Effect of Oxide Coating on Performance of Copper-Zinc Oxide-Based Catalyst for Methanol Synthesis via Hydrogenation of Carbon Dioxide
Abstract
:1. Introduction
2. Results and Discussion
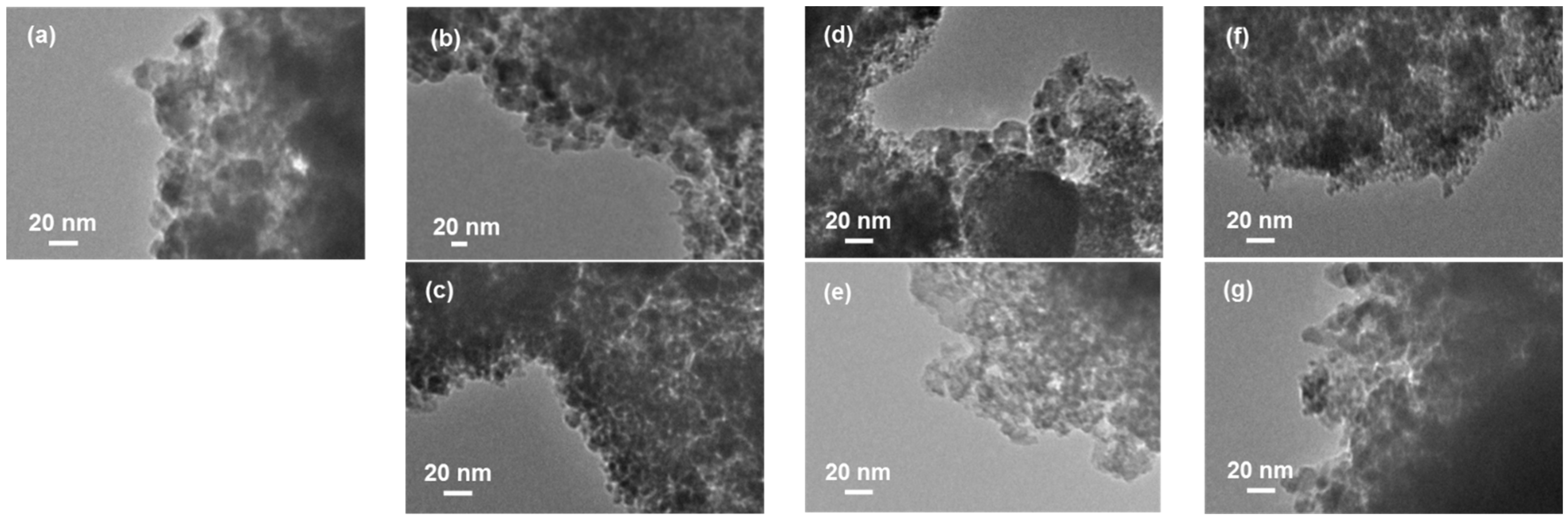
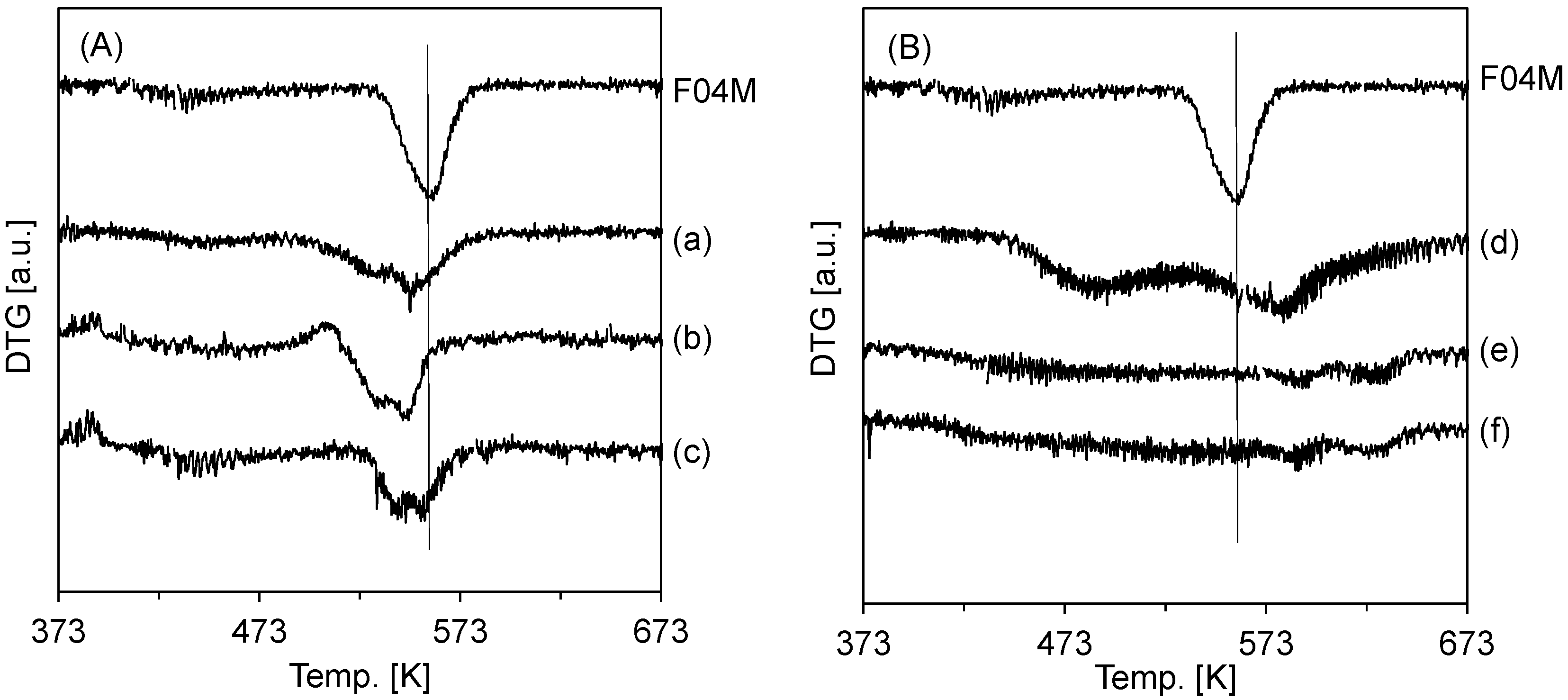
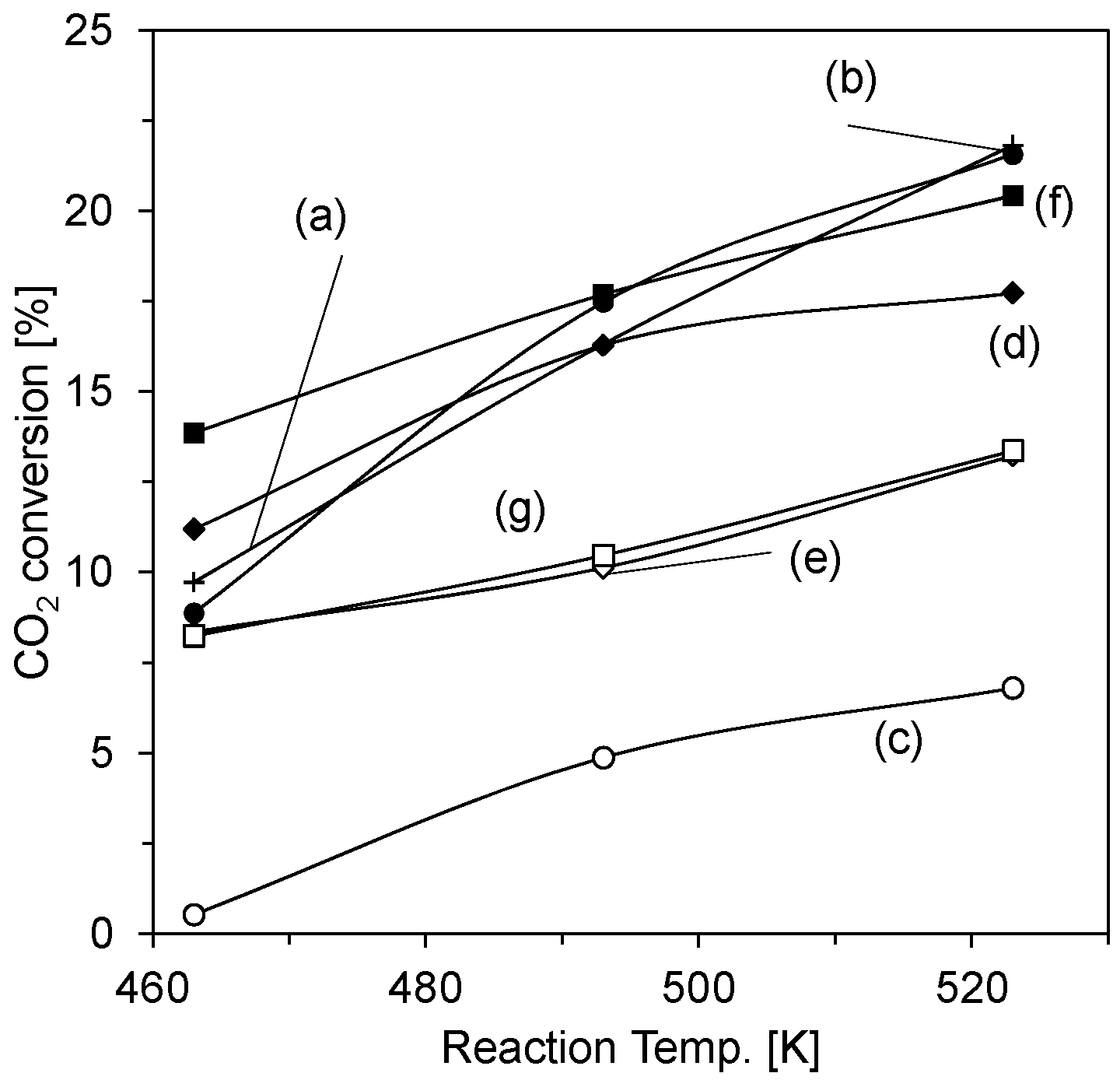
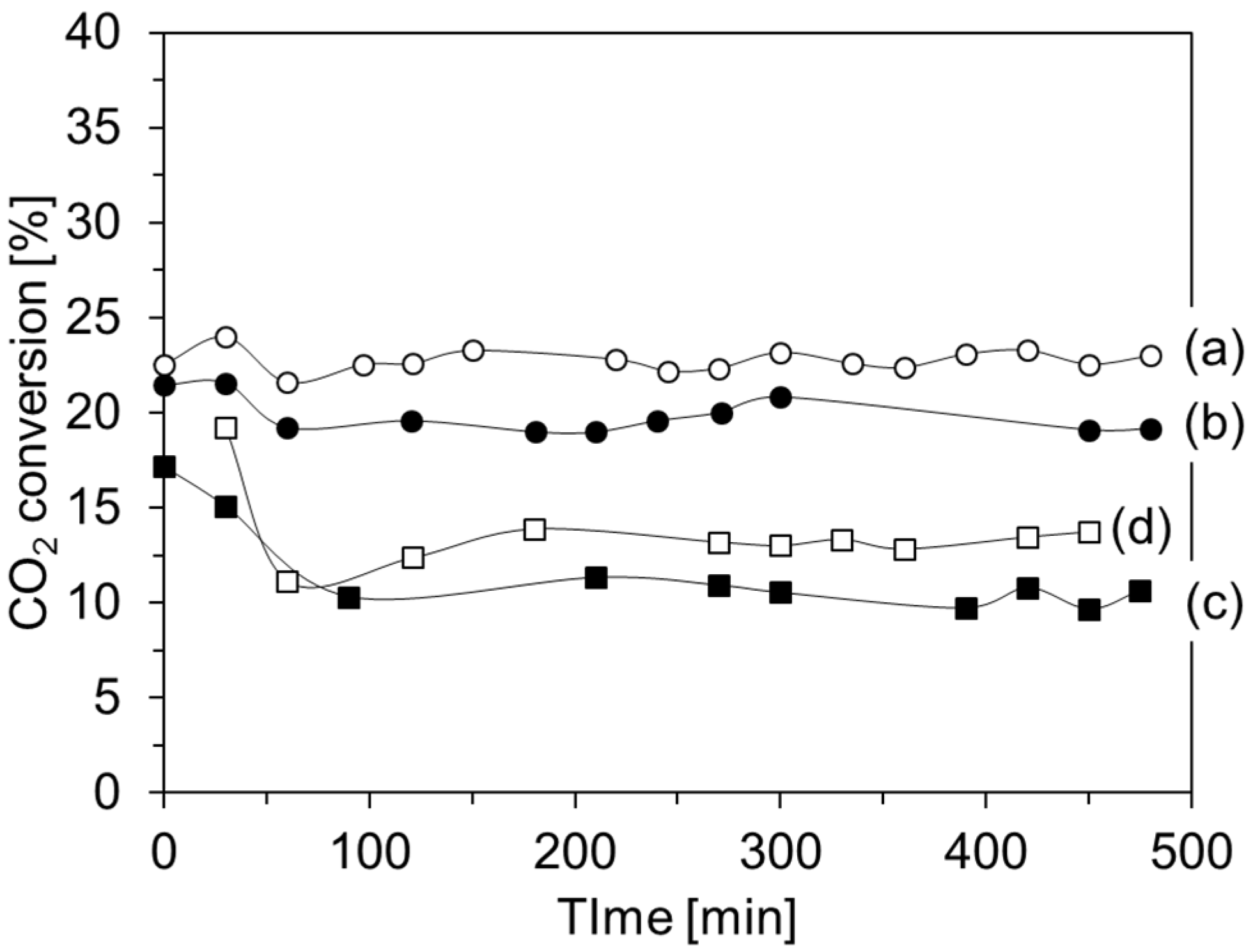
| Catalyst | CO Selectivity (%) | CH3OH Selectivity (%) |
|---|---|---|
| F04M | 62.1 | 37.9 |
| SiO2 coat | 53.1 | 43.9 |
| ZrO2 coat | 61.7 | 38.3 |
| TiO2 coat | 45.3 | 54.7 |
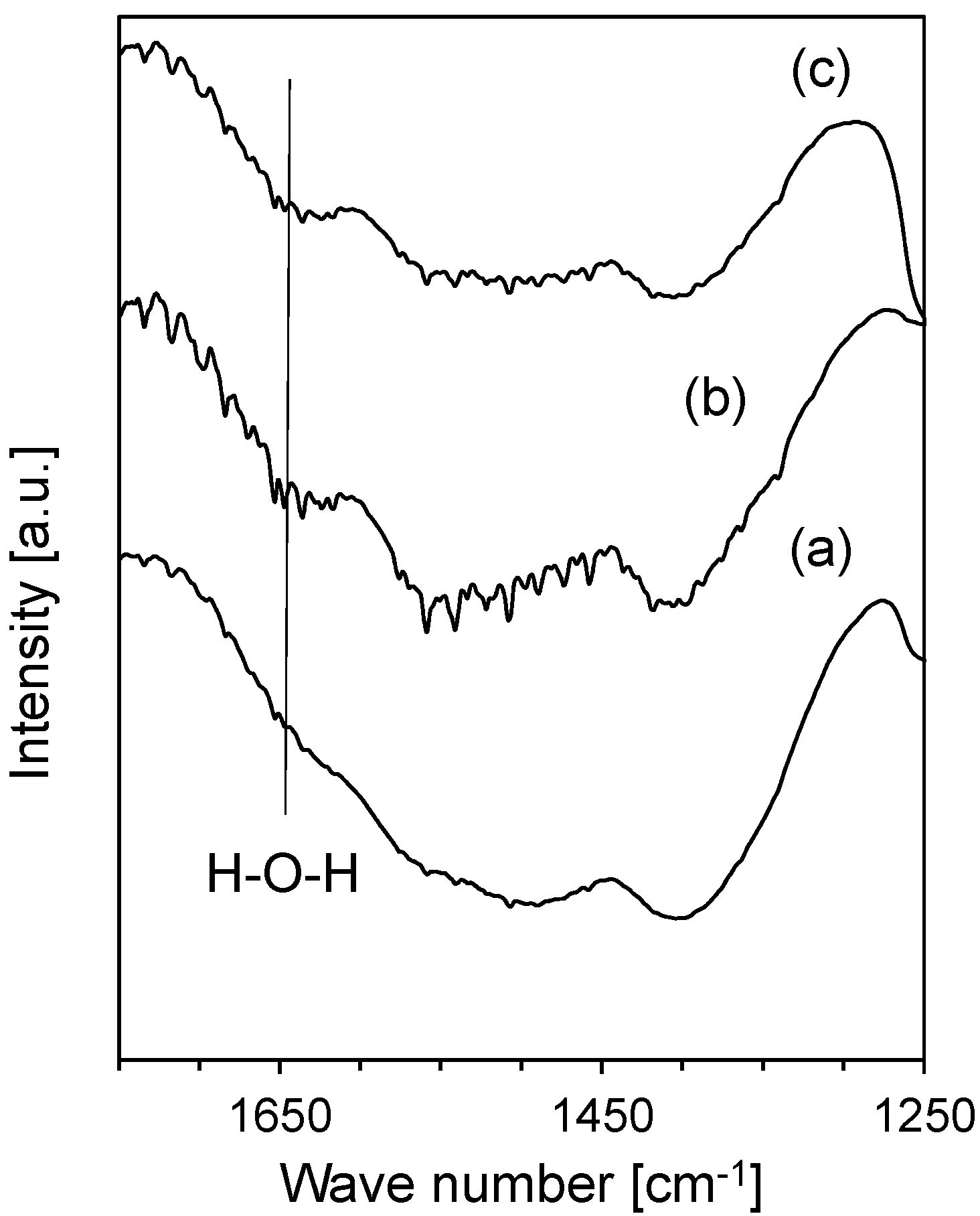
3. Experimental Section
3.1. Catalyst Preparation
3.2. Characterization
3.3. Catalytic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catal. Today 2009, 148, 191–205. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [PubMed]
- Kiler, K. Methanol synthesis. Adv. Catal. 1983, 31, 243–313. [Google Scholar]
- Waugh, K.C. Methanol synthesis. Catal. Today 1992, 15, 51–75. [Google Scholar] [CrossRef]
- Wender, I. Reactions of synthesis gas. Fuel Proc. Tech. 1986, 48, 189–297. [Google Scholar] [CrossRef]
