Influence of pH and Chloride Concentration on the Corrosion Behavior of Unalloyed Copper in NaCl Solution: A Comparative Study Between the Micro and Macro Scales
Abstract
:1. Introduction
2. Results and Discussion
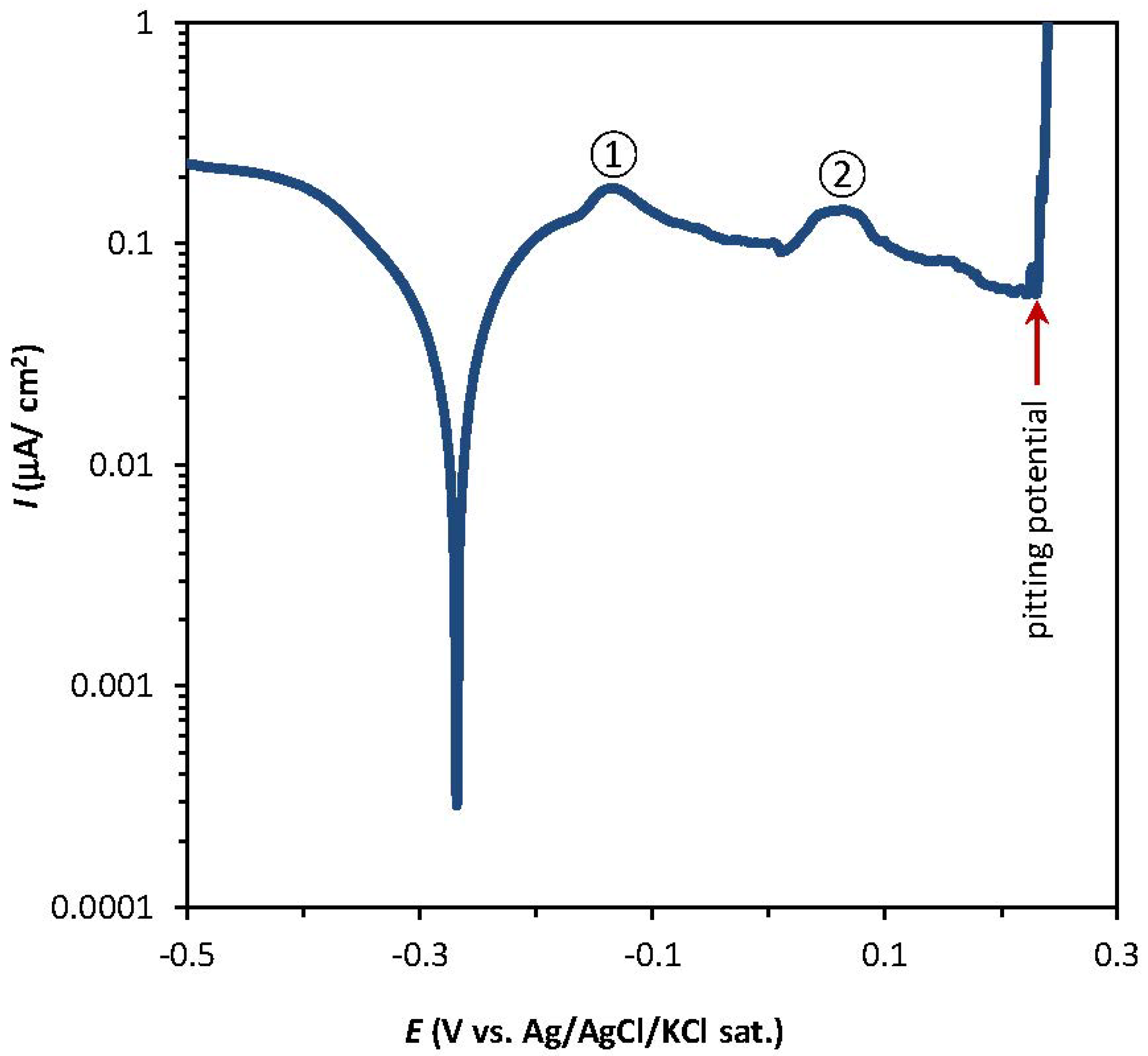
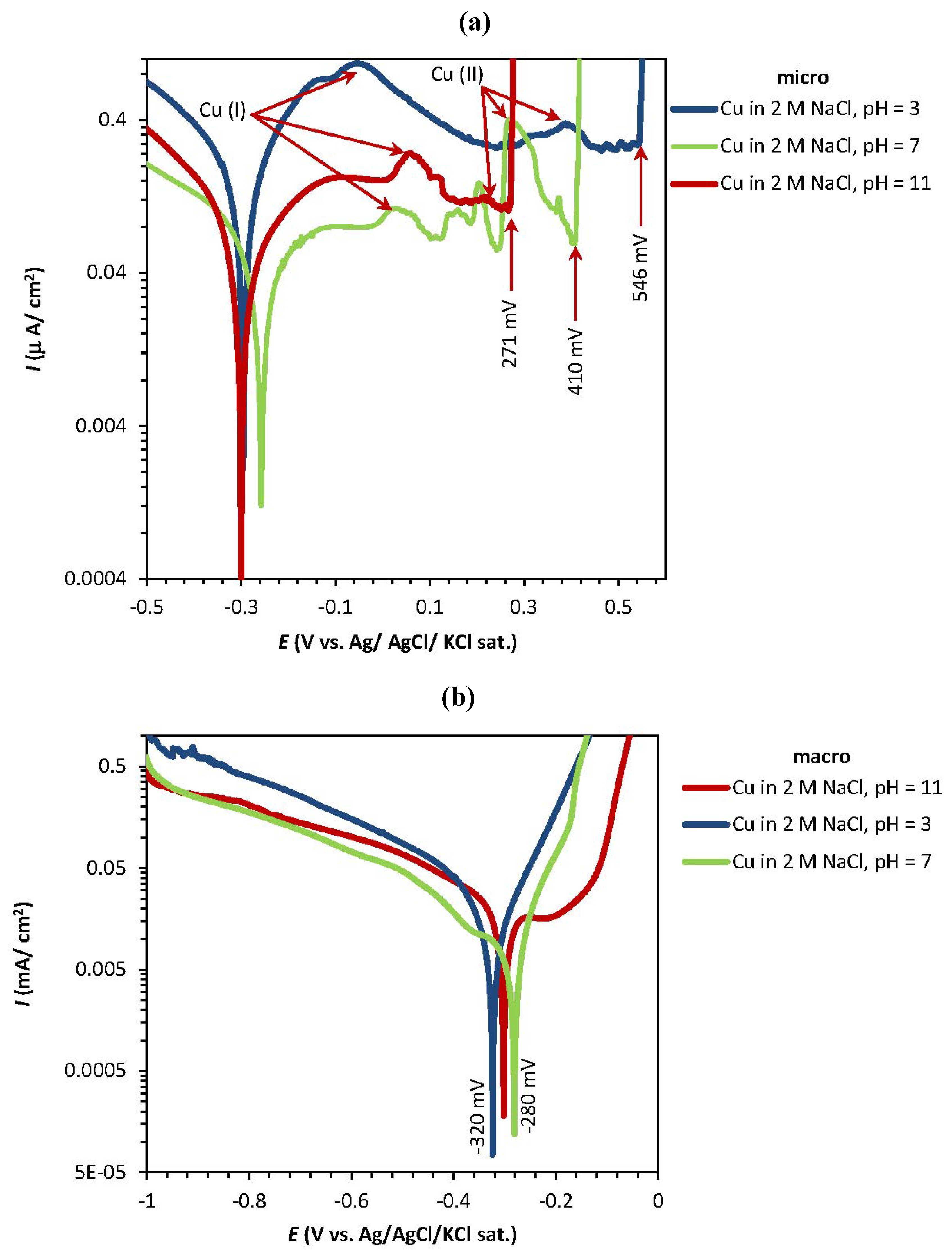
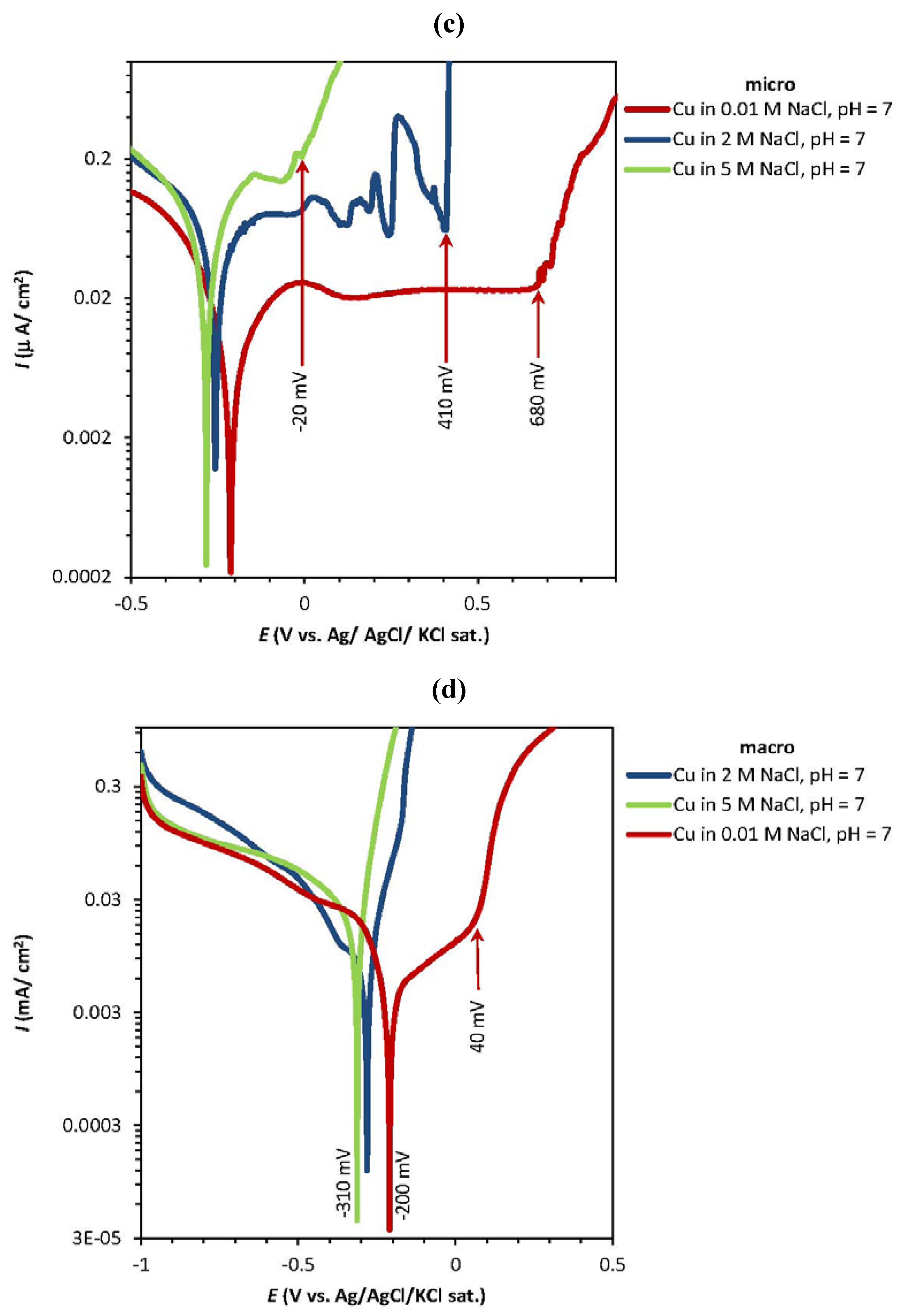
2.1. Potentiodynamic and Potentiostatic Measurements
