Preparation, Characterization and Evaluation of α-Tocopherol Succinate-Modified Dextran Micelles as Potential Drug Carriers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Dex-TOS Conjugate
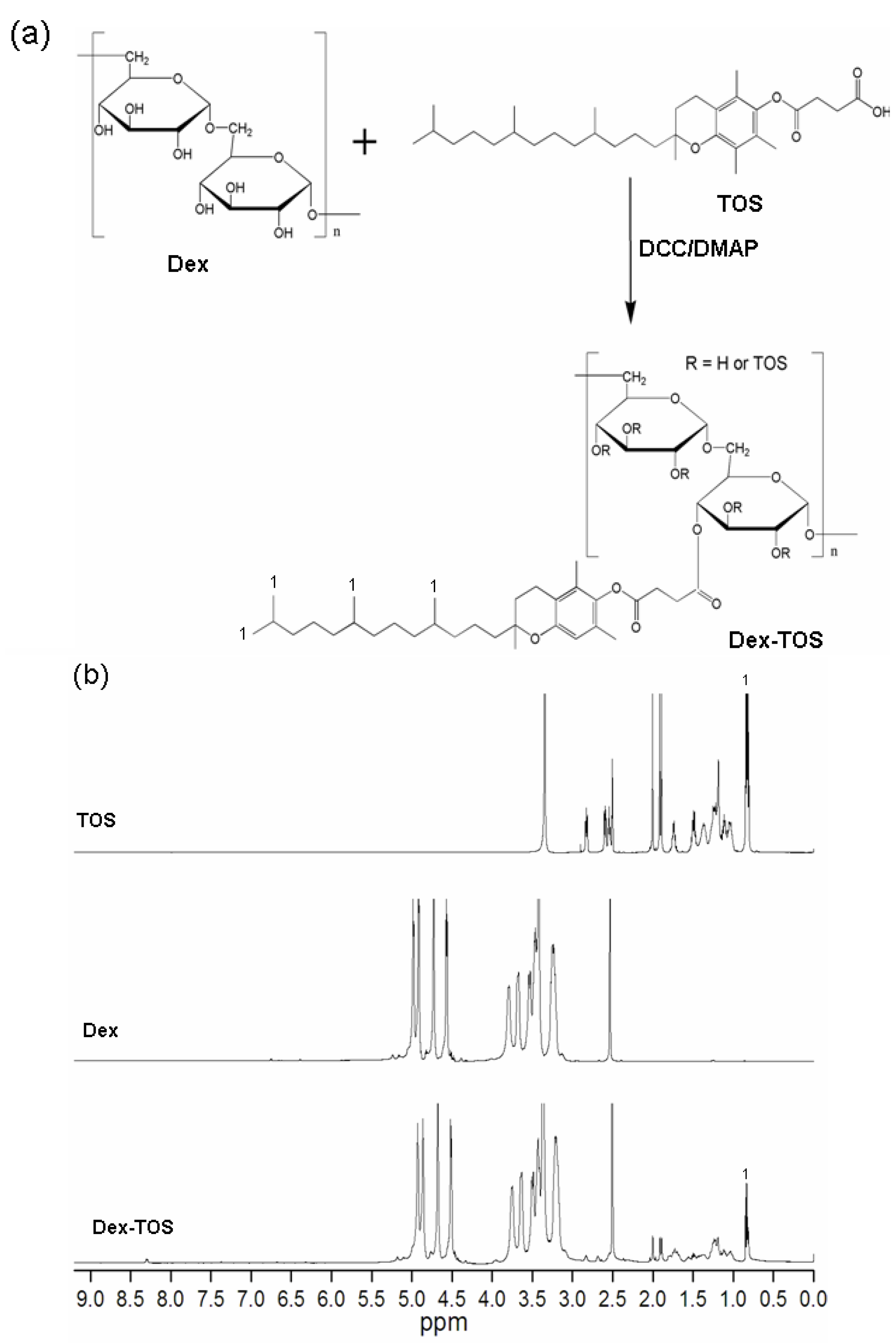
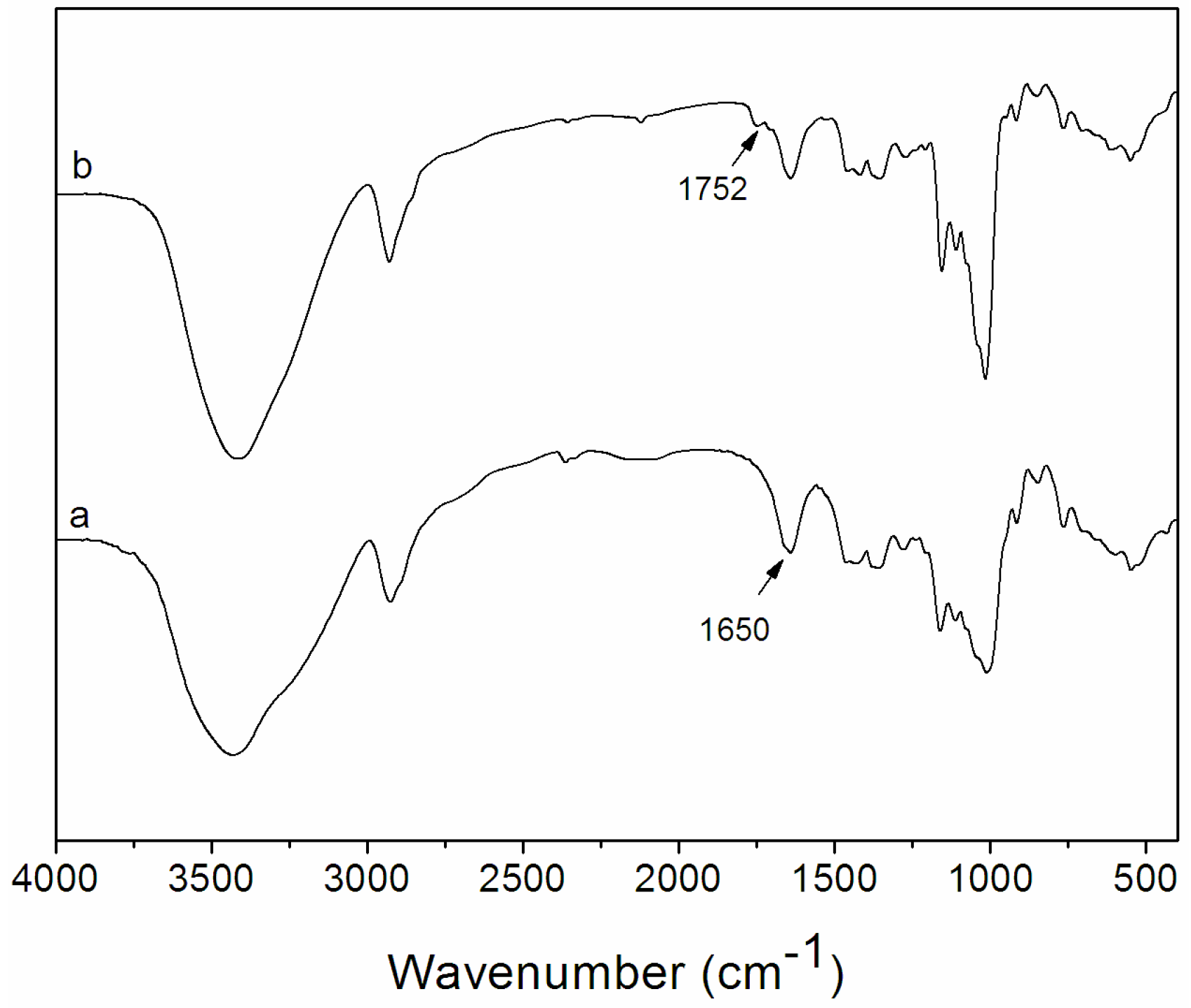
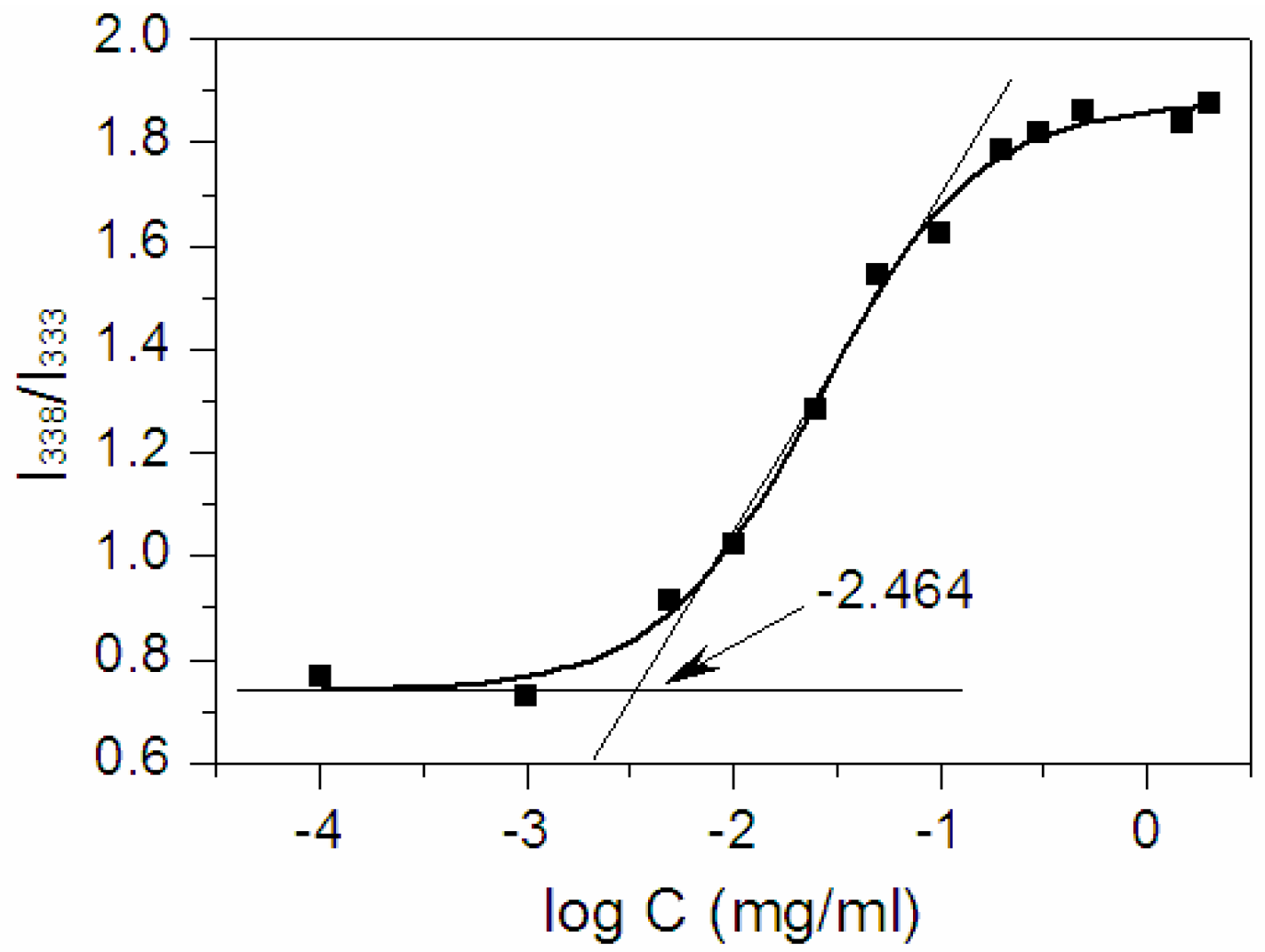
2.2. Determination of Critical Micelle Concentration (CMC)
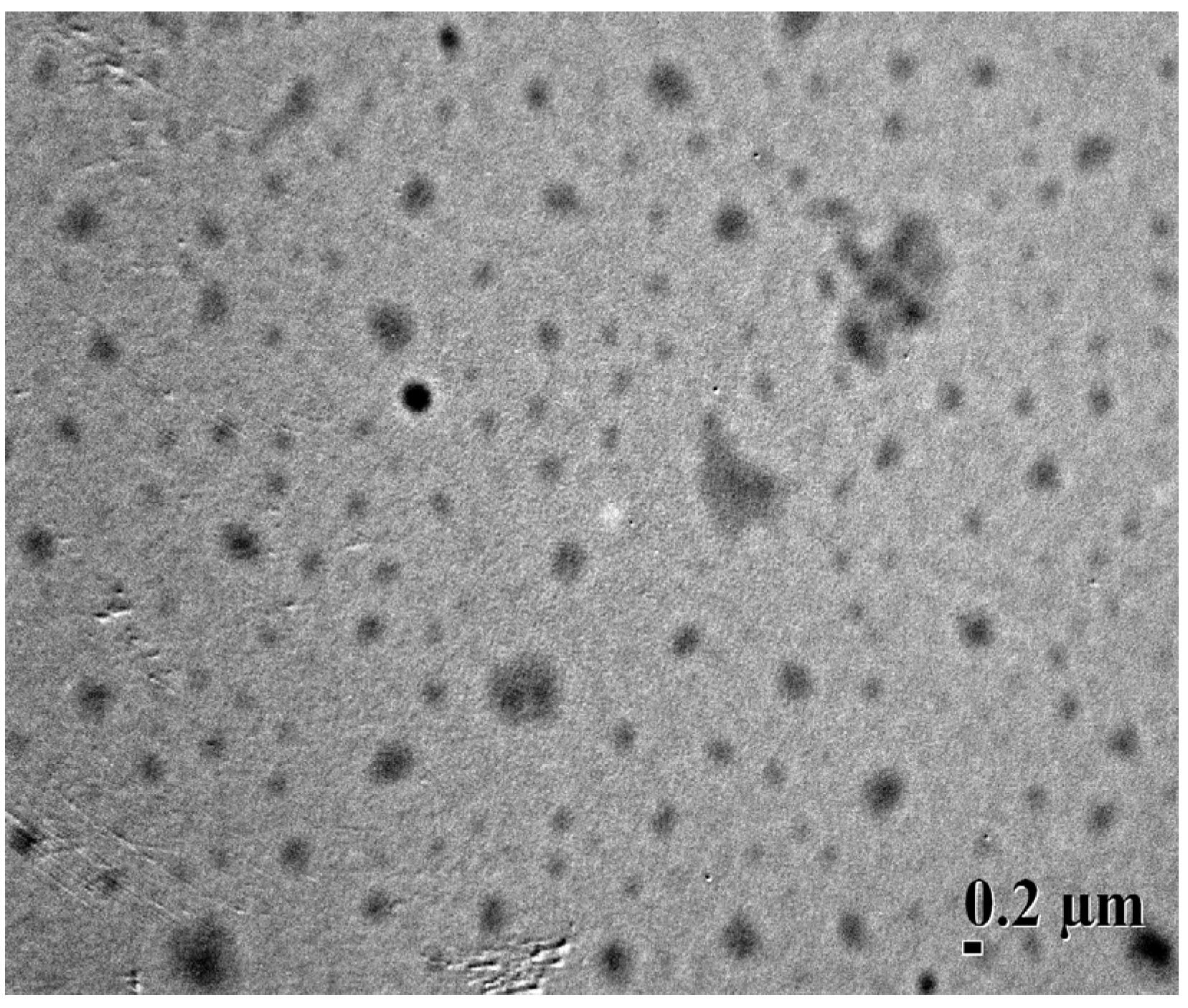
2.3. Characterization of Blank and Dex-TOS/Dox Micelles
| Sample | Drug/Carrier a | LC (%) b | EE (%) c | Size (nm) d | PI e |
|---|---|---|---|---|---|
| Dex-TOS-1/Dox | 0.5/10 | 4.21 | 87.9 | 295 ± 12.3 | 0.223 ± 0.021 |
| Dex-TOS-2/Dox | 1/10 | 6.57 | 70.3 | 310 ± 21.4 | 0.126 ± 0.013 |
| Dex-TOS-3/Dox | 1.5/10 | 8.12 | 58.9 | 325 ± 25.6 | 0.128 ± 0.018 |
2.4. Differential Scanning Calorimetry (DSC)
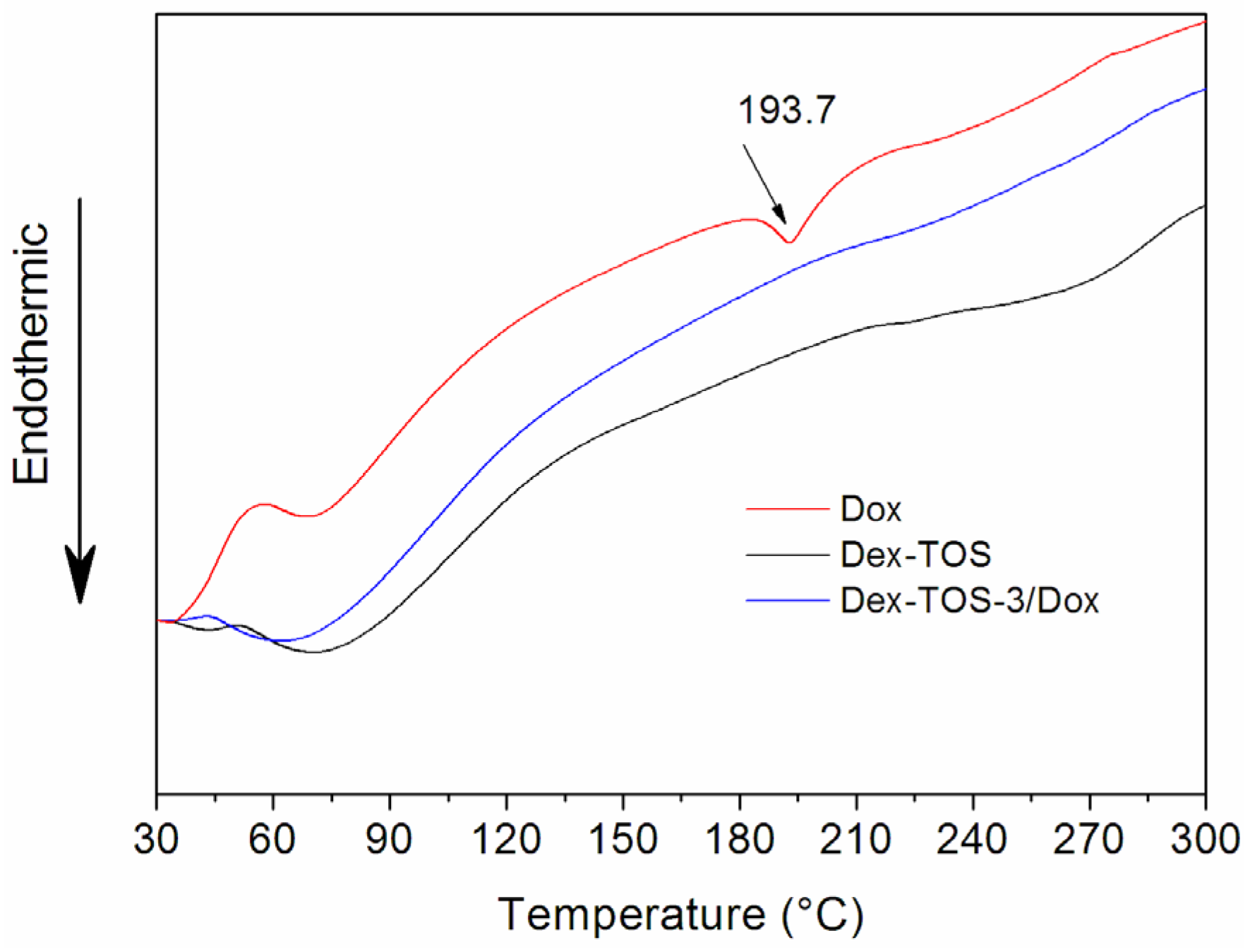
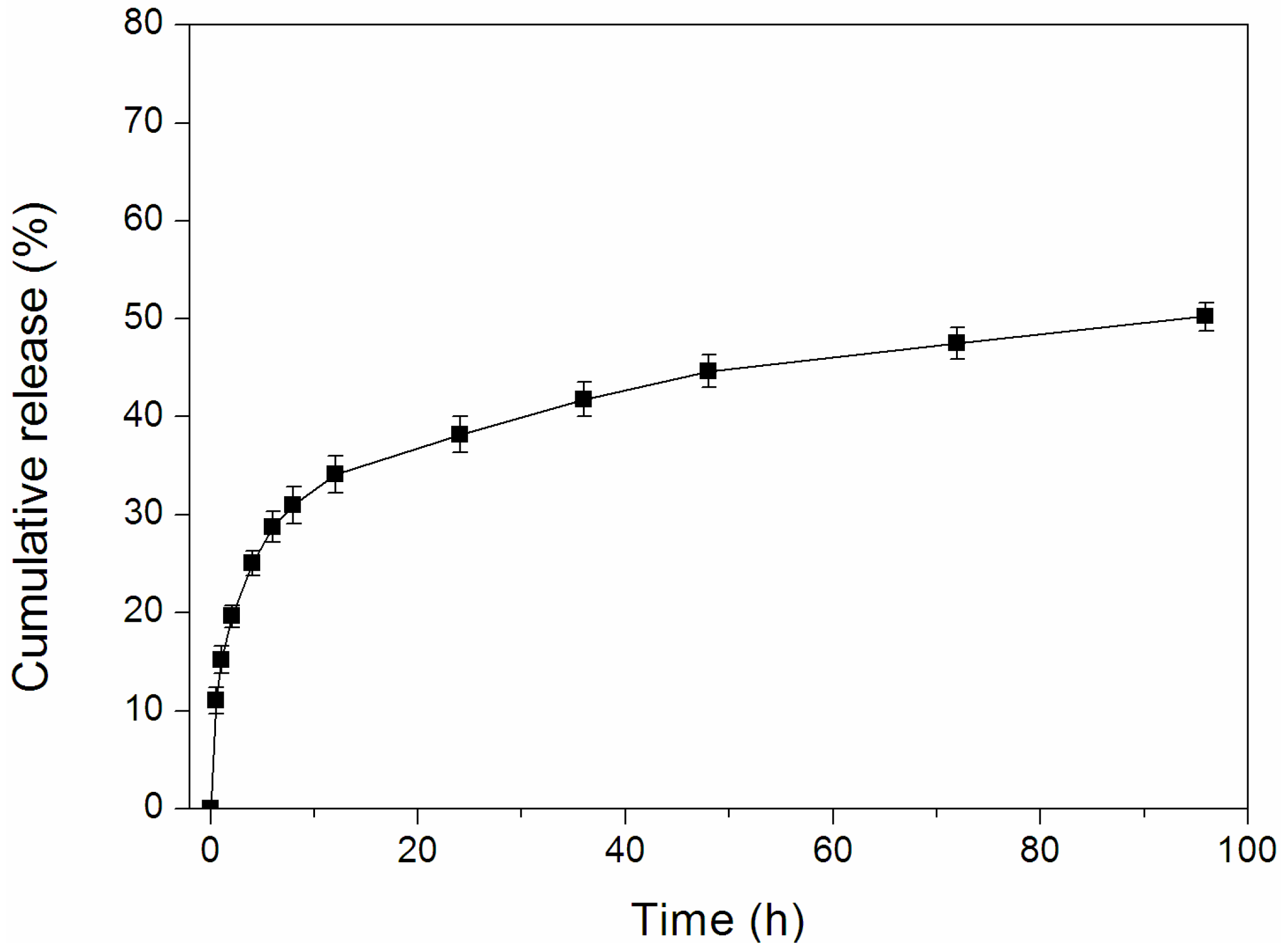
2.5. In Vitro Release of Dox-Loaded Micelles
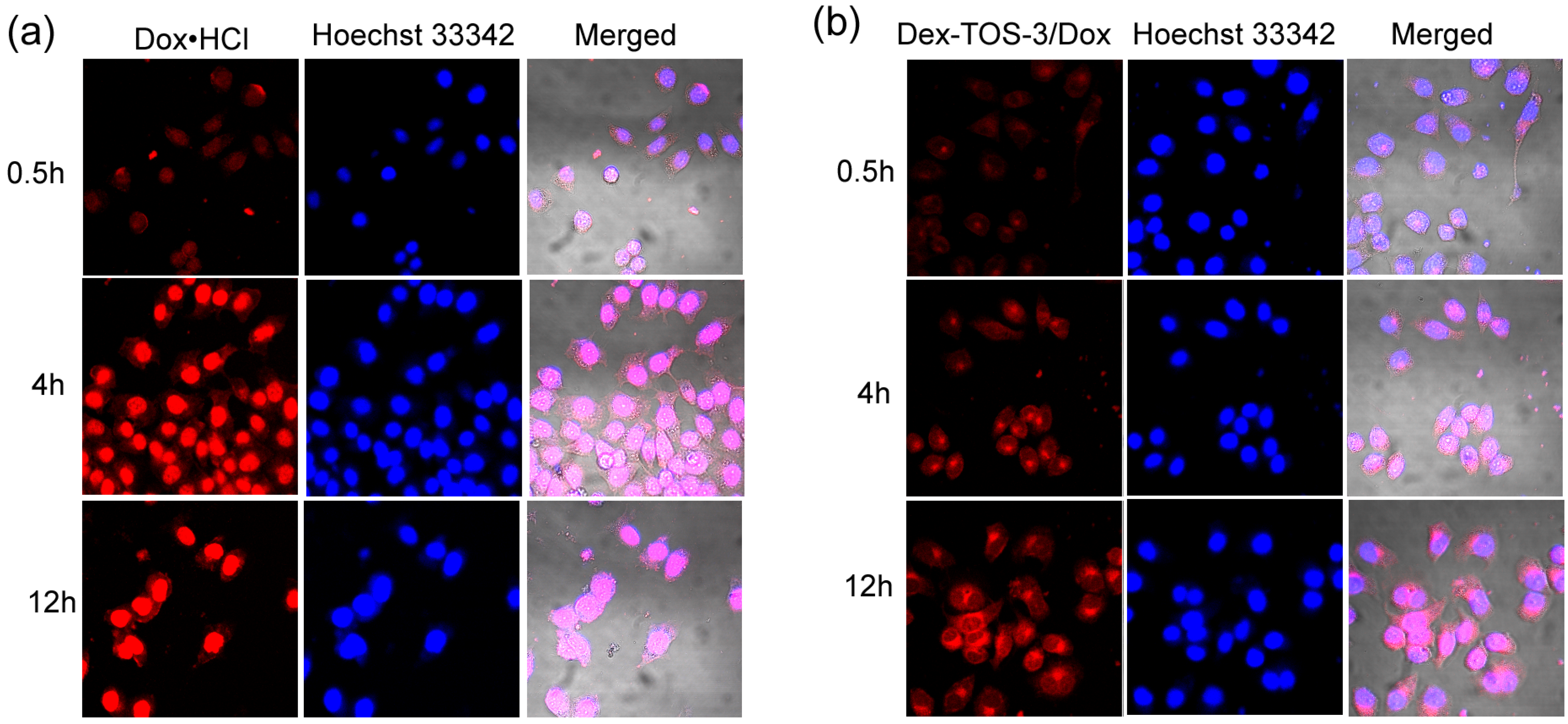
2.6. Confocal Laser Scanning Microscopy (CLSM) Observation
2.7. In Vitro Cytotoxicity Study

3. Experimental Section
3.1. Materials
3.2. Synthesis of Dex-TOS Conjugate
3.3. Determination of Critical Micelle Concentration (CMC)
3.4. Preparation of Dox-loaded Micelles
