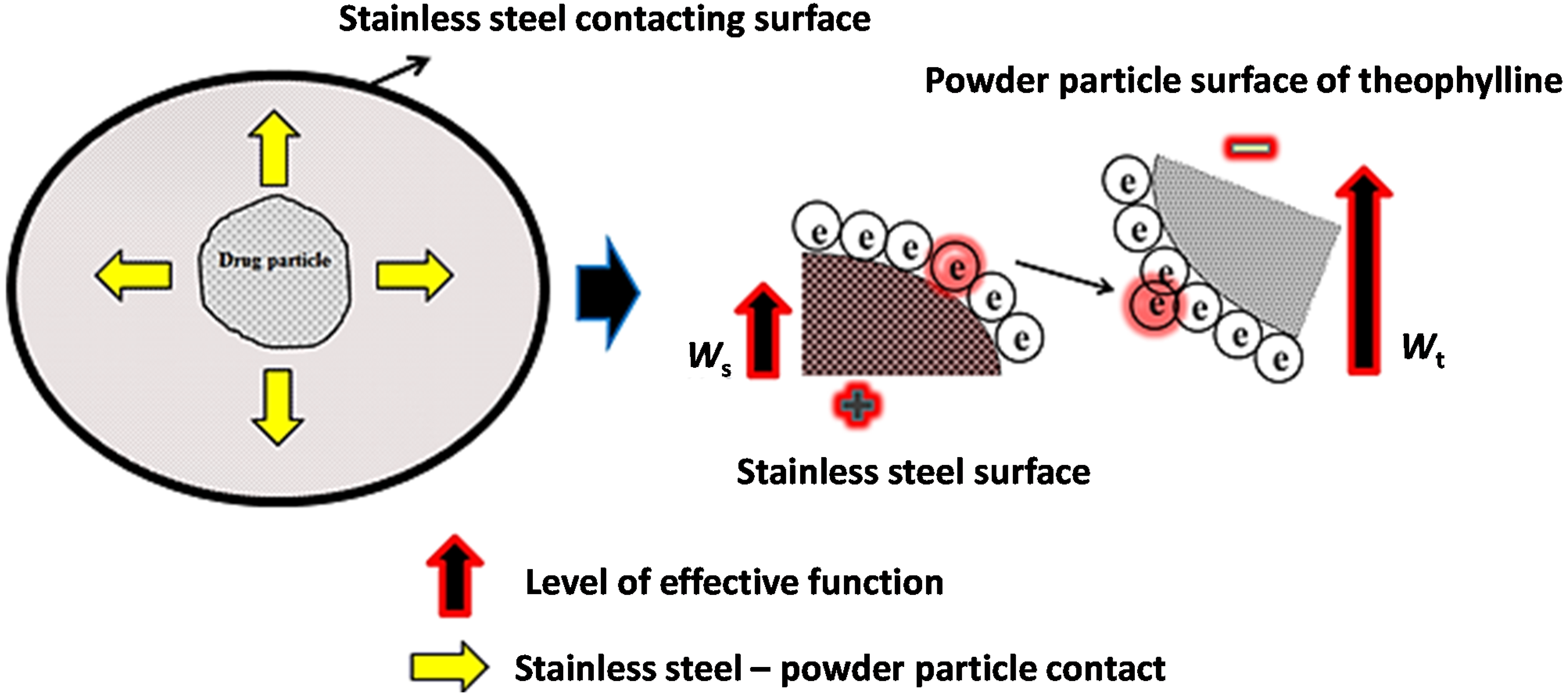
2.2. Tribo-Electric Charging and Surface Adhesion Properties of Theophylline
| Drug | Particle size | Charge to mass ratio (nC/g) | Adhesion (%) |
|---|---|---|---|
| Theophylline (THP) | 38–63 μm | −32.01 (3.8) | 38.42 (5.11) |
2.3. Characterisation of Powder Blends
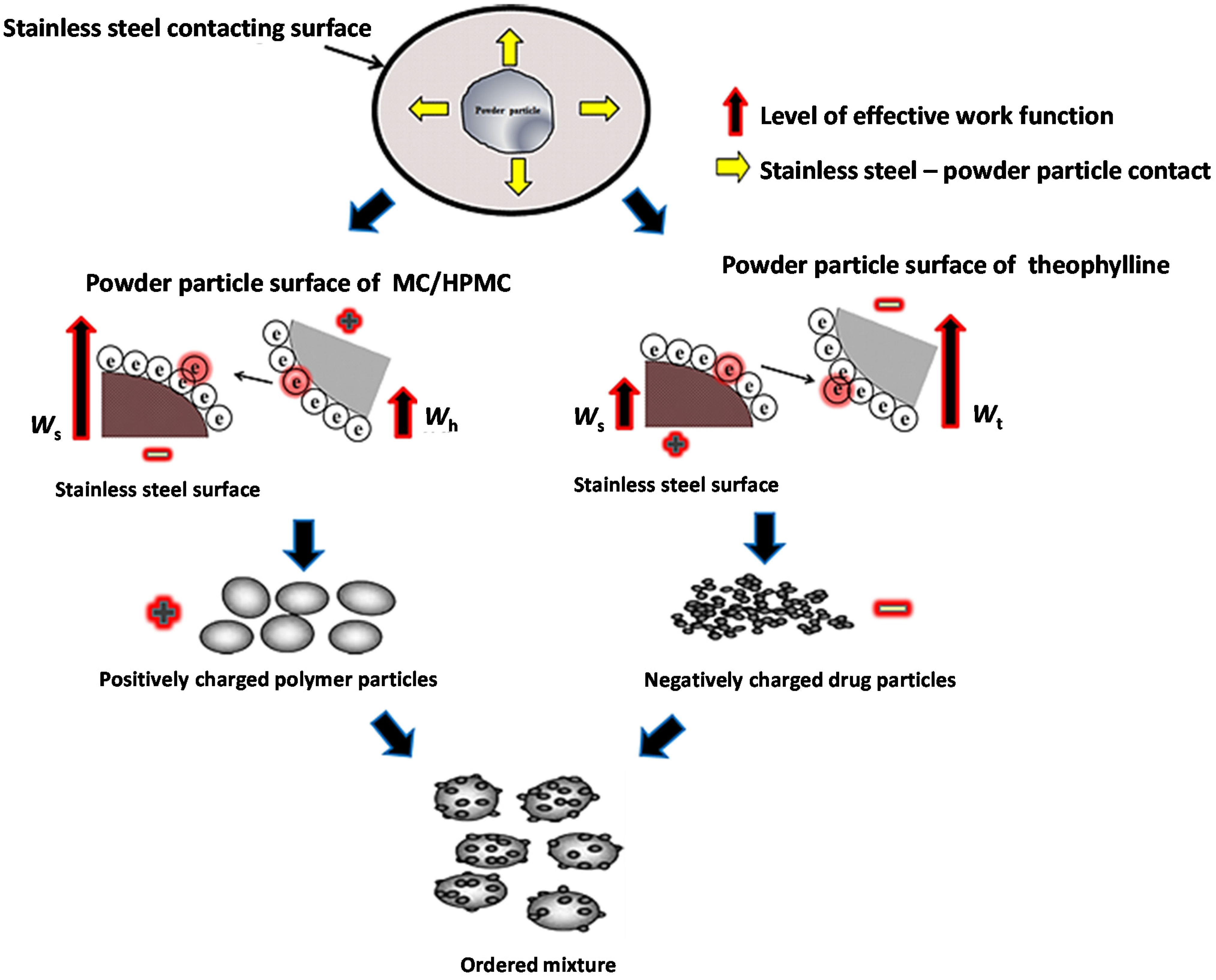
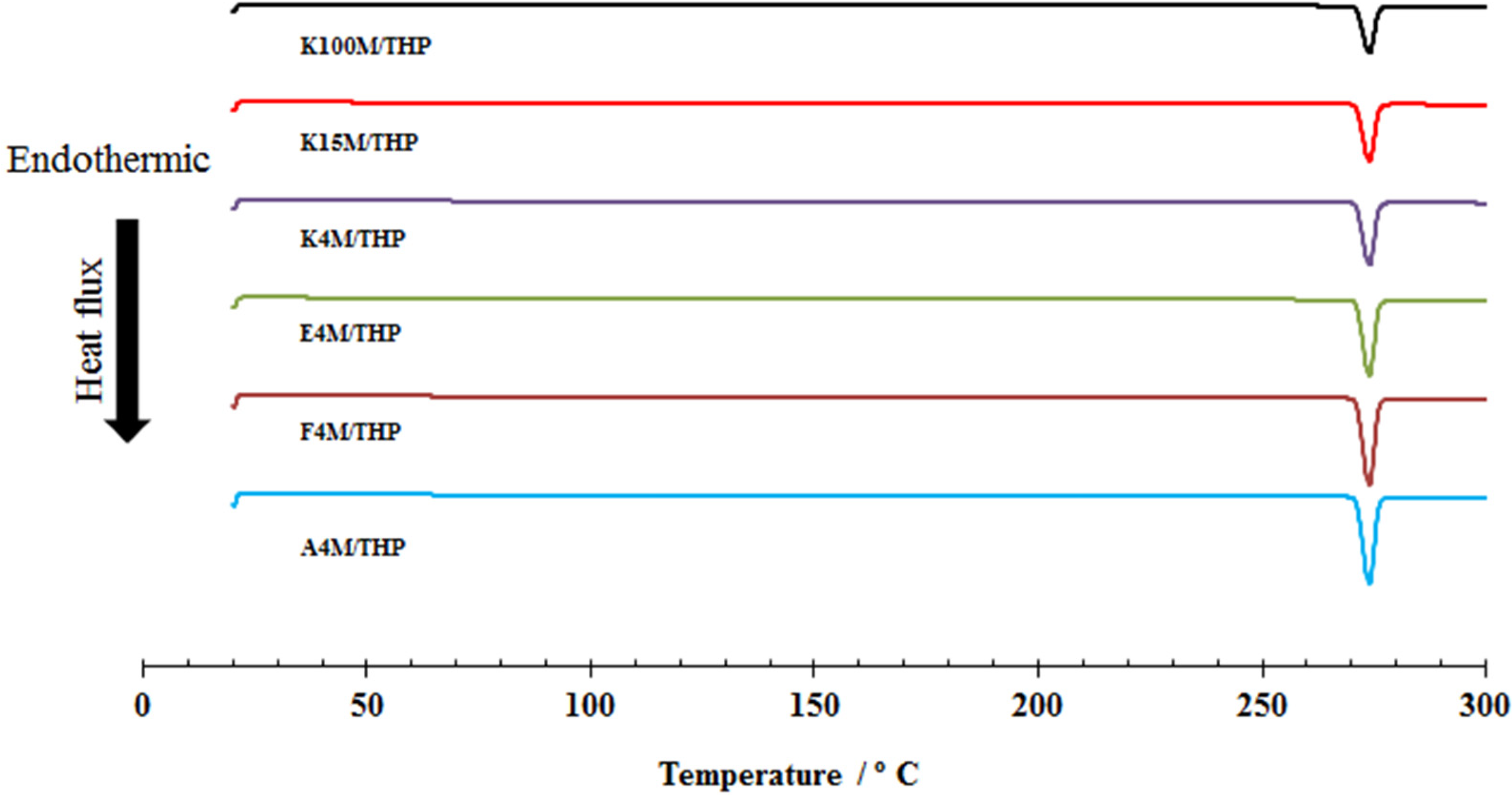

2.4. Tribo-Electric Charging and Surface Adhesion Properties of Powder Blends
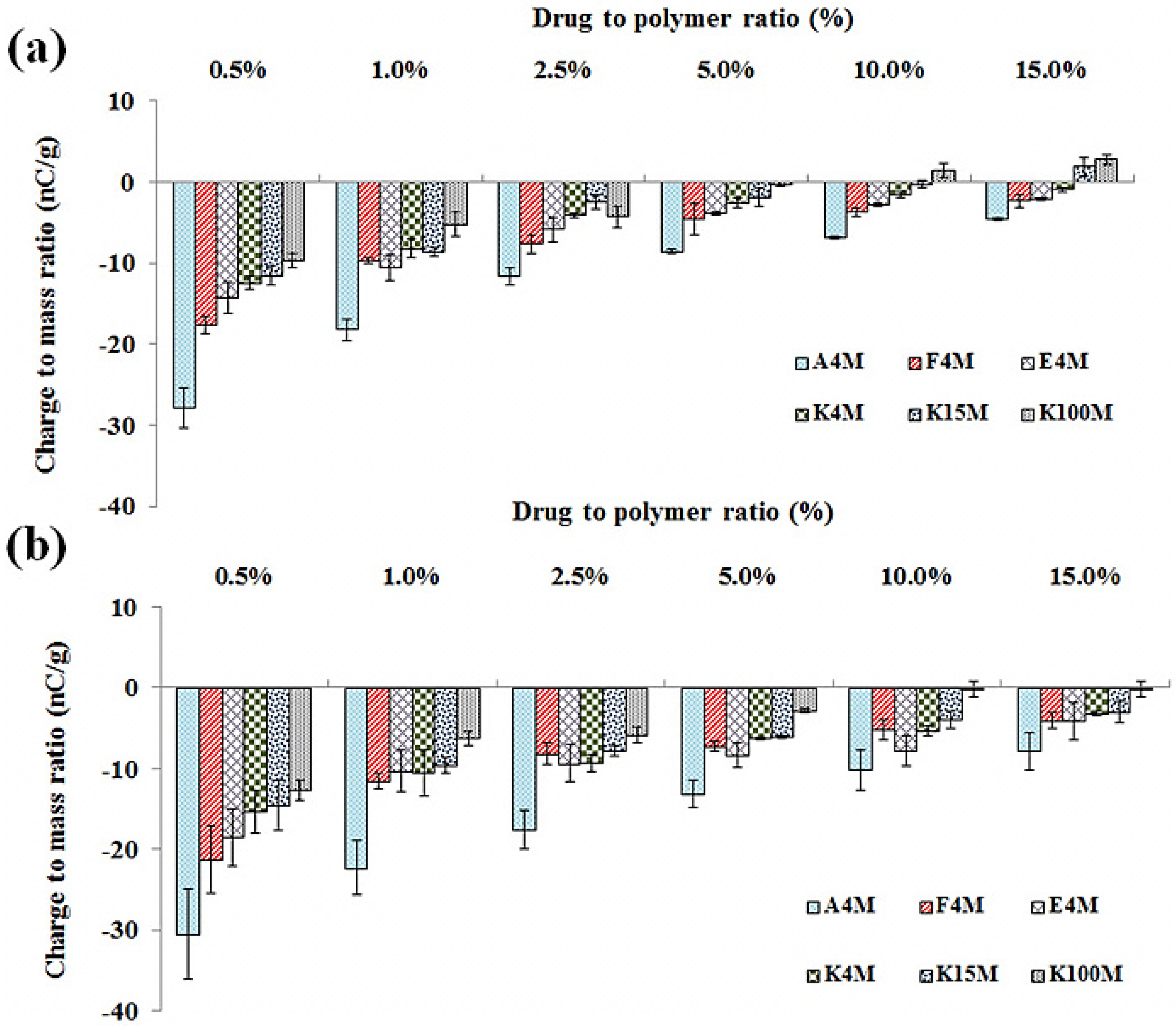
2.4.1. Effect of MC/HPMC Concentration
| Methocel® | Particle size (μm) | Surface adhesion (%) | |||||
|---|---|---|---|---|---|---|---|
| Methocel® concentration (%) | |||||||
| 0.5 | 1 | 2.5 | 5 | 10 | 15 | ||
| A4M | 90–150 | 35.1 (5.27) | 30.2 (4.53) | 28.1 (5.63) | 22.2 (4.89) | 20.3 (4.69) | 18.5 (4.64) |
| 150–250 | 34.6 (5.20) | 28.3 (4.25) | 20.5 (4.12) | 18.6 (4.11) | 15.1 (3.47) | 12.6 (3.17) | |
| F4M | 90–150 | 33.8 (5.08) | 27.5 (4.14) | 22.5 (4.51) | 15.5 (3.43) | 17.3 (3.99) | 14.2 (3.57) |
| 150–250 | 32.2 (4.84) | 21.5 (3.24) | 16.2 (3.25) | 11.4 (2.52) | 10.7 (2.46) | 8.8 (2.22) | |
| E4M | 90–150 | 30.8 (4.62) | 21.3 (3.20) | 16.3 (3.27) | 13.9 (3.08) | 10.5 (2.43) | 9.5 (2.40) |
