Inhibition of Mild Steel Corrosion in Hydrochloric Acid Solution by New Coumarin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
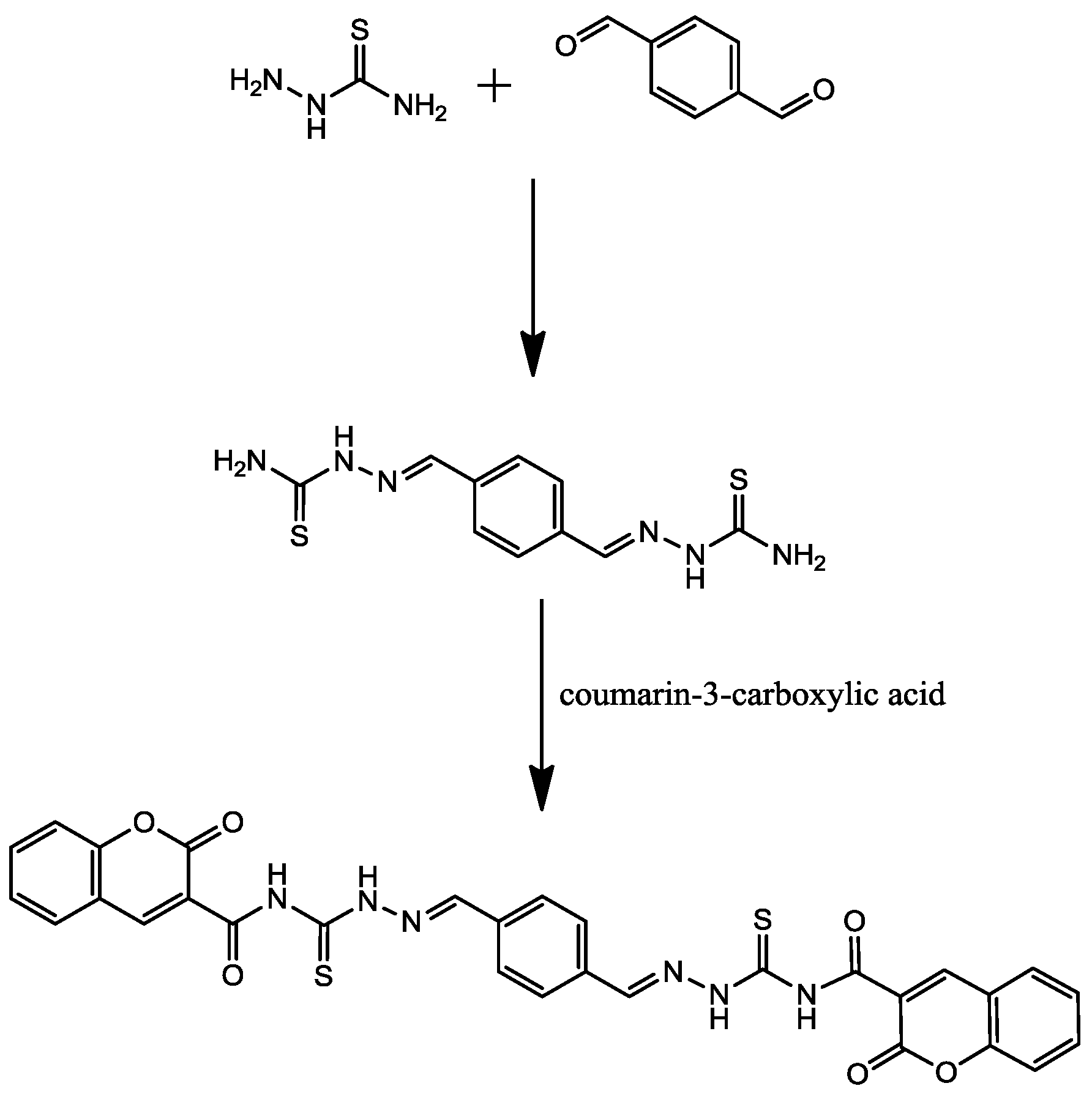
2.2. Electrochemical Measurements
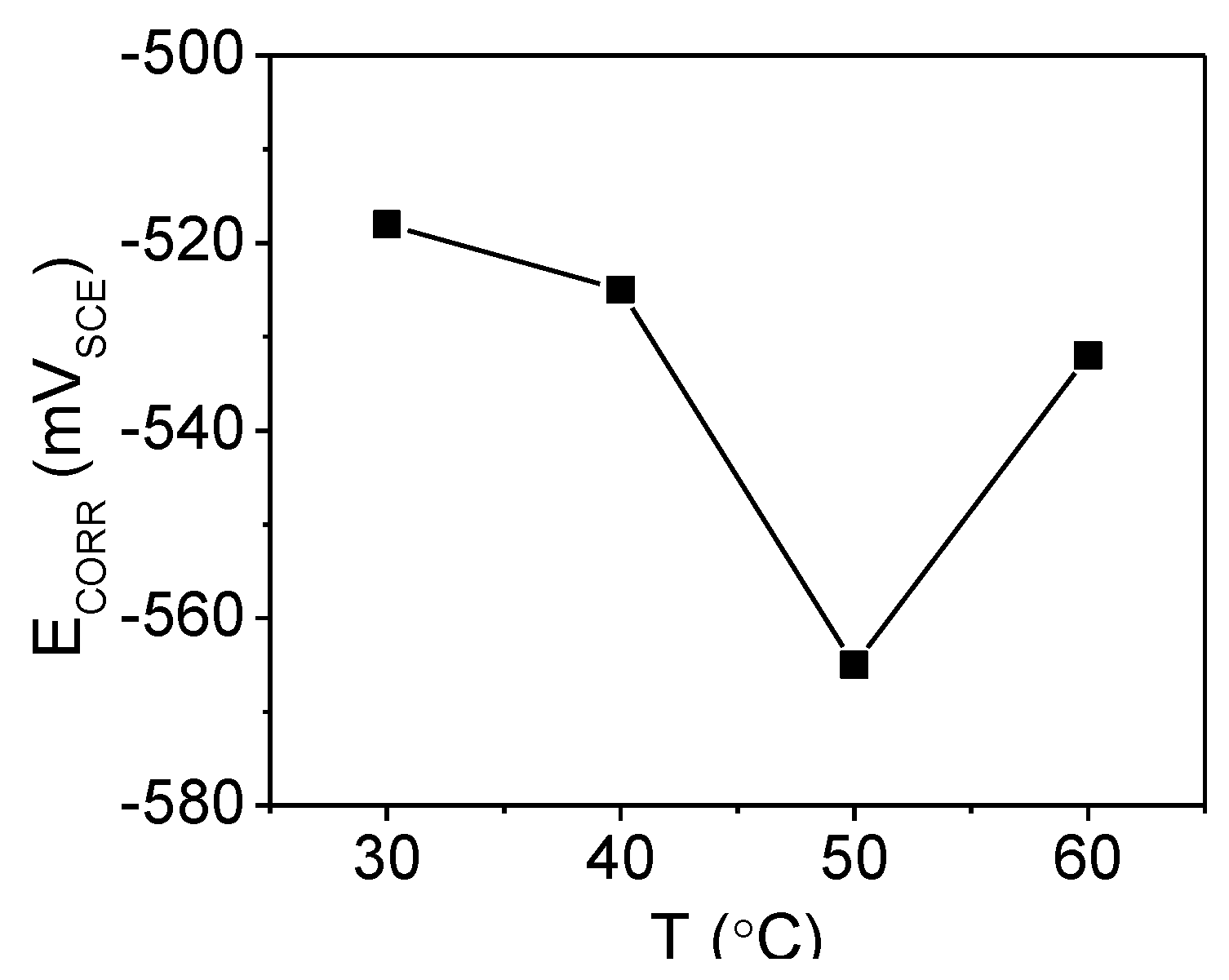
2.2.1. Corrosion Potential (ECORR) Measurements
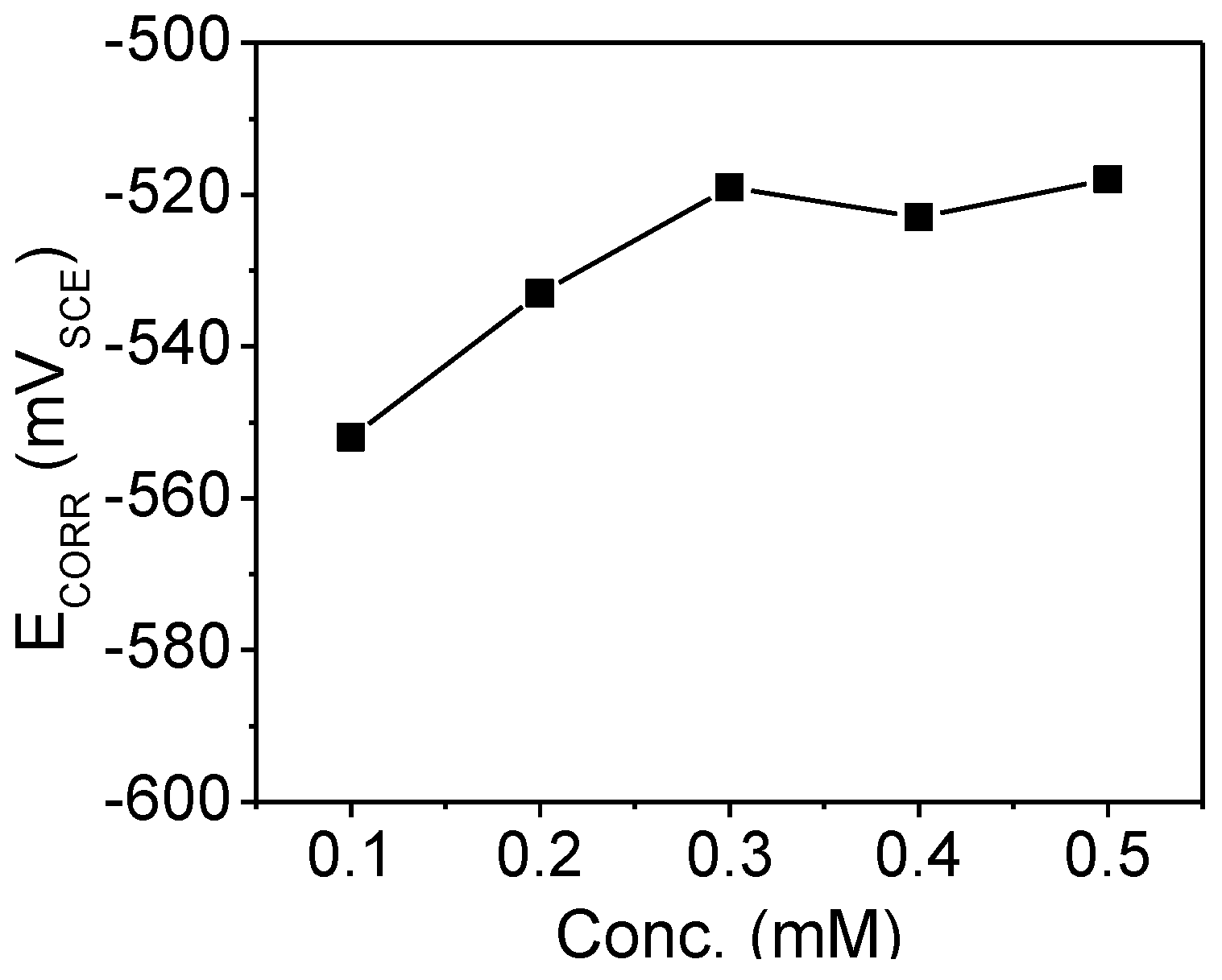
2.2.2. Polarization Measurements

| Conc.(mM) | icorr (μA·cm−2) | ECORR (VSCE) | βa (V·dec−1) | βc (V·dec−1) | IE(%) |
|---|---|---|---|---|---|
| 0 | 660.1 | −0.49 | 0.12 | 0.14 | 0 |
| 0.1 | 19.1 | −0.53 | 0.08 | 0.14 | 97.1 |
| 0.2 | 16.2 | −0.55 | 0.13 | 0.13 | 97.5 |
| 0.3 | 8.76 | −0.52 | 0.06 | 0.09 | 98.6 |
| 0.4 | 7.72 | −0.51 | 0.05 | 0.1 | 98.8 |
| 0.5 | 7.36 | −0.51 | 0.05 | 0.1 | 98.8 |
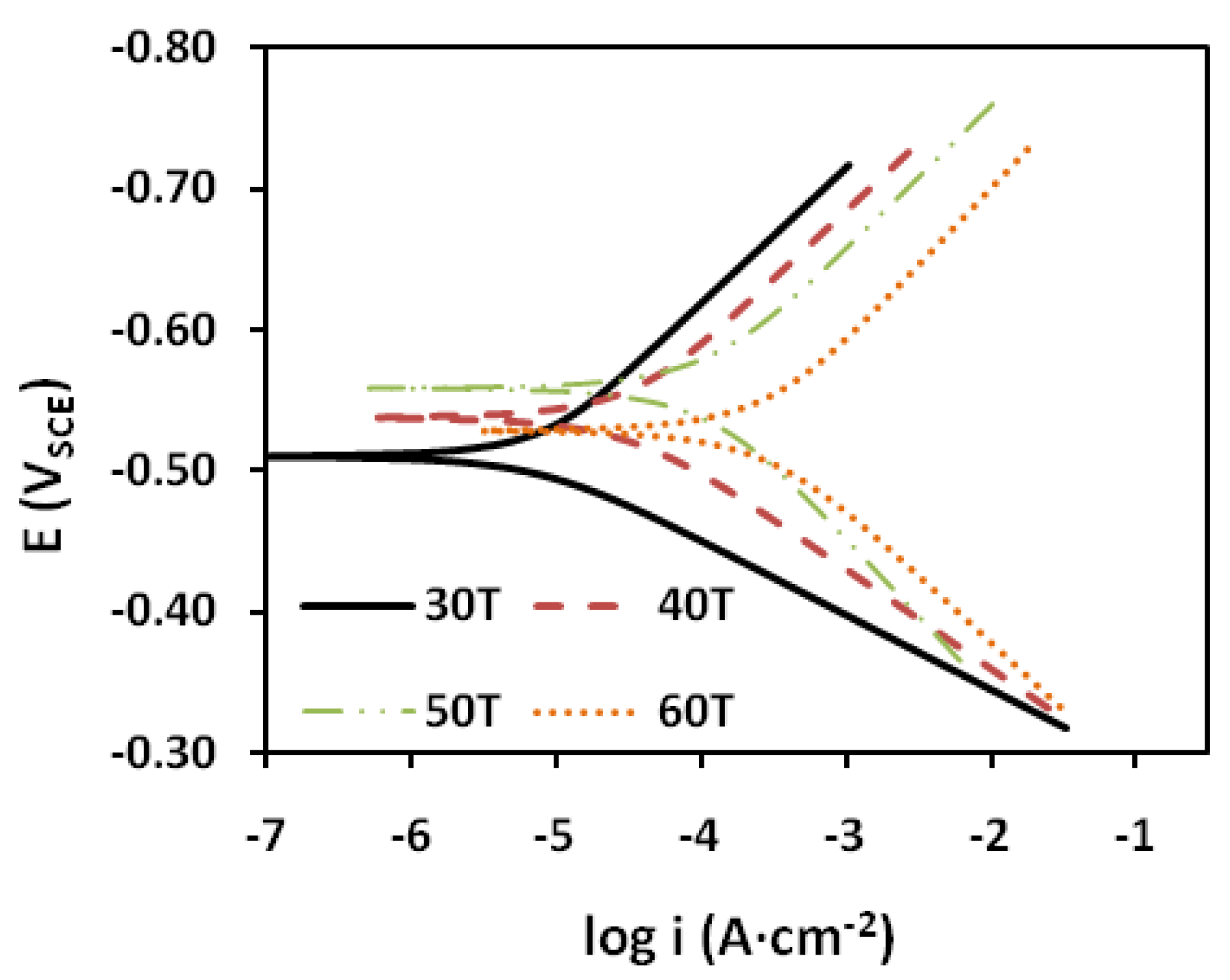
| T (°C) | icorr (μA·cm−2) | ECORR (VSCE) | βa (V·dec−1) | βc (V·dec−1) | IE (%) |
|---|---|---|---|---|---|
| 30 | 7.3 | −0.51 | 0.05 | 0.10 | 98.8 |
| 40 | 35 | −0.538 | 0.07 | 0.10 | 96.6 |
| 50 | 124 | −0.558 | 0.12 | 0.10 | 94.3 |
| 60 | 401 | −0.528 | 0.14 | 0.12 | 93.8 |

2.2.3. Electrochemical Impedance Spectroscopy (EIS) Measurements
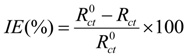
| Conc. (mM) | Rct (ohm·cm2) | IE (%) |
|---|---|---|
| Blank | 42 | – |
| 0.1 | 782 | 94.6 |
| 0.2 | 881 | 95.2 |
| 0.3 | 932 | 95.5 |
| 0.4 | 977 | 95.7 |
