3.1. Highly Tilted Multilayers of Cuticle on the Ridges
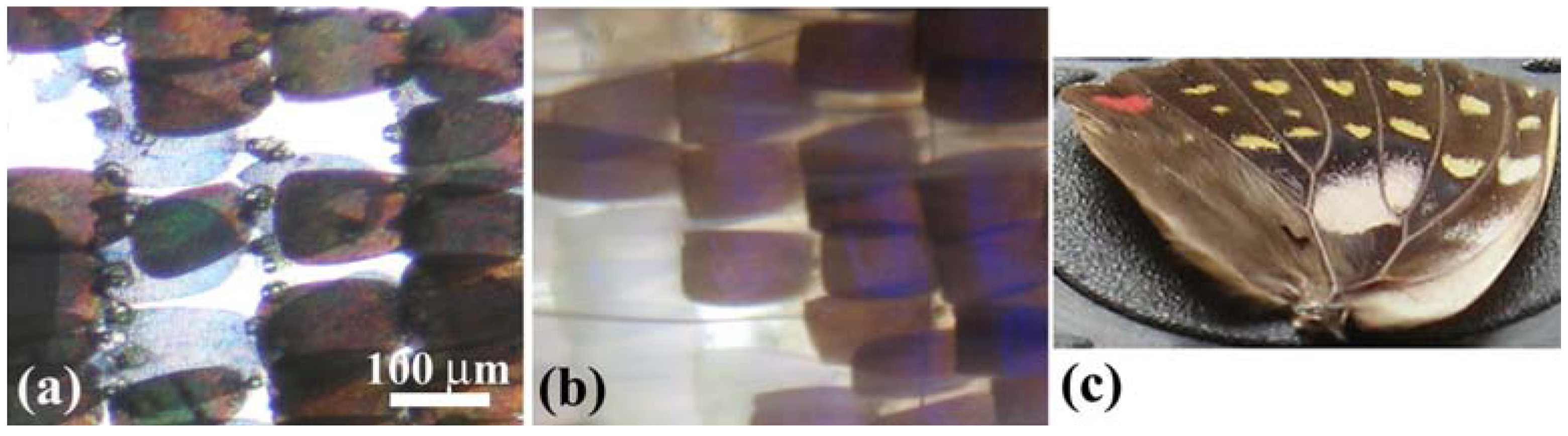
Figure 2(a–d) shows OM images of scales in a vivid blue iridescent area in the forewing of the male
E. mulciber. As seen in a transmission image taken with white light (
Figure 2(a)), these scales have the intrinsic dark brown color due to their melanin-content. When the images of the scales are taken under reflection mode, they exhibit a vivid blue-green iridescence only in parts where the incident light is reflected on the surfaces, as seen in
Figure 2(b–d).
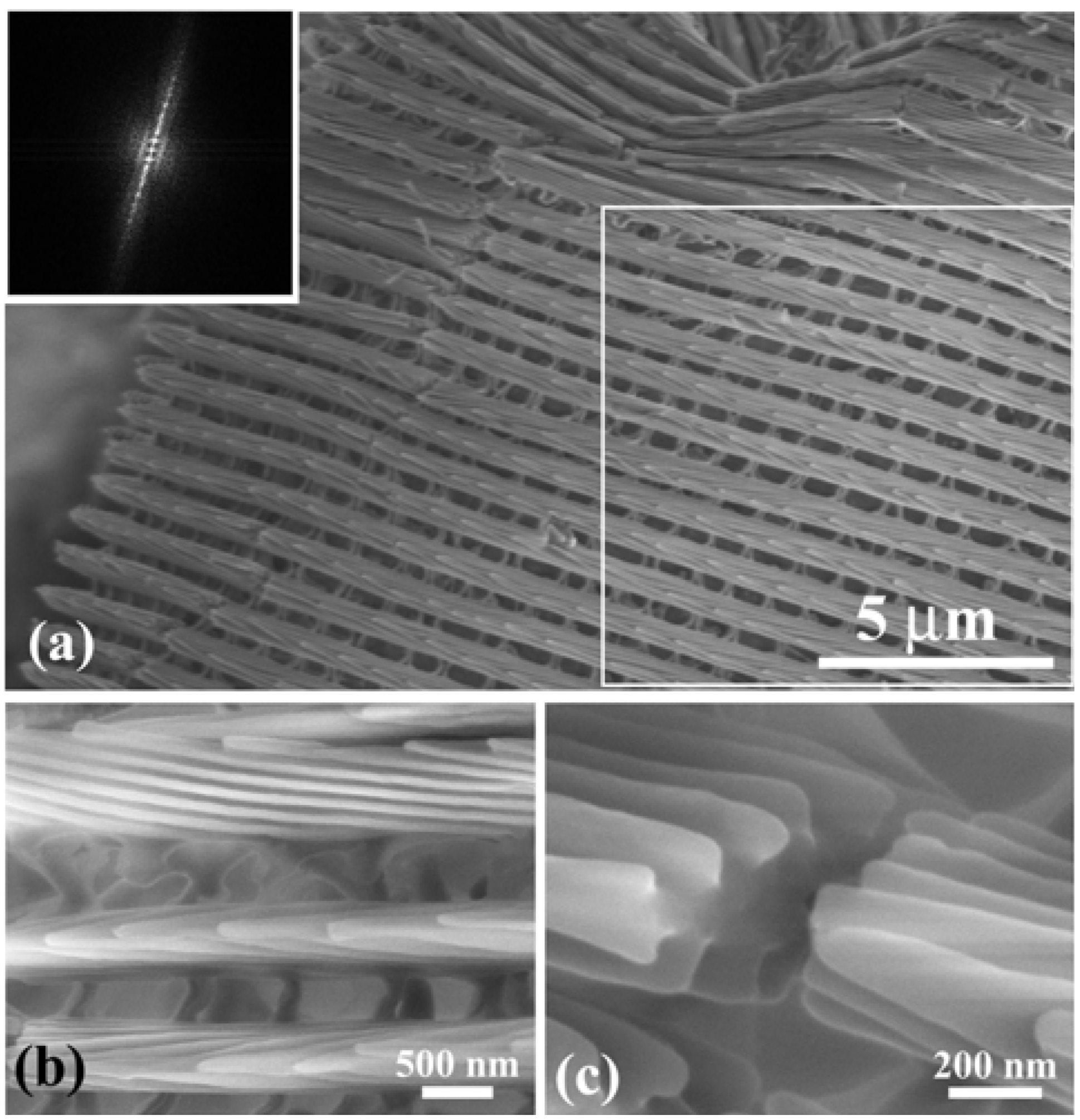
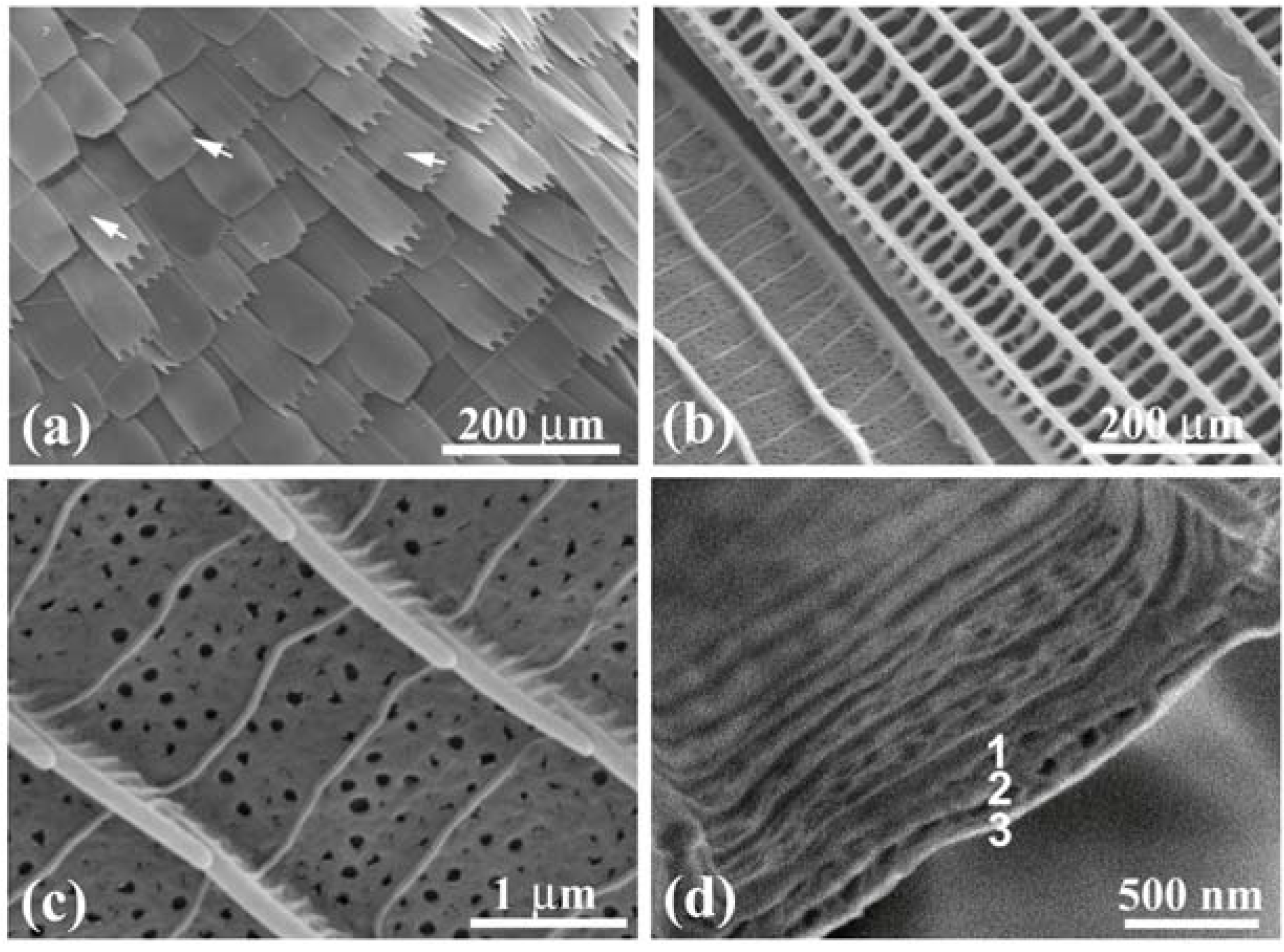
Figure 3(a) shows an SEM image of scales on a vein and the surrounding blue iridescent background. The iridescent blue background comprises two kinds of scales: Broad and narrow scales, which are almost alternately arranged so that the spaces between the broad scales are covered with the narrow scales, as can be clearly seen in
Figure 3(b).
Figure 3(c) shows a top view of the ridges in a narrow scale (left) and a broad scale (right). The ridges run along the length of the scale. The ridge occasionally branches, as indicated by the circles in
Figure 3(b), looking like the edge dislocation in a crystal. This is a kind of growth defect of the living tissue, as previously reported in
S. charonda [
12]. The scales on the vein are almost the same in shape with the narrow scales in the background.
