Corrosion Behavior of Detonation Gun Sprayed Fe-Al Type Intermetallic Coating
Abstract
:1. Introduction
2. Experimental

3. Results and Discussion
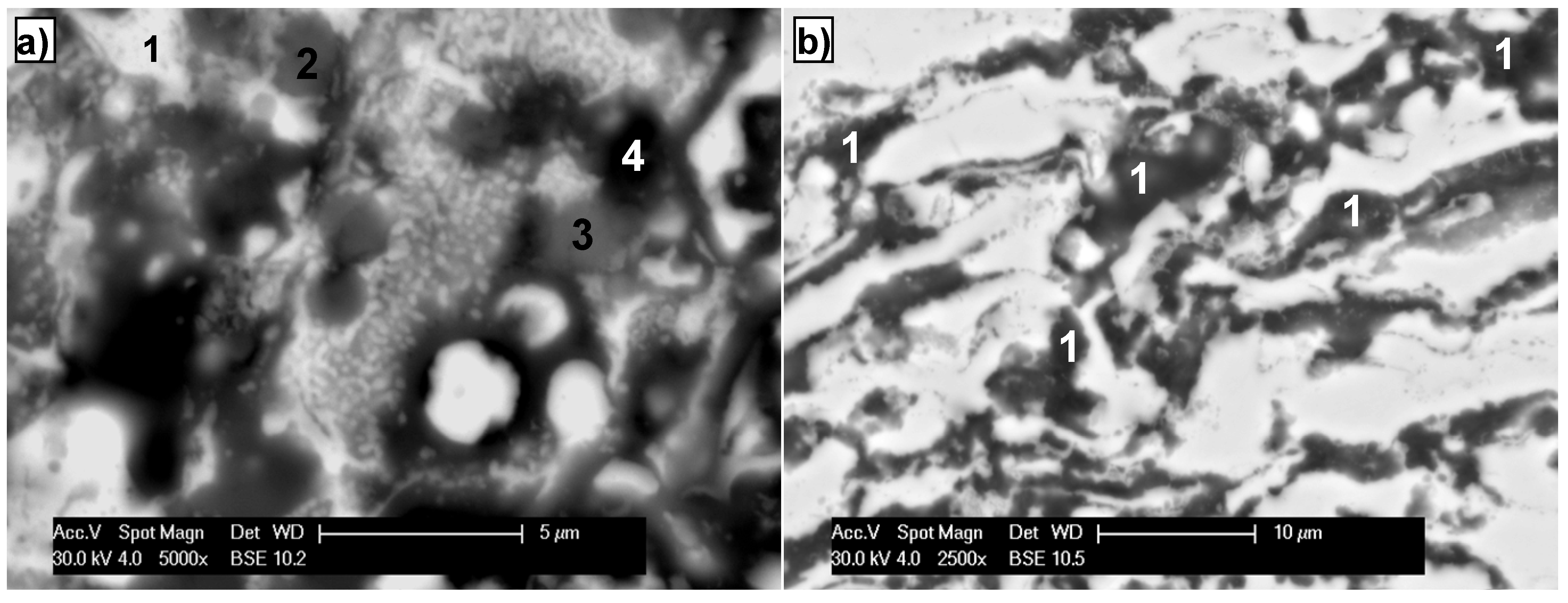
3.1. As-Detonation Gun Sprayed FeAl Coatings Characterization
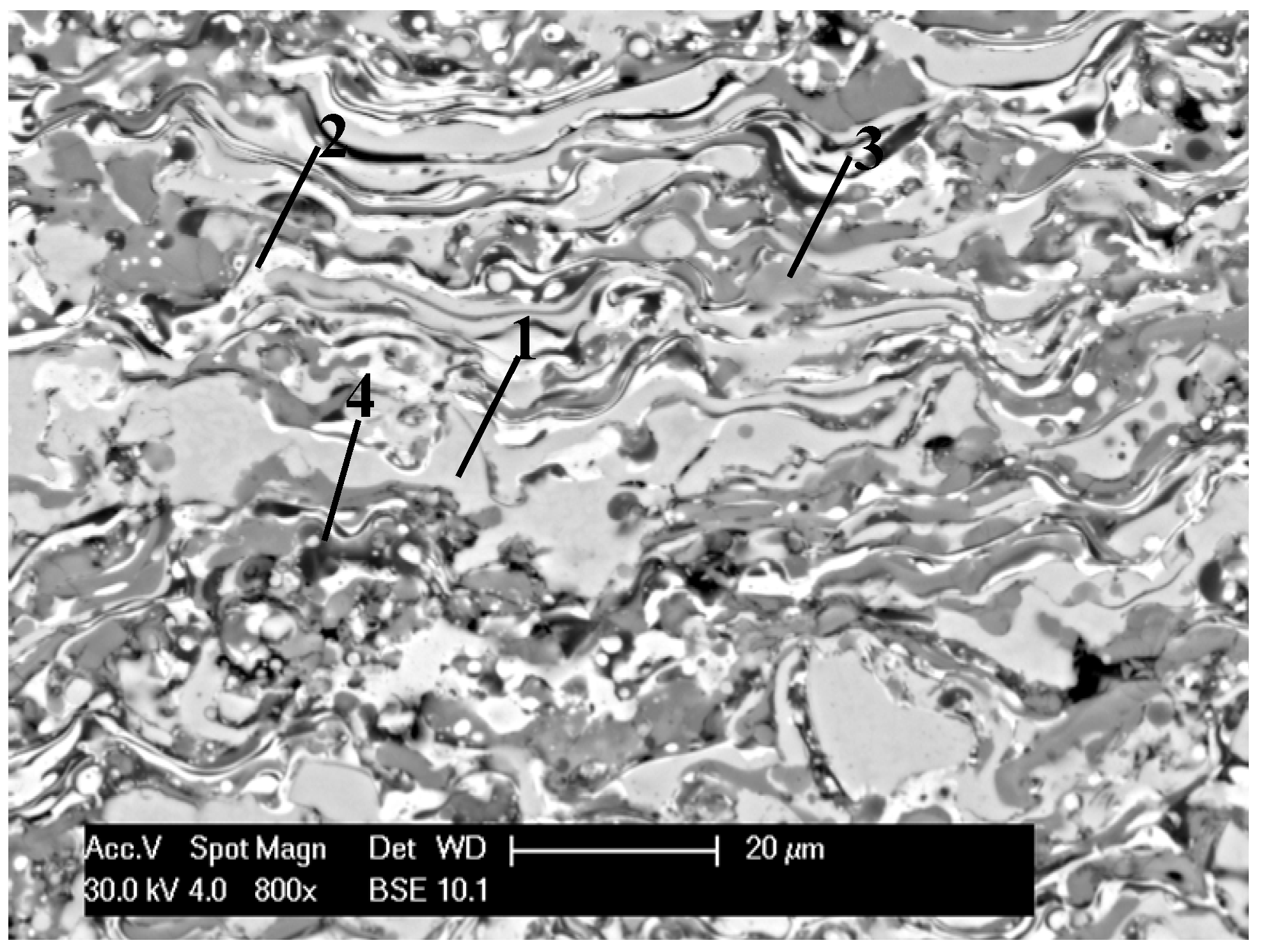
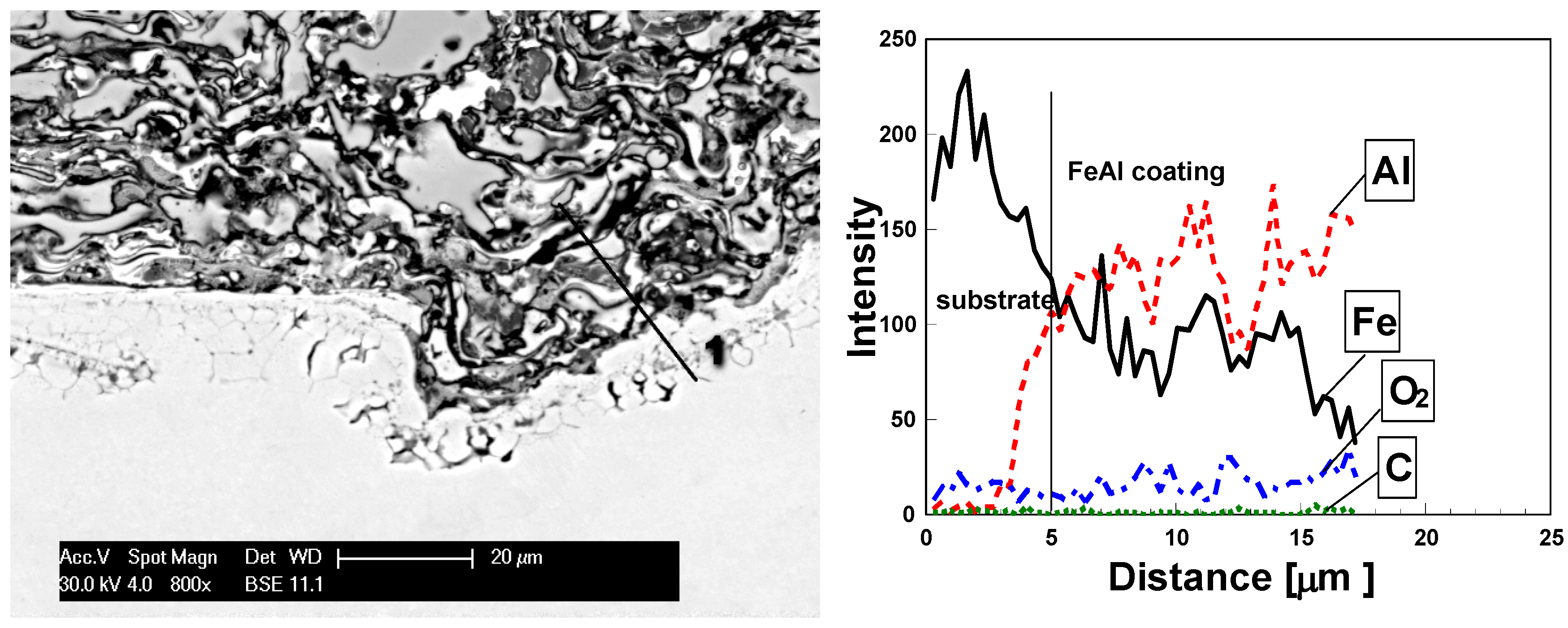