- Wu, J.; Saito, M.; Takeuchi, M.; Watanabe, T. The stability of Cu/ZnO-based catalysts in methanol synthesis from CO2-rich feed and from a CO-rich feed. Appl. Catal. A Gen. 2001, 218, 235–240. [Google Scholar] [CrossRef]
- Batyrev, E.D.; Raveendran Shiju, N.; Rothenberg, G. Exploring the activated state of Cu/ZnO(0001)-Zn, a model catalyst for methanol synthesis. J. Phys. Chem. C 2012, 116, 19335–19341. [Google Scholar] [CrossRef]
- Marschner, F.; Moeller, F.W. Methanol Synthesis. In Applied Industrial Catalysis; Leach, B.E., Ed.; Academic Press: New York, NY, USA, 1983; Volume 2, pp. 215–243. [Google Scholar]
- Arena, F.; Mezzatesta, G.; Zafarana, G.; Trufio, G.; Frusteri, F.; Spadaro, L. How oxide carriers control the catalytic functionality of the Cu/ZnO system in the hydrogenation of CO2 to methanol. Catal. Today 2013, 210, 39–46. [Google Scholar] [CrossRef]
- Arena, F.; Mezzatesta, G.; Zafarana, G.; Trunfio, G.; Frusteri, F.; Spadaro, L. Effects of oxide carriers on surface functionality and process performance of the Cu-ZnO system in the synthesis of methanol via CO2 hydrogenation. J. Catal. 2013, 300, 141–151. [Google Scholar] [CrossRef]