| 0.5 | 1087 | 96.1 |
| T (°C) | Conc. (mM) | Rct (ohm·cm2) | IE (%) |
|---|---|---|---|
| 30 | Blank | 42 | – |
| 30 | 0.5 | 1087 | 96.1 |
| 40 | Blank | 13 | – |
| 40 | 0.5 | 546 | 97.5 |
| 50 | Blank | 7 | – |
| 50 | 0.5 | 195 | 96.4 |
| 60 | Blank | 3 | – |
| 60 | 0.5 | 101 | 97.0 |
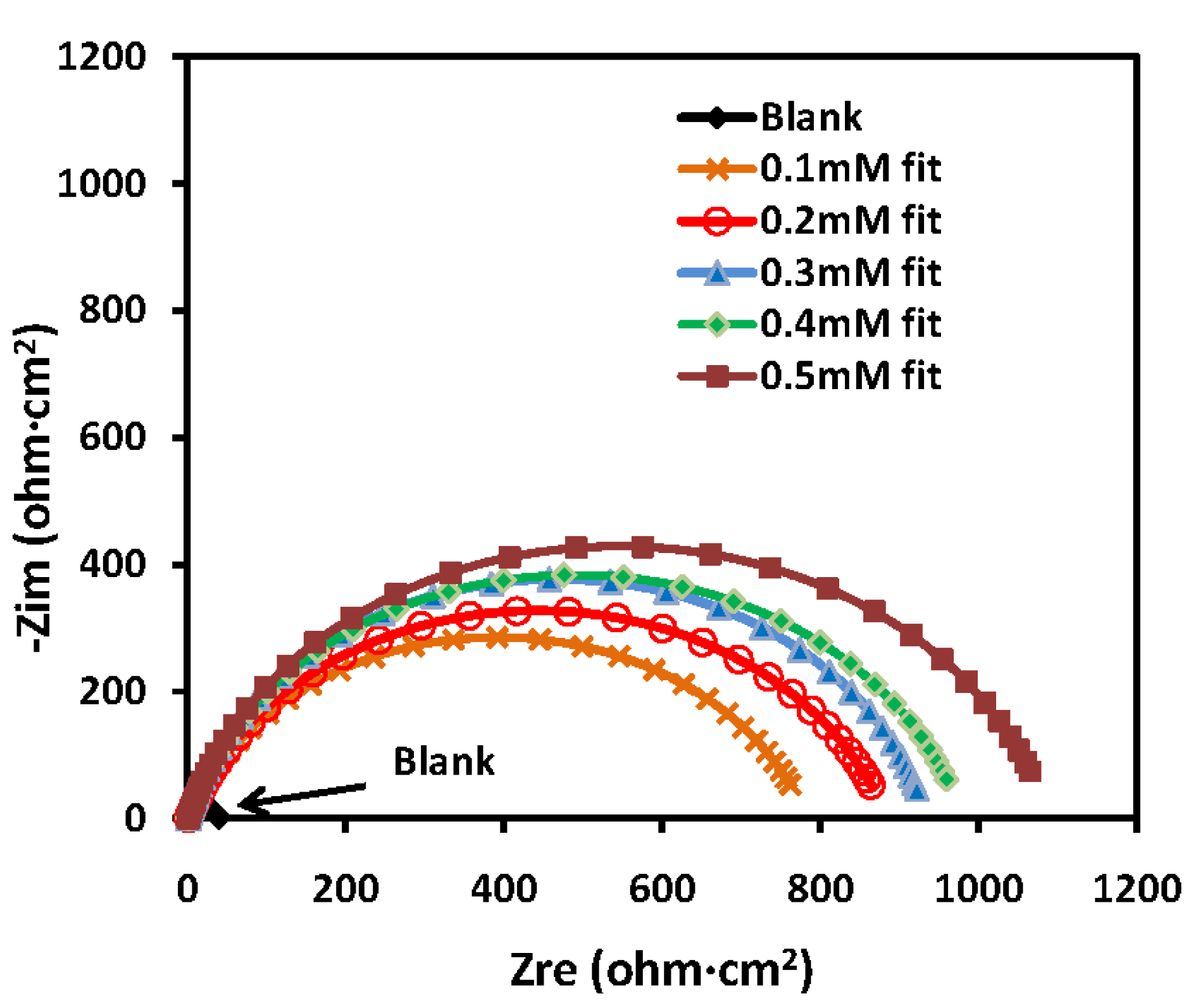
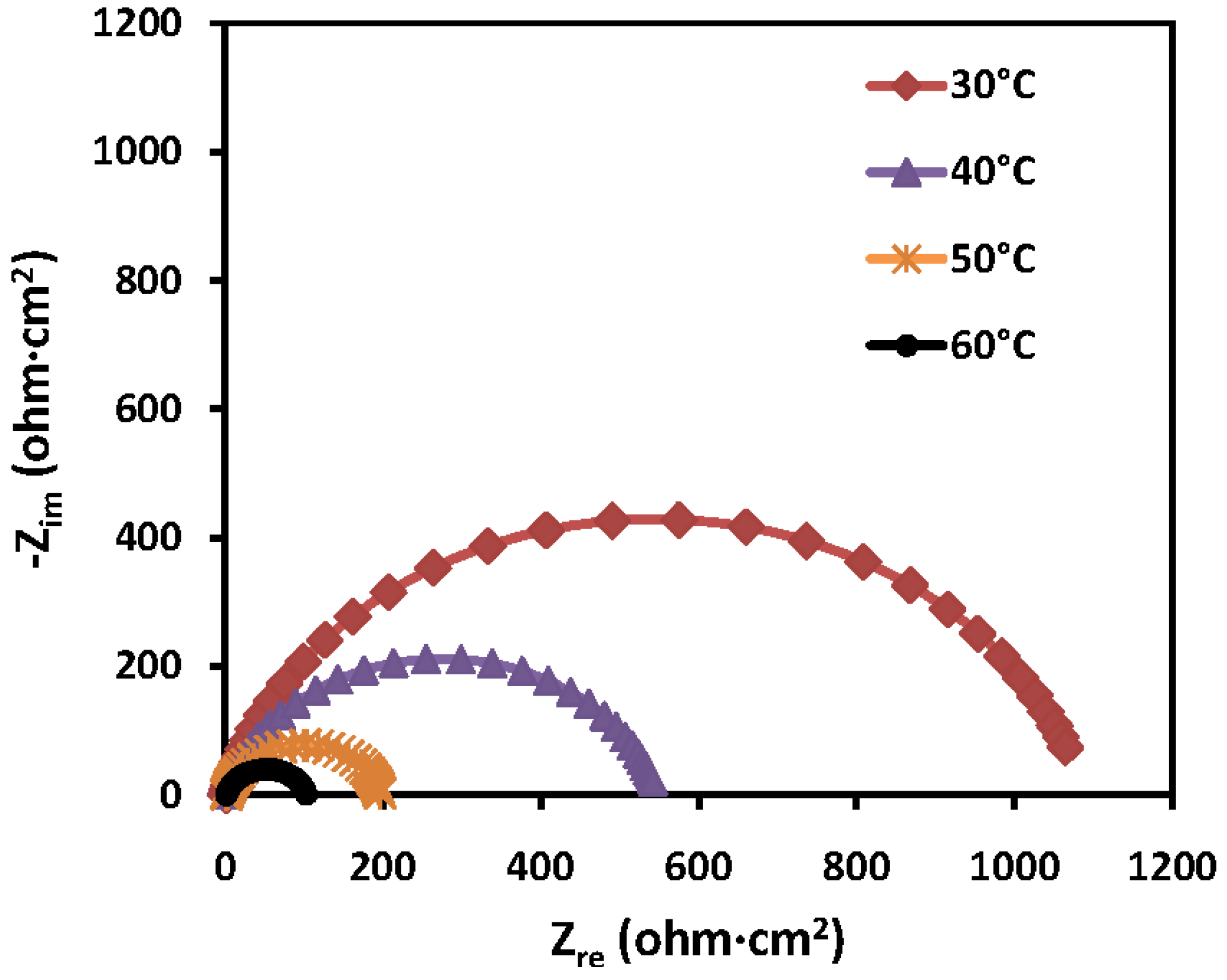
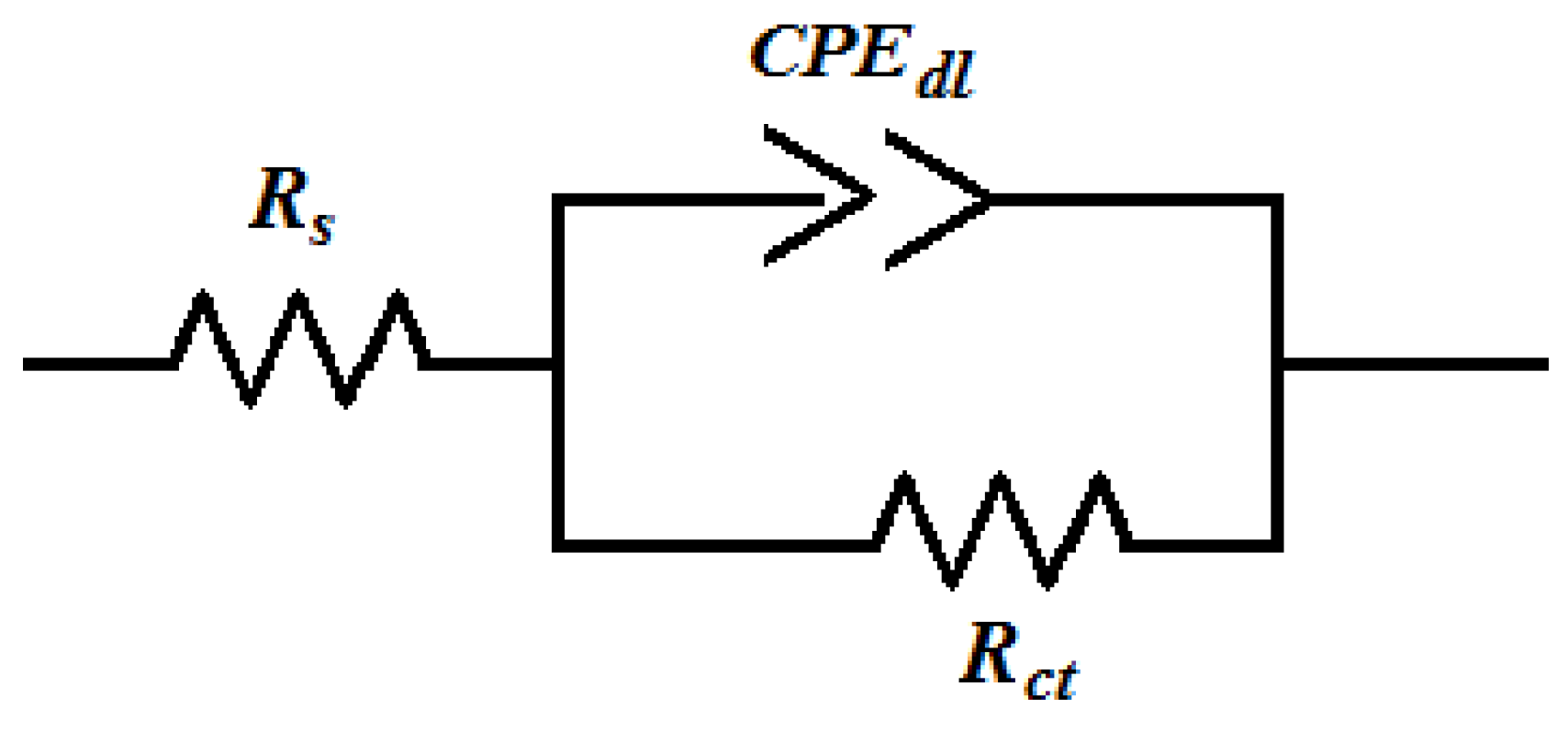
2.2.4. Electrochemical Frequency Modulation
| Conc. (mM) | icorr (μA·cm−2) | βa (V·dec−1) | βc (V·dec−1) | C.R. (mpy) | IE (%) | CF-2 | CF-3 |
|---|---|---|---|---|---|---|---|
| Blank | 81.7 | 0.09 | 0.27 | 90.2 | – | 1.97 | 2.02 |
| 0.1 | 61.0 | 0.05 | 0.06 | 27.5 | 25.3 | 4.56 | 2.49 |
| 0.2 | 43.7 | 0.16 | 0.17 | 20 | 46.4 | 2.79 | 2.11 |
| 0.3 | 22.4 | 0.09 | 0.10 | 10.2 | 72.5 | 1.79 | 1.70 |
| 0.4 | 20.9 | 0.08 | 0.09 | 9.5 | 74.3 | 1.80 | 1.78 |
| 0.5 | 17.8 | 0.08 | 0.09 | 8.1 | 78.2 | 1.34 | 2.42 |
| T (°C) | icorr (μA·cm−2) | βa (V·dec−1) | βc (V·dec−1) | C.R. (mpy) | IE (%) | CF-2 | CF-3 |
|---|---|---|---|---|---|---|---|
| 30 | 17.8 | 0.08 | 0.09 | 8.1 | 97.4 | 1.34 | 2.42 |
| 40 | 37.0 | 0.08 | 0.10 | 16.9 | 96.4 | 2.01 | 4.63 |
| 50 | 128.1 | 0.08 | 0.16 | 58.6 | 94 | 1.91 | 2.99 |
| 60 | 370.6 | 0.09 | 0.15 | 123.7 | 94 | 1.91 | 5.67 |
2.3. Scanning Electron Microscopy (SEM)

3. Experimental