| 150–250 | 27.3 (4.11) | 18.2 (3.29) | 14.3 (2.87) | 11.4 (2.51) | 8.5 (1.97) | 7.5 (1.89) | |
| K4M | 90–150 | 27.3 (5.47) | 17.5 (3.15) | 14.3 (2.88) | 9.6 (2.13) | 8.9 (2.06) | 6.2 (1.55) |
| 150–250 | 20.7 (4.16) | 11.5 (2.07) | 9.7 (1.95) | 7.6 (1.69) | 7.1 (1.64) | 5.5 (1.40) | |
| K15M | 90–150 | 24.3 (4.88) | 18.6 (3.36) | 11.3 (2.26) | 8.8 (1.95) | 7.5 (1.74) | 5.8 (1.47) |
| 150–250 | 14.3 (2.88) | 9.6 (1.74) | 7.3 (1.46) | 6.8 (1.71) | 4.5 (1.05) | 3.8 (0.97) | |
| K100M | 90–150 | 18.3 (3.66) | 9.5 (1.71) | 7.2 (1.44) | 6.2 (1.56) | 5.8 (1.35) | 4.1 (1.05) |
| 150–250 | 8.3 (1.66) | 6.5 (1.17) | 5.2 (1.04) | 3.9 (1.00) | 3.8 (0.89) | 2.90 (0.75) | |
2.4.2. Effect of MC/HPMC Particle Size
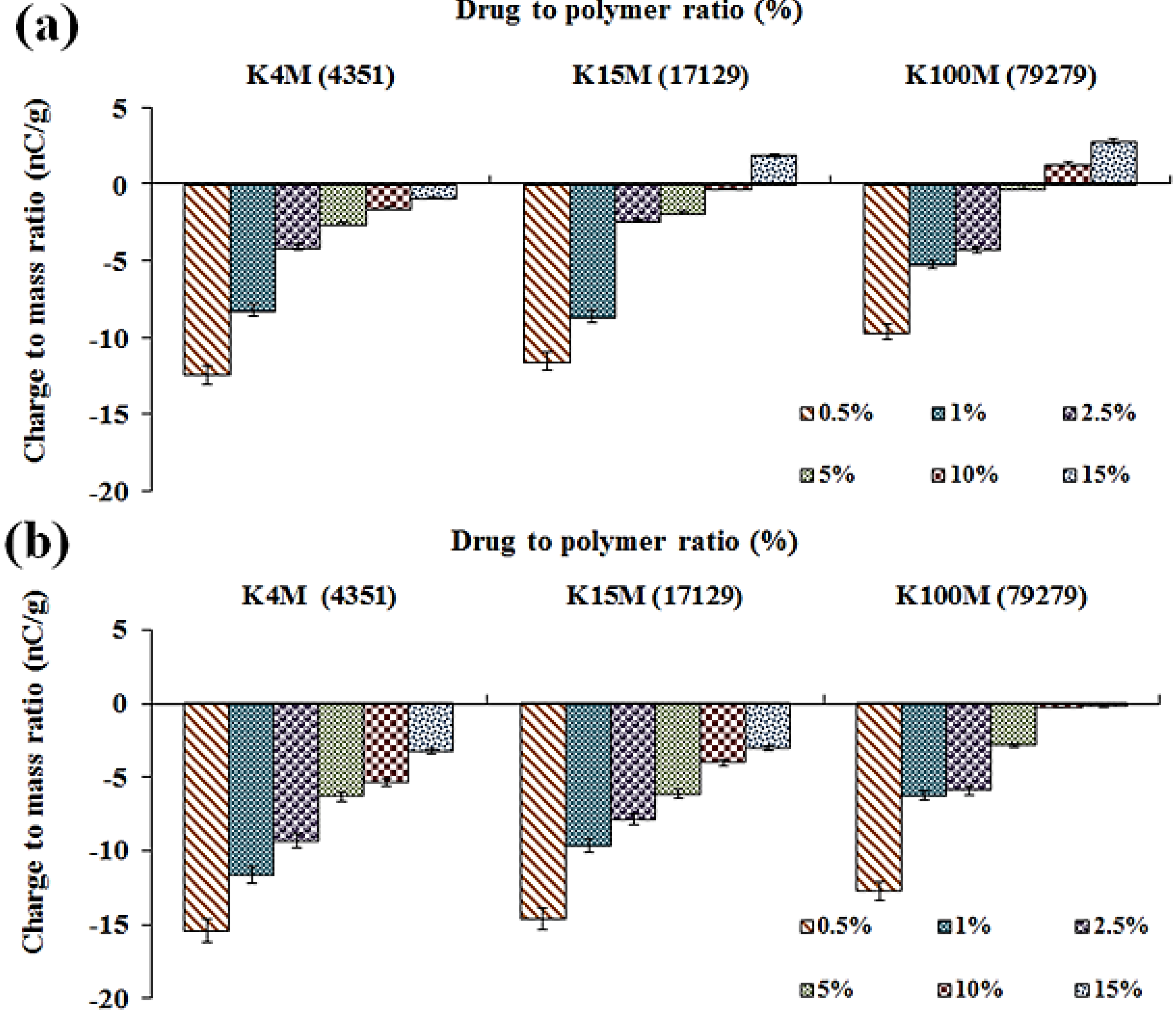
2.4.3. Effect of MC/HPMC Degree of Substitution
2.4.4. Effect of MC/HPMC Molecular Size (Viscosity)
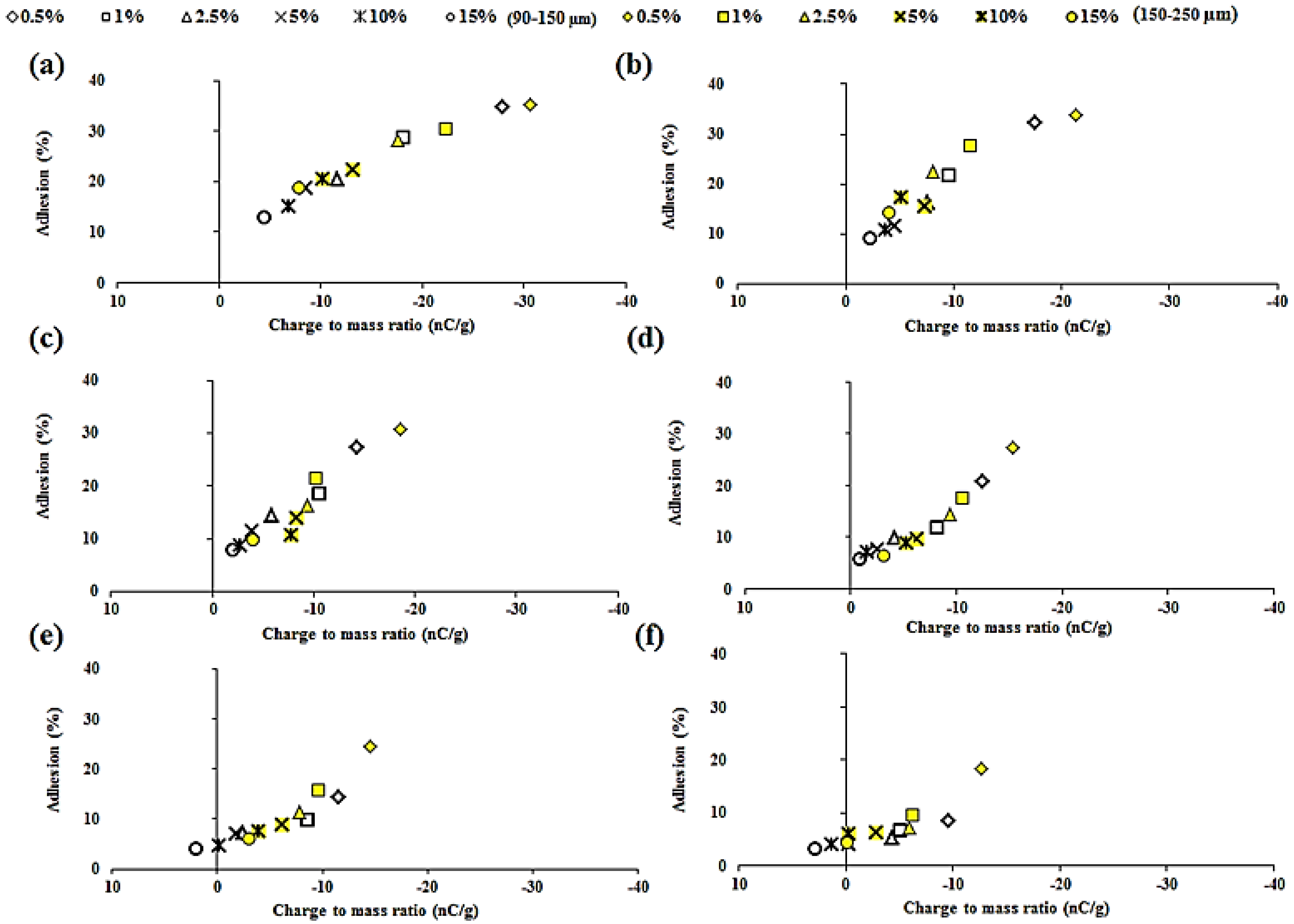
2.5. Relationship between Tribo-Electric Charge and Powder Surface Adhesion
| Powder mixtures | Correlation co-efficient (R2) | |
|---|---|---|
| Particle size (90–150 μm) | Particle size (150–250 μm) | |
| A4M/THP | 0.750 | 0.947 |
| F4M/THP | 0.880 | 0.992 |
| E4M/THP | 0.917 | 0.967 |
| K4M/THP | 0.982 | 0.930 |
| K15M/THP | 0.975 | 0.938 |