3.5. Characterization of Blank and Dex-TOS/Dox Micelles
3.6. DSC Analysis
3.7. In Vitro Release of Dox-loaded Micelles
3.8. Confocal Laser Scanning Microscopy (CLSM) Observation
3.9. In Vitro Cytotoxicity Study
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Y.L.; Zhu, L.; Liu, Z.; Cheng, R.; Meng, F.; Cui, J.H.; Ji, S.J.; Zhong, Z. Reversibly stabilized multifunctional dextran nanoparticles efficiently deliver doxorubicin into the nuclei of cancer cells. Angew. Chem. Int. Ed. 2009, 48, 9914–9918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, Z.; Shi, F.; Xiao, C.; Ding, J.; Zhuang, X.; He, C.; Chen, L.; Chen, X. Redox-sensitive shell-crosslinked polypeptide-block-polysaccharide micelles for efficient intracellular anticancer drug delivery. Macromol. Biosci. 2013, 13, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, S.; Liu, Z.; Dong, C.; Yang, J.; Gong, X.; Chang, J. Upconverting crystal/dextran-g-DOPE with high fluorescence stability for simultaneous photodynamic therapy and cell imaging. Nanotechnology 2014, 25. [Google Scholar] [CrossRef] [PubMed]
- Aumelas, A.; Serrero, A.; Durand, A.; Dellacherie, E.; Leonard, M. Nanoparticles of hydrophobically modified dextrans as potential drug carrier systems. Colloid. Surface. B 2007, 59, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; You, D.G.; Han, H.S.; Deepagan, V.G.; Jeon, S.M.; Suh, Y.D.; Choi, K.Y.; Kim, K.; Kwon, I.C.; Yi, G.