3.1. Synthesis
3.1.1. Synthesis of Thiosemicarbazone 1
3.1.2. Synthesis of PMBH N,N′-((2E,2′E)-2,2′-(1,4-phenylenebis(methanylylidene))bis (hydrazinecarbonothioyl))bis(2-oxo-2H-chromene-3-carboxamide) 2
3.2. Electrochemical Measurements
3.3. Surface Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rani, B.E.A.; Basu, B.B.J. Green inhibitors for corrosion protection of metals and alloys: An overview. Int. J. Corros. 2012, 2, 1–15. [Google Scholar] [CrossRef]
- Shukla, S.K.; Singh, A.; Quraishi, A. Triazines: Efficient corrosion inhibitors for mild steel in hydrochloric acid solution. Int. J. Electrochem. Sci. 2012, 7, 3371–3389. [Google Scholar]
- Bin, X.; Wenzhong, Y.; Ying, L.; Xiaoshuang, Y.; Weinan, G.; Yizhong, C. Experimental and theoretical evaluation of two pyridinecarboxaldehyde thiosemicarbazone compounds as corrosion inhibitors for mild steel in hydrochloric acid solution. Corros. Sci. 2014, 78, 260–268. [Google Scholar]
- Kosari, A.; Moayed, M.H.; Davoodi, A.; Parvizi, R.; Momeni, M.; Eshghi, H.; Moradi, H. Electrochemical and quantum chemical assessment of two organic compounds from pyridine derivatives as corrosion inhibitors for mild steel in HCl solution under stagnant condition and hydrodynamic flow. Corros. Sci. 2014, 78, 138–150. [Google Scholar] [CrossRef]
