Impact of Groundwater Salinity on Bioremediation Enhanced by Micro-Nano Bubbles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.1.1. Generation Method
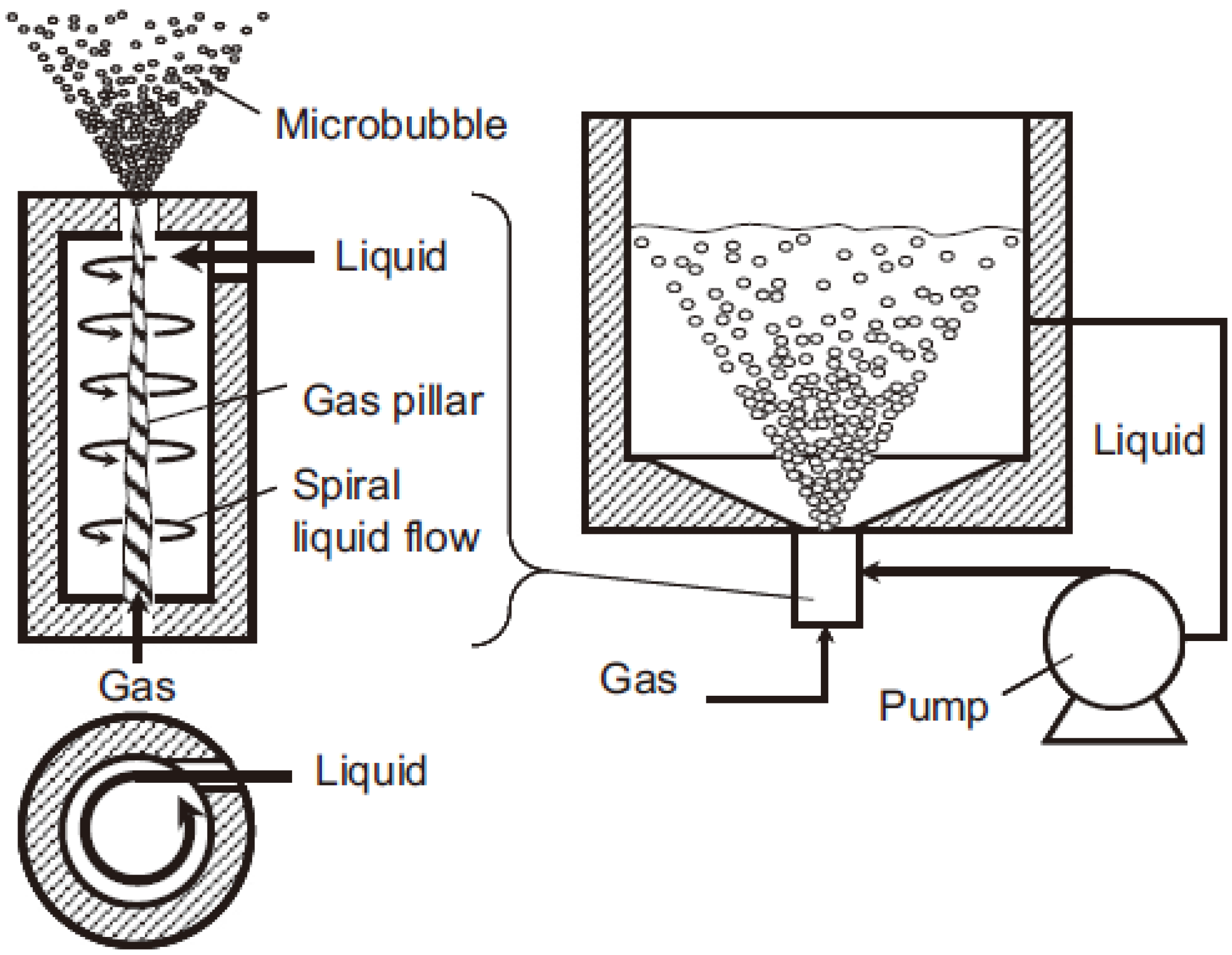
2.1.2. Bubble Size Distribution Analyzer
2.1.3. Dissolved Oxygen Sensor
2.1.4. Zeta Potential Analyzer
2.1.5. Permeameter
2.2. Materials
2.3. Experimental Procedure
| Test group | Water volume | Temperature (°C) | Salinity (g/L) |
|---|---|---|---|
| 1 | 11 L ultrapure water | 20 | 0 |
| 2 | 11 L ultrapure water | 20 | 0.1 |
| 3 | 11 L ultrapure water | 20 | 0.4 |
| 4 | 11 L ultrapure water | 20 | 0.7 |
| 5 | 11 L ultrapure water | 20 | 1.0 |
| 6 | 11 L ultrapure water | 20 | 3.0 |
| 7 | 11 L ultrapure water | 20 | 5.0 |
| 8 | 11 L ultrapure water | 20 | 7.0 |
| 9 | 11 L ultrapure water | 20 | 9.0 |
3. Results and Discussion
3.1. Bubble Size Distribution
| Test group | Average bubble diameter (μm) | Standard deviation (μm) |
|---|---|---|
| 1 | 33.44 | 13.30 |
| 2 | 45.78 | 14.97 |
| 3 | 52.01 | 11.13 |
| 4 | 55.38 | 7.32 |
| 5 | 59.49 | 12.13 |
| 6 | 54.62 | 14.05 |
| 7 | 53.61 | 15.07 |
| 8 | 55.54 | 14.07 |
| 9 | 57.01 | 18.00 |
3.2. Oxygen Transfer Efficiency
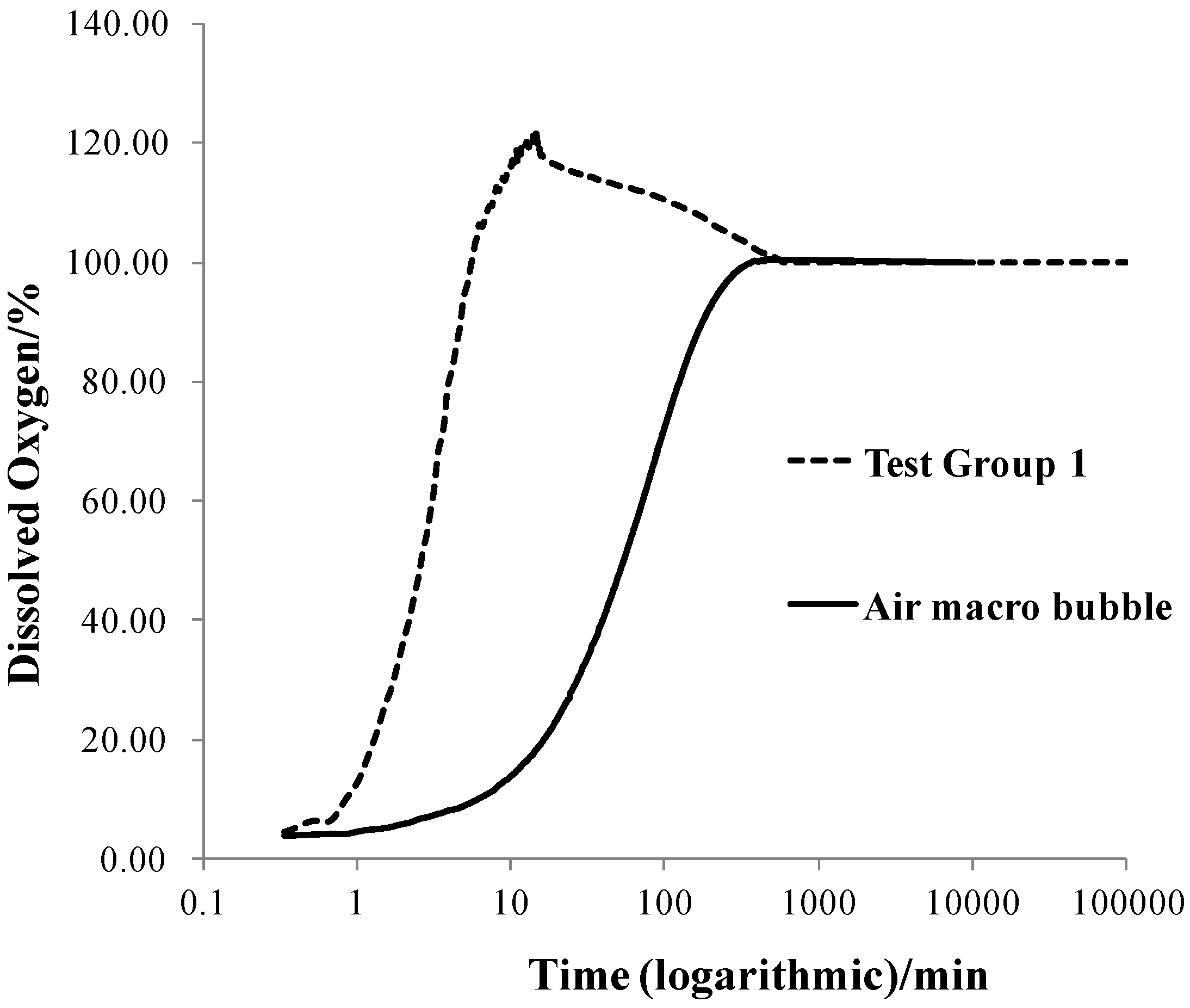
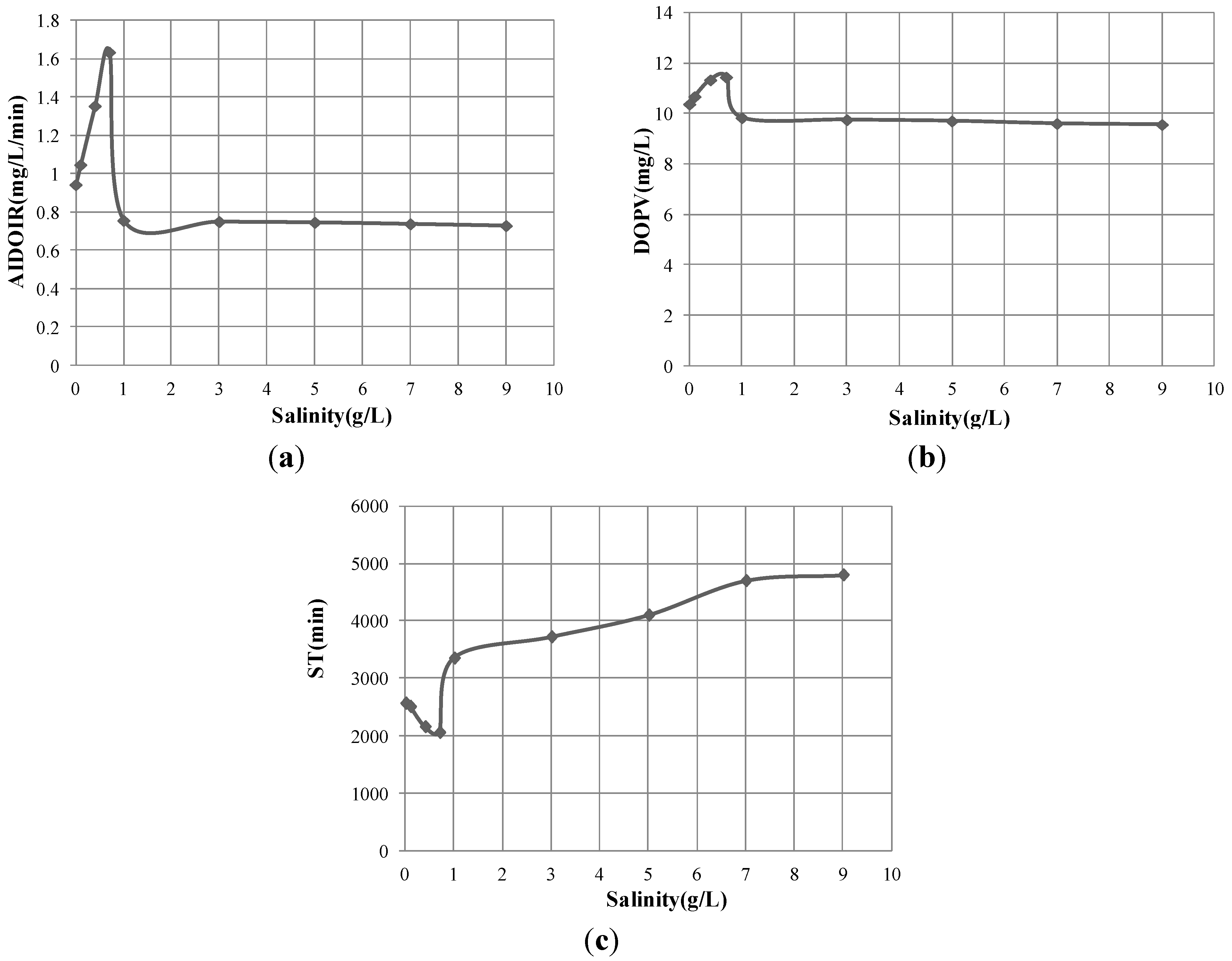
3.3. Zeta Potential
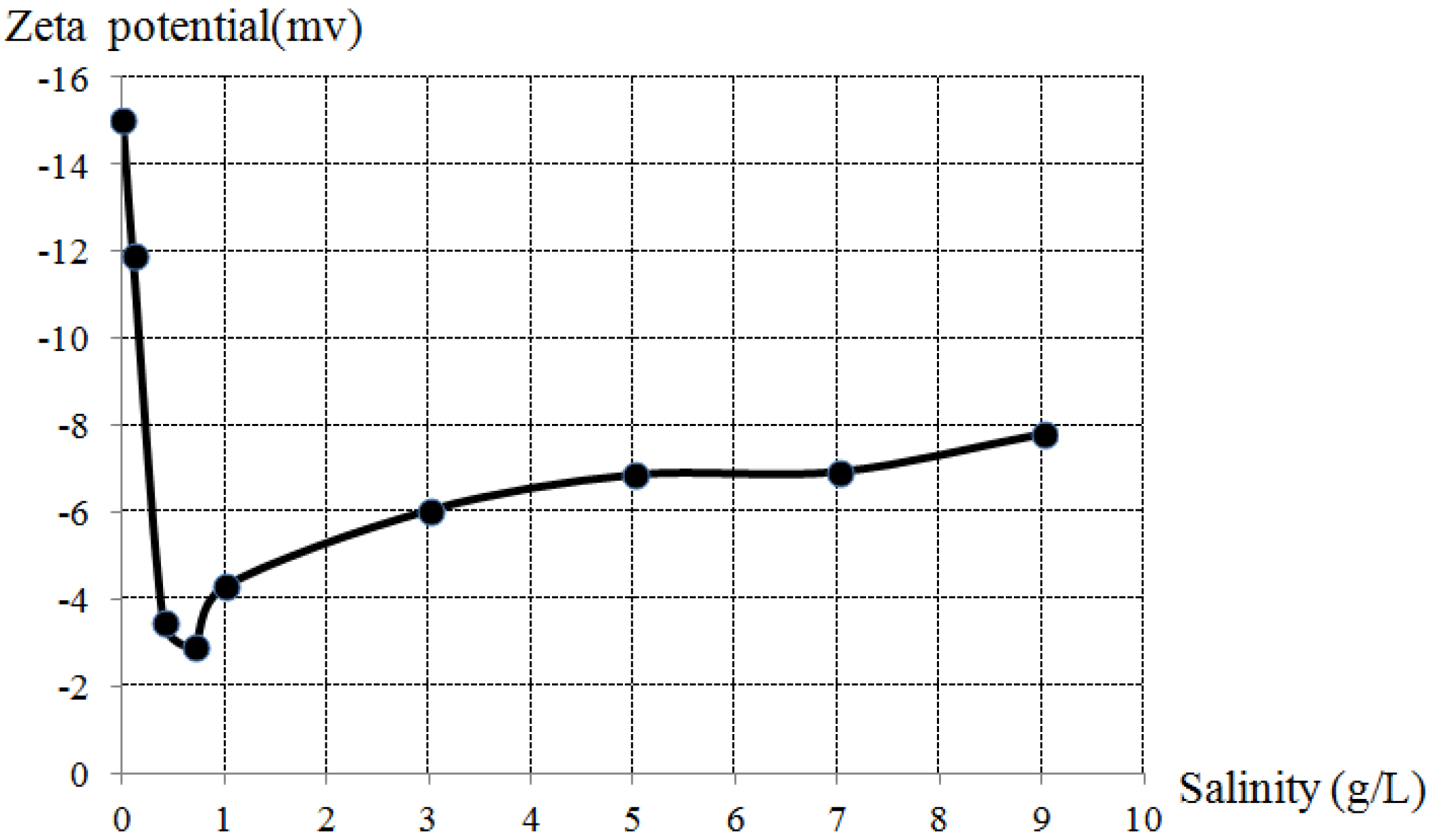
3.4. Discussion
3.5. Permeability Results
| Salinity (g/L) | Hydraulic conductivity (10−6 m/s) | Standard deviation (10−6 m/s) |
|---|---|---|