| K100M/THP | 0.899 | 0.963 |
3. Experimental
3.1. Materials
3.2. Methods
3.2.1. Powder preparation and characterisation
| Methocel® grade | Methoxy (Meo) (% w/w) a | Hydroxypropyl (Hpo) (% w/w) a | Hpo/Meo ratio | Total substitution (% w/w) | Viscosity (cps) a |
|---|---|---|---|---|---|
| A4M | 30 | 0 | 0 | 30 | 4878 |
| F4M | 28.1 | 6.7 | 0.238 | 34.8 | 4031 |
| E4M | 29.0 | 8.3 | 0.286 | 37.3 | 3919 |
| K4M | 22.3 | 8.5 | 0.381 | 30.8 | 4351 |
| K15M | 22.3 | 9.0 | 0.403 | 31.3 | 17129 |
| K100M | 22.5 | 8.9 | 0.395 | 31.4 | 79279 |
3.2.2. Preparation and Storage of Powder Mixtures
3.2.3. Efficiency of Mixing
Content Uniformity of Powder Blends
Differential Scanning Calorimetry (DSC) of Powders
X-ray Diffraction (XRD) of Powders
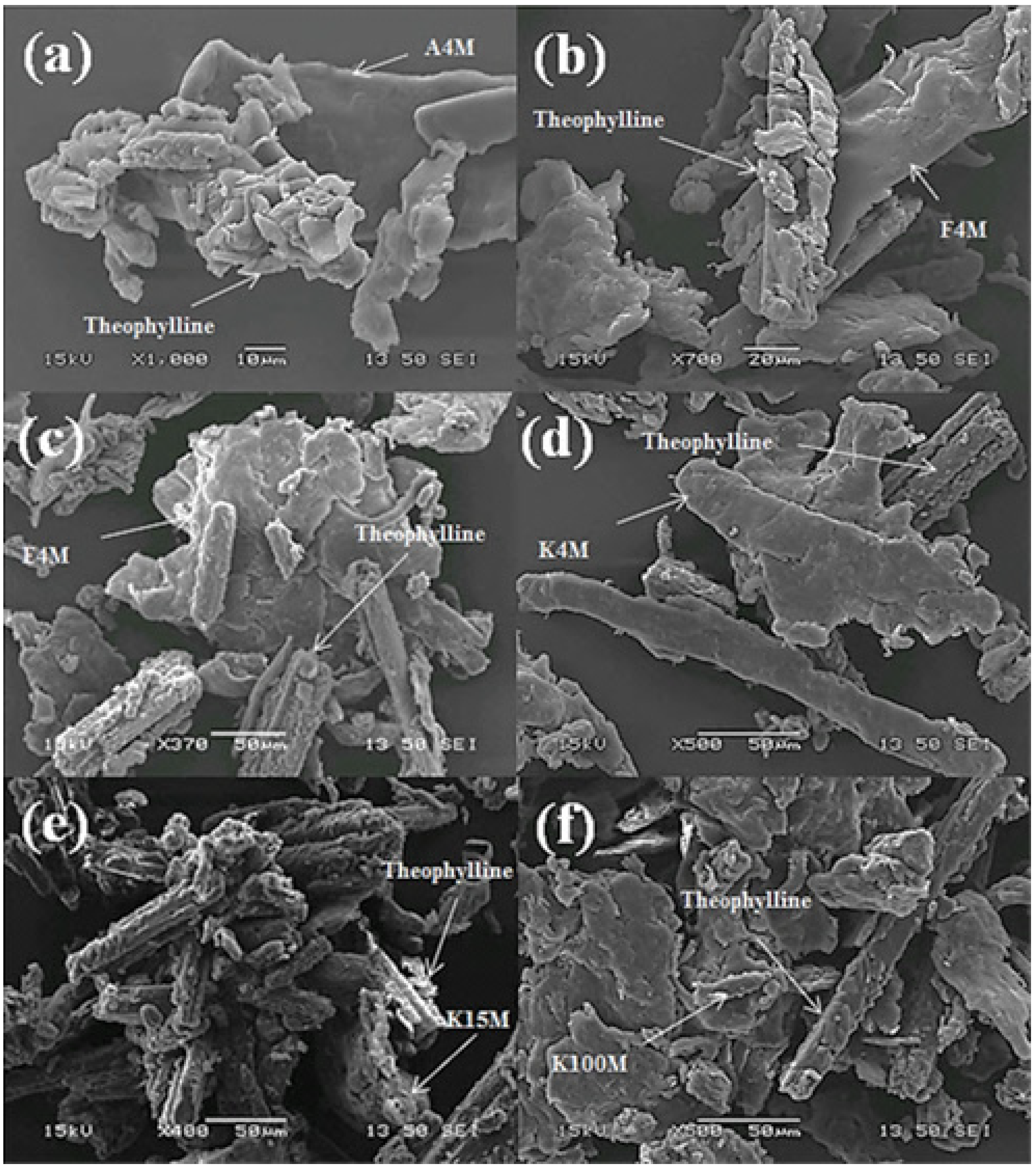
Scanning Electron Microscopy (SEM) of Powder Blends
3.2.4. Tribo-Electrification
3.2.5. Surface Adhesion
3.2.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jivraj, M.; Martini, L.G.; Thomson, C.M. An overview of the different excipients useful for the direct compression of tablets. Pharm. Sci. Technol. Today 2000, 3, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Swarbrick, J. Encyclopedia of Pharmaceutical Technology: Comp-Dry; Informa Healthcare: London, UK, 2007; pp. 671–1434. [Google Scholar]
