Effect of Different Manufacturing Methods on the Conflict between Porosity and Mechanical Properties of Spiral and Porous Polyethylene Terephthalate/Sodium Alginate Bone Scaffolds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Surface Observation of PET Bone Scaffolds: Effect of Number of Layers and Concentration of CaCl2 Solution on the Fiber Arrangement
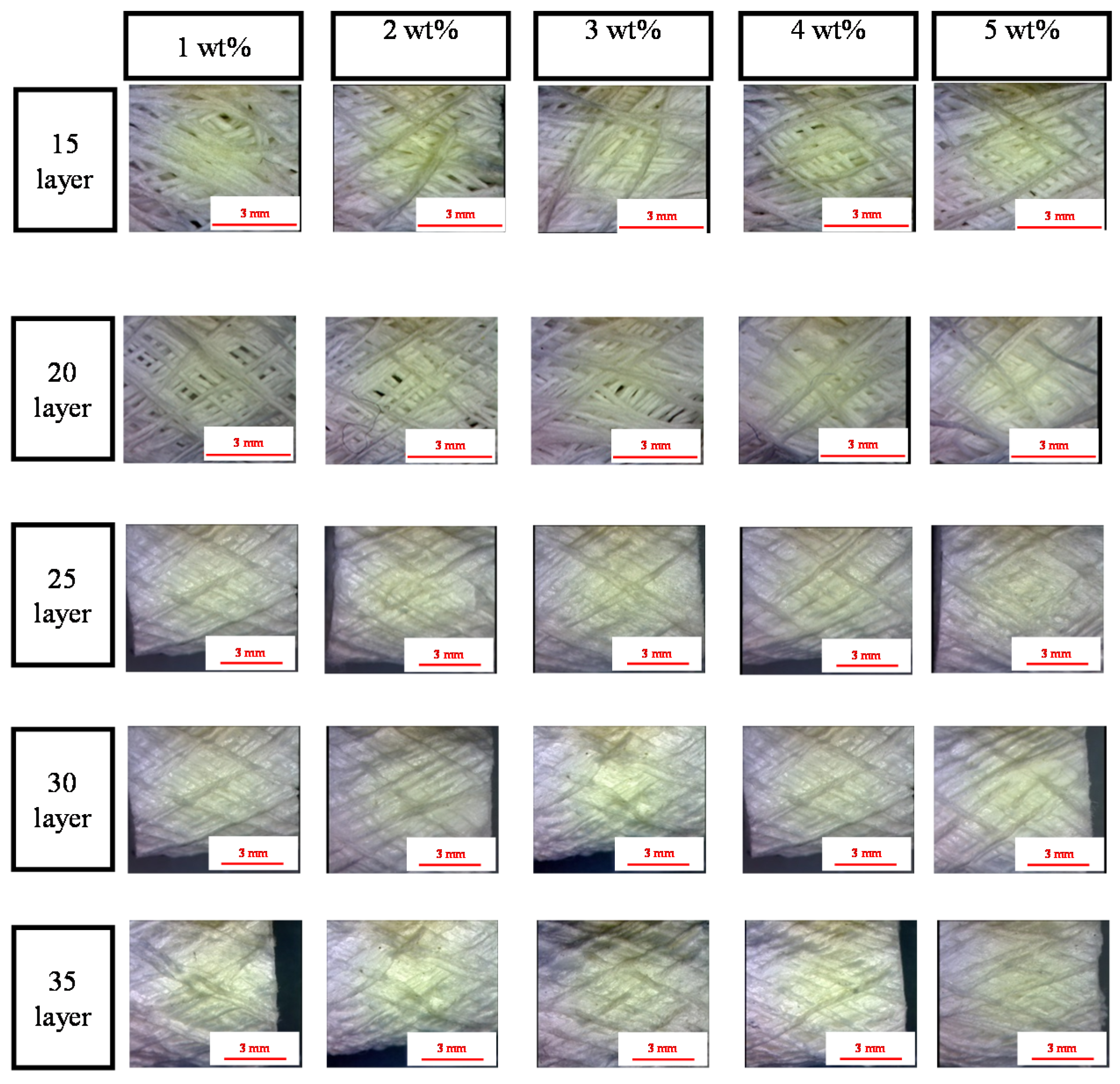
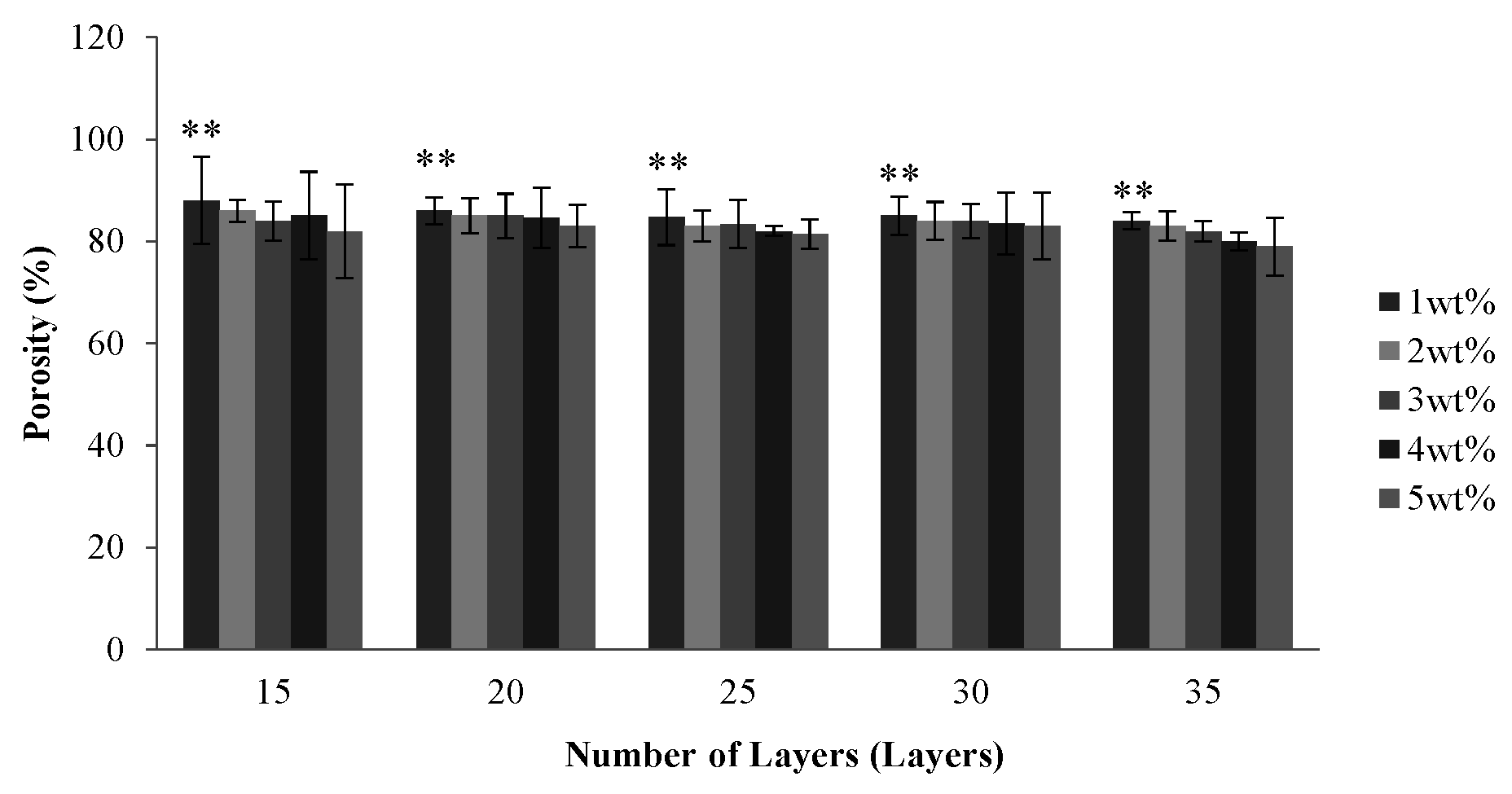
2.2. Effect of Number of Layers and Concentration of CaCl2 Solution on the Porosity of PET Bone Scaffolds
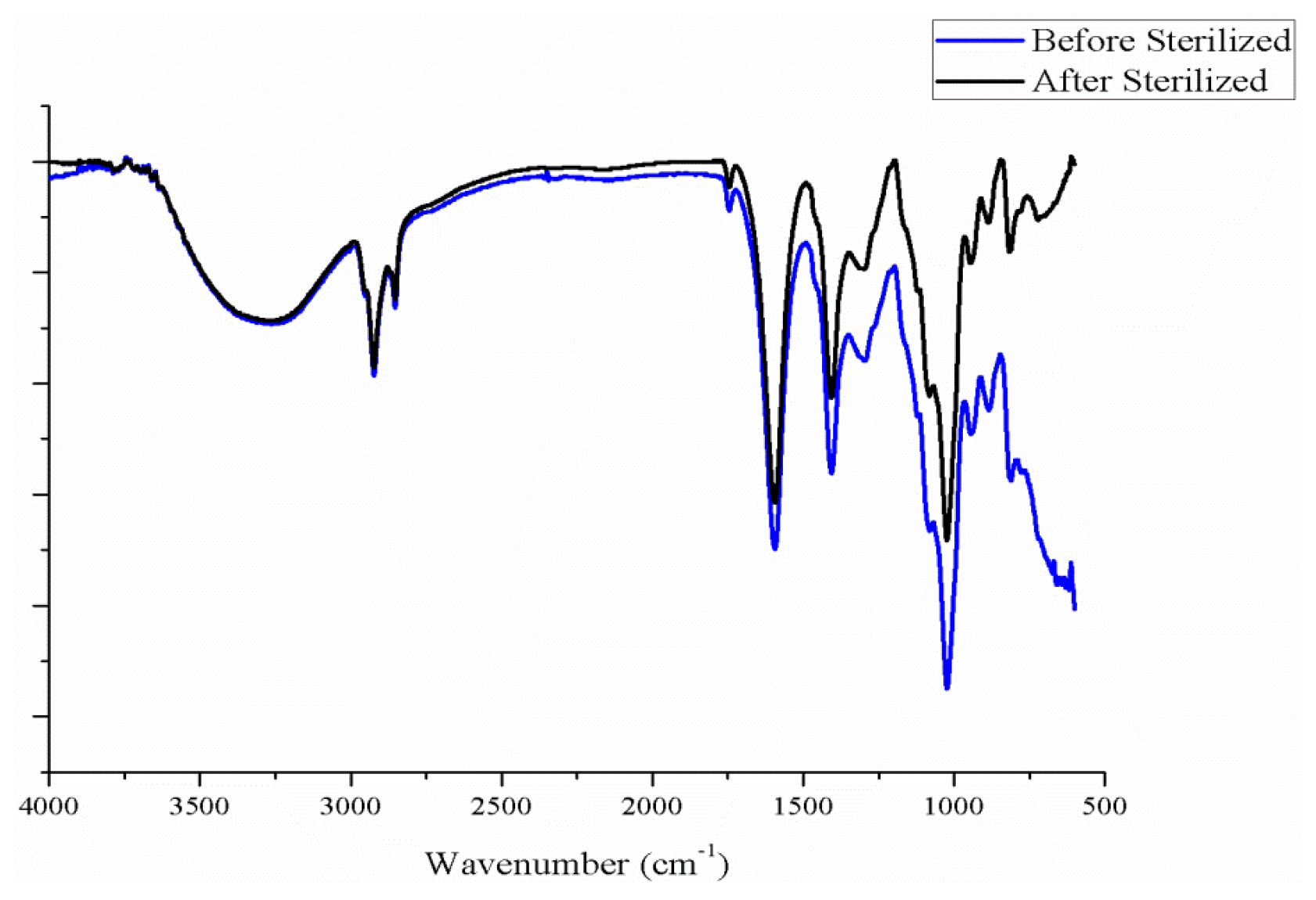
2.3. Effect of Number of Layers and Concentration of CaCl2 Solution on the Hydrophilicity of PET Bone Scaffolds

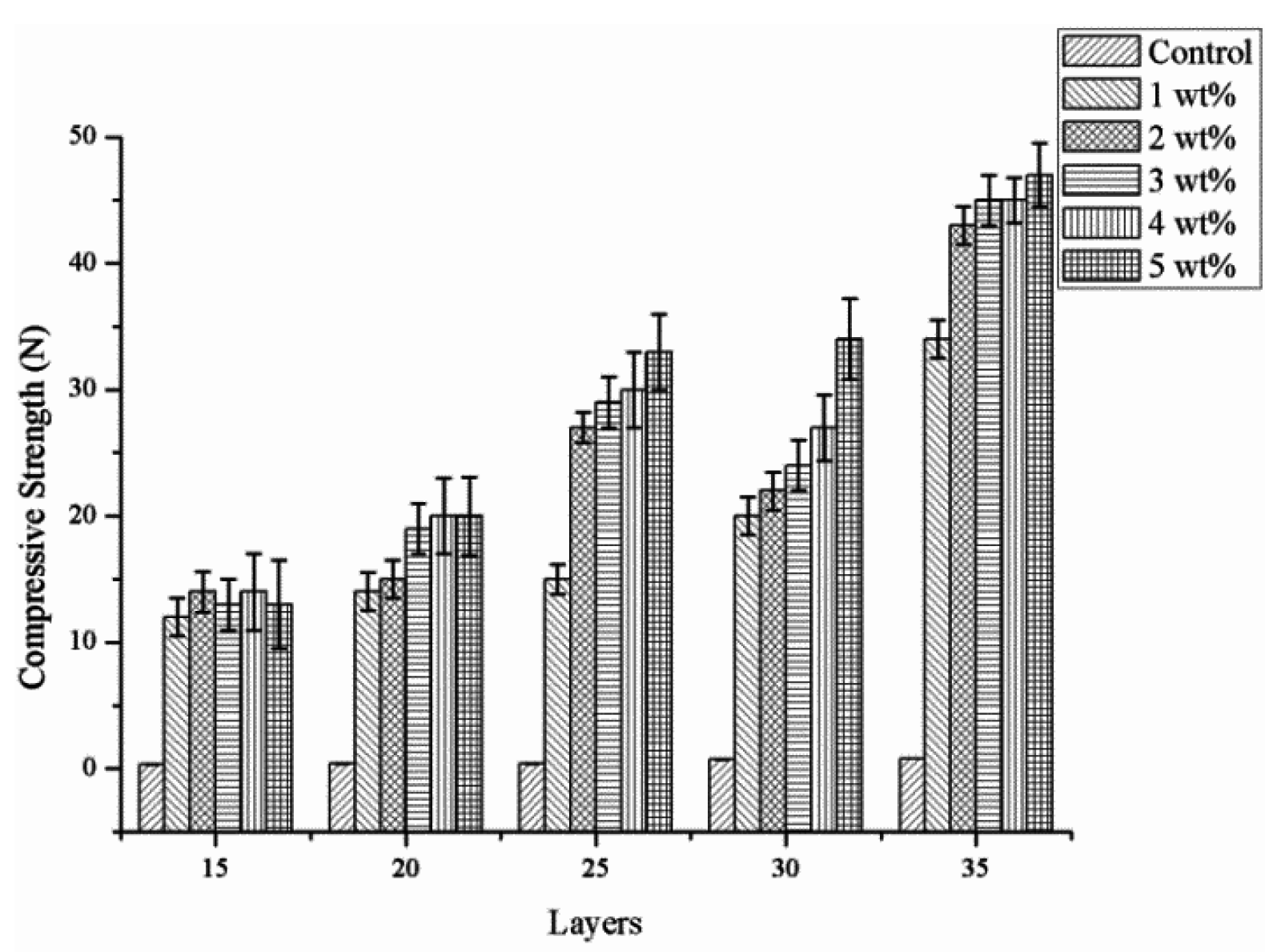