Figure 3(d–g) reveal a multilayered arrangement of cuticles on the ridges, which is similar to that discovered in
A. meliboeus [
8] and that observed in
Morpho Peleides [
26] and
S. charonda [
12]. The scales form a three-dimensional optical diffraction grating.
Figure 3(h) illustrates schematic projections of the grating, which is composed of the grid of the ridges with the spacing
d, the
n multilayered arrangement of cuticles lapped on the ridge, and the surface arrangement of cuticles tilted at
θB and spaced by
D. The
x-axis is defined along the ridges running the length of the scale from the root, the
z-axis is normal to the scale plane and the
y-axis normal to the
x–
z plane. The width of the ridges
d1 and the width of the grooves
d2 as well as the spacing
d and
D are estimated and listed in
Table 1. We also estimated the thickness of the cuticle layers and air gaps to be
tc = ~100 nm and
ta = ~100 nm, respectively, and the tilting angle of
θB to be ~25°. The number of the piled cuticle layers is
n = 3, which is smaller than 7 in the
S. charonda [
12] and 4 in the
A. meliboeus [
8].
Figure 2.
(
a–
f) Optical microscope (OM) images of the scales of the male
E. mulciber, taken at different incident angles of white light. Transmission image (
a) and reflection images of
B scales (
b–
d); Transmission image (
e) and reflection image of
W scales (
f); (
g) Schematic illustration of the selective reflection from cuticle layers piled on the ridge; (
h) Schematic illustration of a blazed diffraction grating (adapted from [
11]).
Figure 2.
(
a–
f) Optical microscope (OM) images of the scales of the male
E. mulciber, taken at different incident angles of white light. Transmission image (
a) and reflection images of
B scales (
b–
d); Transmission image (
e) and reflection image of
W scales (
f); (
g) Schematic illustration of the selective reflection from cuticle layers piled on the ridge; (
h) Schematic illustration of a blazed diffraction grating (adapted from [
11]).
Figure 3.
Scanning electron microscope (SEM) images of scales in the forewing of the male
E. mulciber. (
a) Scales on a vein and
B scales in the blue-iridescent background; (
b)
B scales; (
c) Top view of two
B scales; (
d,
e) Enlarged images of ridges in the
B scale; (
f) Cross-section of ridges on a y-z plane; (
g) Cross-section on an x–z plane; (
h) Schematic of the piled cuticles (adapted from [
11]).
Figure 3.
Scanning electron microscope (SEM) images of scales in the forewing of the male
E. mulciber. (
a) Scales on a vein and
B scales in the blue-iridescent background; (
b)
B scales; (
c) Top view of two
B scales; (
d,
e) Enlarged images of ridges in the
B scale; (
f) Cross-section of ridges on a y-z plane; (
g) Cross-section on an x–z plane; (
h) Schematic of the piled cuticles (adapted from [
11]).
The broad and narrow scales are almost the same in microstructure, and we will call them
B scales hereafter. The scales in the white spots (
W scales) resemble the broad
B scales in shape and structure but not in color. They are transparent white or pearl due to little content of melanin pigment, as seen in
Figure 2(e) which is a transmission OM image. The
W scales also exhibit the iridescent hues when they reflect the incident light, as seen in
Figure 2(f). The parameters of the grating of the
W scales were measured and are shown in
Table 1.
Vukusic
et al. [
8] illustrated that the layer tilt of
θB = 30° causes a 60° portion of the wing’s ‘observation hemisphere’ not to appear leading to an iridescent ‘dark zone’ in
A. meliboeus. The dark zone (2
θB = 50°) corresponds to the
E. mulciber’s scales and is schematically shown in the
Figure 2(g). The dark area in the right wing of the butterfly shown in
Figure 4 is surely in the dark zone. The butterfly can thereby generate a strong flicker contrast with minimal wing movement. They also addressed that the diffraction component appears to combine additively with the interference from the underlying multilayer to produce a broad range of coloration, as well as a limited reverse color change with an angle compared to that associated with conventional flat multilayering [
8]. It may be considered that the grid, which has the spacing of
d along the
y axis and the spacing of
D along the
x axis, and the tilted triple cuticle/air layers with the spacing of
tc + ta form a three-dimensional grating or a monoclinic lattice.
Table 1.
Diffraction grating of the wing scales in butterflies.
Table 1.
Diffraction grating of the wing scales in butterflies.