-R.; et al. Bioreducible carboxymethyl dextran nanoparticles for tumor-targeted drug delivery. Adv. Health. Mater. 2014, 3, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.Z.; Weng, Q.; Yuan, H.; Hu, F.Q. Synthesis and antitumor activity of stearate-g-dextran micelles for intracellular doxorubicin delivery. ACS Nano 2010, 4, 6894–6902. [Google Scholar] [CrossRef] [PubMed]
- Mochida, Y.; Cabral, H.; Miura, Y.; Albertini, F.; Fukushima, S.; Osada, K.; Nishiyama, N.; Kataoka, K. Bundled assembly of helical nanostructures in polymeric micelles loaded with platinum drugs enhancing therapeutic efficiency against pancreatic tumor. ACS Nano 2014, 8, 6724–6738. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Su, Z.; Yao, Y.; Zhang, N. Preparation of pH sensitive Pluronic-docetaxel conjugate micelles to balance the stability and controlled release issues. Materials 2015, 8, 379–391. [Google Scholar] [CrossRef]
- Yu, J.; Xie, X.; Wu, J.; Liu, Y.; Liu, P.; Xu, X.; Yu, H.; Lu, L.; Che, X. Folic acid conjugated glycol chitosan micelles for targeted delivery of doxorubicin: preparation and preliminary evaluation in vitro. J. Biomater. Sci. Polym. Ed. 2013, 24, 606–620. [Google Scholar] [CrossRef]