- Bobina, M.; Kellenberger, A.; Millet, J.; Muntean, C.; Vaszilcsin, N. Corrosion resistance of carbon steel in weak acid solutions in the presence of L-histidine as corrosion inhibitor. Corros. Sci. 2013, 69, 389–395. [Google Scholar] [CrossRef]
- Fragoza-Mar, L.; Olivares-Xometl, O.; Domínguez-Aguilar, M.; Flores, E.; Lozada, P.; Jiménez-Cruz, F. Corrosion inhibitor activity of 1,3-diketone malonates for mild steel in aqueous hydrochloric acid solution. Corros. Sci. 2012, 61, 171–184. [Google Scholar] [CrossRef]
- Hosseini, M.; Mertens, S.F.L.; Ghorbani, M.; Arshadi, M.R. Asymmetrical Schiff bases as inhibitors of mild steel corrosion in sulphuric acid media. Mater. Chem. Phys. 2003, 78, 800–808. [Google Scholar] [CrossRef]
- Yohai, L.; Vázquez, M.; Valcarce, M.B. Phosphate ions as corrosion inhibitors for reinforcement steel in chloride-rich environments. Electrochim. Acta 2013, 102, 88–96. [Google Scholar] [CrossRef]
- Saravanamoorthy, S.; Velmathi, S. Physiochemical interactions of chiral Schiff bases on high carbon steel surface: Corrosion inhibition in acidic media. Prog. Org. Coat. 2013, 76, 1527–1535. [Google Scholar] [CrossRef]
- Nam, N.D.; Bui, Q.V.; Mathesh, M.; Tan, M.Y.J.; Forsyth, M. A study of 4-carboxyphenylboronic acid as a corrosion inhibitor for steel in carbon dioxide containing environments. Corros. Sci. 2013, 76, 257–266. [Google Scholar] [CrossRef]