| Grain area | Remarks | Content (at%) | ||
|---|---|---|---|---|
| Fe | Al | O | ||
| Light grey (1) | FeAl lightly oxidized | 43.5 | 50.1 | 6.4 |
| White (2) | FeAl/Fe3Al lightly oxidized | 54.2 | 38.1 | 7.7 |
| Grey (3) | FeAl strongly oxidized | 36.7 | 57.4 | 16.9 |
| Dark grey (4) | Al2O3 oxide films in FeAl coating | 31.9 | 32.7 | 35.4 |
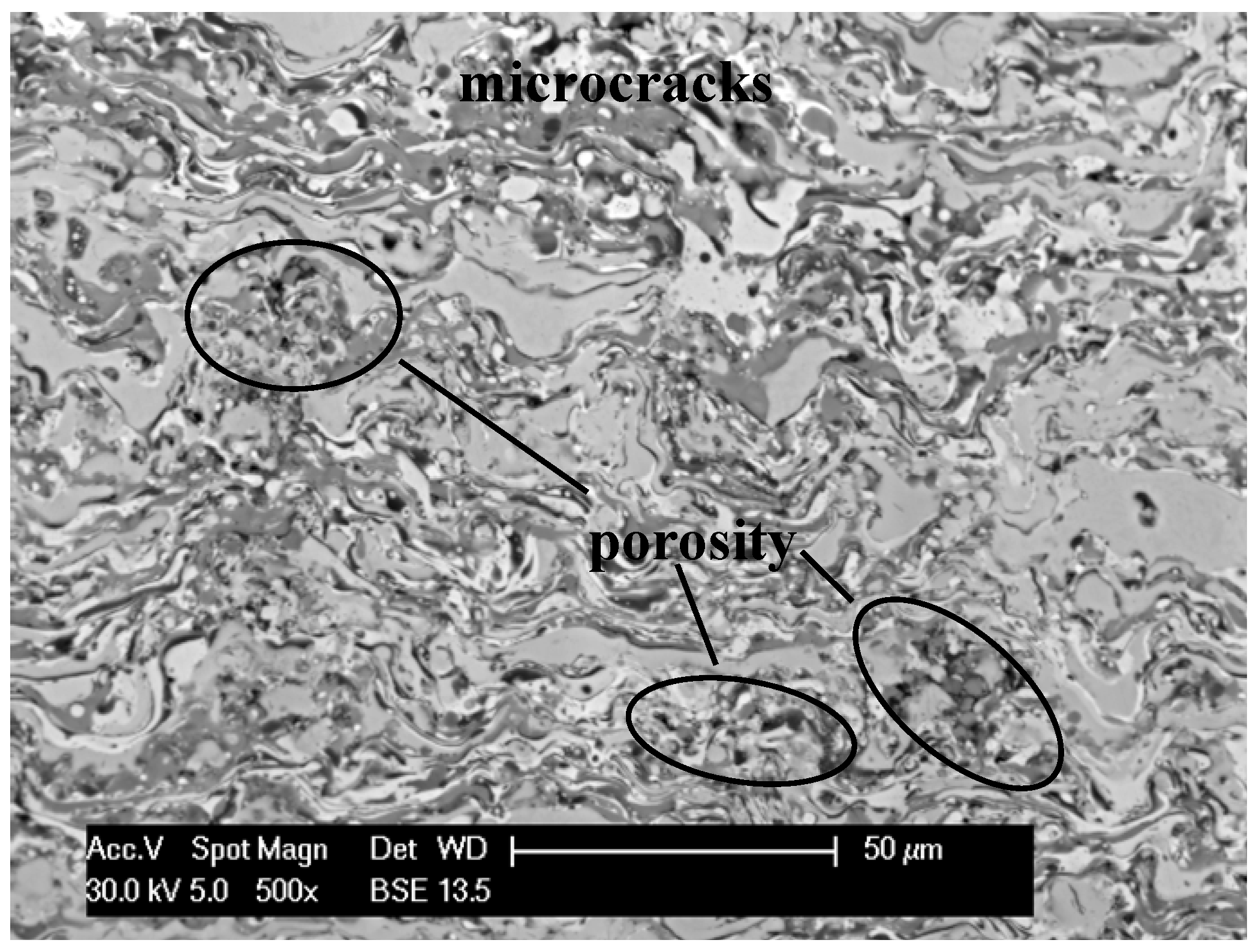
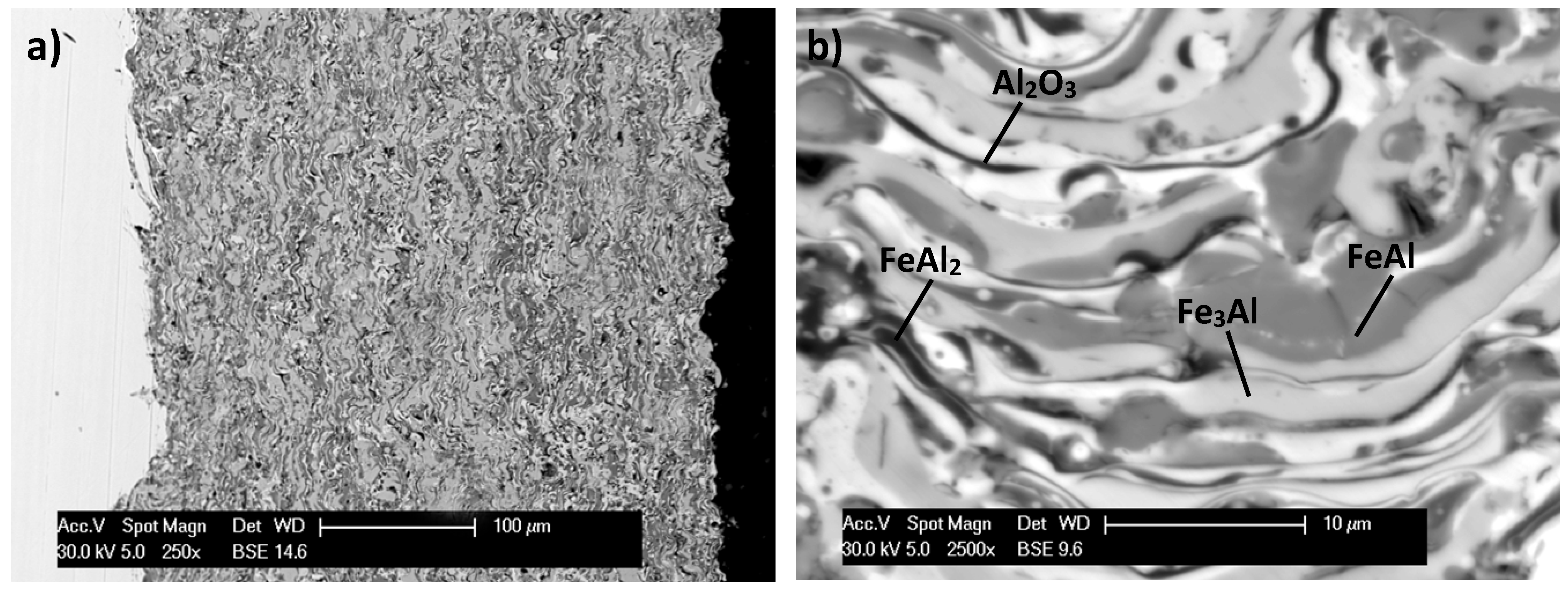
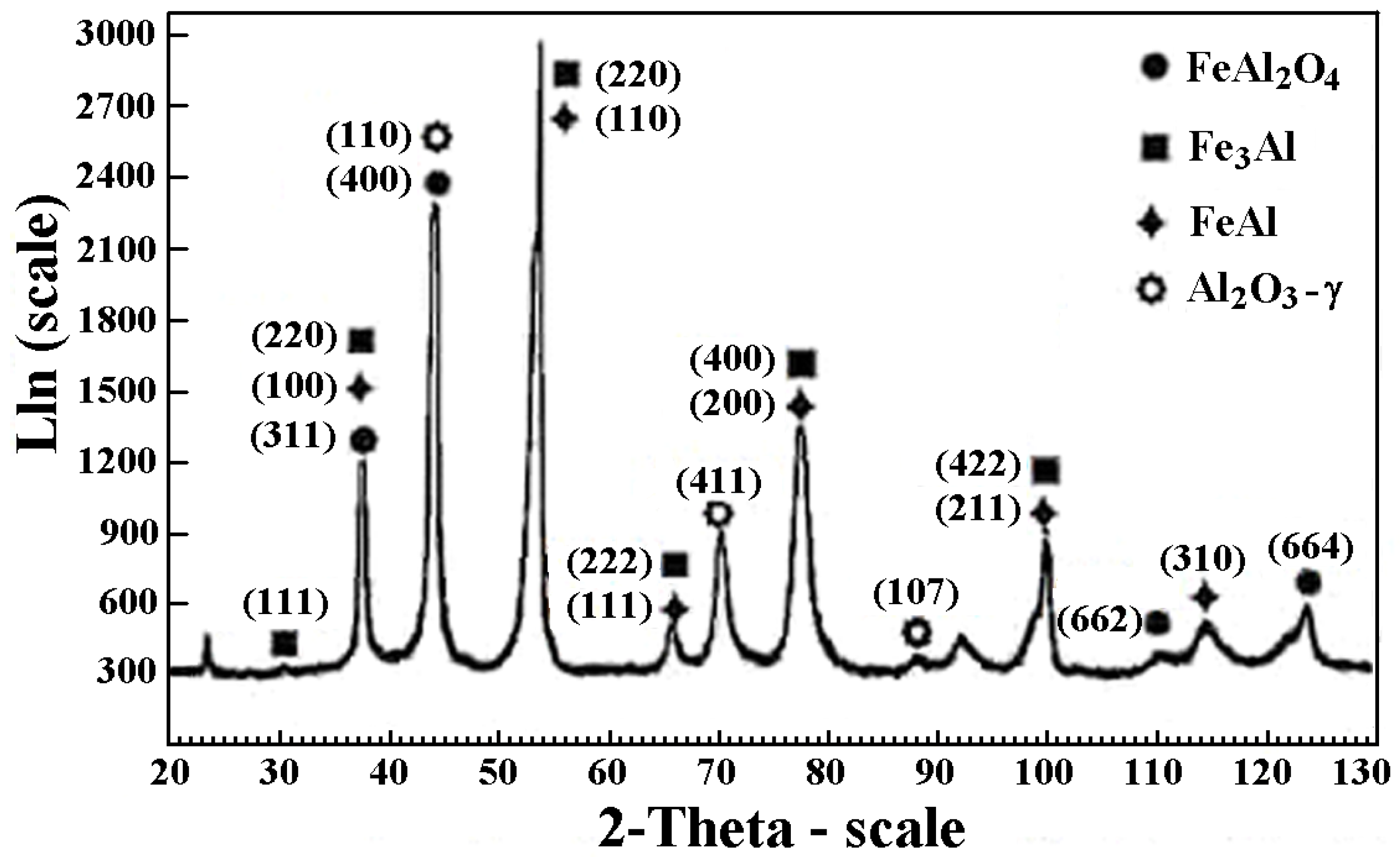

3.2. Electrochemical Corrosion Behavior
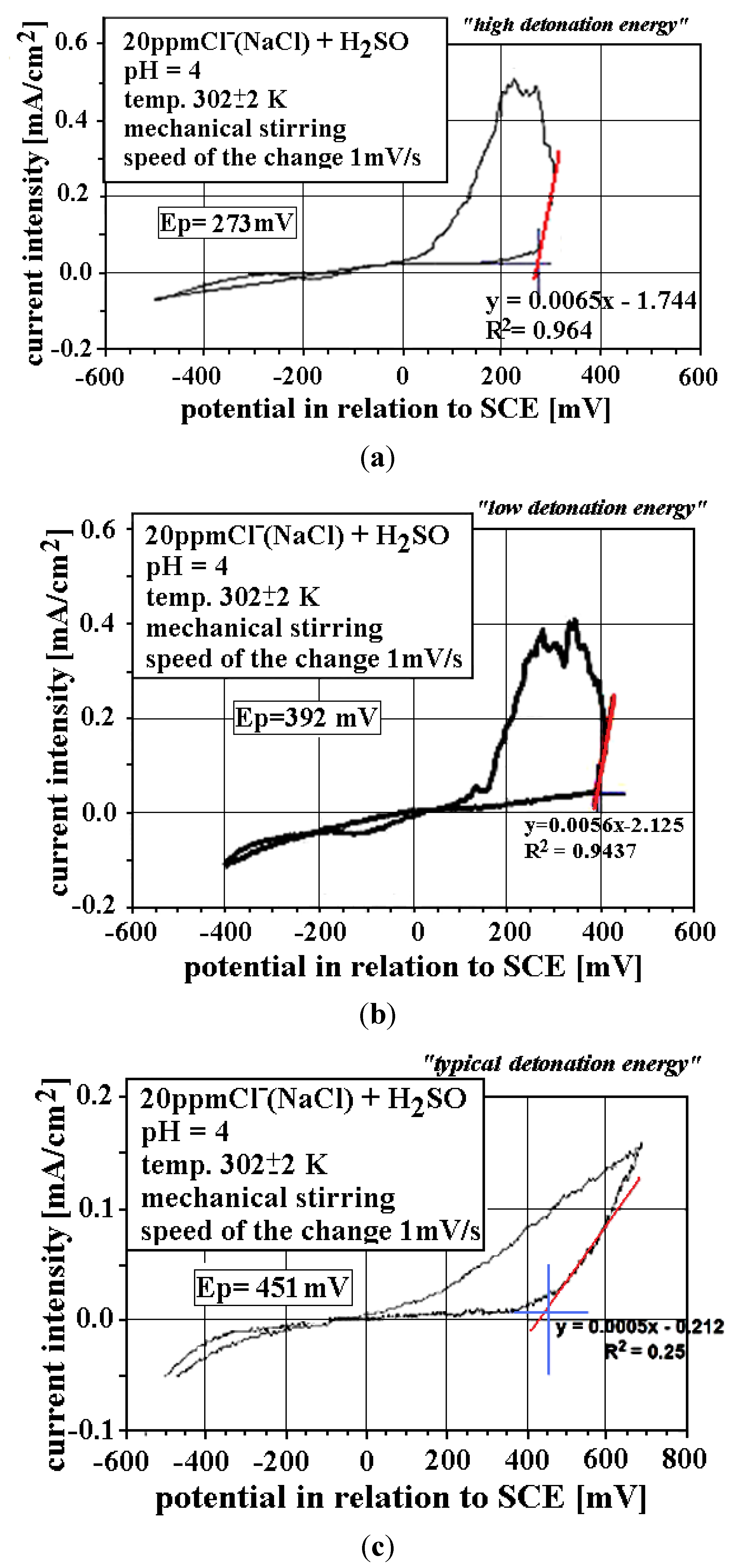
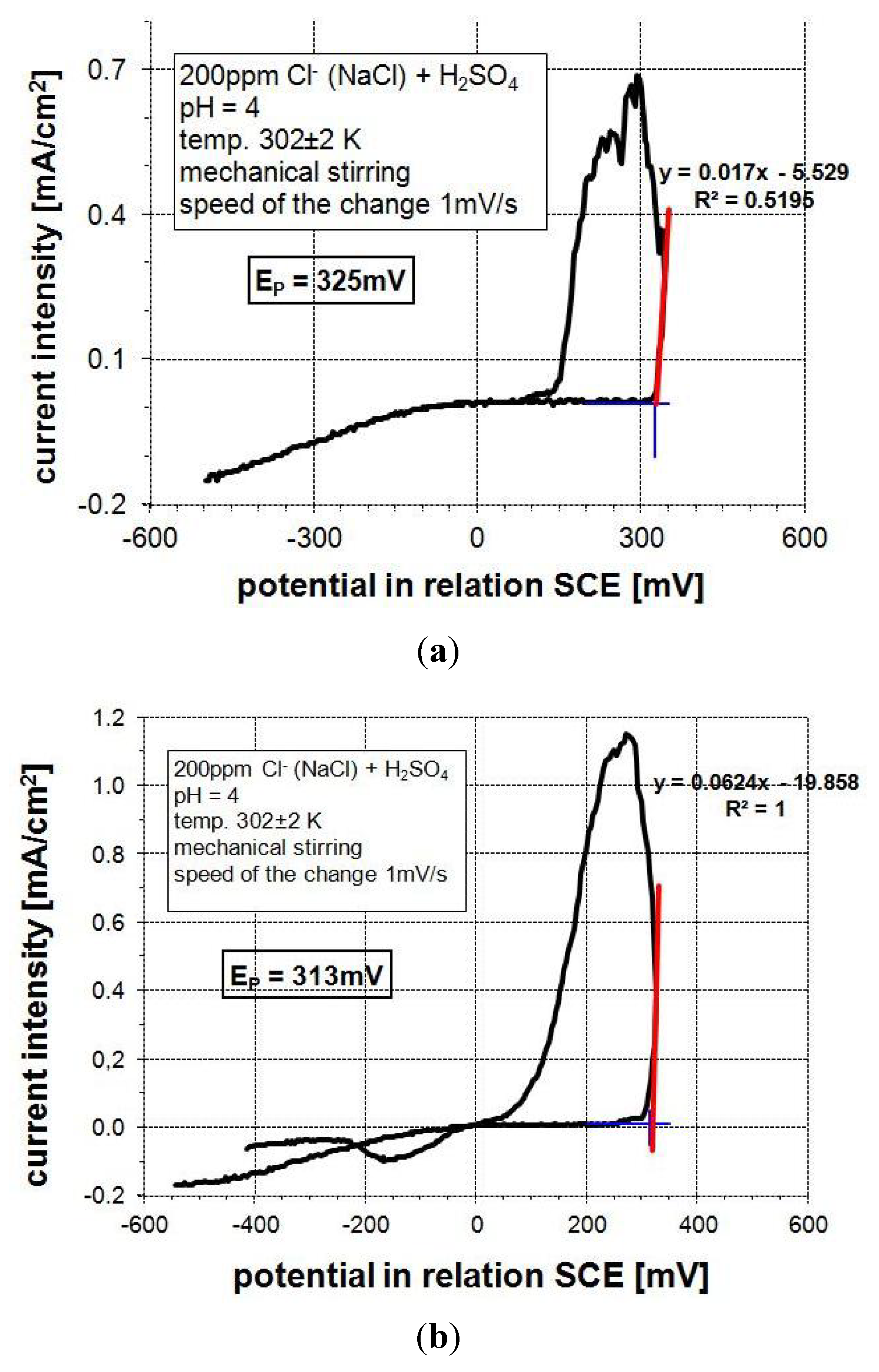
3.3. Corrosion Performance in High Temperature Air Environments