| Buterfly | Scale | d1 (μm) | d2 (μm) | d (μm) | D (μm) | d1/d (%) | n | t (μm) | θB (°) | Reference |
|---|
| E. mulciber | B | 0.3~0.4 | 0.6~0.7 | 0.9~1.1 | ~0.5 | ~35 | 3 | tc = ~0.1 | 25 | |
| W | ~0.25 | 0.8~0.9 | 1.0~1.2 | 0.5~0.8 | ~23 | ta = ~0.1 | [11] |
| B4 | ~0.2 | ~1.3 | ~1.5 | 0.6~ 1.7 | ~13 | 1 | | | |
| S. charonda | B& W | ~0.6 | ~0.3 | ~0.9 | ~1.0 | ~67 | 7 | ~1 | ~8 | [13] |
| B1, W1 & R1 | ~0.4 | ~1.1 | ~1.5 | ~0.8 | ~27 | 2 | ~0.8 | ~10 |
| C. ataxus M | Green-blue | 0.4~0.5 | 2.5 | 3 | 0.8~1.5 | ~15 | 1 | D’ = 0.8~1.2 | [14] |
| F | Violet | ~0.25 | ~2.1 | ~2.3 | ~1.4 | ~11 | 1 | D’ = ~0.9 |
| Dark brown | ~0.3 | ~1.2 | ~1.5 | ~2.2 | ~20 | 1 | D’ = ~0.6 |
Figure 4 reproduces the reflectance in the ultraviolet (UV) and visible region from the different areas in the dorsal wings of the male
E. mulciber. The spectrum from the dark brown scales
B exhibiting iridescent blue has a heap with high reflectance of 4~6% in a range over UV (<380 nm) and violet (380~450 nm), and a valley with lower reflectance below 4% in a range over blue (450~495 nm), green (495~570 nm), yellow (570~590 nm), and orange (590~620 nm). Small peaks can be seen at ~480 and ~240 nm. The vivid blue coloration comes from the multiple cuticle layers on the ridges. For simplicity we assume the incident and reflected rays in the
x-
z plane. From the cuticle-air multilayered arrangement the multiple reflection occurs as dynamical diffraction or coherent reflection between layers, when the following interference condition is satisfied:
where
θa and
θc are the angles of incidence and refraction of the rays to the cuticle layer normal,
na and
nc are the relative refractive index of air and cuticles, respectively. An integer of
m is the order of interference and
λP is the wavelength of the reflected light. Since in the reflectance measurement the incident rays were parallel to the scale plane normal (which is different from the cuticle layer normal as illustrated in
Figure 3(g)),
θa =
θB and then sin
θB/sin
θc =
nc. Taking
nc = 1.55 as an appropriate value for the cuticles [
20,
21],
na = 1,
tc = ~100 nm,
ta = ~100 nm, and
θB = 25°, we can obtain
λp = ~480 nm for
m = 1 and
λp = ~240 nm for
m = 2. These wavelengths correspond to the observed peaks in the spectrum. As a result, this confirms the assumption of
nc = 1.55.
Figure 4.
Reflectance spectrum (UV and visible region) from different areas in the dorsal wings of the male
E. mulciber. Small caves at 375 nm were caused by a change of the incident light source so that they should be neglected. Inset is the spectrum of sunlight (adapted from [
11]).
Figure 4.
Reflectance spectrum (UV and visible region) from different areas in the dorsal wings of the male
E. mulciber. Small caves at 375 nm were caused by a change of the incident light source so that they should be neglected. Inset is the spectrum of sunlight (adapted from [
11]).
According to the dark zone mentioned above, the incident angle
θa which causes the observed reflection, is limited within 90° −
θB = 65° >
θa > −65° = −90° +
θB. Since sin
θa/sin
θc =
nc = 1.55, the wavelength of the reflected rays
λ must be ~510 >
λ > ~335 nm for
m = 1 and ~255 >
λ > ~168 nm for
m = 2, because the wavelength calculated from Equation (1) is
λ=335 nm at
θa = ±65° and
λ = 510 nm at
θa = 0 for
m = 1, and
λ = 168 nm at
θa = ±65° and
λ = 255 nm at
θa = 0 for
m = 2. Hence, human eyes, which respond to wavelengths from about 380 to 790 nm, can see the iridescent reflected rays only in a range from ~510 nm (green) to ~380 nm (violet), which is schematically illustrated in
Figure 2(g). The violet and green hues are observed in reflections from the scales in
Figure 2(b–d) and also
Figure 2(f). The incident (and reflection) angle
θa corresponding to
λ = 380 nm for
m = 1 can be calculated to be ±54.5°. Therefore, besides the dark zone where no reflection geometrically occurs [
8], human invisible zones due to UV reflection appear in an angle of
θ = 10.5° (= 90° − 25° − 54.5°) at both sides of the visible zone, as shown in
Figure 2(g). If the incident ray is not in the
x–
z plane, we can explain the reflection using the Ewald construction for the monoclinic lattice mentioned above. A diffractogram of a
W scale removed from the white patch in the male
S. charonda wing, may be regarded as a cross-section of the reciprocal lattice space used in the Ewald construction.
The tilting of the cuticles on the ridges, indicated in
Figure 3(h), forms a blazed grating. The blazed diffraction grating, illustrated in
Figure 2(h), is designed to obtain high diffraction efficiency for a certain order
m and wavelength [
30]. When the incident light and the
m-th order diffracted light are related by mirror reflection with each other on the facet surfaces, most of the incident energy is concentrated into the
m-th order diffracted light. This satisfies λ = (2
D/
m) sin
θB cos (α−
θB), where
D is the spacing or the grating period and α is the angle between the incident light
i and the grating normal
ns. The angle
θB is called blaze angle. The wavelength for
m = 1 and α =
θB, where the 1st-order diffracted light returns along the same path as the incident light, is called the blaze wavelength
λB, and then
λΒ = 2Dsin
θB. The blaze wavelength represents the blaze characteristics of the grating. For the
B scales
D = ~0.5 μm and
θB = ~25° so that the blaze wavelength is estimated to be
λΒ = ~400 nm. The high diffraction efficiency from this multilayered grating hence would be obtained in a low wavelength range of violet, which may be a reason of the heap of the spectrum in
Figure 4(a). Thus, the reason why the
E. mulciber butterfly wings exhibit vivid iridescent violet hues has been completely elucidated.
The reflectance of a white spot with
W scales, shown in
Figure 4, is greater than that of
B, and exceeds 10%, corresponding to its white hues. Heaps appear with peaks at ~500 and ~250 nm.