- Biswas, S.; Torchilin, V.P. Nanopreparations for organelle-specific delivery in cancer. Adv. Drug. Deliver. Rev. 2014, 66, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Palao-Suay, R.; Aguilar, M.R.; Parra-Ruiz, F.J.; Fernandez-Gutierrez, M.; Parra, J.; Sanchez-Rodriguez, C.; Sanz-Fernandez, R.; Rodriganez, L.; San Roman, J. Anticancer and antiangiogenic activity of surfactant-free nanoparticles based on self-assembled polymeric derivatives of vitamin E: Structure-activity relationship. Biomacromolecules 2015, 16, 1566–1581. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.W.; Yoon, S.M.; Sonn, C.H.; Lee, K.M.; Kim, Y.H. Nano self-assembly of recombinant human gelatin conjugated with alpha-tocopheryl succinate for Hsp90 inhibitor, 17-AAG, delivery. ACS Nano 2011, 5, 3839–3848. [Google Scholar] [CrossRef] [PubMed]
- Duhem, N.; Danhier, F.; Preat, V. Vitamin E-based nanomedicines for anti-cancer drug delivery. J. Control. Release 2014, 182, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Kouhe, T.T.B.; Duhem, N.; Ucakar, B.; Staub, A.; Draoui, N.; Feron, O.; Preat, V. Vitamin E-based micelles enhance the anticancer activity of doxorubicin. Int. J. Pharm. 