2.4. Effect of Number of Layers and Concentration of CaCl2 Solution on the Compressive Strength of PET Bone Scaffolds
2.5. Effect of Number of Layers and Concentration of CaCl2 Solution on the Morphology of the PET/SA Bone Scaffolds
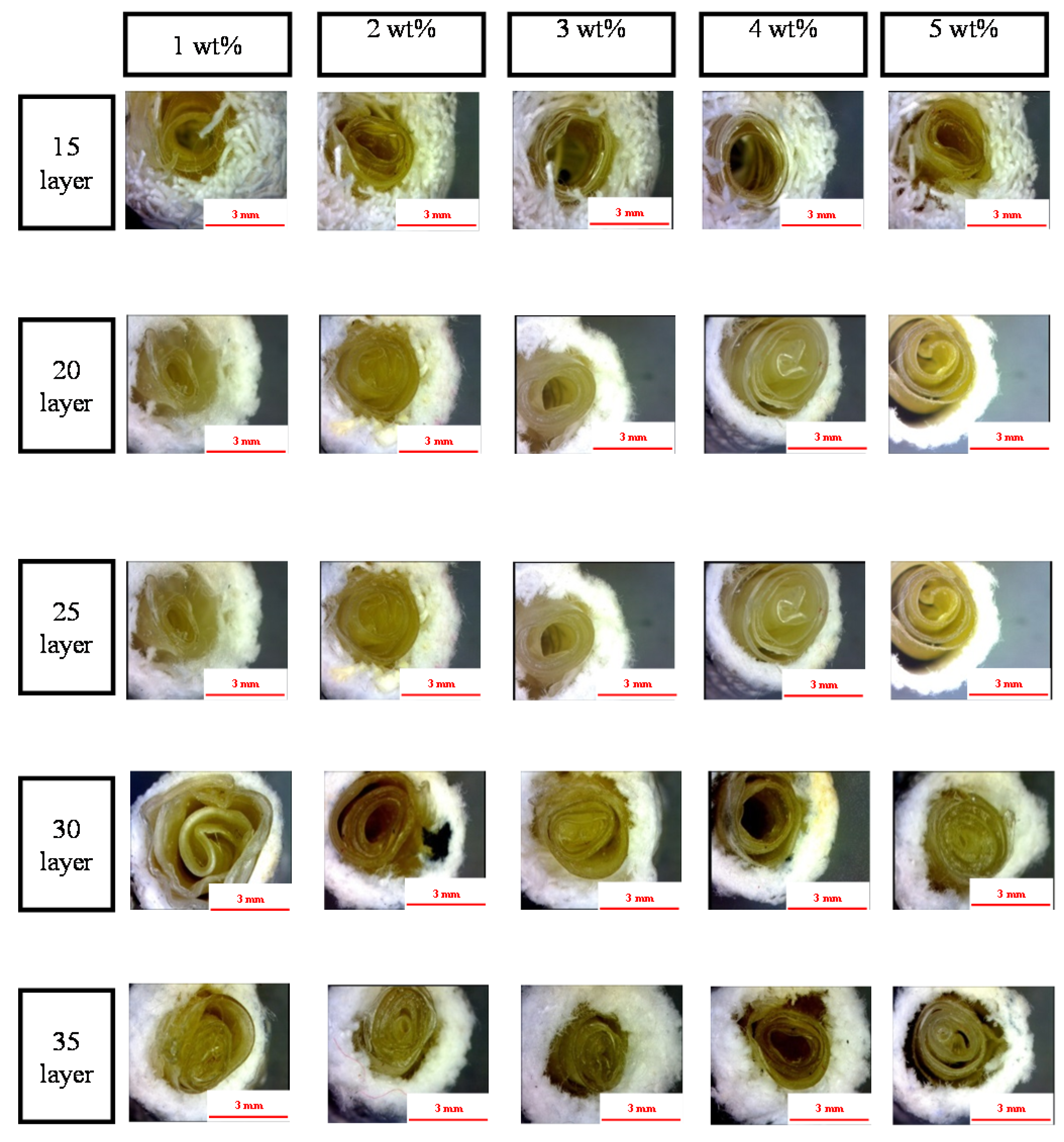
2.6. Effect of Number of Layers and Concentration of CaCl2 Solution on the Porosity of the PET/SA Bone Scaffolds
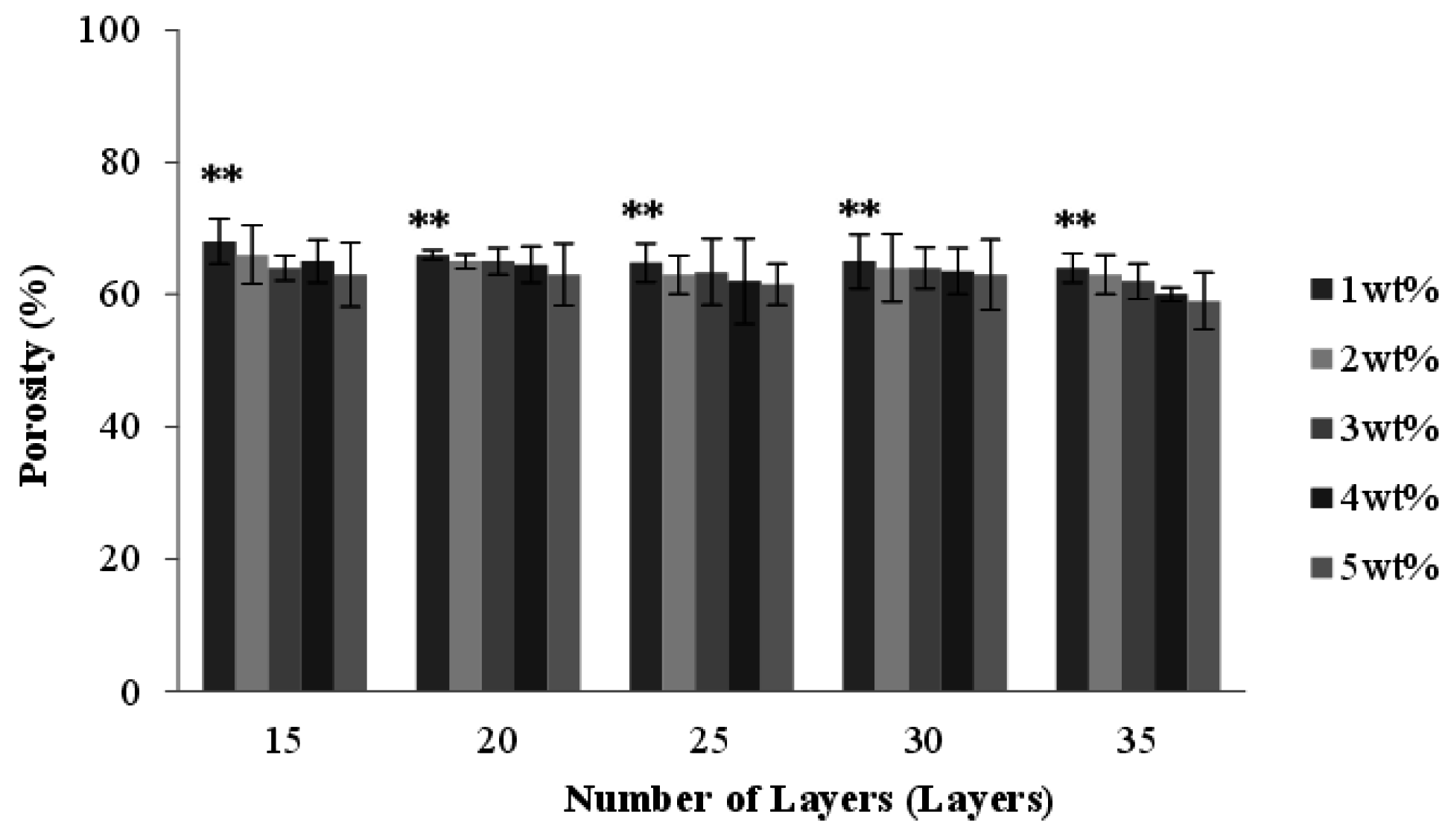
2.7. Effect of Number of Layers and Concentration of CaCl2 Solution on the Hydrophilicity of PET/SA Bone Scaffolds
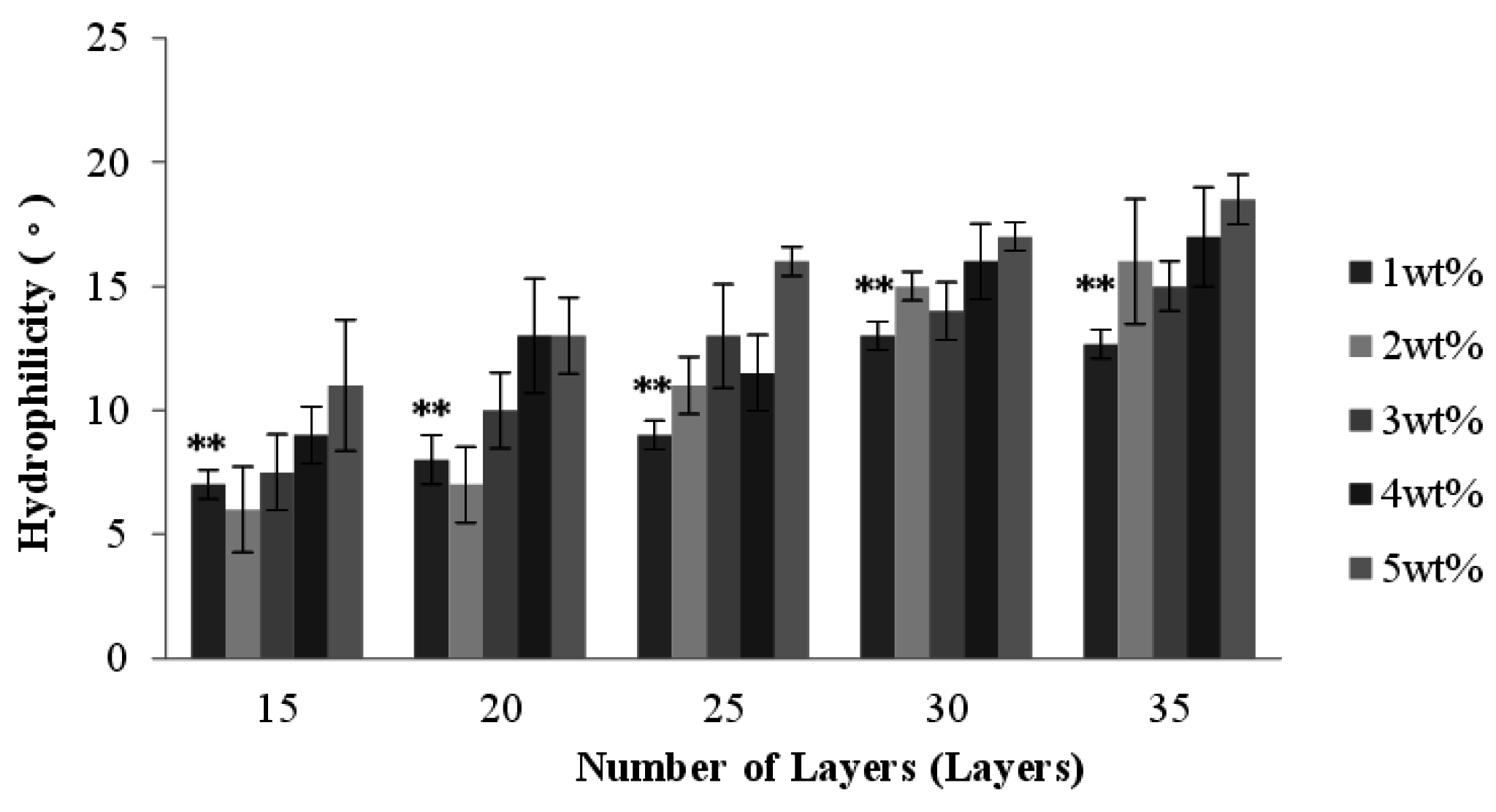
2.8. Effect of Number of Layers and Concentration of CaCl2 Solution on the Compressive Strength of PET/SA Bone Scaffolds
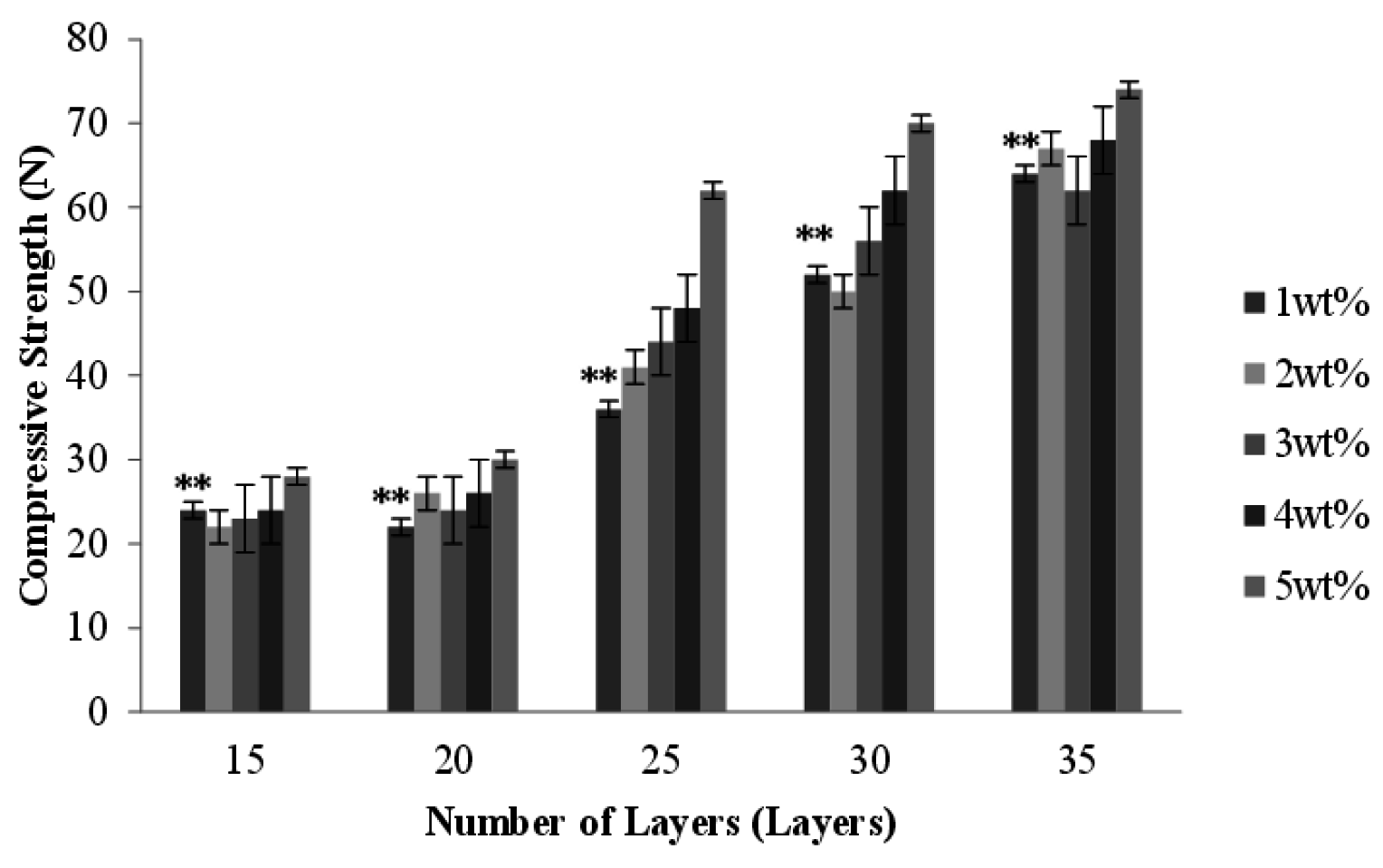
2.9. Cytotoxicity Assay
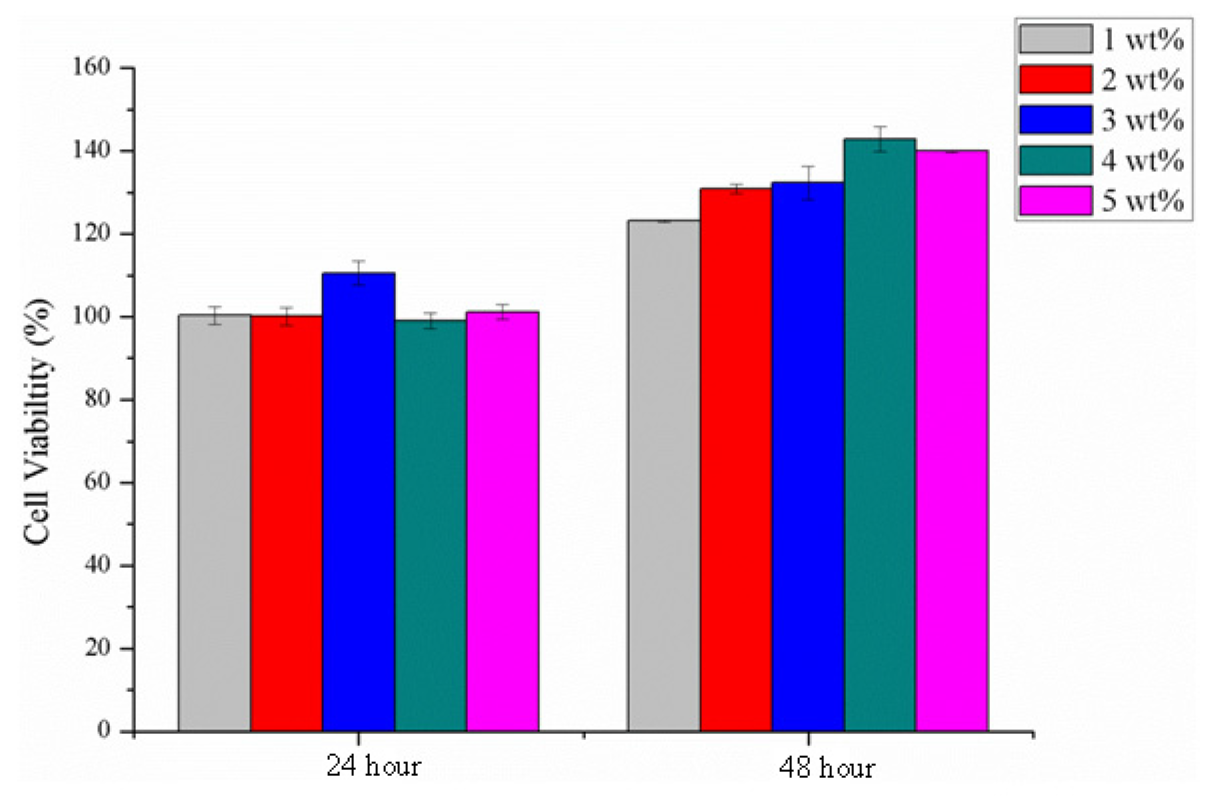
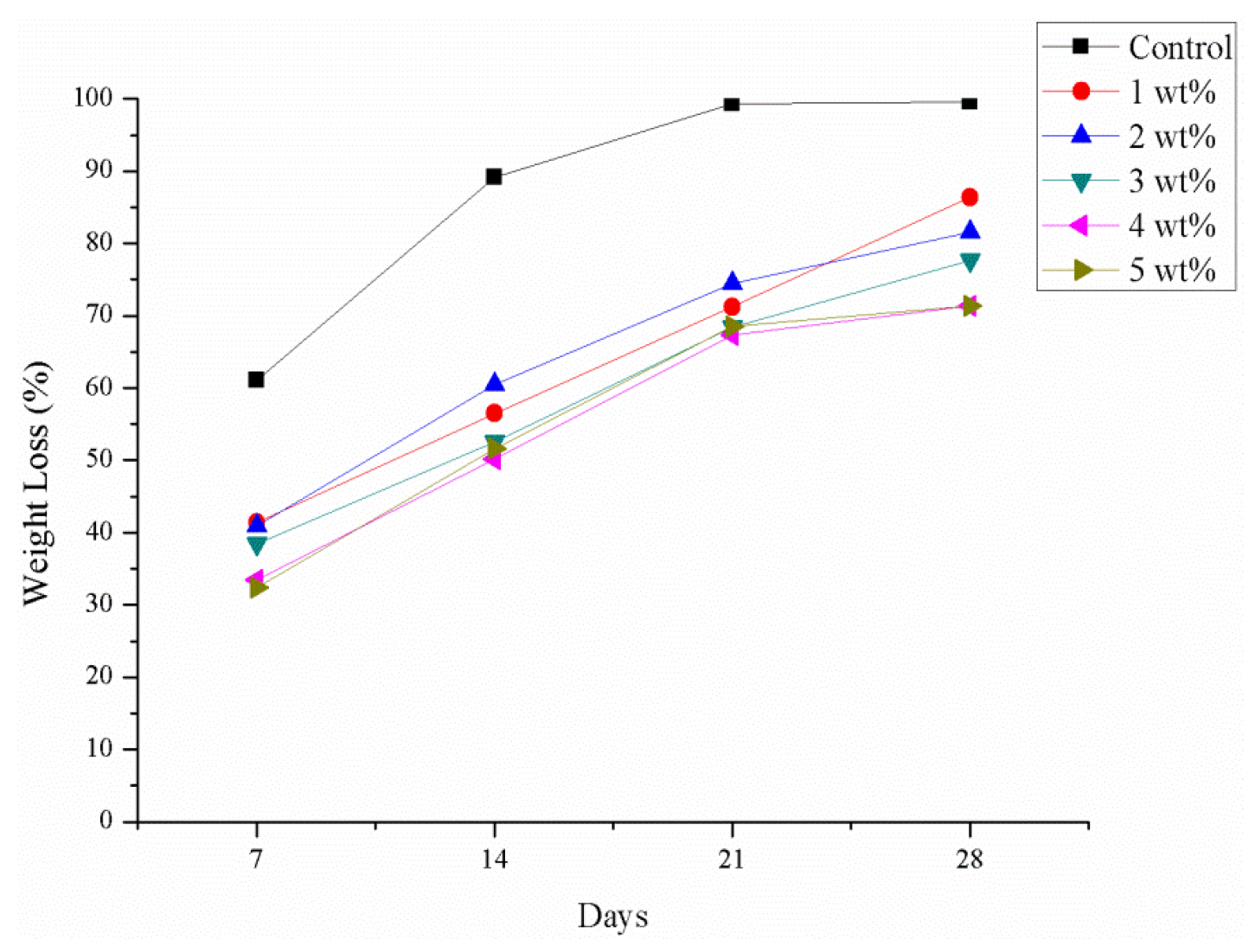