- Wen, H.; Park, K. Oral Controlled Release Formulation Design and Drug Delivery: Theory to Practice; Wiley: Weinheim, Germany, 2011. [Google Scholar]
- Harper, W.R. Contact and Frictional Electrification; Clarendon Press: Gloucestershire, UK, 1967. [Google Scholar]
- Chang, J.S.; Kelly, A.J.; Crowley, J.M. Handbook of Electrostatic Processes; Taylor & Francis: Abingdon, UK, 1995. [Google Scholar]
- Wong, J.; Kwok, P.C.L.; Chan, H.-K. Electrostatics in pharmaceutical solids. Chem. Eng. Sci. 2015, 125, 225–237. [Google Scholar] [CrossRef]
- Cross, J. Electrostatics: Principles, Problems and Applications; Adam Hilger: Bristol, UK, 1987. [Google Scholar]
- Matsusaka, S.; Maruyama, H.; Matsuyama, T.; Ghadiri, M. Triboelectric charging of powders: A review. Chem. Eng. Sci. 2010, 65, 5781–5807. [Google Scholar] [CrossRef] [Green Version]
- Šupuk, E.; Hassanpour, A.; Ahmadian, H.; Ghadiri, M.; Matsuyama, T. Tribo-electrification and associated segregation of pharmaceutical bulk powders. KONA Powder Part. J. 2011, 29, 208–223. [Google Scholar] [CrossRef]