2014, 476, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Duhem, N.; Rolland, J.; Riva, R.; Guillet, P.; Schumers, J.M.; Jerome, C.; Gohy, J.F.; Preat, V. Tocol modified glycol chitosan for the oral delivery of poorly soluble drugs. Int. J. Pharm. 2012, 423, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Mandracchia, D.; Tripodo, G.; Latrofa, A.; Dorati, R. Amphiphilic inulin-d-α-tocopherol succinate (INVITE) bioconjugates for biomedical applications. Carbohyd. Polym. 2014, 103, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Tripodo, G.; Pasut, G.; Trapani, A.; Mero, A.; Lasorsa, F.M.; Chlapanidas, T.; Trapani, G.; Mandracchia, D. Inulin-d-α-tocopherol succinate (INVITE) nanomicelles as a platform for effective intravenous administration of curcumin. Biomacromolecules 2015, 16, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Wang, A.T.; Yang, Z.Z.; Liu, Y.J.; Qi, X.R. Enhance cancer cell recognition and overcome drug resistance using hyaluronic acid and α-tocopheryl succinate based multifunctional nanoparticles. Mol. Pharm. 2015, 12, 2189–2202. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, Z.; Lv, S.; Song, W.; Hong, H.; Jing, X.; Zhang, Y.; Chen, X. Cisplatin crosslinked pH-sensitive nanoparticles for efficient delivery of doxorubicin. Biomaterials 2014, 35, 3851–3864. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.B.; Li, H.; Zhu, X.X.; Woo, H.G. Self-aggregated nanoparticles composed of periodate-oxidized dextran and cholic acid: Preparation, stabilization and in-vitro drug release. J. Chem. Technol. Biot. 2006, 81, 746–754. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, P.X. Polymeric core-shell assemblies mediated by host-guest interactions: Versatile nanocarriers for drug delivery. Angew. Chem. Int. Ed. 2009, 48, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, L.; Mandracchia, D.; Sorrenti, M.; Colombo, L.; Serra, M.; Tripodo, G. In-solution structural considerations by 1H NMR and solid-state thermal properties of inulin-d-α-tocopherol succinate (INVITE) micelles as drug delivery systems for hydrophobic drugs. Macromol. Chem. Phys. 2014, 215, 2084–2096. [Google Scholar] [CrossRef]
- Liang, N.; Sun, S.; Li, X.; Piao, H.; Piao, H.; Cui, F.; Fang, L. α-Tocopherol succinate-modified chitosan as a micellar delivery system for paclitaxel: Preparation, characterization and in vitro/in vivo evaluations. Int. J. Pharm. 2012, 423, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wang, Y.; He, X.; Zhang, Z.; Yin, Q.; Chen, Y.; Yu, H.; Huang, Y.; Chen, L.; Xu, M.; et al. Codelivery of sorafenib and curcumin by directed self-assembled nanoparticles enhances therapeutic effect on hepatocellular carcinoma. Mol. Pharm. 