2.10. Degradation Test
3. Experimental
3.1. Materials
3.2. Preparation of PET Bone Scaffolds
3.3. Preparation of SA Membranes
3.4. Preparation of PET/SA Bone Scaffolds
3.5. Tests
3.5.1. Surface Observation of PET and PET/SA Bone Scaffolds
3.5.2. Porosity
PET Bone Scaffolds
PET/SA Bone Scaffolds
3.5.3. Hydrophilicity of PET and PET/SA Bone Scaffolds
3.5.4. Compressive Strength of PET and PET/SA Bone Scaffolds
3.5.5. Cytotoxicity Assay
3.5.6. Degradation Test
3.5.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Valente, J.F.A.; Valente, T.A.M.; Alves, P.; Ferreira, P.; Silva, A.; Correia, I.J. Alginate based scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2012, 32, 2596–2603. [Google Scholar] [CrossRef]
- Aflori, M.; Drobota, M.; Dimitriu, D.G.; Stoica, I.; Simionescu, B.; Harabagiu, V. Collagen immobilization on polyethylene terephthalate surface after helium plasma treatment. Mater. Sci. Eng. B 2013, 178, 1303–1310. [Google Scholar] [CrossRef]
- Saito, T.; Takemoto, M.; Fukuda, A.; Kuroda, Y.; Fujibayashi, S.; Neo, M.; Honjoh, D.; Hiraide, T.; Kizuki, T.; Kokubo, T.; et al. Effect of titania-based surface modification of polyethylene terephthalate on bone-implant bonding and pen-implant tissue reaction. Acta Biomater. 2011, 7, 1558–1569. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.V.D.; Ramachandran, R.; Chennazhi, K.P.; Tamura, H.; Nair, S.V.; Jayakumar, R. Fabrication of alginate/nano TiO2 needle composite scaffolds for tissue engineering applications. Carbohydr. Polym. 2011, 83, 858–864. [Google Scholar] [CrossRef]
