Enhanced Stability of Calcium Sulfate Scaffolds with 45S5 Bioglass for Bone Repair
Abstract
:1. Introduction
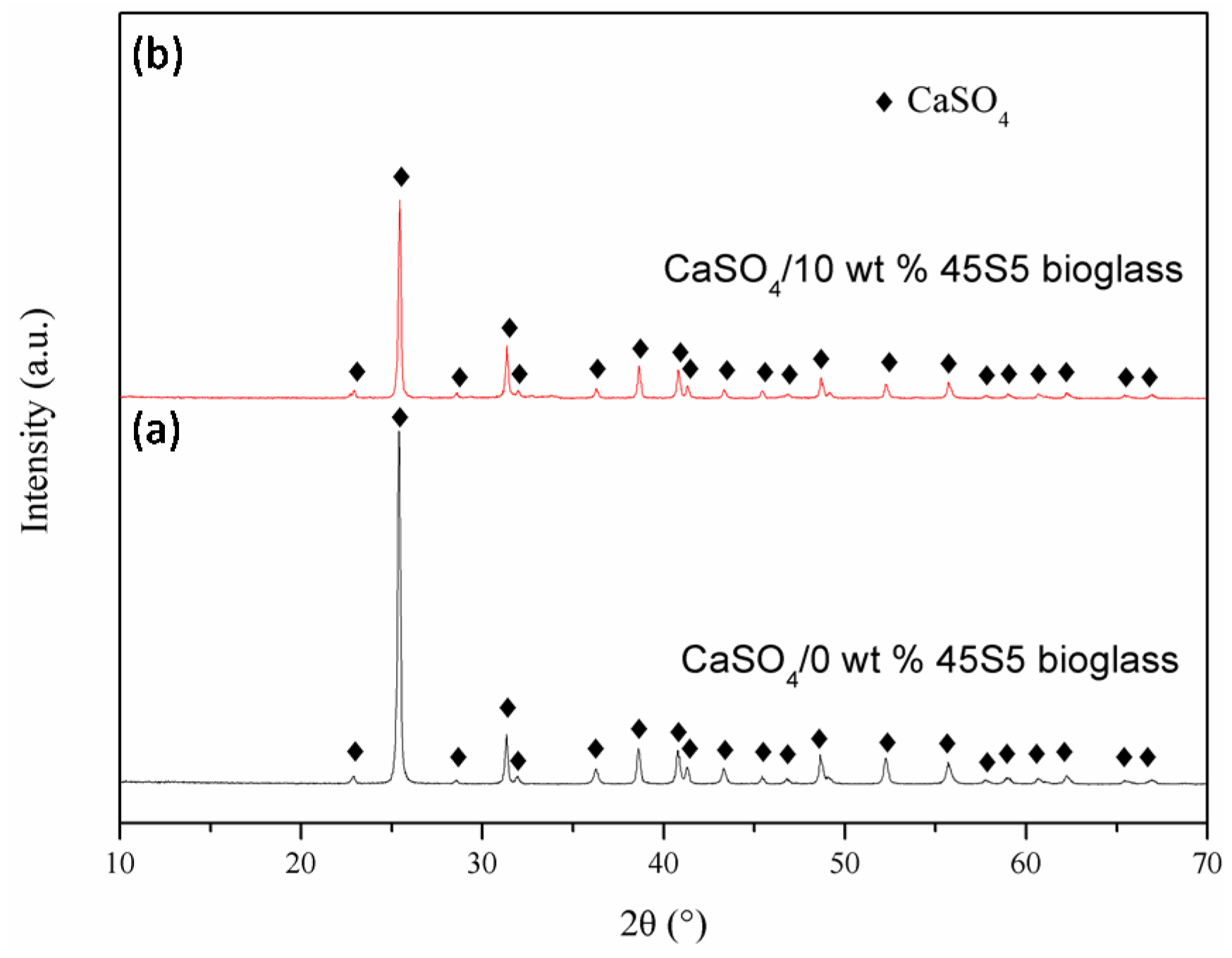
2. Results and Discussion
2.1. Fabrication of the Scaffolds
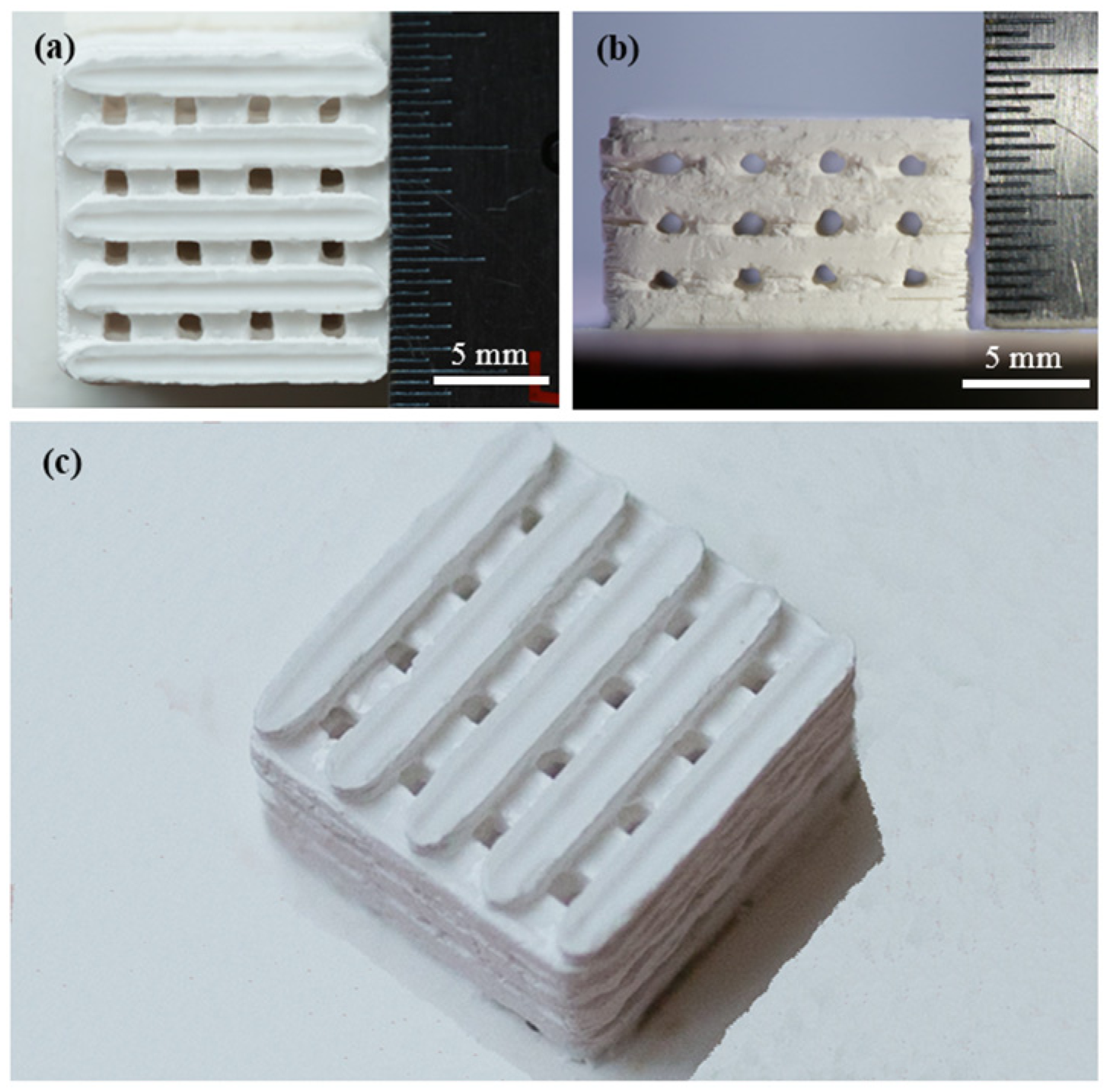
| Scan Speed (mm·min−1) | Laser Power (W) | Layer Thickness (mm) | Scan Spacing (mm) | Spot Diameter (mm) |
|---|---|---|---|---|
| 100 | 7.0 | 0.1 | 3.0 | 1.0 |
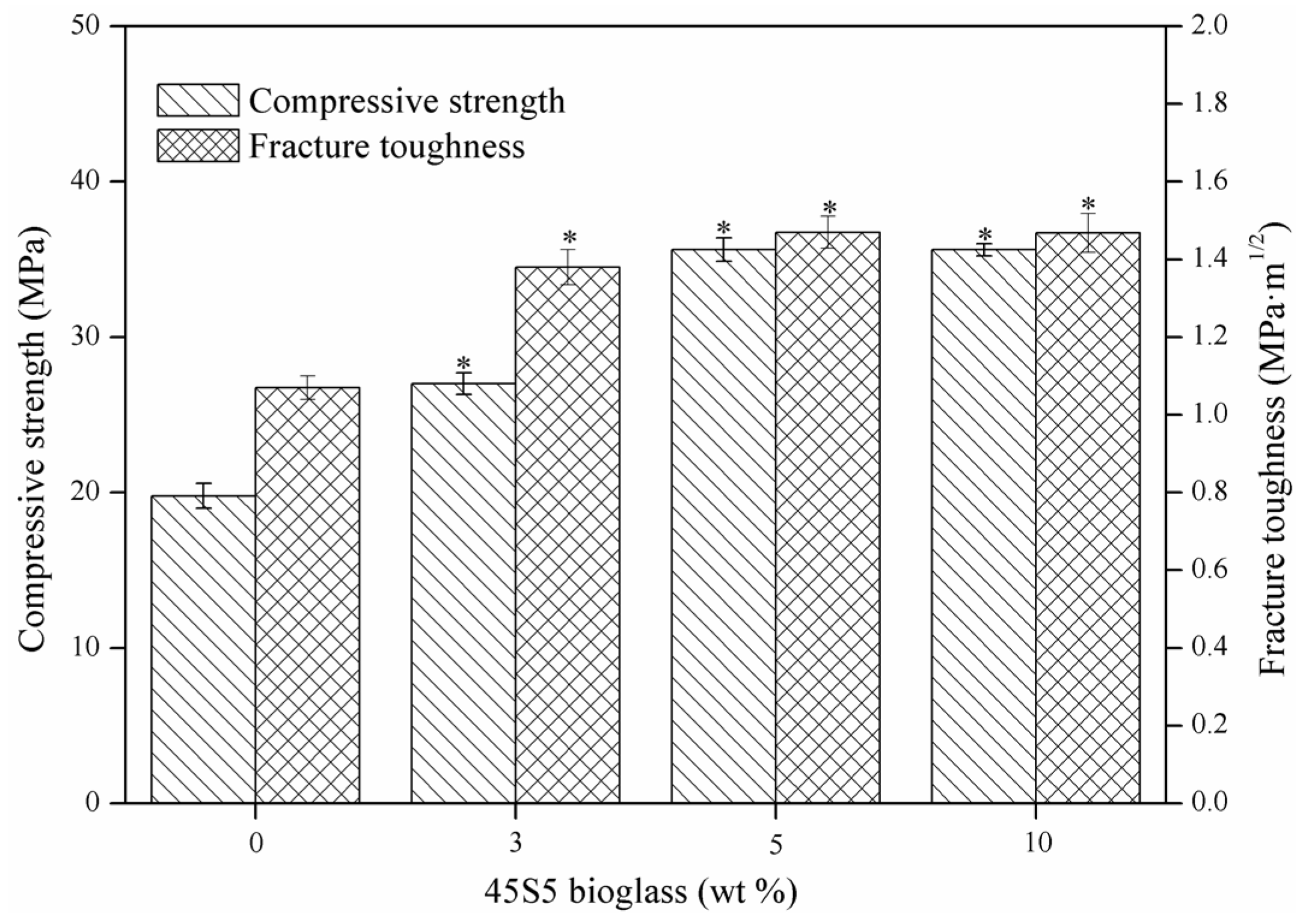
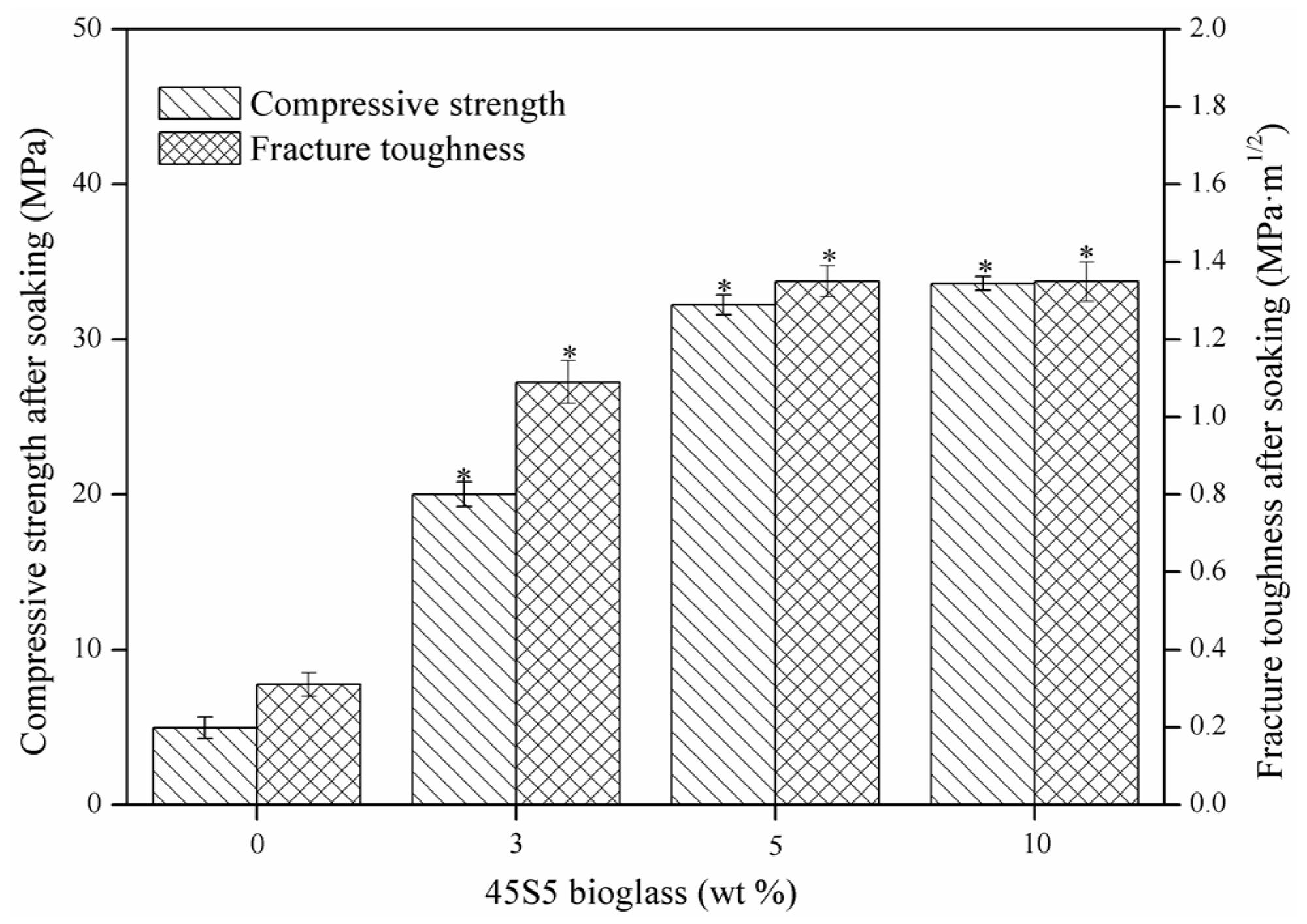
2.2. Mechanical Properties
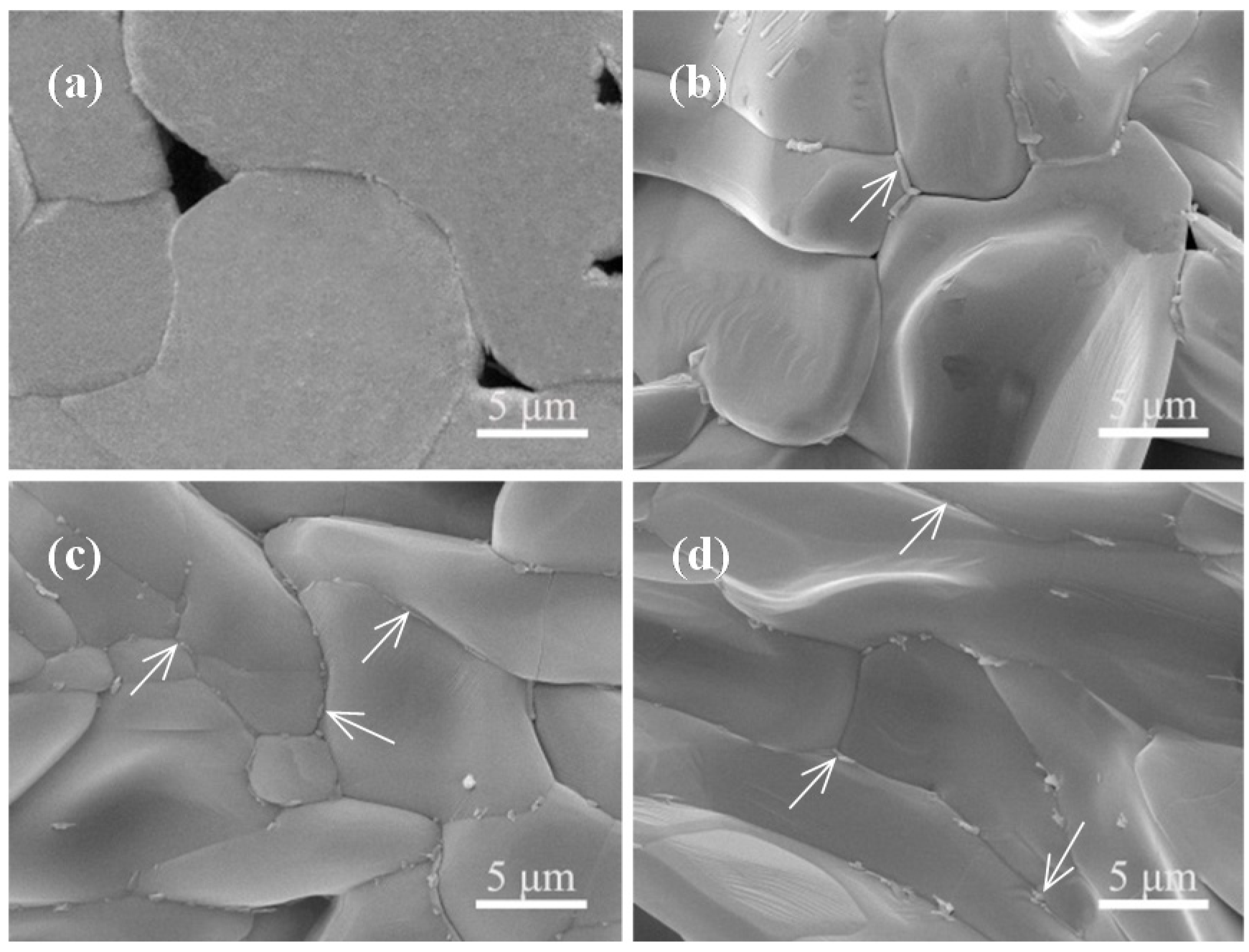
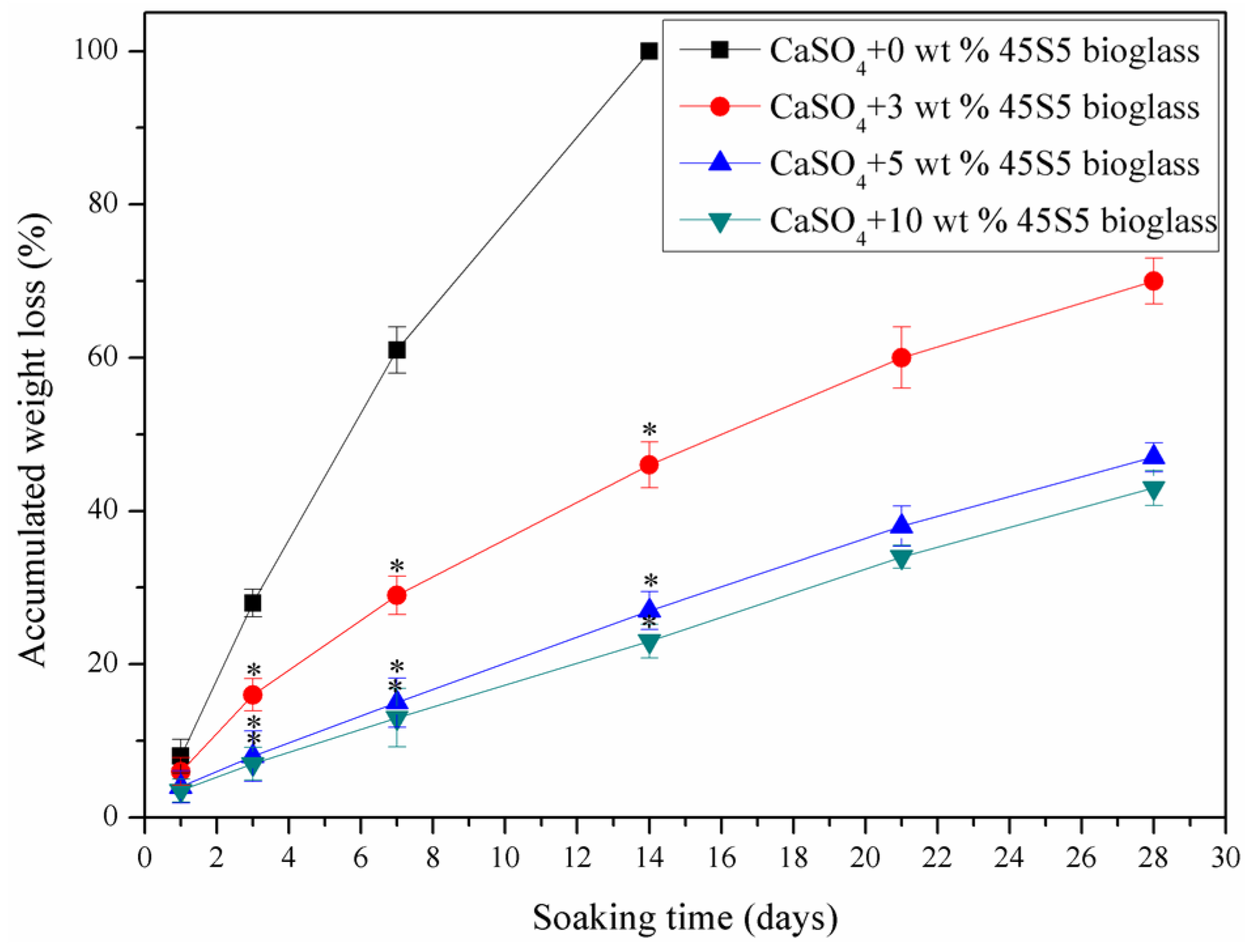
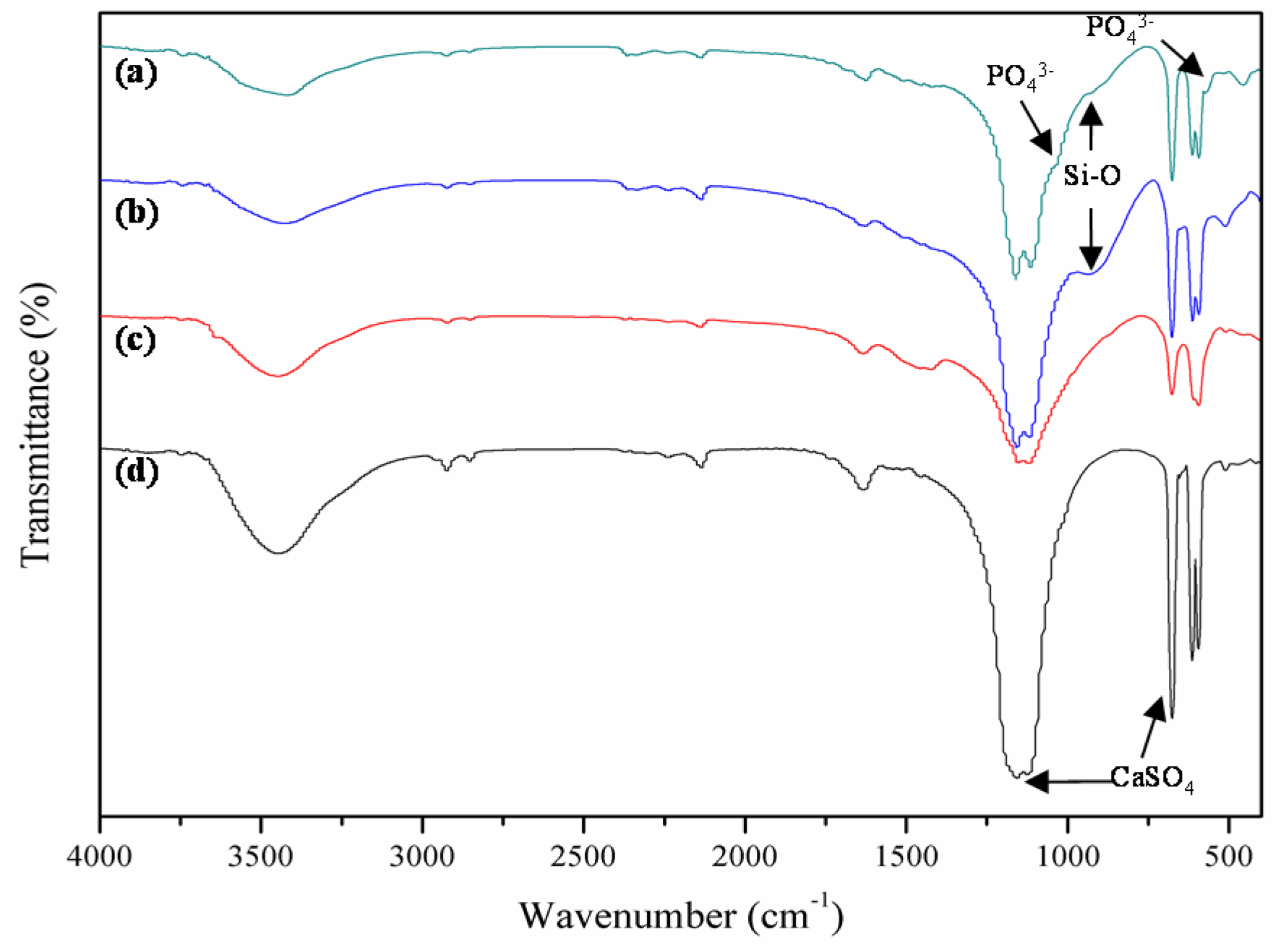
2.3. Stability
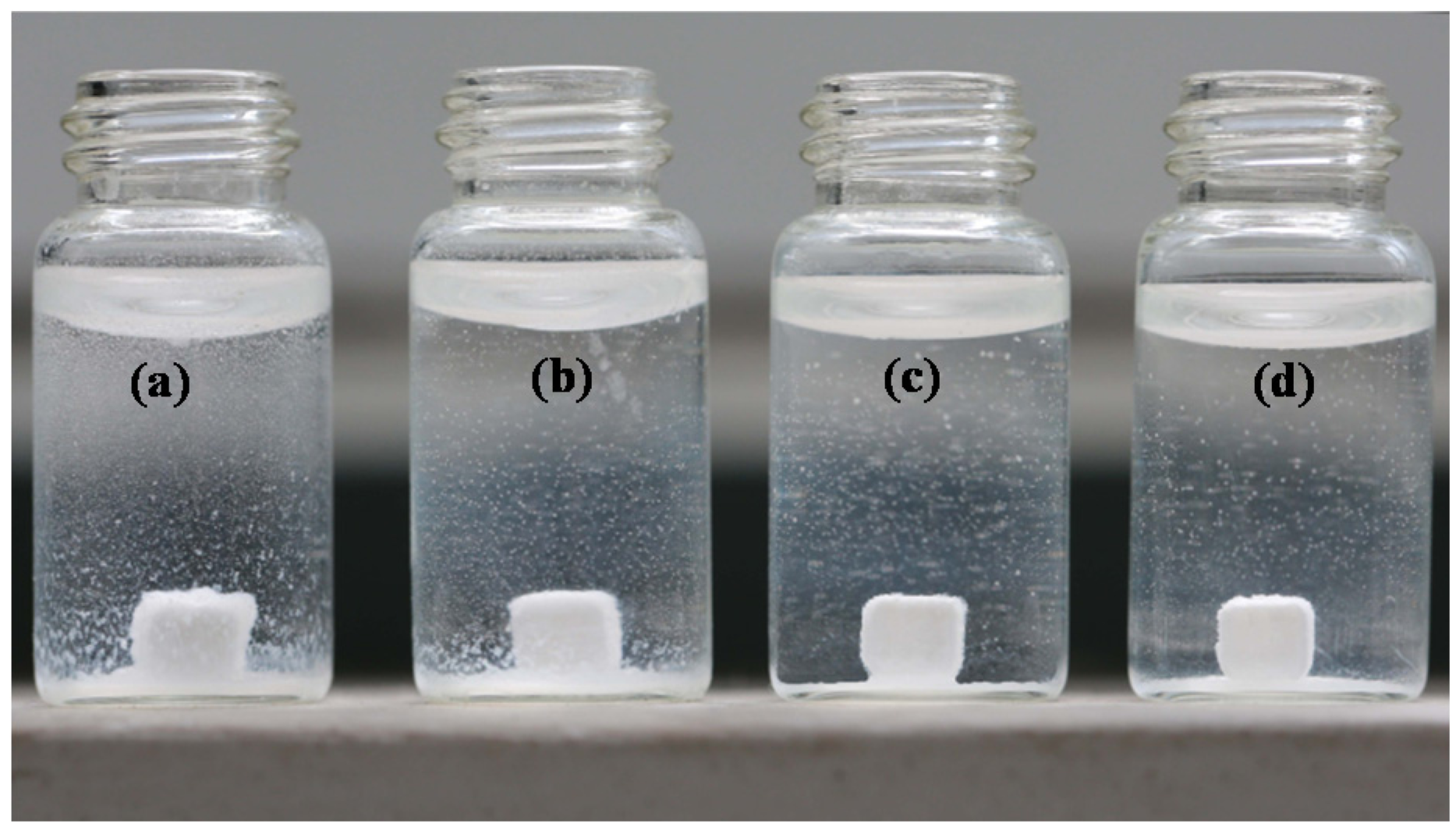
2.4. Bioactivity
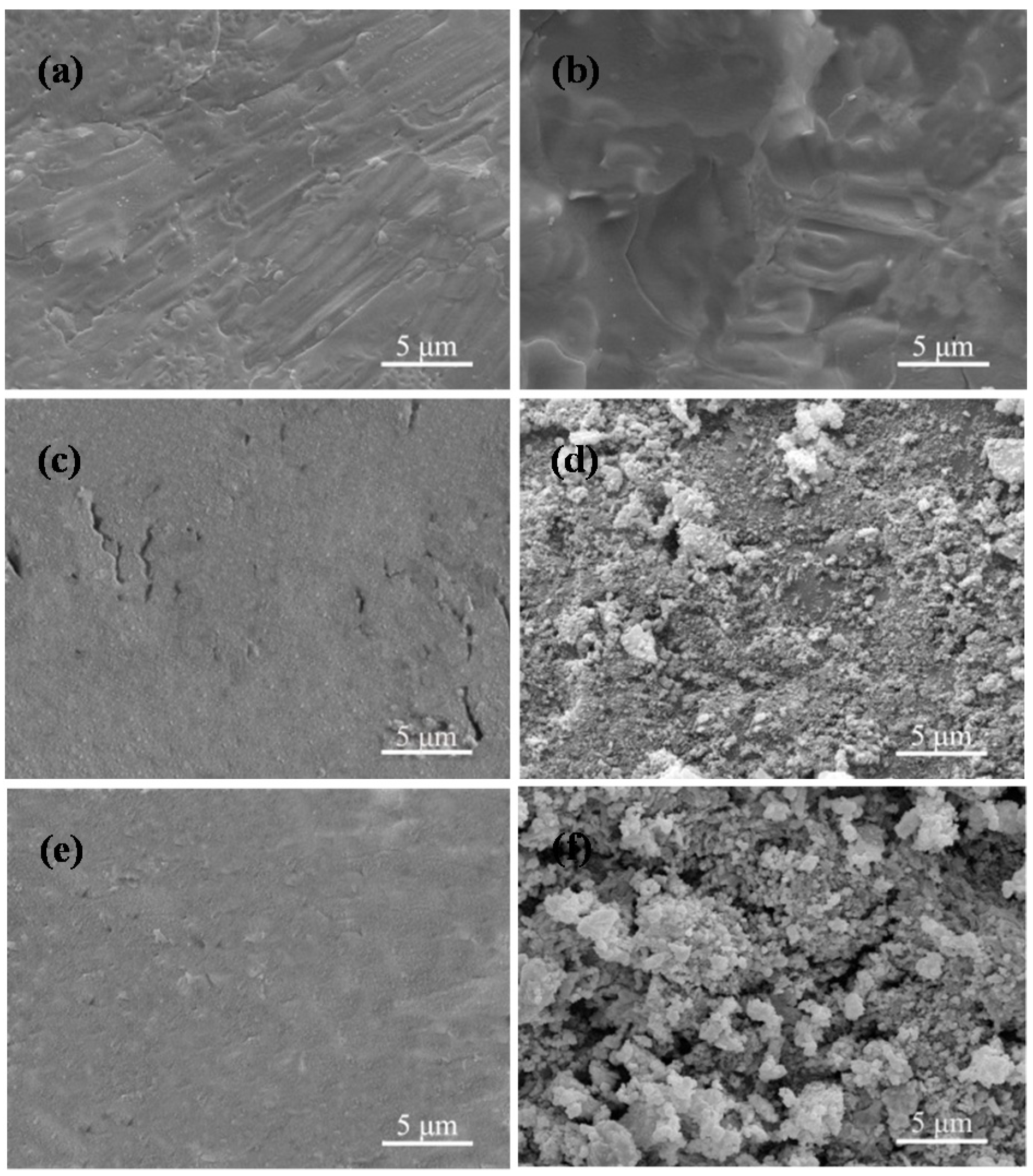
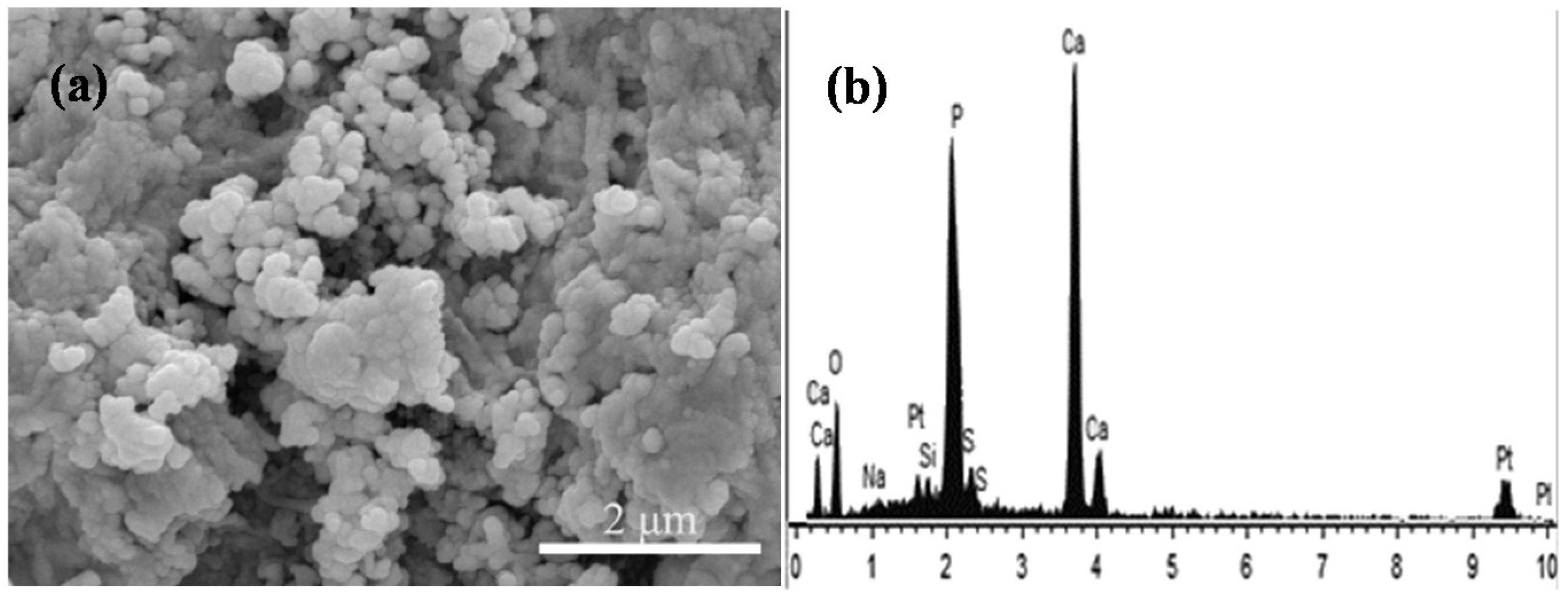
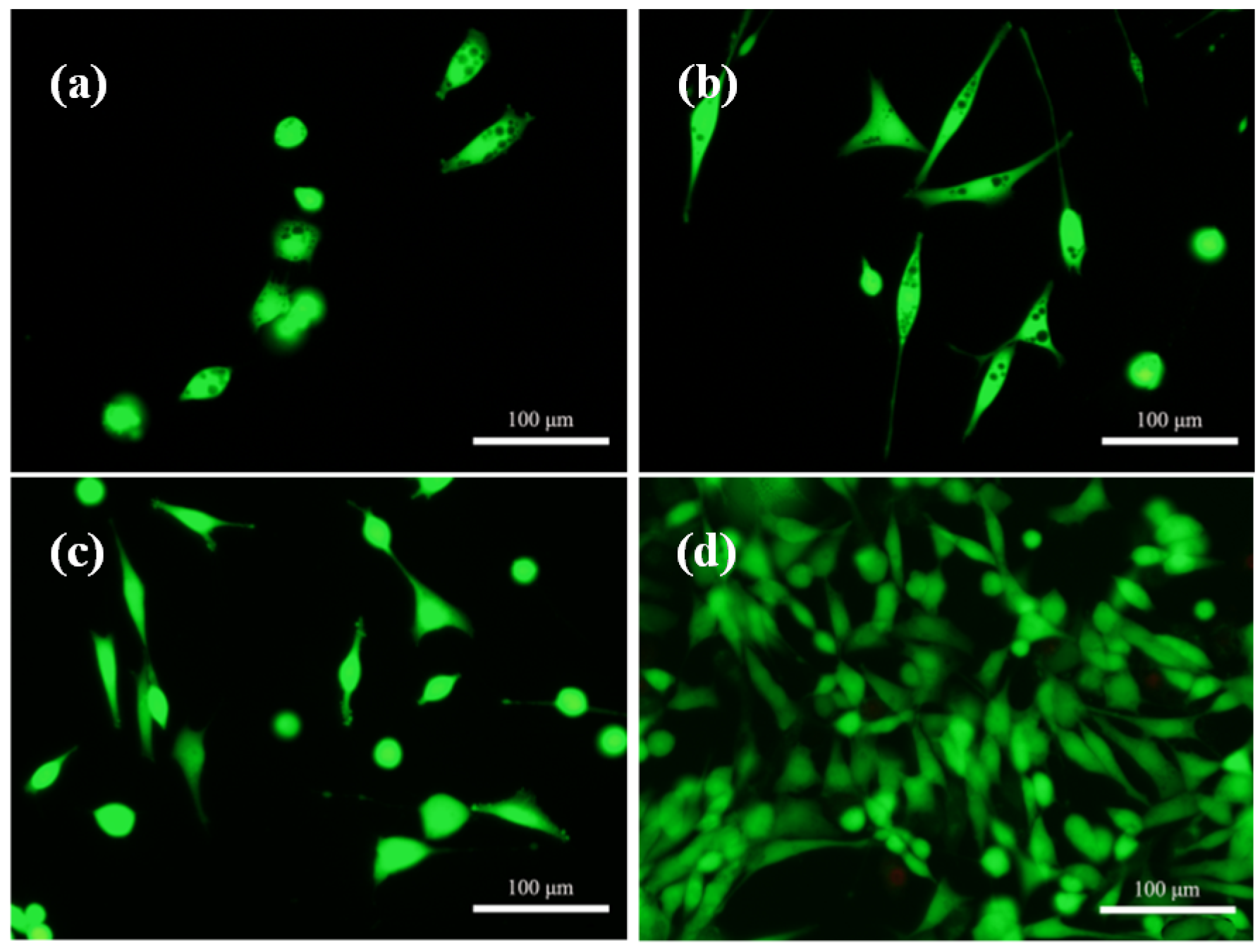
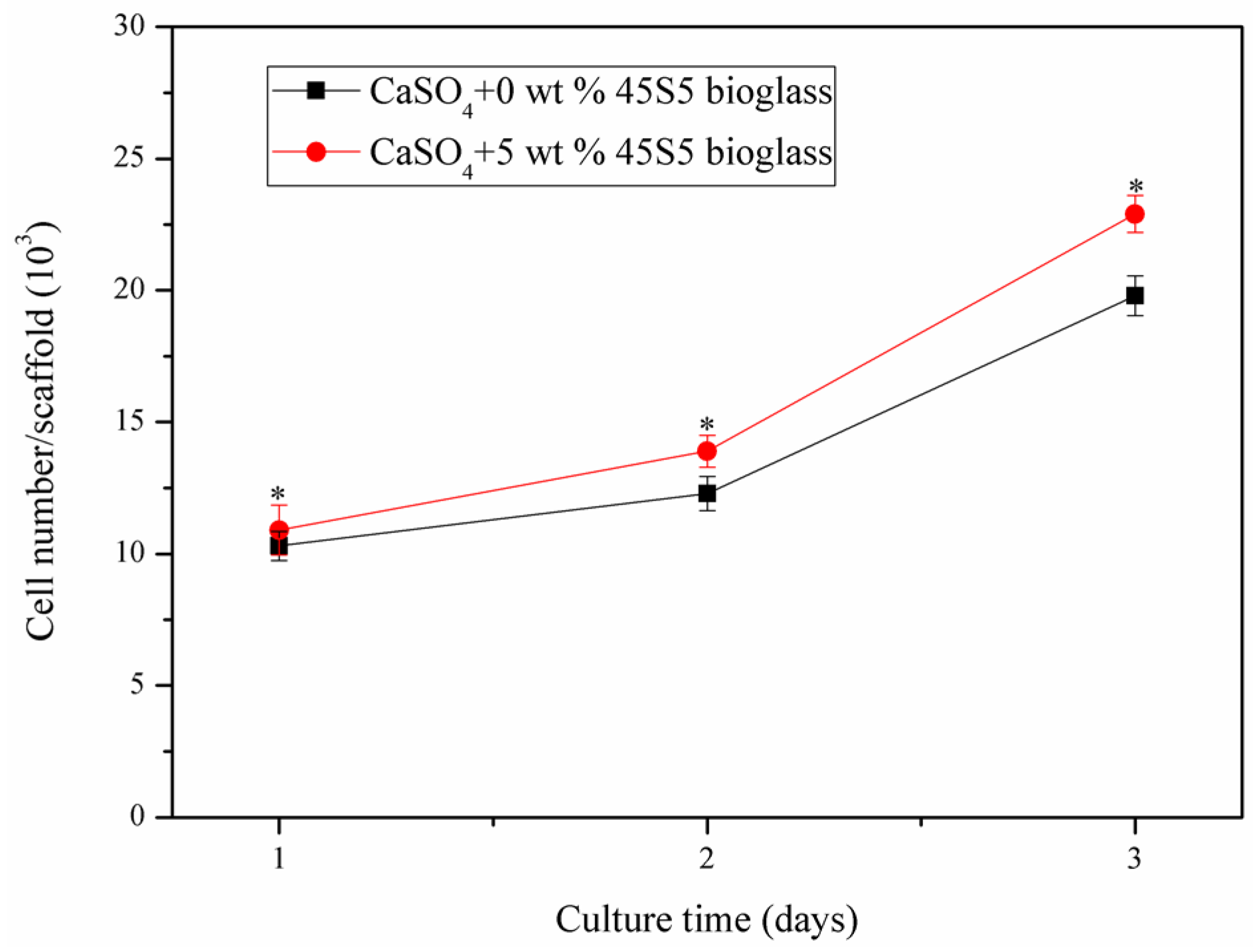
2.5. Cell Attachment and Proliferation

3. Materials and Methods
3.1. Scaffolds Preparation
3.2. Microstructure
3.3. Mechanical Test
3.4. The Stability and Weight Loss
3.5. In Vitro Bioactivity
3.6. Cell Attachment and Proliferation