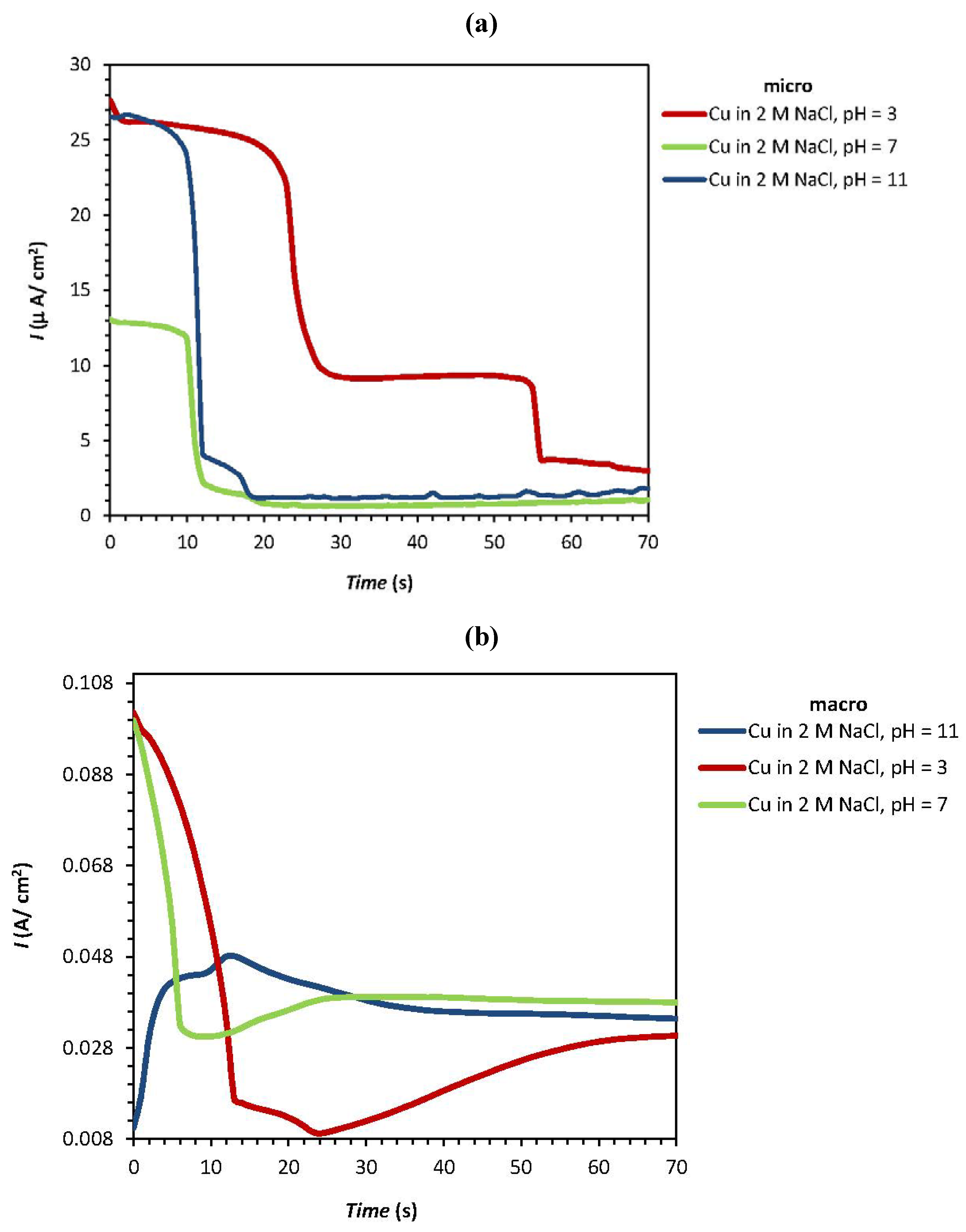
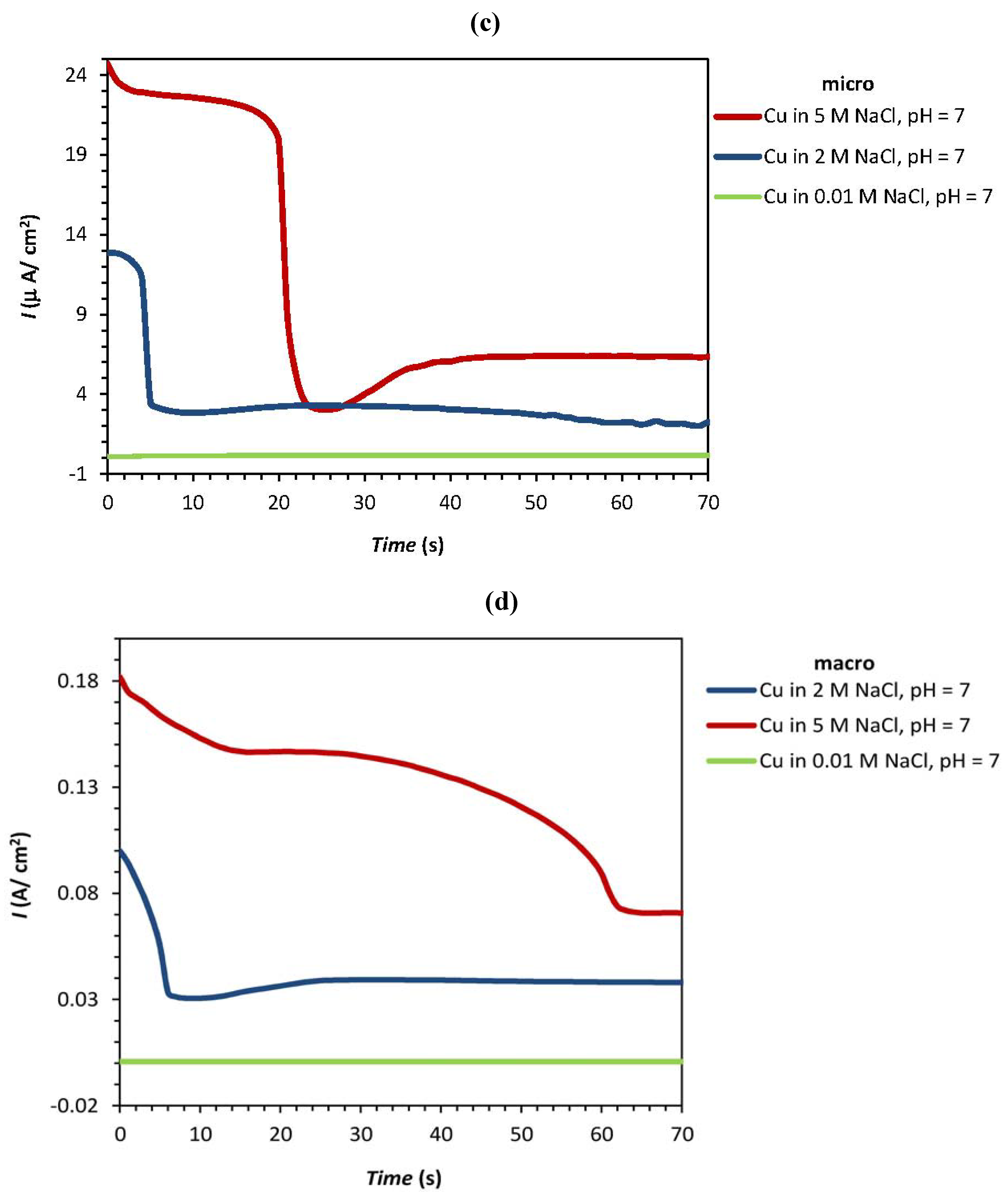
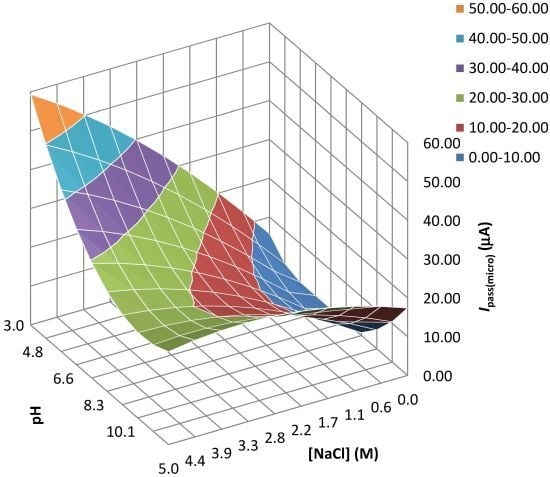
| Experiment | F1: [NaCl] (M) | F2: pH | Epit (micro) (V) | Ecorr(macro) (V) | Ipass(micro) (µA/cm2) | Ipass(macro) (A/cm2) |
|---|---|---|---|---|---|---|
| 1 | 0 | 2 | 0.186 | −0.302 | 26.5 | 0.0105 |
| 2 | 0 | −2 | 0.167 | −0.324 | 27.15 | 0.102 |