2015, 12, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Fluorescence microscopy to follow the targeting of liposomes and micelles to cells and their intracellular fate. Adv. Drug Deliver. Rev. 2005, 57, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xie, X.; Zheng, M.; Yu, L.; Zhang, L.; Zhao, J.; Jiang, D.; Che, X. Fabrication and characterization of nuclear localization signal-conjugated glycol chitosan micelles for improving the nuclear delivery of doxorubicin. Int. J. Nanomed. 2012, 7, 5079–5090. [Google Scholar] [CrossRef]
- Yu, J.; Xie, X.; Xu, X.; Zhang, L.; Zhou, X.; Yu, H.; Wu, P.; Wang, T.; Che, X.; Hu, Z. Development of dual ligand-targeted polymeric micelles as drug carriers for cancer therapy in vitro and in vivo. J. Mater. Chem. B 2014, 2, 2114–2126. [Google Scholar] [CrossRef]
- Cui, C.; Xue, Y.N.; Wu, M.; Zhang, Y.; Yu, P.; Liu, L.; Zhuo, R.X.; Huang, S.W. Cellular uptake, intracellular trafficking, and antitumor efficacy of doxorubicin-loaded reduction-sensitive micelles. Biomaterials 2013, 34, 3858–3869. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lai, Q.; Wang, W.; Wu, Y.; Zhang, C.; Liu, Y.; Yuan, Z. Functional alginate nanoparticles for efficient intracellular release of doxorubicin and hepatoma carcinoma cell targeting therapy. Int. J. Pharm. 