- Sing, D.N.; Dey, A.K. Localized coating failure of epoxy-coated Aluminium alloy 2024-T3 in 0.5 M NaCl solutions: Correlation between coating degradation, blister formation and local chemistry within blisters. Corros. Sci. 2007, 49, 594–619. [Google Scholar] [CrossRef]
- Banerjee, G.; Mahotra, S.N. Contribution to adsorption of aromatic amines on mild steel surface from HCl solutions by impedance, UV, and Raman spectroscopy. Corrosion 1992, 48, 10–15. [Google Scholar] [CrossRef]
- Arab, S.T.; Noor, E.A. Inhibition of acid corrosion of steel by some S-alkylisothiouronium iodides. Corrosion 1993, 49, 122–129. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Zaky, M.F. Inhibition effect of novel nonionic surfactants on the corrosion of carbon steel in acidic medium. Corros. Sci. 2010, 52, 1333–1341. [Google Scholar] [CrossRef]
- Xianghong, L.; Guannan, M. Tween-40 as corrosion inhibitor for cold rolled steel in sulphuric acid: Weight loss study, electrochemical characterization, and AFM. Appl. Surf. Sci. 2005, 252, 1254–1265. [Google Scholar] [CrossRef]
- Scendo, M.; Uznanska, J. Inhibition effect of 1-butyl-4-methylpyridinium tetrafluoroborate on the corrosion of copper in phosphate solutions. Int. J. Corros. 2011, 1, 1–12. [Google Scholar]