B and
W scales have almost the same microstructure and exhibit similar peaks in the reflectance spectra. Therefore it may be considered that the vivid blue coloration of
B is caused by melanin in the cuticle layers. According to Ou-Yang
et al. [
31], the typical absorbance spectrum of soluble eumelanin includes a linear increase of absorbance from 800 to 600 nm and an exponential increase of absorbance from 600 to 300 nm. In any case the strong reflection is caused from areas satisfying the interference condition, where the semitransparent brown cuticle layers also work as internal optical feedback reflectors. The parts that are out of the interference condition look dark brown due to the absorption of shorter wavelength rays in the incident rays (see
Figure 2(b–d)), reducing scattering light and working only as a dark background to make the reflection light stand out.
The reflectance spectra of other areas are also shown in
Figure 4. The scales in these areas were observed by SEM. The grey patch marked by
B3 has long flat fiber scales like sea tangles. The scales in the prominent pale brown costal area
B4 are dark brown with deep splits on the end and exhibit no iridescence with monolayer cuticle arrangement (see
Table 1). The scales in the
B5 area are dark brown, similar to
B and
W scales but not the same because of a double-layers arrangement of cuticles on the ridges. The spectrum of
B5 indicates that the double-layer arrangement is not enough to reflect observable interference light. The details have been reported in [
11].
Next, we brief the investigation on the
S. charonda butterfly, comparing it with and complimenting the information found on
E. mulciber mentioned above.
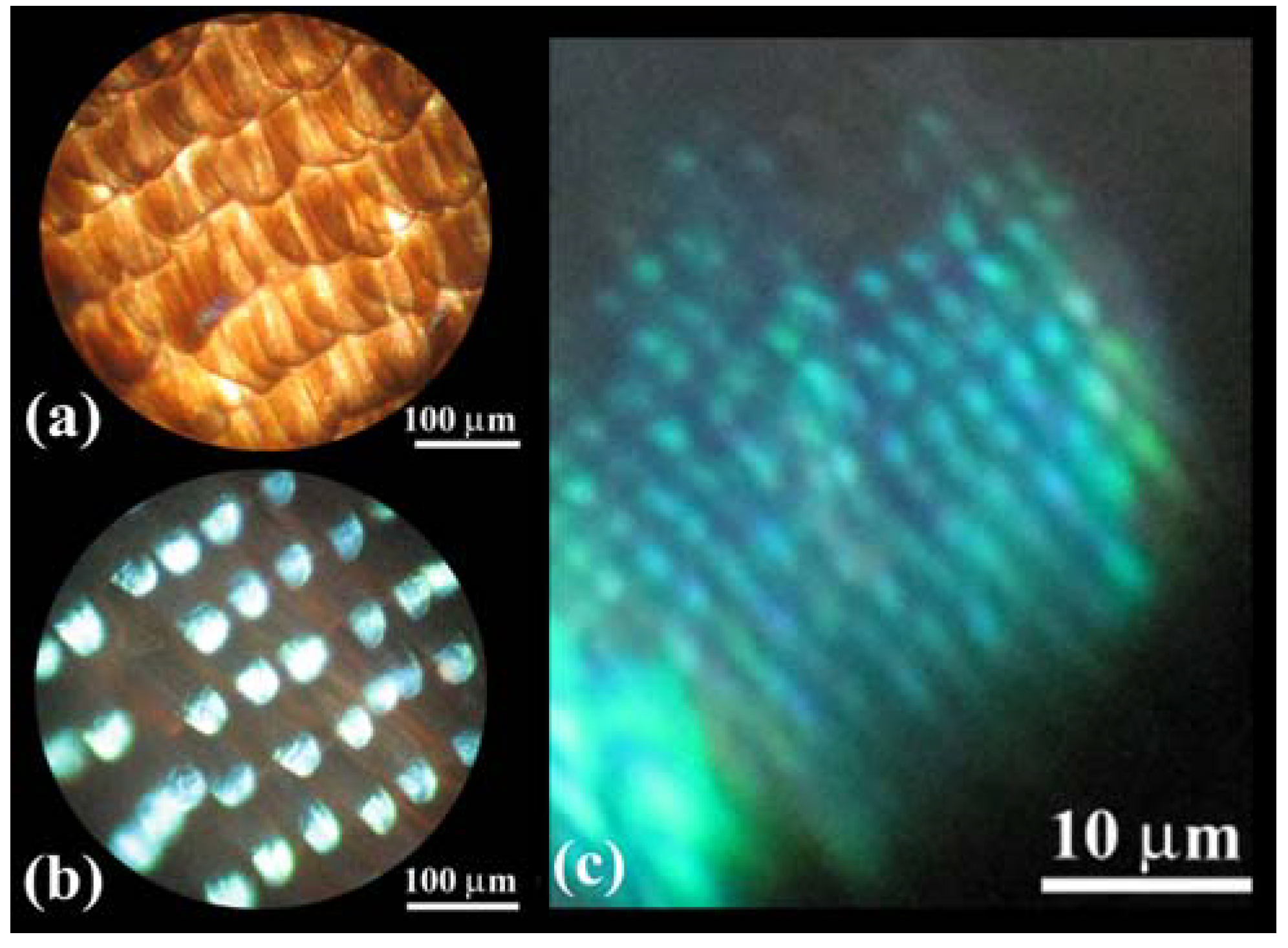
Figure 5(a) shows a transmission OM image of scales on the border between the purple-blue iridescent area (
B) and white patch (
W) in a forewing of the male butterfly.