- Saito, M.; Fujitani, T.; Takeuchi, M.; Watanabe, T. Development of copper/zinc oxide-based multicomponent catalysts for methanol synthesis from carbon dioxide and hydrogen. Appl. Catal. A Gen. 1996, 138, 311–318. [Google Scholar] [CrossRef]
- Arena, F.; Barbera, K.; Italiano, G.; Bonura, G.; Spadaro, L.; Frusteri, F. Synthesis, characterization and activity pattern of Cu-ZnO/ZrO2 catalysts in the hydrogenation of carbon dioxide to methanol. J. Catal. 2007, 249, 185–194. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Wu, J.; Luo, S.; Toyir, J.; Saito, M.; Takeuchi, M.; Watanabe, T. Optimization of preparation conditions and improvement of stability of Cu/ZnO-based multicomponent catalysts for methanol synthesis from CO2 and H2. Catal. Today 1998, 45, 215–220. [Google Scholar] [CrossRef]
- Wu, J.; Saito, M.; Mabuse, H. Activity and stability of Cu/ZnO/Al2O3 catalyst promoted with B2O3 for methanol synthesis. Catal. Lett. 2000, 68, 55–58. [Google Scholar] [CrossRef]
- Zha, F.; Ding, J.; Chang, Y.; Ding, J.; Wang, J.; Ma, J. Cu-Zn-Al oxide cores packed by metal-doped amorphous silica-alumina membrane for catalyzing the hydrogenation of carbon dioxide to dimethyl ether. Ind. Eng. Chem. Res. 2012, 51, 345–352. [Google Scholar] [CrossRef]
- Mosser, C.; Mosser, A.; Romeo, M.; Petit, S.; Decarreau, A. Natural and synthetic copper phyllosilicates studied by XPS. Clays Clay Min. 1992, 40, 593–599. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Lu, G. Studies on the active species and on dispersion of Cu in Cu/SiO2 and Cu/Zn/SiO2 for hydrogen production via methanol partial oxidation. Int. J. Hydrog. Energy 2003, 28, 151–158. [Google Scholar] [CrossRef]
- Subasri, R.; Malathi, R.; Jyothirmayi, A.; Hebalkar, N.Y. Synthesis and characterization of CuO-hybrid silica nano composite coating on SS 304. Ceram. Int. 2012, 38, 5731–5740. [Google Scholar] [CrossRef]
- Chary, K.V.R.; Sagar, G.V.; Srikanth, C.S.; Rao, V.V. Characterization and catalytic functionalities of copper oxide catalysts supported on zirconia. J. Phys. Chem. B 2007, 111, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Kurr, P.; Kasatkin, I.; Girgsdies, F.; Trunschke, A.; Schlögl, R.; Ressler, T. Microstructural