- Eddy, N.O.; Ebenso, E.E. Adsorption and inhibitive properties of ethanol extracts of Musa sapientum peels as a green corrosion inhibitor for mild steel in H2SO4. Afr. J. Pure Appl. Chem. 2008, 2, 046–054. [Google Scholar]
- Laamari, M.R.; Benzakour, J.; Berrekhis, F.; Bakasse, M.; Villemin, D. Adsorption and kinetic studies of piperidin-1-yl-phosphonic acid as a corrosion inhibitor of iron in sulphuric acid medium. J. Mater. Environ. Sci. 2012, 3, 485–496. [Google Scholar]
- Wang, L. Inhibiting effect of 2-mercaptopyrimidine on the corrosion of a low carbon steel in phosphoric acid. Corros. Sci. 2001, 43, 1637–1644. [Google Scholar] [CrossRef]
- Sekine, I.; Nakahata, Y.; Tanabe, H. The corrosion inhibition of mild steel by ascorbic and folic acids. Corros. Sci. 1988, 28, 987–1001. [Google Scholar] [CrossRef]
- Fengling, X.U.; Baorong, H.O.U. Triazole derivatives as corrosion inhibitors for mild steel in hydrochloric acid solution. Acta Metall. Sin. 2009, 22, 247–254. [Google Scholar] [CrossRef]
- Manahan, S.E. Environmental Chemistry, 6th ed; Lewis: Boca Raton, FL, USA, 1994. [Google Scholar]
- Popova, A.; Christov, M. Evaluation of impedance measurements on mild steel corrosion in acid media in the presence of heterocyclic compounds. Corros. Sci. 2006, 48, 3208–3221. [Google Scholar] [CrossRef]