Figure 5(b) is a reflection image. The observed brown scales
B and transparent scales
W seem very similar in optical property to the
B and
W scales of the male
E. mulciber, respectively.
Figure 5.
(
a,
b) OM images of scales on the border between the purple-blue iridescent area and white patch in a forewing of the male
S. charonda. Transmission image (
a) and reflection image (
b); (
c) The hindwing taken at a low glancing angle under sunlight (adapted from [
13]).
Figure 5.
(
a,
b) OM images of scales on the border between the purple-blue iridescent area and white patch in a forewing of the male
S. charonda. Transmission image (
a) and reflection image (
b); (
c) The hindwing taken at a low glancing angle under sunlight (adapted from [
13]).
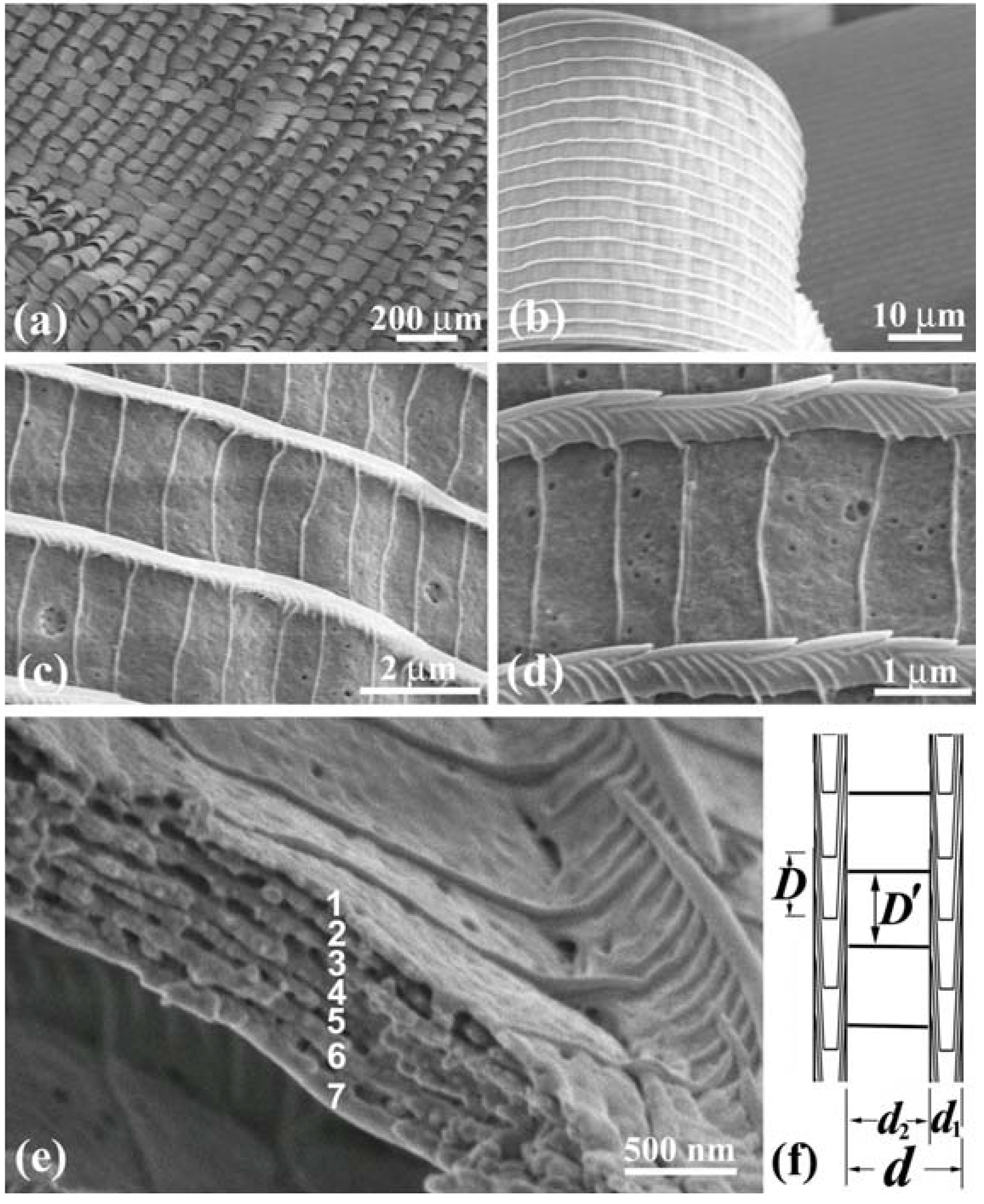
Figure 6 shows SEM images of the
W scales that were removed from the wings. During the preparation they were bent and broken, and thus allowed us to observe the side and broken cross-section as well as the top of the cuticles lapped on the ridges. The images reveal the highly tilted, multilayered arrangement and the three-dimensional grating structure of the
W scale. The SEM observation also showed that the
B and
W scales are the same in microstructure. The difference between the
B and
W scales is only the content of melanin. The parameters of the diffraction grating of these scales are shown in
Table 1 [
13]. The
B and
W scales of the male
S. charonda have seven cuticle layers piled on the ridges, that is
n = 7. They also have the wide ridges, which is recognized from a ratio
d1/
d of ~2/3 as compared with ~1/3 for the
B scales of
E. mulciber (see
Table 1). The seven cuticle layers on the wide ridges would cause a stronger iridescence—due to multiple interference—than found with
E mulciber (
n = 3) and
A. meliboeus (
n = 4). For the scales of
S. charonda, the tilting angle of cuticle layers or the blaze angle is
θB = 8° so that the dark zone shown in
Figure 3(g) is within 2
θB~16°. A photograph of the hindwing taken at a low glancing angle under sunlight is shown in
Figure 5(c), which clearly indicates the dark zone.