- Šupuk, E.; Zarrebini, A.; Reddy, J.P.; Hughes, H.; Leane, M.M.; Tobyn, M.J.; Peter, T.B.; Ghadiri, M.; Supuk, E. Tribo-electrification of active pharmaceutical ingredients and excipients. Powder Technol. 2012, 217, 427–434. [Google Scholar] [CrossRef]
- Venables, H.J.; Wells, J. Powder mixing. Drug Dev. Ind. Pharm. 2001, 27, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Duff, N.; Lacks, D.J. Particle dynamics simulations of triboelectric charging in granular insulator systems. J. Electrost. 2008, 66, 51–57. [Google Scholar] [CrossRef]
- Maderuelo, C.; Zarzuelo, A.; Lanao, J.M. Critical factors in the release of drugs from sustained release hydrophilic matrices. J. Controll. Release 2011, 154, 2–19. [Google Scholar] [CrossRef]
- Ghori, M.U.; Ginting, G.; Smith, A.M.; Conway, B.R. Simultaneous quantification of drug release and erosion from hypromellose hydrophilic matrices. Int. J. Pharm. 2014, 465, 406–412. [Google Scholar] [CrossRef]
- Ghori, M.U.; Šupuk, E.; Conway, B.R. Tribo-electric charging and adhesion of cellulose ethers and their mixtures with flurbiprofen. Eur. J. Pharm. Sci. 2014, 65, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Asare-Addo, K.; Kaialy, W.; Levina, M.; Rajabi-Siahboomi, A.; Ghori, M.U.; Šupuk, E. The influence of agitation sequence