- Morais, D.S.; Rodrigues, M.A.; Silva, T.I.; Lopes, M.A.; Santos, M.; Santos, J.D.; Botelho, C.M. Development and characterization of novel alginate-based hydrogels as vehicles for bone substitutes. Carbohydr. Polym. 2013, 95, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Lim, J.; Teoh, S.H. Review: Development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol. Adv. 2013, 31, 688–705. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.W.; Yao, C.H.; Chen, Y.S.; Lu, C.T.; Chen, W.C.; Yen, K.C.; Lin, J.H. PLA/β-TCP complex tubes: The mechanical properties and applications of artificial bone. J. Biomater. Sci. Polym. 2012, 23, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.L.; Gaspar, V.M.; Serra, I.R.; Diogo, G.S.; Fradique, R.; Silva, A.P.; Correia, I.J. Bioactive polymeric-ceramic hybrid 3D scaffold for application in bone tissue regeneration. Mater. Sci. Eng. C 2013, 33, 4460–4469. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, X. Preparation, characterization and in vitro analysis of novel structured nanofibrous scaffolds for bone tissue engineering. Acta Biomater. 2010, 6, 3004–3012. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Ingle, N.P.; Montero, G.A.; Kim, S.H.; King, M.W. Bioresorbable elastomeric vascular tissue engineering scaffolds via melt spinning and electrospinning. Acta Biomater. 2010, 6, 1958–1967. [Google Scholar] [CrossRef] [PubMed]
- American Society for Testing Materials. Standard Test Method for Compressive Properties of Polymer Matrix Composite Materials Using a Combined Loading Compression (CLC) Test Fixture; ASTM