| 0.0 | 5.00 | 2.16 |
| 0.1 | 4.84 | 0.73 |
| 0.4 | 4.80 | 1.19 |
| 0.7 | 4.76 | 2.28 |
| 1.0 | 4.61 | 0.41 |
| 3.0 | 4.53 | 0.33 |
| 5.0 | 4.72 | 1.76 |
| 7.0 | 4.56 | 1.35 |
| 9.0 | 4.66 | 3.07 |
4. Conclusions
- Water salinity had no influence on MNBs size.
- The greater the dissolved oxygen enhancement, the shorter the bubble stagnation time was achieved at the salinity of 0.7 g/L compared to those at other salinities.
- The lowest zeta potential value was obtained as –2.9 mV at the salinity of 0.7 g/L.
- Water salinity had no impact on the MNBs water permeability of sand.
Acknowledgments
Conflicts of Interest
References
- Pardieck, D.L.; Bouwer, E.J.; Stone, A.T. Hydrogen peroxide use to increase oxidant capacity for in situ bioremediation of contaminated soils and aquifers: A review. J. Contam. Hydrol. 1992, 9, 221–242. [Google Scholar] [CrossRef]
- Farhadian, M.; Vachelard, C.; Duchez, D.; Larroche, C. In situ bioremediation of monoaromatic pollutants in groundwater: A review. Bioresour. Technol. 2008, 99, 5296–5308. [Google Scholar] [CrossRef] [PubMed]
- Van Stempvoort, D.; Biggar, K. Potential for bioremediation of petroleum hydrocarbons in groundwater under cold climate conditions: A review. Cold Reg. Sci. Technol. 2008, 53, 16–41. [Google Scholar] [CrossRef]