| 3a | 0 | 0 | 0.410 | −0.281 | 13.1 | 0.099 |
| 4 | 1.414 | −1.414 | 0.374 | −0.310 | 44.35 | 0.142 |
| 5 | 2 | 0 | −0.063 | −0.313 | 24.7 | 0.182 |
| 6a | 0 | 0 | 0.364 | −0.287 | 12.9 | 0.0864 |
| 7a | 0 | 0 | 0.373 | −0.280 | 12.9 | 0.0946 |
| 8 | −1.414 | 1.414 | 0.539 | −0.247 | 7.83 | 0.0314 |
| 9 | −1.414 | −1.414 | 0.311 | −0.239 | 9 | 0.0327 |
| 10 | −2 | 0 | 0.781 | −0.210 | 0.102 | 0.0007 |
| 11 | 1.414 | 1.414 | 0.241 | −0.332 | 22.65 | 0.163 |
| 12a | 0 | 0 | 0.396 | −0.262 | 13.25 | 0.080 |
| Coded value (−2) | 0.01 | 3 | – | – | – | – |
| Coded value (−1) | 0.59 | 4.1 | – | – | – | – |
| Coded value (0) | 2 | 7 | – | – | – | – |
| Coded value (+1) | 3.828 | 9.8 | – | – | – | – |
| Coded value (+2) | 5 | 11 | – | – | – | – |