D6641/D6641M-09; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- International Organization for Standardization. Biological Evaluation of Medical Devices—Part 5: Tests for in vitro Cytotoxicity; ISO 10993-5:2009; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, C.-W.; Huang, C.-L.; Chen, C.-K.; Liu, C.-F.; Wen, S.-P.; Lin, J.-H. Effect of Different Manufacturing Methods on the Conflict between Porosity and Mechanical Properties of Spiral and Porous Polyethylene Terephthalate/Sodium Alginate Bone Scaffolds. Materials 2015, 8, 8768-8779. https://doi.org/10.3390/ma8125488
Lou C-W, Huang C-L, Chen C-K, Liu C-F, Wen S-P, Lin J-H. Effect of Different Manufacturing Methods on the Conflict between Porosity and Mechanical Properties of Spiral and Porous Polyethylene Terephthalate/Sodium Alginate Bone Scaffolds. Materials. 2015; 8(12):8768-8779. https://doi.org/10.3390/ma8125488
Chicago/Turabian StyleLou, Ching-Wen, Chien-Lin Huang, Chih-Kuang Chen, Chi-Fan Liu, Shih-Peng Wen, and Jia-Horng Lin. 2015. "Effect of Different Manufacturing Methods on the Conflict between Porosity and Mechanical Properties of Spiral and Porous Polyethylene Terephthalate/Sodium Alginate Bone Scaffolds" Materials 8, no. 12: 8768-8779. https://doi.org/10.3390/ma8125488
APA StyleLou, C.-W., Huang, C.-L., Chen, C.-K., Liu, C.-F., Wen, S.-P., & Lin, J.-H. (2015). Effect of Different Manufacturing Methods on the Conflict between Porosity and Mechanical Properties of Spiral and Porous Polyethylene Terephthalate/Sodium Alginate Bone Scaffolds. Materials, 8(12), 8768-8779. https://doi.org/10.3390/ma8125488