- Liu, F.G.; Du, M.; Zhang, J.; Qiu, M. Electrochemical behavior of Q235 steel in saltwater saturated with carbon dioxide based on new imidazoline derivative inhibitor. Corros. Sci. 2009, 51, 102–109. [Google Scholar] [CrossRef]
- Li, W.H.; He, Q.; Pei, C.L.; Hou, B.R. Some new triazole derivatives as inhibitors for mild steel corrosion in acidic medium. J. Appl. Electrochem. 2008, 38, 289–295. [Google Scholar] [CrossRef]
- Musa, A.Y.; Mohamad, A.B.; Kadhum, A.A.H.; Takriff, M.S. Corrosion inhibition of mild steel in 1.0 M HCL by amino compound: Electrochemical and DFT studies. Metall. Mater. Trans. A 2012, 43, 3379–3386. [Google Scholar] [CrossRef]
- Sorkhabi, H.; Asghari, E.; Ejbari, P. Electrochemical studies of adsorption and inhibitive performance of basic yellow 28 dye on mild steel corrosion in acid solutions. Acta Chim. Slov. 2011, 58, 270–277. [Google Scholar]
- Soltani, N.; Tavakkoli, N.; Khayatkashani, M.; Jalali, M.R.; Mosavizade, A. Green approach to corrosion inhibition of 304 stainless steel in hydrochloric acid solution by the extract of Salvia officinalis leaves. Corros. Sci. 2012, 62, 122–135. [Google Scholar] [CrossRef]
- De-Souza, F.S. Caffeic acid as a green corrosion inhibitor for mild steel. Corros. Sci. 2009, 51, 642–649. [Google Scholar] [CrossRef]