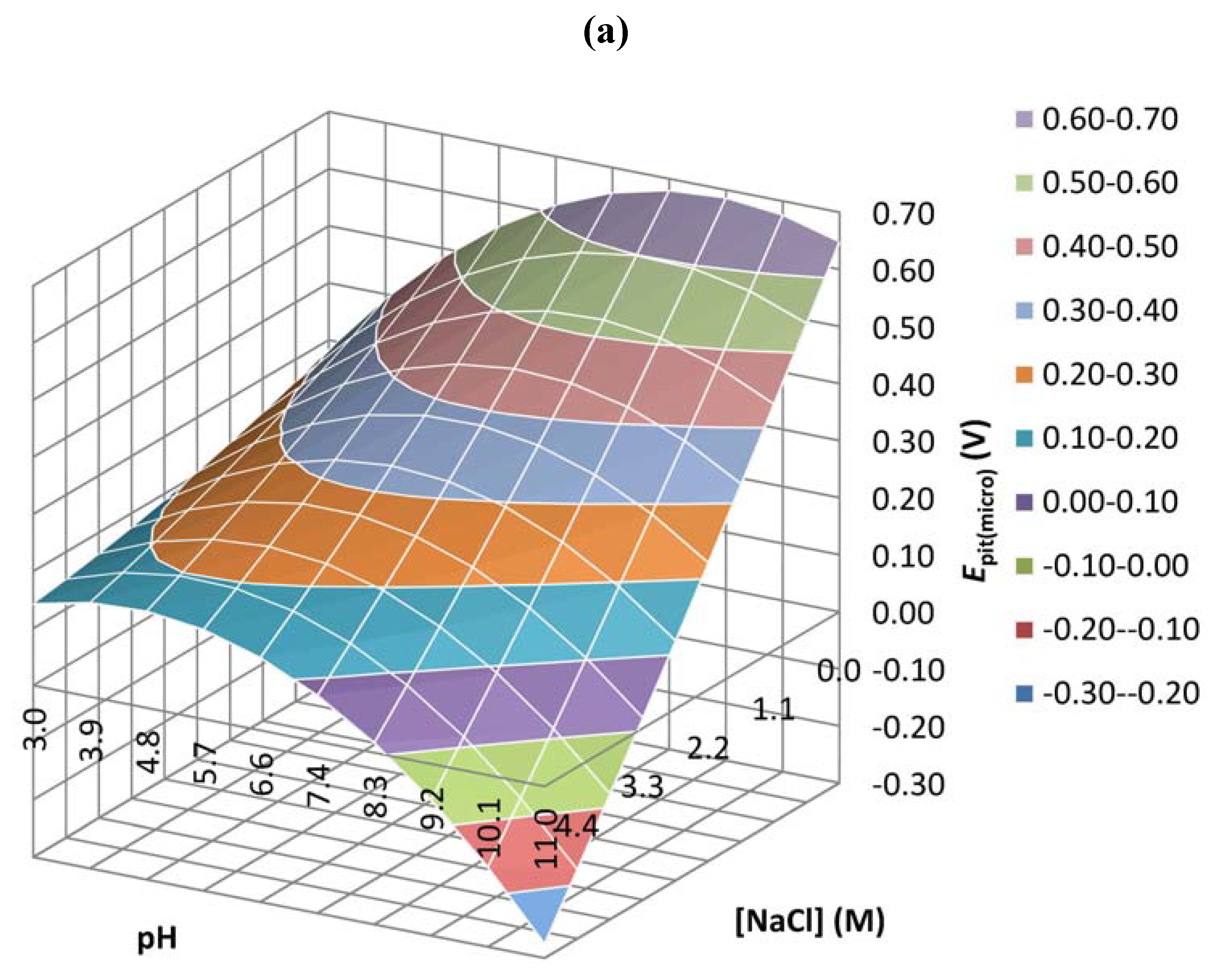
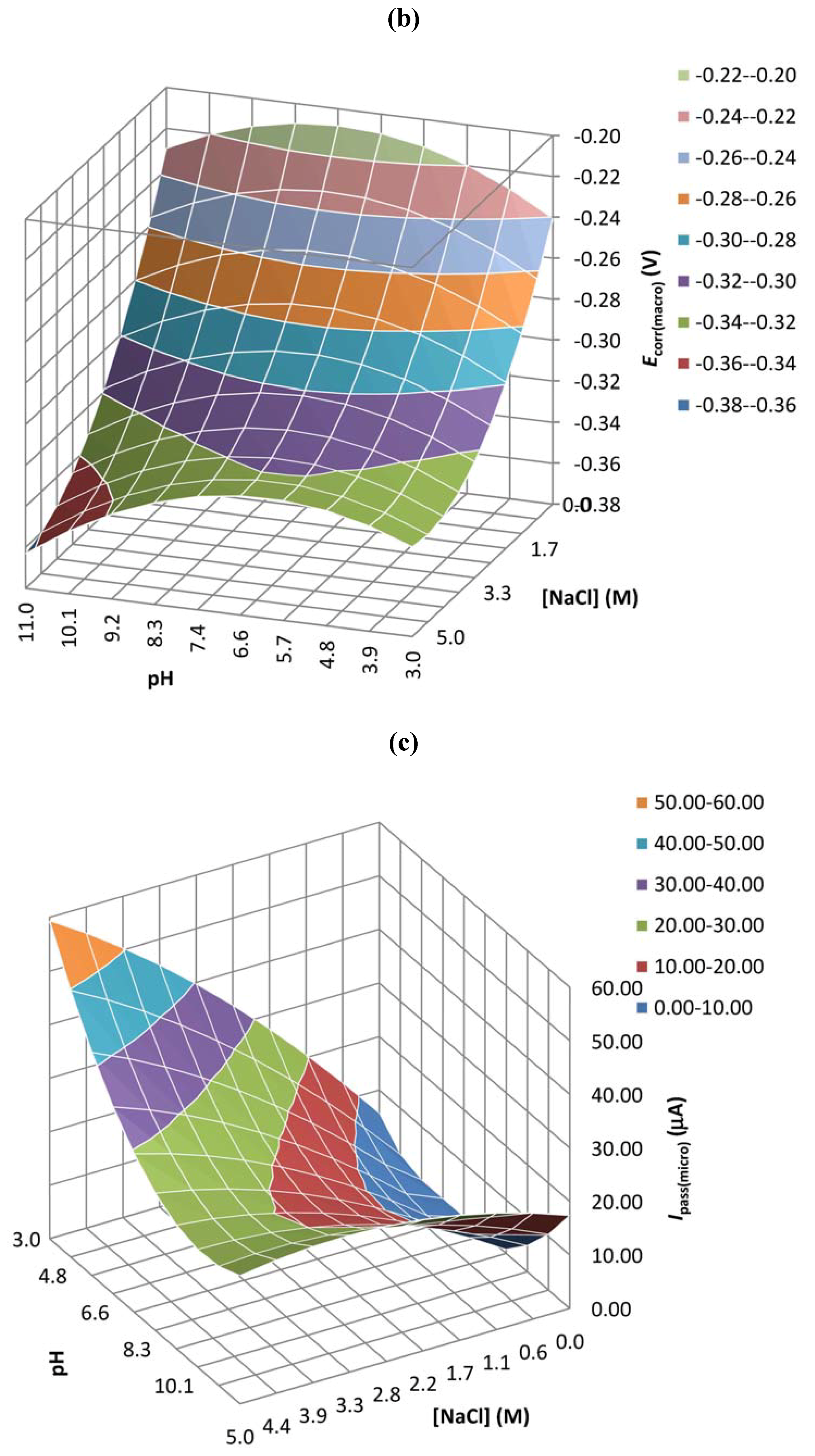
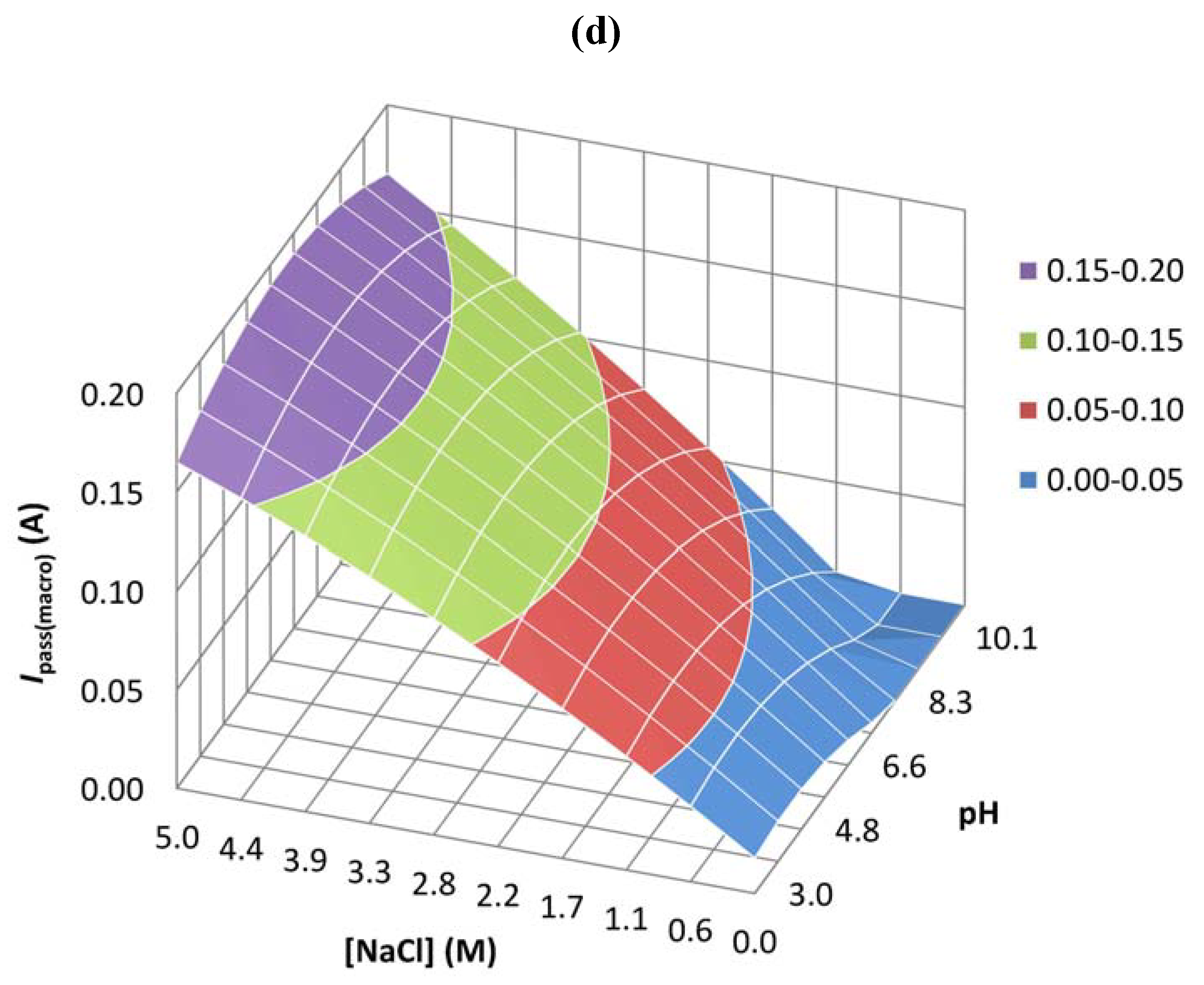
| Coefficient | Epit(micro) | Ipass(micro) | Ecorr(macro) | Ipass(macro) |
|---|---|---|---|---|
| b0 | 0.647 | 0.029 | 0.000 | 0.905 |
| b1 | 0.910 | 0.003 | 0.021 | 0.238 |
| b2 | 0.119 | 0.005 | 0.017 | 0.479 |
| b3 | 0.894 | 0.200 | 0.024 | 0.632 |