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Benders, K.E.M.; Weeren, P.R.; Badylak, S.F.; Saris, D.B.F.; Dhert, W.J.A.; Malda, J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends. Biotechnol. 2013, 31, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lim, J.; Teoh, S.H. Review: Development of clinically relevant scaffolds or vascularised bone tissue engineering. Biotechnol. Adv. 2013, 31, 688–705. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pinto, A.R.; Reis, R.L.; Neves, N.M. Scaffolds based bone tissue engineering: The role of chitosan. Tissue Eng. Part B Rev. 2011, 17, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Vitale-Brovarone, C. Three-dimensional glass-derived scaffolds for bone tissue engineering: Current trends and forecasts for the future. J. Biomed. Mater. Res. A 2011, 97, 514–535. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Deng, Y.; Feng, P.; Mao, Z.; Li, P.; Yang, B.; Deng, J.; Gao, Y.; Shuai, C.; Peng, S. Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int. J. Mol. Sci. 2014, 15, 4714–4732. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; You, C.; Kim, K.H. Combined effect of a microporous layer and type I collagen coating on a biphasic calcium phosphate scaffold for bone tissue engineering. Materials 2015, 8, 1150–1161. [Google Scholar] [CrossRef]
- Moussa, M.; Carrel, J.P.; Scherrer, S.; Cattani-Lorente, M.; Wiskott, A.; Durual, S. Medium-term function of a 3D printed TCP/HA structure as a new osteoconductive scaffold for vertical bone augmentation: A simulation by BMP-2 activation. Materials 2015, 8, 2174–2190. [Google Scholar] [CrossRef]
- Rodriguez, L.C.; Chari, J.; Aghyarian, S.; Gindri, I.M.; Kosmopoulos, V.; Rodrigues, D.C. Preparation and characterization of injectable brushite filled-poly(methyl methacrylate) bone cement. Materials 2014, 7, 6779–6795. [Google Scholar] [CrossRef]
- Toloue, S.M.; Chesnoiu-Matei, I.; Blanchard, S.B. A clinical and histomorphometric study of calcium sulfate compared with freeze-dried bone allograft for alveolar ridge preservation. J. Periodontol. 2012, 83, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Kutkut, A.; Andreana, S.; Kim, H.; Monaco, J.E. Extraction socket preservation graft before implant placement with calcium sulfate hemihydrate and platelet-rich plasma: A clinical and histomorphometric study in humans. J. Periodontol. 