- Hermas, A.A.; Morad, M.S. A comparative study on the corrosion behaviour of 304 austenitic stainless steel in sulfamic and sulfuric acid solutions. Corros. Sci. 2008, 50, 2710–2717. [Google Scholar] [CrossRef]
- ASTM G1-03. In Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens; ASTM International: West Conshohocken, PA, USA, 2003.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kadhum, A.A.H.; Mohamad, A.B.; Hammed, L.A.; Al-Amiery, A.A.; San, N.H.; Musa, A.Y. Inhibition of Mild Steel Corrosion in Hydrochloric Acid Solution by New Coumarin. Materials 2014, 7, 4335-4348. https://doi.org/10.3390/ma7064335
Kadhum AAH, Mohamad AB, Hammed LA, Al-Amiery AA, San NH, Musa AY. Inhibition of Mild Steel Corrosion in Hydrochloric Acid Solution by New Coumarin. Materials. 2014; 7(6):4335-4348. https://doi.org/10.3390/ma7064335
Chicago/Turabian StyleKadhum, Abdul Amir H., Abu Bakar Mohamad, Leiqaa A. Hammed, Ahmed A. Al-Amiery, Ng Hooi San, and Ahmed Y. Musa. 2014. "Inhibition of Mild Steel Corrosion in Hydrochloric Acid Solution by New Coumarin" Materials 7, no. 6: 4335-4348. https://doi.org/10.3390/ma7064335
APA StyleKadhum, A. A. H., Mohamad, A. B., Hammed, L. A., Al-Amiery, A. A., San, N. H., & Musa, A. Y. (2014). Inhibition of Mild Steel Corrosion in Hydrochloric Acid Solution by New Coumarin. Materials, 7(6), 4335-4348. https://doi.org/10.3390/ma7064335