| b4 | 0.189 | 0.002 | 0.018 | 0.240 |
| b5 | 0.260 | 0.002 | 0.496 | 0.540 |
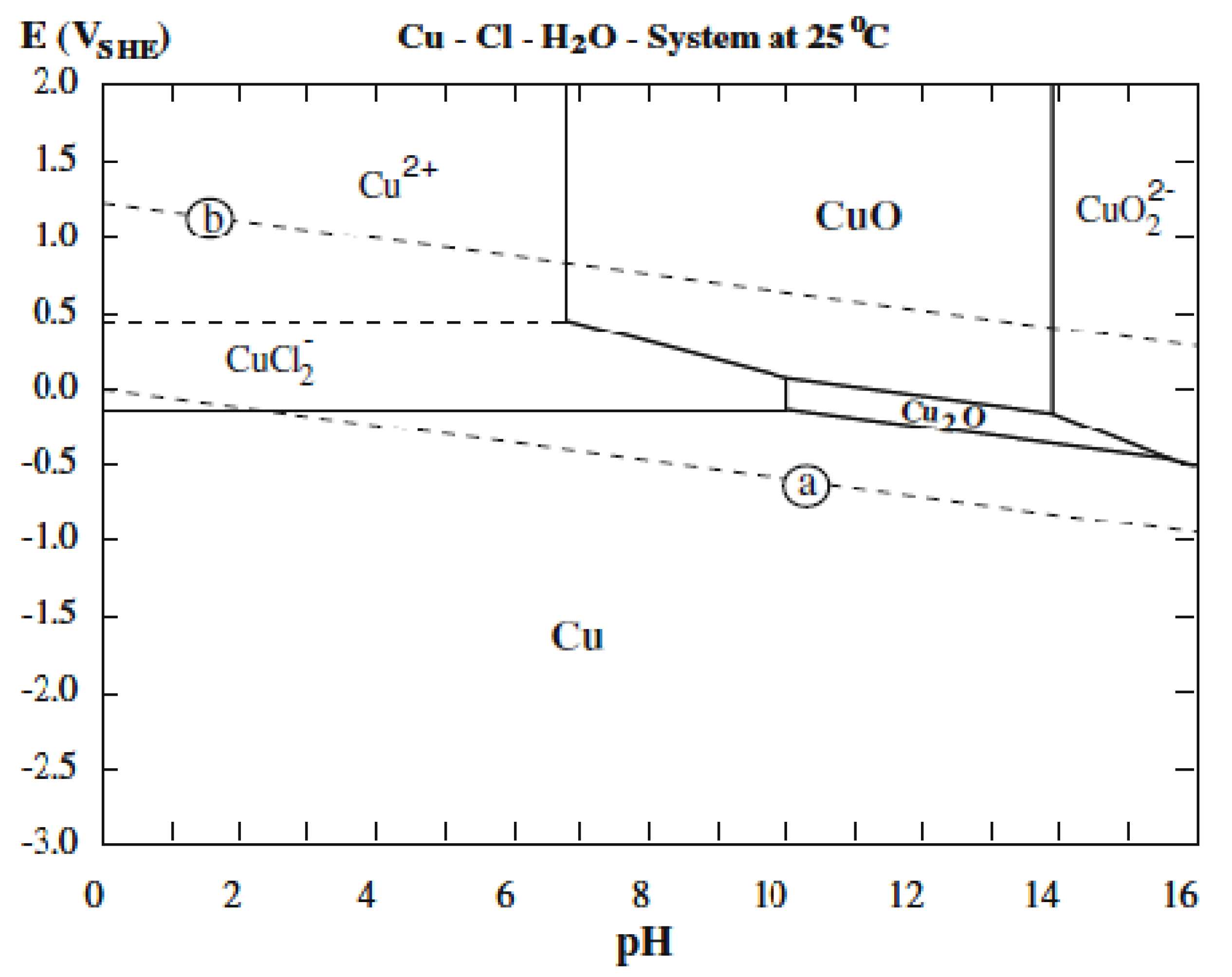
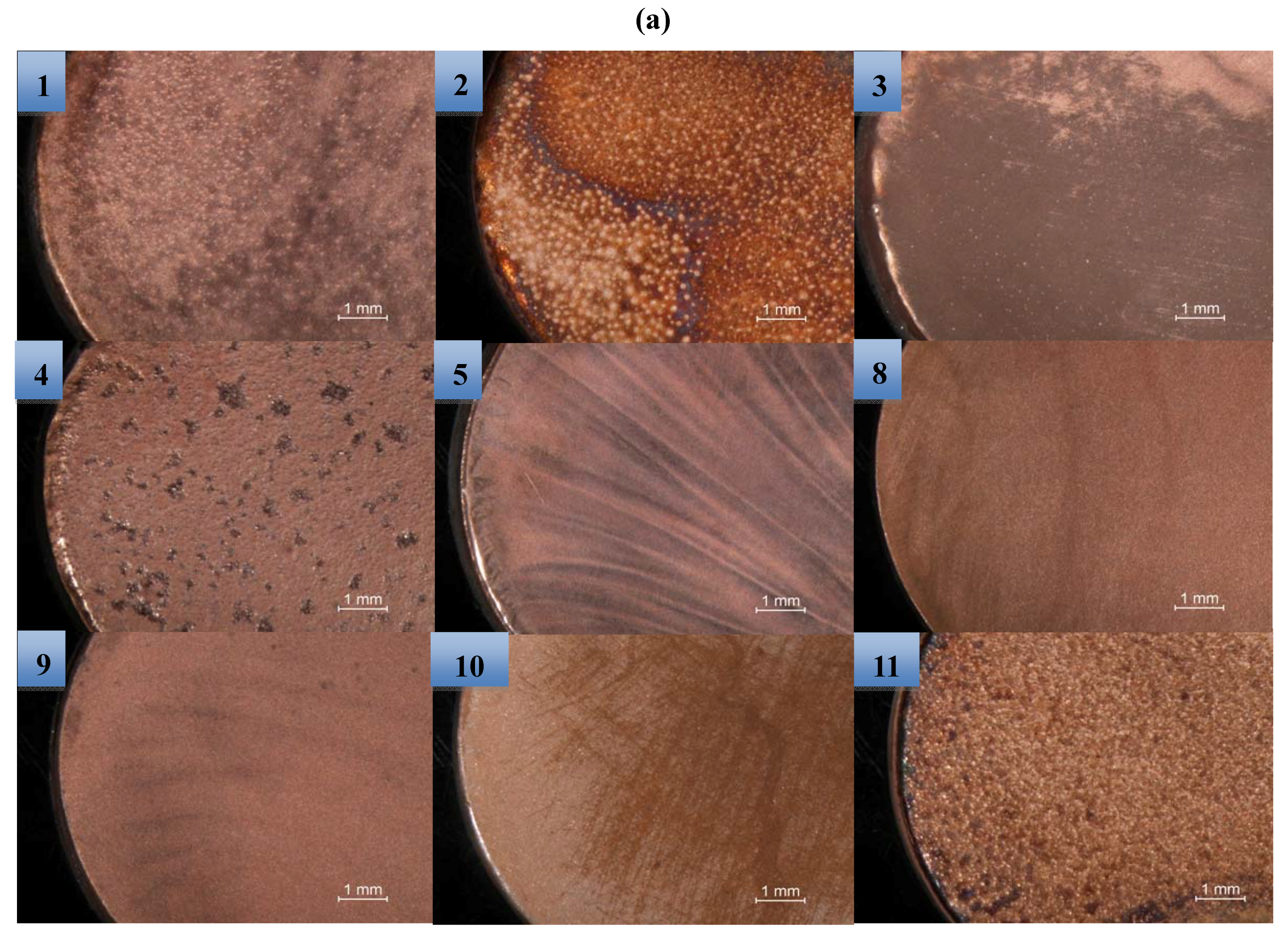
2.2. Surface Morphology
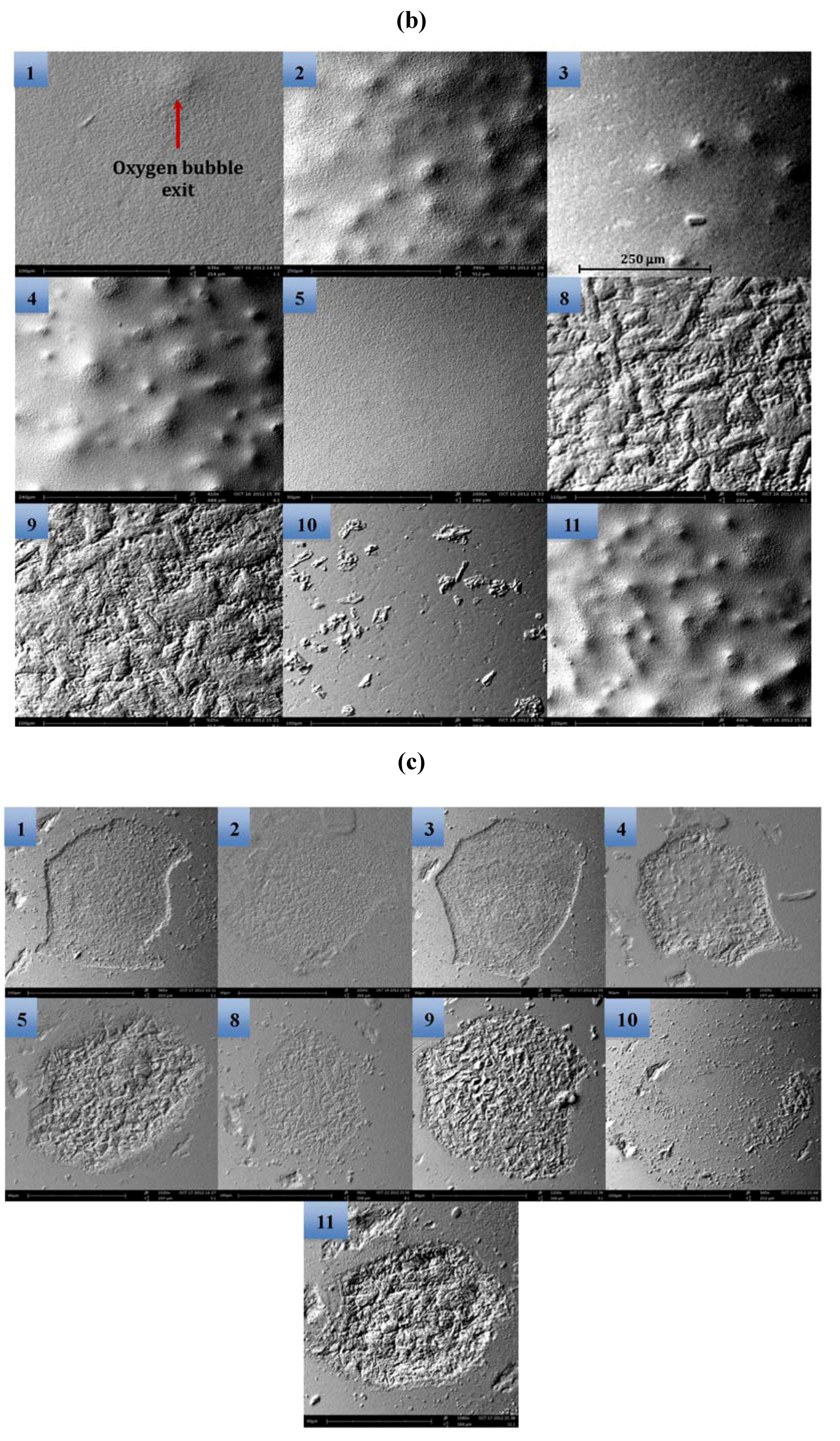
2.3. Electrochemical Impedance Spectroscopy (EIS)
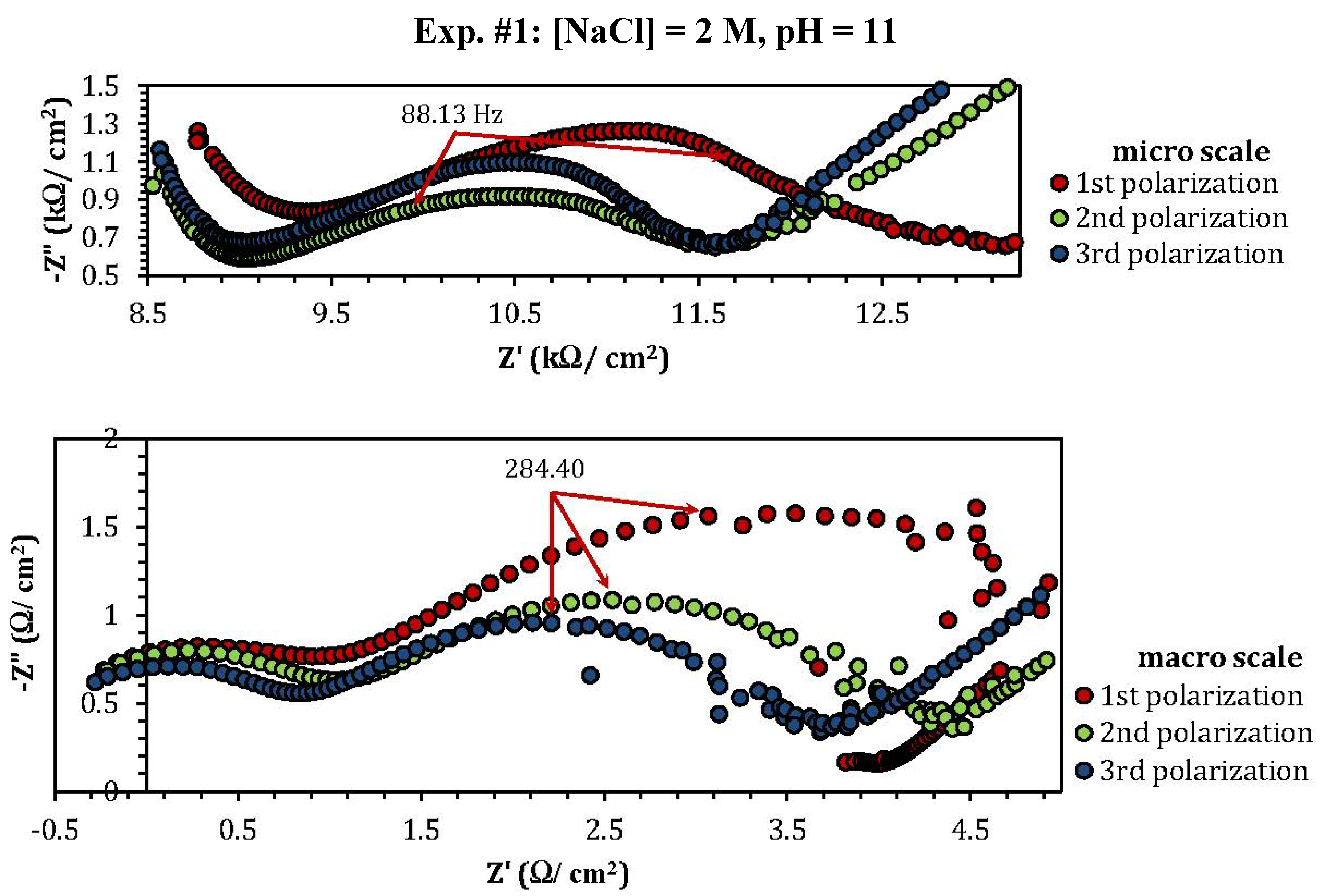
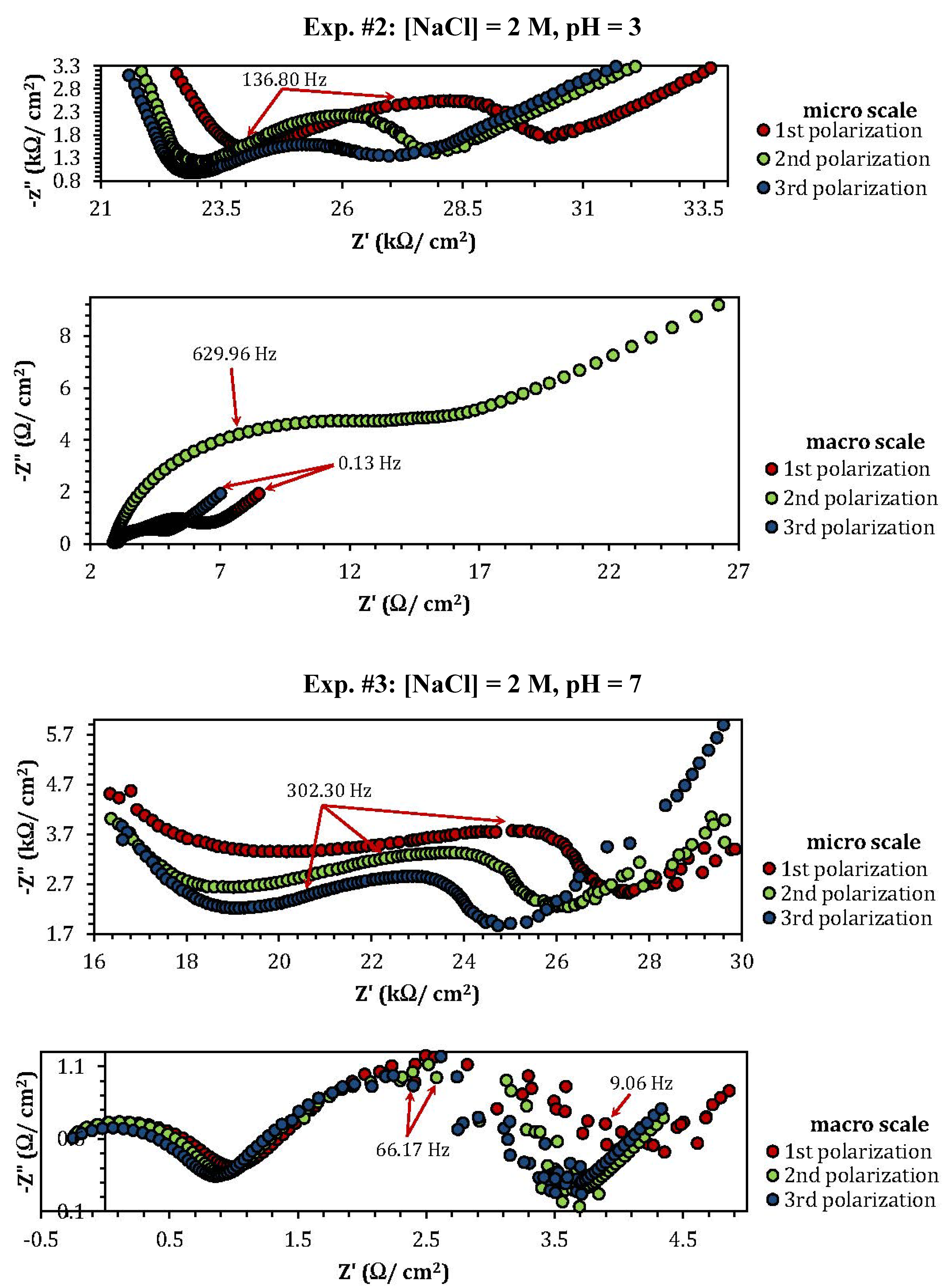
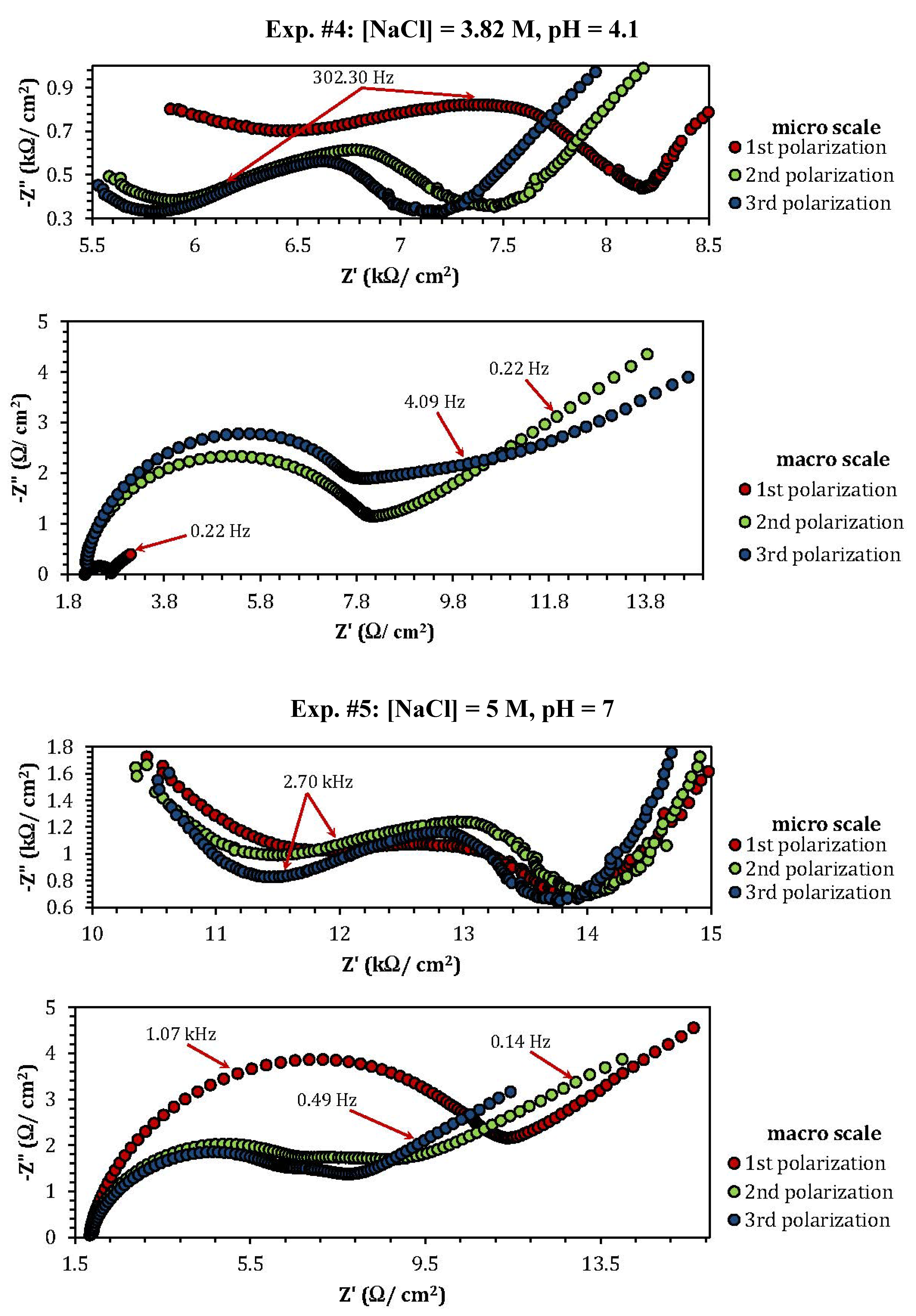