- Wu, W.-M.; Carley, J.; Fienen, M.; Mehlhorn, T.; Lowe, K.; Nyman, J.; Luo, J.; Gentile, M.E.; Rajan, R.; Wagner, D. Pilot-scale in situ bioremediation of uranium in a highly contaminated aquifer. 1. Conditioning of a treatment zone. Environ. Sci. Technol. 2006, 40, 3978–3985. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-M.; Carley, J.; Gentry, T.; Ginder-Vogel, M.A.; Fienen, M.; Mehlhorn, T.; Yan, H.; Caroll, S.; Pace, M.N.; Nyman, J. Pilot-scale in situ bioremedation of uranium in a highly contaminated aquifer. 2. Reduction of U(VI) and geochemical control of U(VI) bioavailability. Environ. Sci. Technol. 2006, 40, 3986–3995. [Google Scholar] [CrossRef] [PubMed]
- Margesin, R.; Schinner, F. Biodegradation and bioremediation of hydrocarbons in extreme environments. Appl. Microbiol. Biotechnol. 2001, 56, 650–663. [Google Scholar] [CrossRef] [PubMed]
- Nikolopoulou, M.; Kalogerakis, N. Enhanced bioremediation of crude oil utilizing lipophilic fertilizers combined with biosurfactants and molasses. Marine Pollut. Bull. 2008, 56, 1855–1861. [Google Scholar] [CrossRef]
- Ayotamuno, M.; Kogbara, R.; Ogaji, S.; Probert, S. Bioremediation of a crude-oil polluted agricultural-soil at port harcourt, nigeria. Appl. Energy 2006, 83, 1249–1257. [Google Scholar] [CrossRef]
- Robinson, C.; Barry, D.; McCarty, P.L.; Gerhard, J.I.; Kouznetsova, I. Ph control for enhanced reductive bioremediation of chlorinated solvent source zones. Sci. Total Environ. 2009, 407, 4560–4573. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Katayama, A. Approach for estimating microbial growth and the biodegradation of hydrocarbon contaminants in subsoil based on field measurements: 1. Model development and verification. Environ. Sci. Technol. 2009, 44, 767–773. [Google Scholar] [CrossRef]