2012, 83, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, X.; Zhang, L.; Ai, H.; Cui, F. Improvement on the performance of bone regeneration of calcium sulfate hemihydrate by adding mineralized collagen. Tissue Eng. Part A 2010, 16, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Chen, Z.; Liu, X.; Liu, H.; Lian, X.; Wang, X.; Cui, F. Mechanical properties and in vitro bioactivity of injectable and self-setting calcium sulfate/nano-HA/collagen bone graft substitute. J. Mech. Behav. Biomed. 2012, 12, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.C.; Hung, S.H.; Chen, W.L.; Chen, C.K.; Lin, J.L.; Ju, C.P. Properties of TTCP/DCPA/CSH cement immersed in Hanks’ solution. J. Med. Biol. Eng. 2012, 32, 201–204. [Google Scholar] [CrossRef]
- Erdemli, O.; Captug, O.; Bilgili, H.; Orhan, D.; Tezcaner, A.; Keskin, D. In vitro and in vivo evaluation of the effects of demineralized bone matrix or calcium sulfate addition to polycaprolactone-bioglass composites. J. Mater. Sci. Mater. Med. 2010, 21, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, H.; Liu, X.; Cui, F. Injectable calcium sulfate/mineralized collagen-based bone repair materials with regulable self–setting properties. J. Biomed. Mater. Res. A 2011, 99, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; So-Ra, S.; Lee, B.T. Fabrication and characterization of ZrO2–CaO–P2O5–Na2O–SiO2 bioactive glass ceramics. J. Mater. Sci. 2013, 48, 1863–1872. [Google Scholar] [CrossRef]