and ionic strength on in vitro drug release from hypromellose (E4M and K4M) ER matrices-The use of the USP III apparatus. Colloids Surf. B Biointerfaces 2013, 104, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, C.; Bonferoni, M.C.; Caramella, C.; Lennholm, H.; Nyström, C. Characterisation of particle properties and compaction behaviour of hydroxypropyl methylcellulose with different degrees of methoxy/hydroxypropyl substitution. Eur. J. Pharm. Sci. 1999, 9, 171–184. [Google Scholar] [CrossRef] [PubMed]
- York, P. Solid-state properties of powders in the formulation and processing of solid dosage forms. Int. J. Pharm. 1983, 14, 1–28. [Google Scholar] [CrossRef]
- Kwek, J.W.; Vakarelski, I.U.; Ng, W.K.; Heng, J.Y.Y.; Tan, R.B.H. Novel parallel plate condenser for single particle electrostatic force measurement in atomic force microscopy. Colloids Surf. A Physicochem. Eng. Asp. 2011, 385, 206–212. [Google Scholar] [CrossRef]
- Mäki, R.; Suihko, E.; Rost, S.; Heiskanen, M.; Murtomaa, M.; Lehto, V.P.; Ketolainen, J. Modifying drug release and tablet properties of starch acetate tablets by dry powder agglomeration. J. Pharm. Sci. 2007, 96, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Saharan, V.A.; Kukkar, V.; Kataria, M.; Kharb, V.; Choudhury, P. Ordered