- Song, D.; Kitamura, M.; Katayama, A. Approach for estimating microbial growth and biodegradation of hydrocarbon contaminants in subsoil based on field measurements: 2. Application in a field lysimeter experiment. Environ. Sci. Technol. 2010, 44, 6795–6801. [Google Scholar] [CrossRef] [PubMed]
- Nash, H.; McCall, G.J.H. Groundwater Quality; Chapman & Hall: London, UK, 1995. [Google Scholar]
- Rhykerd, R.L.; Weaver, R.W.; McInnes, K.J. Influence of salinity on bioremediation of oil in soil. Environ. Pollut. 1995, 90, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Børresen, M.; Rike, A. Effects of nutrient content, moisture content and salinity on mineralization of hexadecane in an arctic soil. Cold Reg. Sci. Technol. 2007, 48, 129–138. [Google Scholar] [CrossRef]
- Qin, X.; Tang, J.; Li, D.; Zhang, Q. Effect of salinity on the bioremediation of petroleum hydrocarbons in a saline? Alkaline soil. Lett. Appl. Microbiol. 2012, 55, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Bazire, A.; Diab, F.; Jebbar, M.; Haras, D. Influence of high salinity on biofilm formation and benzoate assimilation by pseudomonas aeruginosa. J. Ind. Microbiol. Biotechnol. 2007, 34, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Badi, H.N.; Sorooshzadeh, A. Evaluating potential of borage (borago officinalis l.) in bioremediation of saline soil. Afr. J. Biotechnol. 2011, 10, 146–153. [Google Scholar]