Figure 6.
(
a) SEM image of a
W scale removed from the white patch in the male
S. charonda wing. The inset is a computer diffractogram from the area enclosed by the square; (
b) The side view and top view of the cuticles piled on the ridges; (
c) The cross-sectional view of cuticle layers.
B scales in the purple-blue iridescent area have the same microstructure (adapted from [
13]).
Figure 6.
(
a) SEM image of a
W scale removed from the white patch in the male
S. charonda wing. The inset is a computer diffractogram from the area enclosed by the square; (
b) The side view and top view of the cuticles piled on the ridges; (
c) The cross-sectional view of cuticle layers.
B scales in the purple-blue iridescent area have the same microstructure (adapted from [
13]).
The
B1,
W1, and
R1 scales, which are named for the scales in the dark brown area, the yellowish patches and the small orange spots, respectively, have the same microstructure (
Table 1). The difference is ascribed to the species and quantity of the contained melanin; perhaps brown-black dihydroxyindole eumelanin and red-brown benzothiazine pheomelanin. The small ratio of
d1/
d = ~0.27 and the number of the piled cuticle layers of
n = 2 may be insufficient to develop the iridescence hues. Rather, the incident light, particularly UV radiation, may be absorbed and converted to heat energy by melanin. The female butterflies have bigger brown wings without iridescent scales, which significantly reduce the penetration of light due to reflection. The females thereby can receive more heat energy, to be used for breeding, from sunshine than the male butterflies. Thus, the wing structure of the butterflies may give us a hint to design photochemical crystal devices for internal conversion or radiationless de-excitation where the UV radiation is transformed into heat [
13].
Imafuku
et al. [
32] measured wing colors of
Chrysozephyrus butterflies with a spectrophotometer, and reported that the dorsal wing surface of a male
C. ataxus shows a strong reflectance when the specimen is tilted and that it appears green to the human eye, reflecting UV as well as green light.
Table 2 was made using their results, especially the reflectance/wavelength curves displayed in
Figure 7 in their paper. The green hues in
Figure 1(c) correspond to the reflection of a very broad peak at 547 nm with a full-width at half-maximum (FWHM) of ~125 nm. In the measurement shown in
Table 2, the incident light was applied to a wing piece, and the reflected light that returned in the same course was measured [
32]. This means that the reflectance measured was from the surfaces whose normal is parallel to the incident light. If the groove plates cause multi-reflection between the layers with air gaps, the reflection is observed when the following interference condition is satisfied:
We can take
nc = 1.55 for the layer substance as mentioned in
Section 3.1 and
na = 1. Assuming
m = 1 for the observed reflection peak of
λP = 547 nm, (1.55
tc + ta) = 274 nm, and the corresponding wavelengths of the reflection for
m = 2 and
m = 3 must be 274 nm and 182 nm, respectively. However, the observed peaks did appear neither at 274 nm nor at 182 nm in the spectrum measured by Imafuku
et al. [
32]. This suggests that the multiple layers in the groove plates cannot be considered as the structure creating the multiple interference but they must be incoherent with each other. This is natural because their surfaces may not be completely parallel to each other. Then, we consider the interference for one layer, where the reflection takes place under the following condition:
Figure 7.
OM images of the dorsal wing scales of the male
C. ataxus taken by white light. (
a) Transmission image; (
b) Reflection image; (
c) Enlarged image of a scale in (b) (adapted from [
14]).
Figure 7.
OM images of the dorsal wing scales of the male
C. ataxus taken by white light. (
a) Transmission image; (
b) Reflection image; (
c) Enlarged image of a scale in (b) (adapted from [
14]).
Table 2.
Wavelength λ
P, FWHM and reflectance
rP of the peaks on the reflectance spectra, which were measured for the iridescent wings of the male and female
C. ataxus butterflies by Imafuku
et al. [
32].
Table 2.
Wavelength λP, FWHM and reflectance rP of the peaks on the reflectance spectra, which were measured for the iridescent wings of the male and female C. ataxus butterflies by Imafuku et al. [32].
| Sex | λP (nm) | FWHM (nm) | rP (%) | λP (nm) | FWHM (nm) | rP (%) | λP (nm) | FWHM (nm) | rP (%) |
|---|
| Male | 257 * | 35 * | 25 * | 341 ± 5 | 89 * | 39 * | 547 ± 4 | 125 * | 36 * |
| Female | 252 * | 30 * | 13 * | 395 | 134 * | 43 * | | | |
Taking
m = 1 for
λP = 547 nm and
m = 2 for
λP = 341 nm, we obtain thicknesses of
tc = 265 and 275 nm, respectively. We thereby acquire a reasonable thickness of the flat groove layers of
t = ~270 nm. Although we could not estimate the thickness from
Figure 8(e) because the layer surfaces were not perpendicular to the imaging plane of SEM, it is not completely inconsistent with the images. The observed UV peak of
λP = ~257 nm is then regarded as the reflection of
m = 3. Thus, each layer reflects incoherently or kinematically with others, like a particle of the mosaic structure in X-ray diffraction, and the reflectance intensity is a simple sum of the reflection from each layer. Imafuku
et al. [
32] mentioned that the wing surface shows a strong reflectance when the specimen is tilted. This is simply explained as a geometric result of the increase of the area on curled scales that is normal to the incident light and of the number of the scales irradiated by the incident light as the specimen is tilted. When we observe the wing obliquely at a glancing angle of
θ, the strong reflection is obtained if the diffraction condition
is satisfied, where
θa = 90° −
θ and sin
θa/sin
θc =
nc. Since −1 ≤ sin
θa =
nc sin
θc ≤ 1 and 0 ≤ cos
θc ≤ 1, taking
t = ~270 nm the satisfied wavelength is calculated as ~558 ≥ λ ≥ ~426 nm for
m = 1 and ~335 nm ≥λ ≥ ~256 nm for
m = 2. The human eye perceives only the reflected light in a range of ~558 (green) > λ > ~426 nm (violet) in the glancing angle 180° ≥
θ ≥ 0. Each scale is highly curved as can be seen in
Figure 8, so that some part of the scale satisfies the interference condition and gives a strong reflection, as seen in
Figure 7(b). That is the color of the male
C. ataxus butterfly in
Figure 1(c).