2013, 451, 1–11. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Zhou, Y.; Chen, W.; Ren, J.; Zhang, L.; Lu, L.; Luo, G.; Huang, H. Preparation, Characterization and Evaluation of α-Tocopherol Succinate-Modified Dextran Micelles as Potential Drug Carriers. Materials 2015, 8, 6685-6696. https://doi.org/10.3390/ma8105332
Yu J, Zhou Y, Chen W, Ren J, Zhang L, Lu L, Luo G, Huang H. Preparation, Characterization and Evaluation of α-Tocopherol Succinate-Modified Dextran Micelles as Potential Drug Carriers. Materials. 2015; 8(10):6685-6696. https://doi.org/10.3390/ma8105332
Chicago/Turabian StyleYu, Jingmou, Yufeng Zhou, Wencong Chen, Jin Ren, Lifang Zhang, Lu Lu, Gan Luo, and Hao Huang. 2015. "Preparation, Characterization and Evaluation of α-Tocopherol Succinate-Modified Dextran Micelles as Potential Drug Carriers" Materials 8, no. 10: 6685-6696. https://doi.org/10.3390/ma8105332
APA StyleYu, J., Zhou, Y., Chen, W., Ren, J., Zhang, L., Lu, L., Luo, G., & Huang, H. (2015). Preparation, Characterization and Evaluation of α-Tocopherol Succinate-Modified Dextran Micelles as Potential Drug Carriers. Materials, 8(10), 6685-6696. https://doi.org/10.3390/ma8105332