mixing: Mechanism, process and applications in pharmaceutical formulations. Asian J. Pharm. Sci. 2008, 3, 240–259. [Google Scholar] [CrossRef]
- Engers, D.A.; Fricke, M.N.; Newman, A.W.; Morris, K.R. Triboelectric charging and dielectric properties of pharmaceutically relevant mixtures. J. Electrost. 2007, 65, 571–581. [Google Scholar] [CrossRef]
- Murtomaa, M.; Laine, E. Electrostatic measurements on lactose-glucose mixtures. J. Electrost. 2000, 48, 155–162. [Google Scholar] [CrossRef]
- Gallo, C.; Lama, W. Some charge exchange phenomena explained by a classical model of the work function. J. Electrost. 1976, 2, 145–150. [Google Scholar] [CrossRef]
- Lacks, D.J.; Sankaran, R.M. Contact electrification of insulating materials. J. Phys. D Appl. Phys. 2011, 44, 453001. [Google Scholar] [CrossRef]
- Davies, D. Charge generation on dielectric surfaces. J. Phys. D Appl. Phys. 1969, 2, 1533. [Google Scholar] [CrossRef]
- British Pharmacopoeia. In British Pharmacopoeia Commission; Stationery Office: London, UK, 2012.
- Šupuk, E.; Seiler, C.; Ghadiri, M. Analysis of a simple test device for tribo-electric charging of bulk powders. Part. Part. Syst. Charact. 2009, 26, 7–16. [Google Scholar] [CrossRef]