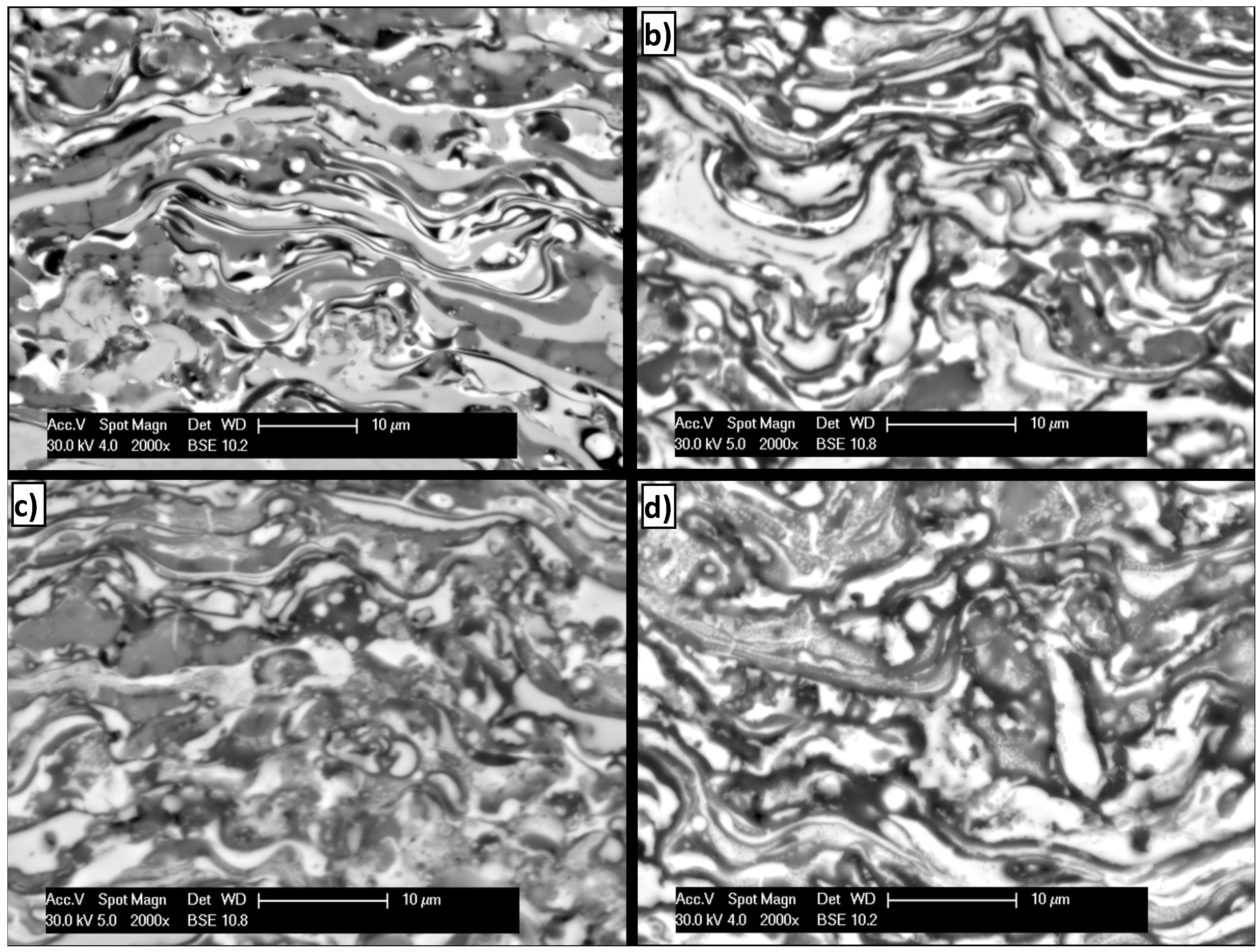

4. Conclusions
- (1)
- Limited chemical interactions between detonation gas products and coating material can not be avoided in the D-gun spraying conditions.
- (2)
- High stability of the intermetallic powders particles allows conserving phase composition, but high content of aluminum particles are being covered by thin oxide films (mainly alumina) during flight to the treated surface.
- (3)
- At the minimal thickness, oxide film and layer coating structure, presence of the oxides does not affect the mechanical properties of the material, but is advantageous in the aspect of corrosion resistance.
- (4)
- Electrochemical corrosion resistance of the sprayed FeAl coatings at optimal spraying conditions is higher for low porosity coating than for bulk Fe-Al and austenitic valve steel Cr21Mn9Ni4. It allows concluding that the coatings may find applications in aggressive environments containing acids.