- Agarwal, A.; Ng, W.J.; Liu, Y. Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere 2011, 84, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Ushikubo, F.Y.; Furukawa, T.; Nakagawa, R.; Enari, M.; Makino, Y.; Kawagoe, Y.; Shiina, T.; Oshita, S. Evidence of the existence and the stability of nano-bubbles in water. Colloids Surf. A Physicochem. Eng. Aspects 2010, 361, 31–37. [Google Scholar] [CrossRef]
- Jenkins, K.B.; Michelsen, D.L.; Novak, J.T. Application of oxygen microbubbles for in situ biodegradation of p-xylene-contaminated groundwater in a soil column. Biotechnol. Prog. 1993, 9, 394–400. [Google Scholar] [CrossRef]
- Turner, W. Microbubble persistence in fresh water. J. Acoustical Soc. Am. 1961, 33, 1223–1233. [Google Scholar] [CrossRef]
- Ohgaki, K.; Khanh, N.Q.; Joden, Y.; Tsuji, A.; Nakagawa, T. Physicochemical approach to nanobubble solutions. Chem. Eng. Sci. 2010, 65, 1296–1300. [Google Scholar] [CrossRef]
- Takahashi, M. Base and technological application of micro-bubble and nano-bubble. Mater. Integr. 2009, 22, 2–19. [Google Scholar]
- McTaggart, H. The electrification at liquid-gas surfaces. Lond. Edinb. Dublin Philosoph. Mag. J. Sci. 1914, 27, 297–314. [Google Scholar] [CrossRef]
- Xu, Q.; Nakajima, M.; Ichikawa, S.; Nakamura, N.; Shiina, T. A comparative study of microbubble generation by mechanical agitation and sonication. Innov. Food Sci. Emerg. Technol. 2008, 9, 489–494. [Google Scholar] [CrossRef]