- Malavasi, G.; Pedone, A.; Menziani, M.C. Study of the structural role of gallium and aluminum in 45S5 bioactive glasses by molecular dynamics simulations. J. Phys. Chem. B 2013, 117, 4142–4150. [Google Scholar] [CrossRef] [PubMed]
- Schickle, K.; Zurlinden, K.; Bergmann, C.; Lindner, M.; Kirsten, A.; Laub, M.; Telle, R.; Jennissen, H.; Fischer, H. Synthesis of novel tricalcium phosphate-bioactive glass composite and functionalization with rhBMP-2. J. Mater. Sci. Mater. Med. 2011, 22, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Majhi, M.R.; Pyare, R.; Singh, S.P. Studies on preparation and characterizations of CaO–Na2O–SiO2–P2O5 bioglass ceramics substituted with Al2O3, TiO2 and ZrO2. J. Biomater. Tissue Eng. 2012, 2, 154–169. [Google Scholar] [CrossRef]
- Liu, J.; Hu, H.; Li, P.; Shuai, C.; Peng, S. Fabrication and characterization of porous 45S5 glass scaffolds via direct selective laser sintering. Mater. Manuf. Process. 2013, 28, 610–615. [Google Scholar] [CrossRef]
- Li, Y.; Cai, S.; Xu, G.; Shen, S.; Zhang, M.; Zhang, T.; Sun, X. Synthesis and characterization of a phytic acid/mesoporous 45S5 bioglass composite coating on a magnesium alloy and degradation behavior. RSC Adv. 2015, 5, 25708–25716. [Google Scholar] [CrossRef]
- Labbaf, S.; Tsigkou, O.; Müller, K.H.; Stevens, M.M.; Porter, A.E.; Jones, J.R. Spherical bioactive glass particles and their interaction with human mesenchymal stem cells in vitro. Biomaterials 2011, 32, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.P.; Tsung, Y.C.; Hsu, H.C.; Tuan, W.H.; Lai, P.L. Addition of a small amount of glass to improve the degradation behavior of calcium sulfate bioceramic. Ceram. Int. 2015, 41, 1155–1162. [Google Scholar] [CrossRef]
- Kuo, S.T.; Wu, H.W.; Tuan, W.H. Resorbable calcium sulfates with tunable degradation rate. J. Asian Ceram. Soc. 2013, 1, 102–107. [Google Scholar] [CrossRef]
- Wang, C.W.; Chiang, T.Y.; Chang, H.C.; Ding, S.J. Physicochemical properties and osteogenic activity of radiopaque calcium silicate-gelatin cements. J. Mater. Sci. Mater. Med. 2014, 25, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Cabanas, M.V.; Rodriguez-Lorenzo, L.M.; Vallet-Regi, M. Setting behavior and in vitro bioactivity of hydroxyapatite/calcium sulfate cements. Chem. Mater. 2002, 14, 3550–3555. [Google Scholar] [CrossRef]