- (5)
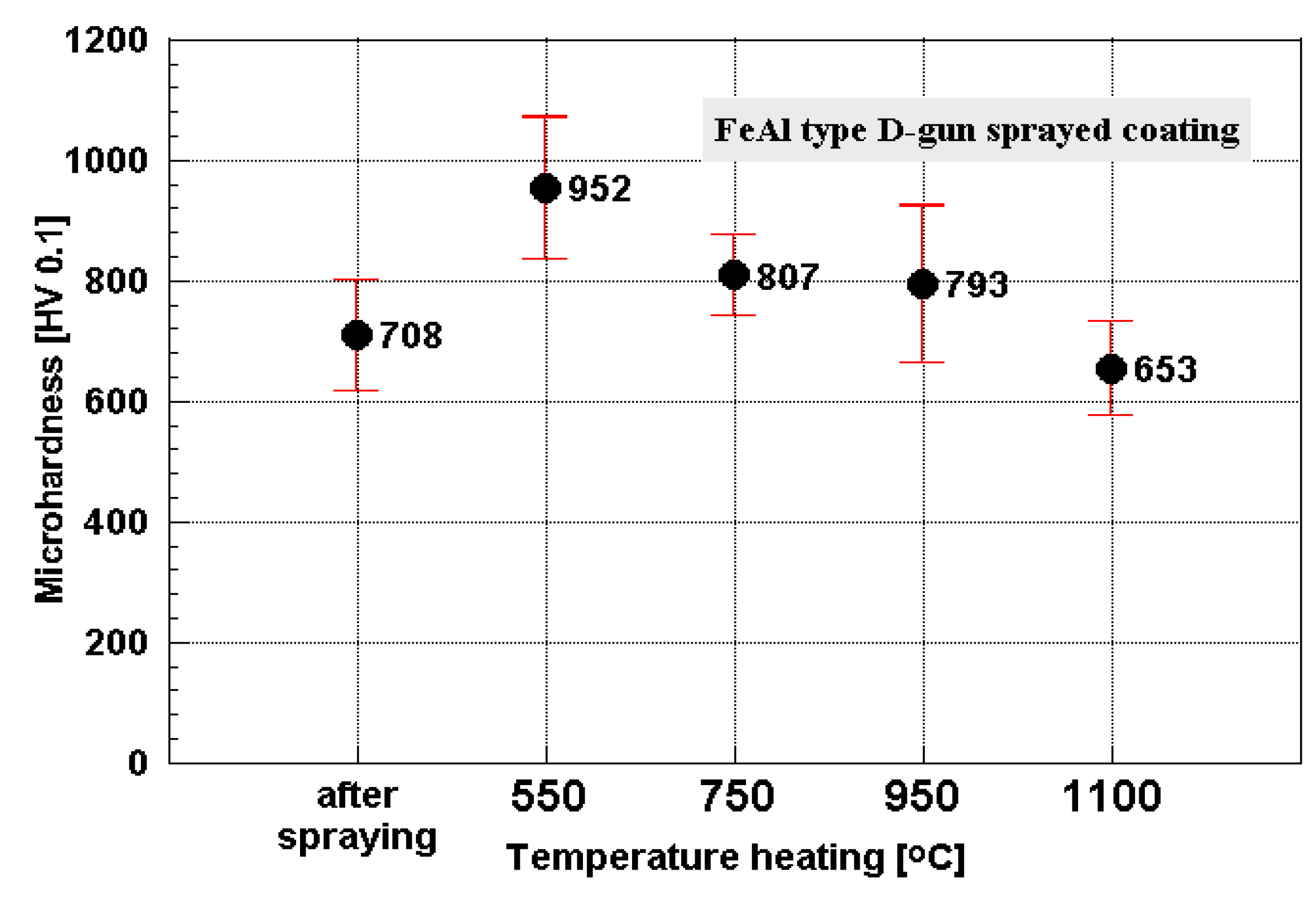
- An important attribute of the fabricated coatings is their corrosion resistance and thermal stability in high temperatures. After heating at temperatures range from 550 to 1100 °C for 10 hours, negligible changes in phase composition and coating morphology were found. Strengthening, controlled by micro-hardness measurements, was fully conserved.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jóźwik, P.; Bojar, Z. Influence of heat treatment on the structure and mechanical properties of Ni3Al-based alloys. Arch. Metall. Mater. 2010, 55, 271–279. [Google Scholar]
- Jóźwik, P.; Bojar, Z.; Grabowski, R. Catalytic activity of Ni3Al foils in methanol decomposition. Mater. Sci. Forum. 2010, 636–637, 895–900. [Google Scholar] [CrossRef]
- Podrez-Radziszewska, M.; Jóźwik, P. Influence of heat treatment on resistance to electrochemical corrosion of the strain-hardened strips made of the Ni3Al phase based alloys. Arch. Civ. Mech. Eng. 2011, 11, 1011–1021. [Google Scholar] [CrossRef]
- Matysik, P.; Jóźwiak, S.; Czujko, T. Characterization of low-symmetry structures from phase equilibrium of Fe-Al system—Microstructures and mechanical properties. Materials 2015, 8, 914–931. [Google Scholar] [CrossRef]
- Durejko, T.; Lipiński, S.; Bojar, Z.; Bystrzycki, J. Processing and characterization of graded metal/intermetallic materials: The example of Fe/FeAl intermetallics. Mater. Des. 2011, 32, 2827–2834. [Google Scholar] [CrossRef]
- Durejko, T.; Ziętala, M.; Polkowski, W.; Czujko, T. Thin wall tubes with Fe3Al/SS316L graded structure obtained by using laser engineered net shaping technology. Mater. Des. 2014, 63, 766–774. [Google Scholar] [CrossRef]
- Senderowski, C.; Bojar, Z. Corrosion behaviour of detonation gun sprayed Fe-Al type intermetallic coating. Eur. Corros. Congr. Eurocorr. 2010, 324, 611–612. [Google Scholar]
- Pawłowski, A.; Senderowski, C.; Bojar, Z.; Faryna, M. Detonation deposited Fe-Al coatings Part I: Morphology of the Ni(Al) and Cr(Ni) transition layers and coatings of Fe-Al type sprayed onto carbon steel substrate. Arch. Metall. Mater. 2010, 55, 1061–1071. [Google Scholar]
- Deevi, S.C.; Sikka, V.K. Nickel and iron aluminides: An overview on properties, processing, and application. Intermetallics 1996, 4, 357–375. [Google Scholar] [CrossRef]
- Guilemany, J.M.; Cinca, N.; Dosta, S.; Lima, C.R.C. High-temperature oxidation of Fe40Al coatings obtained by HVOF thermal spray. Intermetallics 2007, 15, 1384–1394. [Google Scholar] [CrossRef]
- Senderowski, C. Nanocomposite Fe-Al intermetallic coating obtained by Gas detonation spraying of milled self-decomposing powder. J. Therm. Spray Tech. 2014, 23, 1124–1134. [Google Scholar] [CrossRef]
- Senderowski, C.; Pawłowski, A.; Bojar, Z.; Wołczyński, W.; Faryna, M.; Morgiel, J.; Major, Ł. TEM microstructure of Fe-Al coatings detonation sprayed onto steel substrate. Arch. Metall. Mater. 2010, 55, 373–381. [Google Scholar]
- Senderowski, C.; Bojar, Z.; Wołczyński, W.; Pawłowski, A. Microstructure characterization of D-gun sprayed Fe-Al intermetallic coatings. Intermetallics 2010, 18, 1405–1409. [Google Scholar] [CrossRef]
- Senderowski, C.; Zasada, D.; Durejko, T.; Bojar, Z. Characterization of as-synthesized and mechanically milled Fe-Al powders produced by the self-disintegration method. Powder Technol. 2014, 263, 96–103. [Google Scholar] [CrossRef]
- Yin, B.; Liu, G.; Zhou, H.; Chen, J.; Yan, F. Microstructures and properties of plasma sprayed FeAl/CeO2/ZrO2 nano-composite coating. Appl. Surf. Sci. 2010, 256, 4176–4184. [Google Scholar] [CrossRef]
- Yang, D.M.; Tian, B.H. Microstructure and mechanical properties of FeAl coating deposited by low pressure plasma spray. Appl. Mech. Mater. 2013, 333–335, 1916–1920. [Google Scholar] [CrossRef]