- Bowley, W.W.; Hammond, G.L. Controlling factors for oxygen transfer through bubbles. Ind. Eng. Chem. Process Des. Dev. 1978, 17, 2–8. [Google Scholar] [CrossRef]
- Hensirisak, P.; Parasukulsatid, P.; Agblevor, F.; Cundiff, J.; Velander, W. Scale-up of microbubble dispersion generator for aerobic fermentation. Appl. Biochem. Biotechnol. 2002, 101, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Hoage, J.B.; Messer, L.A. Apparatus for Aeration without Significant Agitation to Deplete and Biodegrade Sludge. U.S. Patent US6,884,353 B2, 8 October 2002. [Google Scholar]
- Park, J.-S.; Kurata, K. Application of microbubbles to hydroponics solution promotes lettuce growth. HortTechnology 2009, 19, 212–215. [Google Scholar]
- Onari, H. Fisheries experiment of cultivated shells using micro-bubble techniques. J. Heat Trans. Soc. Jpn. 2001, 40, 2–7. [Google Scholar]
- Takahashi, M.; Chiba, K.; Pan, L. Free-radical generation from collapsing microbubbles in the absence of a dynamic stimulus. J. Phys. Chem. B 2007, 111, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, K.; Pandit, A. Effect of cavitation on chemical disinfection efficiency. Water Res. 2004, 38, 2249–2258. [Google Scholar] [CrossRef]
- Terasaka, K.; Hirabayashi, A.; Nishino, T.; Fujioka, S.; Kobayashi, D. Development of microbubble aerator for waste water treatment using aerobic activated sludge. Chem. Eng. Sci. 2011, 66, 3172–3179. [Google Scholar] [CrossRef]