- Orsini, G.; Ricci, J.; Scarano, A.; Pecora, G.; Petrone, G.; Iezzi, G.; Piattelli, A. Bone-defect healing with calcium-sulfate particles and cement: An experimental study in rabbit. J. Biomed. Mater. Res. B 2004, 68, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Huan, Z.; Chang, J. Self-setting properties and in vitro bioactivity of calcium sulfate hemihydrate-tricalcium silicate composite bone cements. Acta Biomater. 2007, 3, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Hesaraki, S.; Hasan Barounian, M.; Farhangdoust, S.; Khorami, M.; Zamanian, A.; Borhan, S. Mechanical and in vitro biological properties of hydroxyapatite bioceramics reinforced with strontium-containing nano-bioactive glass. Curr. Nanosci. 2012, 8, 612–622. [Google Scholar] [CrossRef]
- Veljović, D.; Zalite, I.; Palcevskis, E.; Smiciklas, I.; Petrović, R.; Janaćković, D. Microwave sintering of fine grained HAP and HAP/TCP bioceramics. Ceram. Int. 2010, 36, 595–603. [Google Scholar] [CrossRef]
- Borhan, S.; Hesaraki, S.; Ahmadzadeh-Asl, S. Evaluation of colloidal silica suspension as efficient additive for improving physicochemical and in vitro biological properties of calcium sulfate-based nanocomposite bone cement. J. Mater. Sci. Mater. Med. 2010, 21, 3171–3181. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuai, C.; Zhou, J.; Wu, P.; Gao, C.; Feng, P.; Xiao, T.; Deng, Y.; Peng, S. Enhanced Stability of Calcium Sulfate Scaffolds with 45S5 Bioglass for Bone Repair. Materials 2015, 8, 7498-7510. https://doi.org/10.3390/ma8115398
Shuai C, Zhou J, Wu P, Gao C, Feng P, Xiao T, Deng Y, Peng S. Enhanced Stability of Calcium Sulfate Scaffolds with 45S5 Bioglass for Bone Repair. Materials. 2015; 8(11):7498-7510. https://doi.org/10.3390/ma8115398
Chicago/Turabian StyleShuai, Cijun, Jianhua Zhou, Ping Wu, Chengde Gao, Pei Feng, Tao Xiao, Youwen Deng, and Shuping Peng. 2015. "Enhanced Stability of Calcium Sulfate Scaffolds with 45S5 Bioglass for Bone Repair" Materials 8, no. 11: 7498-7510. https://doi.org/10.3390/ma8115398
APA StyleShuai, C., Zhou, J., Wu, P., Gao, C., Feng, P., Xiao, T., Deng, Y., & Peng, S. (2015). Enhanced Stability of Calcium Sulfate Scaffolds with 45S5 Bioglass for Bone Repair. Materials, 8(11), 7498-7510. https://doi.org/10.3390/ma8115398