characterization of Cu/ZnO/Al2O3 catalysts for methanol steam reforming—A comparative study. Appl. Catal. A Gen. 2008, 348, 153–164. [Google Scholar] [CrossRef]
- Guo, X.; Mao, D.; Lu, G.; Wang, S.; Wu, G. The influence of La doping on the catalytic behavior of Cu/ZrO2 for methanol synthesis from CO2 hydrogenation. J. Mol. Catal. A Chem. 2011, 345, 60–68. [Google Scholar] [CrossRef]
- Jeong, H.; Cho, C.H.; Kim, T.H. Effect of Zr and pH in the preparation of Cu/ZnO catalysts for the methanol synthesis by CO2 hydrogenation. React. Kinet. Mech. Catal. 2012, 106, 435–443. [Google Scholar] [CrossRef]
- Tagawa, T.; Nomura, N.; Shimakage, M.; Goto, S. Effect of supports on copper catalysts for methanol synthesis from CO2 + H2. Res. Chem. Intermed. 1995, 21, 193–202. [Google Scholar] [CrossRef]
- Schilke, T.C.; Fisher, I.A.; Bell, A.T. In situ infrared study of methanol synthesis from CO2/H2 on titania and zirconia promoted Cu/SiO2. J. Catal. 1999, 184, 144–156. [Google Scholar] [CrossRef]