- Ohnari, H.; Saga, T.; Watanabe, K.; Maeda, K.; Matsuo, K. High functional characteristics of micro-bubbles and water purification. Resour. Process. 1999, 46, 238–244. [Google Scholar] [CrossRef]
- Kukizaki, M.; Goto, M. Size control of nanobubbles generated from shirasu-porous-glass (SPG) membranes. J. Membr. Sci. 2006, 281, 386–396. [Google Scholar] [CrossRef]
- Tasaki, T.; Wada, T.; Fujimoto, K.; Kai, S.; Ohe, K.; Oshima, T.; Baba, Y.; Kukizaki, M. Degradation of methyl orange using short-wavelength uv irradiation with oxygen microbubbles. J. Hazard. Mater. 2009, 162, 1103–1110. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, H.; Hu, L.; Xia, Z. Impact of Groundwater Salinity on Bioremediation Enhanced by Micro-Nano Bubbles. Materials 2013, 6, 3676-3687. https://doi.org/10.3390/ma6093676
Li H, Hu L, Xia Z. Impact of Groundwater Salinity on Bioremediation Enhanced by Micro-Nano Bubbles. Materials. 2013; 6(9):3676-3687. https://doi.org/10.3390/ma6093676
Chicago/Turabian StyleLi, Hengzhen, Liming Hu, and Zhiran Xia. 2013. "Impact of Groundwater Salinity on Bioremediation Enhanced by Micro-Nano Bubbles" Materials 6, no. 9: 3676-3687. https://doi.org/10.3390/ma6093676
APA StyleLi, H., Hu, L., & Xia, Z. (2013). Impact of Groundwater Salinity on Bioremediation Enhanced by Micro-Nano Bubbles. Materials, 6(9), 3676-3687. https://doi.org/10.3390/ma6093676