- Canakci, A.; Erdemir, F.; Varol, T.; Dalmış, R.; Ozkaya, S. Effects of a new pre-milling coating process on the formation and properties of an Fe-Al intermetallic coating. Powder Technol. 2014, 268, 110–117. [Google Scholar] [CrossRef]
- Wołczyński, W.; Senderowski, C.; Morgiel, J.; Garzeł, G. D-gun sprayed Fe–Al single particle solidification. Arch. Metall. Mater. 2014, 59, 209–217. [Google Scholar]
- Senderowski, C.; Bojar, Z.; Wołczyński, W.; Roy, G.; Czujko, T. Residual stresses determined by the modified Sachs method within a gas detonation sprayed coatings of the Fe-Al intermetallic. Arch. Metall. Mater. 2007, 52, 569–578. [Google Scholar]
- Formanek, B.; Szymanski, K.; Szczucka-Lasota, B.; Wlodarczyk, A. New generation of protective coatings intended for the power industry. J. Mater. Process. Technol. 2005, 164, 850–855. [Google Scholar] [CrossRef]
- Szczucka-Lasota, B.; Formanek, B.; Hernas, A. Growth of corrosion products on thermally sprayed coatings with FeAl intermetallic phases in aggressive environments. J. Mater. Process. Technol. 2005, 164, 930–934. [Google Scholar] [CrossRef]
- Senderowski, C.; Bojar, Z. Influence of Detonation gun spraying conditions on the quality of Fe-Al intermetallic protective coatings in the presence of NiAl and NiCr interlayers. J. Therm. Spray Tech. 2009, 18, 435–447. [Google Scholar] [CrossRef]
- Pawłowski, A.; Senderowski, C.; Bojar, Z.; Bonarski, J.; Major, Ł. Detonation deposited Fe-Al coatings Part III: Morphology of the Ni(Cr) and Ni(Al) interlayers and Fe-Al coating sprayed onto the 045 steel substrate. Arch. Metall. Mater. 2011, 56, 263–269. [Google Scholar]
- Ji, G.; Elkedim, O.; Grosdidier, T. Deposition and corrosion resistance of HVOF sprayed nanocrystalline iron aluminide coatings. Surf. Coat. Tech. 2005, 190, 406–416. [Google Scholar] [CrossRef]
- Guilemany, J.M.; Lima, C.R.C.; Cinca, N.; Miguel, J.R. Studies of Fe–40Al coatings obtained by high velocity oxy-fuel. Surf. Coat. Tech. 2006, 201, 2072–2079. [Google Scholar] [CrossRef]
- Senderowski, C.; Bojar, Z. Gas detonation spray forming of Fe-Al coatings in the presence of interlayer. Surf. Coat. Tech. 2008, 202, 3538–3548. [Google Scholar] [CrossRef]
- Pawłowski, A.; Senderowski, C.; Wołczyński, W.; Morgiel, J.; Major, Ł. Detonation deposited Fe-Al coatings, Part II: Transmission electron microscopy of interlayers and Fe-Al intermetallic coating detonation sprayed onto the 045 steel substrate. Arch. Metall. Mater. 2011, 56, 71–79. [Google Scholar]
- Pawłowski, A.; Czeppe, T.; Major, L.; Senderowski, C. Structure morphology of Fe-Al coating detonation sprayed onto carbon steel substrate. Arch. Metall. Mater. 2009, 54, 783–788. [Google Scholar]
- Senderowski, C. Structure and Properties of Gas Detonation Spraying Coatings Based on Iron-Aluminium Intermetallics. Ph.D. Thesis, Military University of Technology, Warsaw, Poland, 2002. [Google Scholar]
- Guilemany, J.M.; Cinca, N.; Dosta, S.; Benedetti, A.V. Corrosion behaviour of thermal sprayed nitinol coatings. Corros. Sci. 2009, 51, 171–180. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senderowski, C.; Chodala, M.; Bojar, Z. Corrosion Behavior of Detonation Gun Sprayed Fe-Al Type Intermetallic Coating. Materials 2015, 8, 1108-1123. https://doi.org/10.3390/ma8031108
Senderowski C, Chodala M, Bojar Z. Corrosion Behavior of Detonation Gun Sprayed Fe-Al Type Intermetallic Coating. Materials. 2015; 8(3):1108-1123. https://doi.org/10.3390/ma8031108
Chicago/Turabian StyleSenderowski, Cezary, Michal Chodala, and Zbigniew Bojar. 2015. "Corrosion Behavior of Detonation Gun Sprayed Fe-Al Type Intermetallic Coating" Materials 8, no. 3: 1108-1123. https://doi.org/10.3390/ma8031108
APA StyleSenderowski, C., Chodala, M., & Bojar, Z. (2015). Corrosion Behavior of Detonation Gun Sprayed Fe-Al Type Intermetallic Coating. Materials, 8(3), 1108-1123. https://doi.org/10.3390/ma8031108