- Xiao, J.; Mao, D.; Guo, X.; Yu, J. Effect of TiO2, ZrO2, and TiO2-ZrO2 on the performance of CuO-ZnO catalyst for CO2 hydrogenation to methanol. Appl. Surf. Sci. 2015, 338, 146–153. [Google Scholar] [CrossRef]
- Takeushi, M.; Martra, G.; Coluccia, S.; Anpo, M. Evaluation of hydrophilic/hydrophobic properties and wettability of oxide surfaces. Hyomen Kagaku 2009, 30, 148–156. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umegaki, T.; Kojima, Y.; Omata, K. Effect of Oxide Coating on Performance of Copper-Zinc Oxide-Based Catalyst for Methanol Synthesis via Hydrogenation of Carbon Dioxide. Materials 2015, 8, 7738-7744. https://doi.org/10.3390/ma8115414
Umegaki T, Kojima Y, Omata K. Effect of Oxide Coating on Performance of Copper-Zinc Oxide-Based Catalyst for Methanol Synthesis via Hydrogenation of Carbon Dioxide. Materials. 2015; 8(11):7738-7744. https://doi.org/10.3390/ma8115414
Chicago/Turabian StyleUmegaki, Tetsuo, Yoshiyuki Kojima, and Kohji Omata. 2015. "Effect of Oxide Coating on Performance of Copper-Zinc Oxide-Based Catalyst for Methanol Synthesis via Hydrogenation of Carbon Dioxide" Materials 8, no. 11: 7738-7744. https://doi.org/10.3390/ma8115414