There is a report on the curled scales in the wing of
Chrysiridia rhipheus (the Madagascan sunset moth) [
20]. From SEM observation of a thin width of
d1 = 0.4 μm and a large separation of
d2 = 3.5 μm of the ridge it is assumed that the reflection of the scale is mostly due to the basal layer (in grove) of the scale, and from cross-sectional transmission electron microscopy that the basal layer consists of air-cuticle alternate layers all over the scale. It concluded that this groove structure together with multilayer optical interference produces an unusual optical effect through an inter-scale reflection mechanism; thereby the wing color changes depending on light polarization. As a result, the coloration of the male
C. ataxus dorsal wing is completely different from that of the
Chrysiridia rhipheus although they have similar multiple layers on the basal flat areas between the ridges. It should be noted that the vivid coloration of
C. ataxus is not ascribed to the multiple interference between the piled layers but to the interference between the top and bottom surfaces of each layer. The rays reflected from different layers are incoherent with each other.
Figure 8.
SEM images of the dorsal scales of the male
C. ataxus. (
a) Law-magnified image of curled scales; (
b) A curled scale; (
c) Ridges and cross ribs in the scale; (
d) Enlarged image of the ridges and ribs; (
e) Cross-section of a groove enclosed by the ridges and ribs, revealing seven piled layers; (
f) Schematic of the fine structure of the scale (adapted from [
14]).
Figure 8.
SEM images of the dorsal scales of the male
C. ataxus. (
a) Law-magnified image of curled scales; (
b) A curled scale; (
c) Ridges and cross ribs in the scale; (
d) Enlarged image of the ridges and ribs; (
e) Cross-section of a groove enclosed by the ridges and ribs, revealing seven piled layers; (
f) Schematic of the fine structure of the scale (adapted from [
14]).
Figure 9(a) shows scales in a violet mark in the female’s dorsal forewing. A vein is observed near the upper right corner. Some scales in the cell are curled and the others are almost flat exhibiting the slit top tails. The image in
Figure 9(b) comprises two scales: One has flat layers in the areas enclosed by the ridges and ribs (left) and the other has no layers (right). The former is a scale exhibiting violet hues, and the latter is a scale on the vein and similar to the scales exhibiting dark brown (
Table 1).
Figure 9(c) shows an enlarged image of the violet scale, whose structure is similar to that of the male’s scales in
Figure 8 although it has many holes. As shown in
Table 1, the ratio
d1/
d is small and
n = 1 so that the contribution of the cuticles on the ridges to the color hues would be small. As seen in
Figure 9(d), the groove plates take the multilayered structure of triple layers. We then estimated the thickness of the layers, similar to the calculation for the male wing, using the data shown in
Table 2 and Equation (3). The observed strong intensity of
λP = 395 nm with
rP = 43% can be regarded as the first order reflection (
m = 1) from the layer with a thickness of
tc = ~191 nm. We do not take
tc = ~319 nm for
m = 2 because no reflection corresponding to
m = 1 appeared at 659 nm in the experiment [
32]. The small thickness of the layers is confirmed by 1.5 kV SEM image in
Figure 9(a), where the underlying scales are recorded through their upper scales as indicated by arrowheads. An FWHM as large as134 nm is attributed to a large variation of the thickness among layers and the curl of the scales. In any case the possible reflection in the overall angle 180° ≥
θ ≥ 0 occurs only for the rays in wavelength ranges of ~400 ≥ λ ≥ ~300 nm (
m = 1) and ~240 ≥ λ ≥ ~180 nm (
m = 2). Hence, the female wing’s marks look violet in the range of human visibility.
We thus gave a reasonable explanation for the coloration of the male and female
C. ataxus butterfly’s wings on the basis of our SEM observation and optical data reported by Imafuku
et al. [
32].
Figure 9.
SEM images of the dorsal scales of the female
C. ataxus. (
a) Law-magnified image; (
b) Ridges and cross ribs in a violet scale (left) and a dark brown scale (right); (
c) Enlarged image of the ridges and ribs in the violet scale; (
d) Cross-section of a groove plate enclosed by the ridges and ribs, revealing triple piled layers (adapted from [
14]).
Figure 9.
SEM images of the dorsal scales of the female
C. ataxus. (
a) Law-magnified image; (
b) Ridges and cross ribs in a violet scale (left) and a dark brown scale (right); (
c) Enlarged image of the ridges and ribs in the violet scale; (
d) Cross-section of a groove plate enclosed by the ridges and ribs, revealing triple piled layers (adapted from [
14]).