- Šupuk, E.; Ghori, M.U.; Asare-Addo, K.; Laity, P.R.; Panchmatia, P.M.; Conway, B.R. The influence of salt formation on electrostatic and compression properties of flurbiprofen salts. Int. J. Pharm. 2013, 458, 118–127. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghori, M.U.; Šupuk, E.; Conway, B.R. Tribo-electrification and Powder Adhesion Studies in the Development of Polymeric Hydrophilic Drug Matrices. Materials 2015, 8, 1482-1498. https://doi.org/10.3390/ma8041482
Ghori MU, Šupuk E, Conway BR. Tribo-electrification and Powder Adhesion Studies in the Development of Polymeric Hydrophilic Drug Matrices. Materials. 2015; 8(4):1482-1498. https://doi.org/10.3390/ma8041482
Chicago/Turabian StyleGhori, Muhammad U., Enes Šupuk, and Barbara R. Conway. 2015. "Tribo-electrification and Powder Adhesion Studies in the Development of Polymeric Hydrophilic Drug Matrices" Materials 8, no. 4: 1482-1498. https://doi.org/10.3390/ma8041482
APA StyleGhori, M. U., Šupuk, E., & Conway, B. R. (2015). Tribo-electrification and Powder Adhesion Studies in the Development of Polymeric Hydrophilic Drug Matrices. Materials, 8(4), 1482-1498. https://doi.org/10.3390/ma8041482