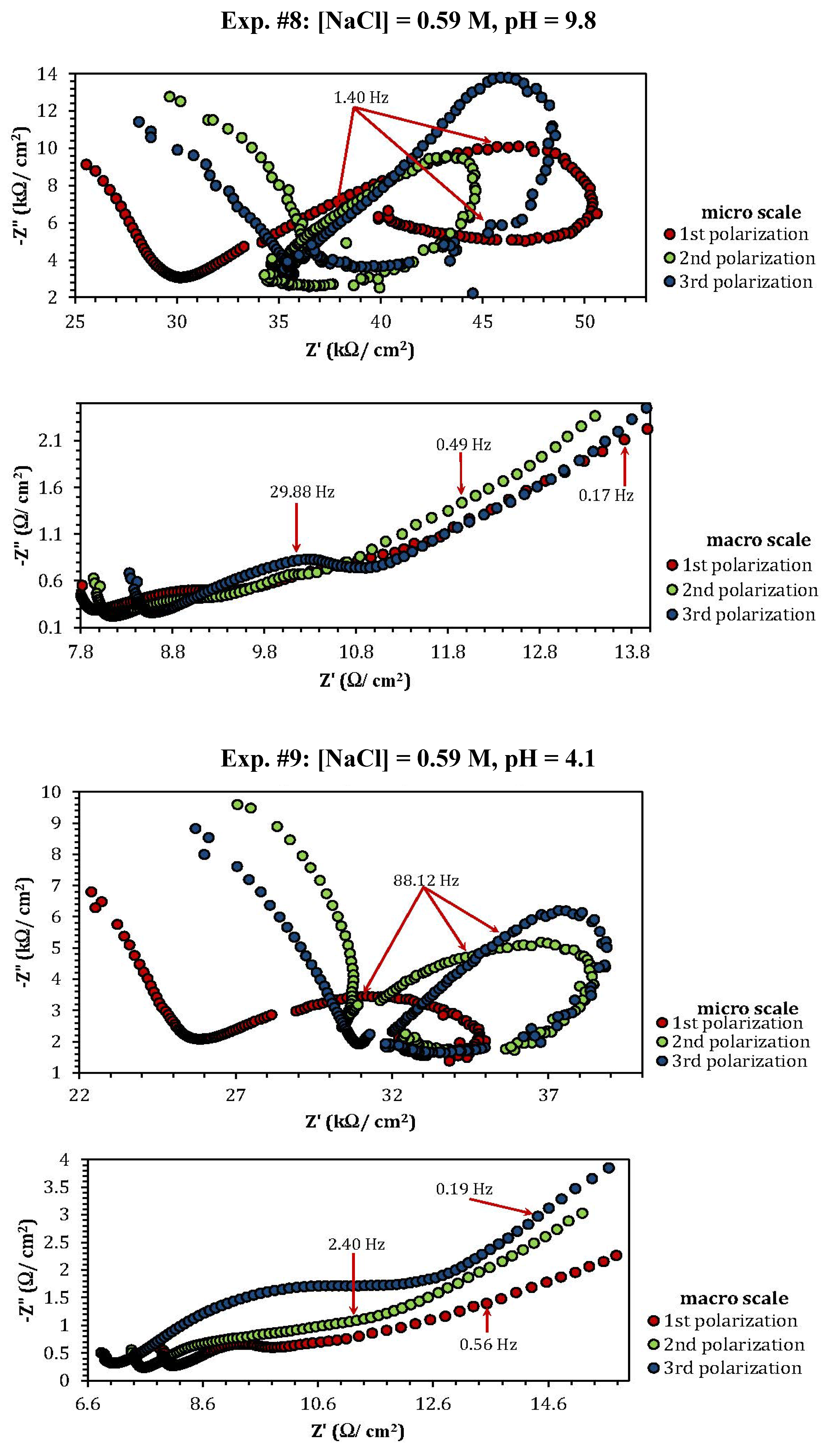
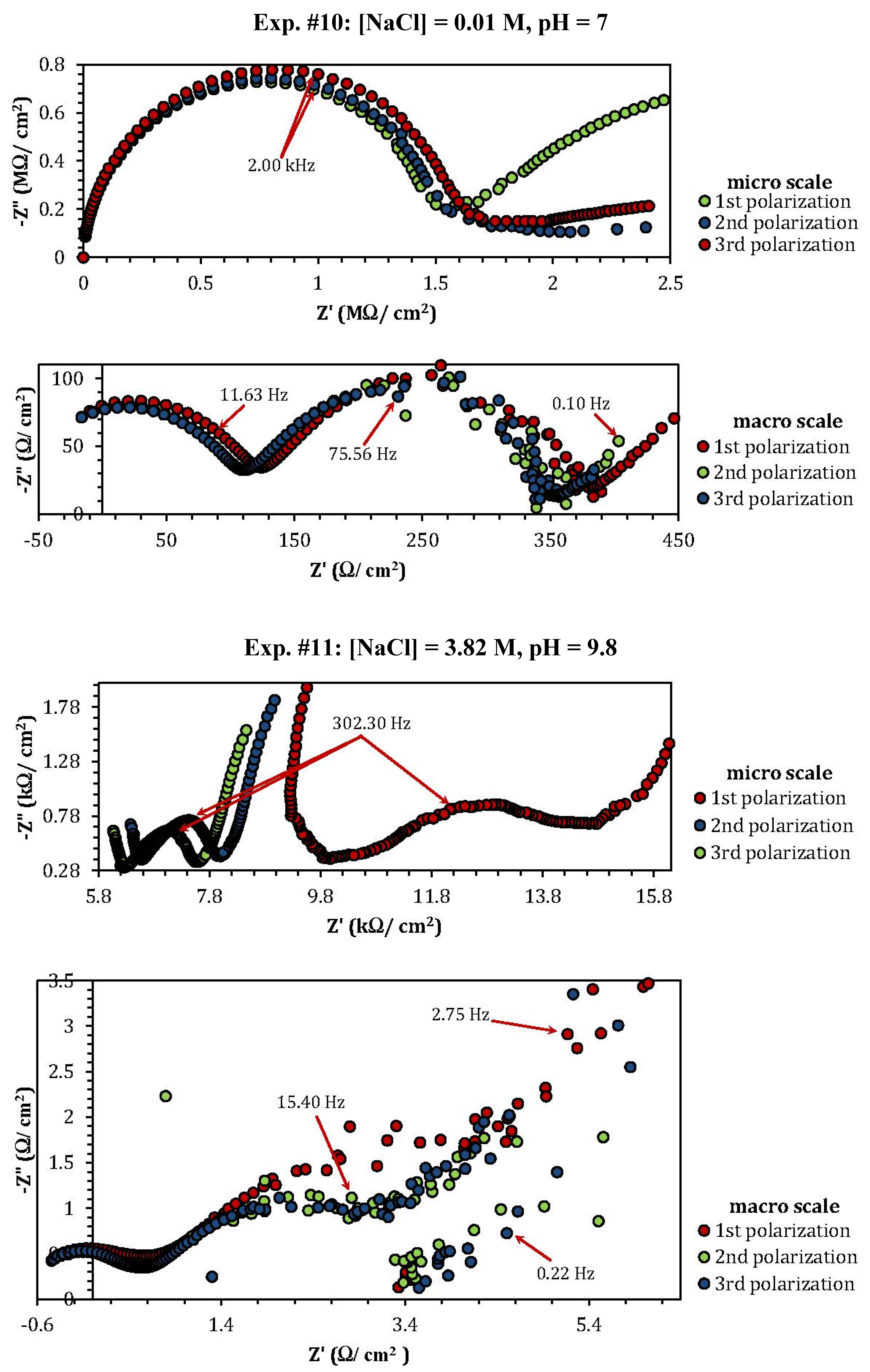
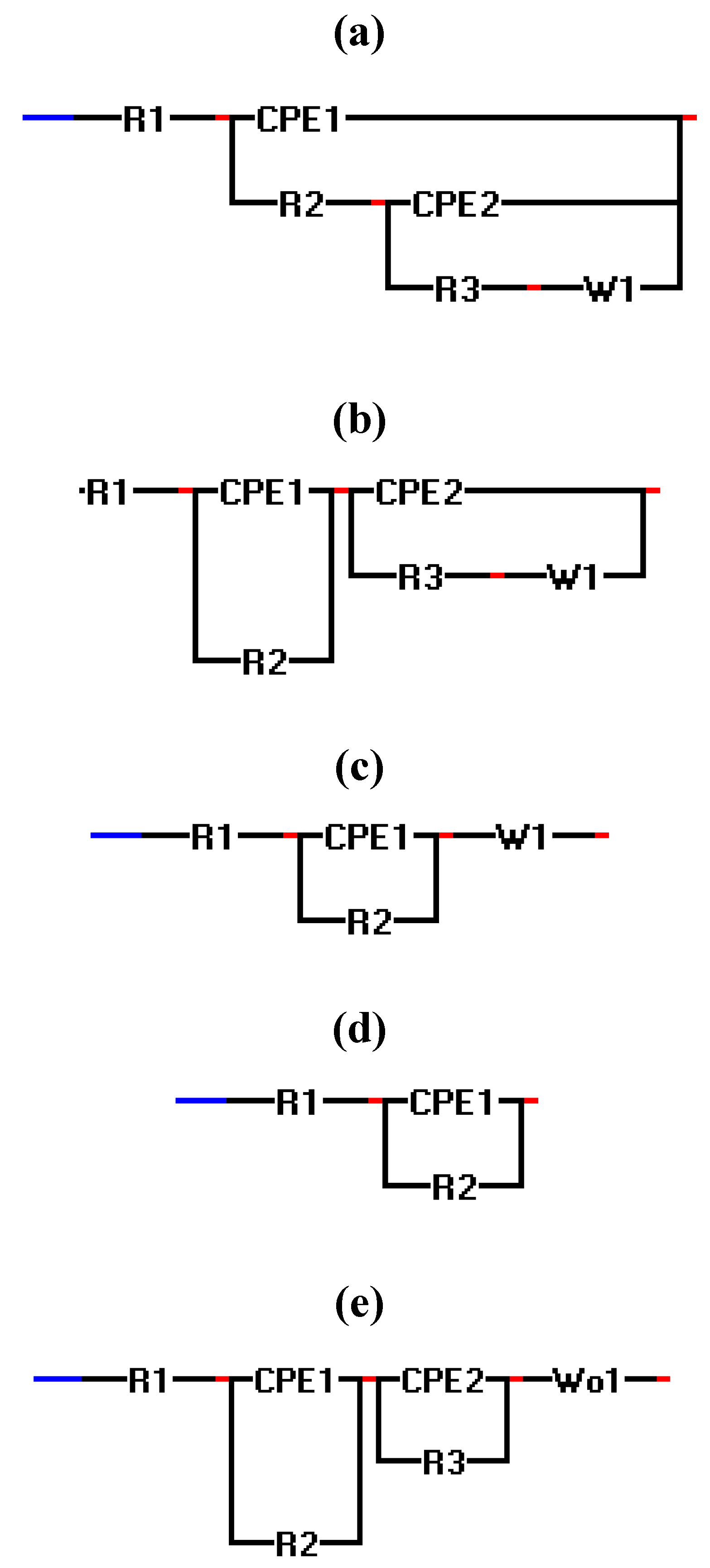
| Experiment | Polarization 1 | Polarization 2 | Polarization 3 |
|---|---|---|---|
| 1–5(micro scale) | a | a | a |
| 10(micro scale) | c | d | d |
| 11(micro scale) | a | a | b |
| 2,5,8,9(macro scale) | a | a | a |
| 4(macro scale) | c | a | a |
| 1,3,10,11(macro scale) | e | e | e |
| Experiment | Z"micro scale (kΩ) | Z"macro scale (Ω) |
|---|---|---|
| 1 | 1.26 | 1.57 |
| 2 | 2.53 | 0.8 |
| 3 | 3.76 | 1.17 |
| 4 | 0.8 (min) | 0.1 (min) |
| 5 | 1.06 | 3.86 |
| 8 | 10 | 0.48 |
| 9 | 3.5 | 0.75 |
| 10 | 0.7 (MΩ) (max) | 102 (max) |
| 11 | 0.88 | 1.75 |
3. Experimental Section
3.1. Chemicals
3.2. Specimen and Surface Preparation
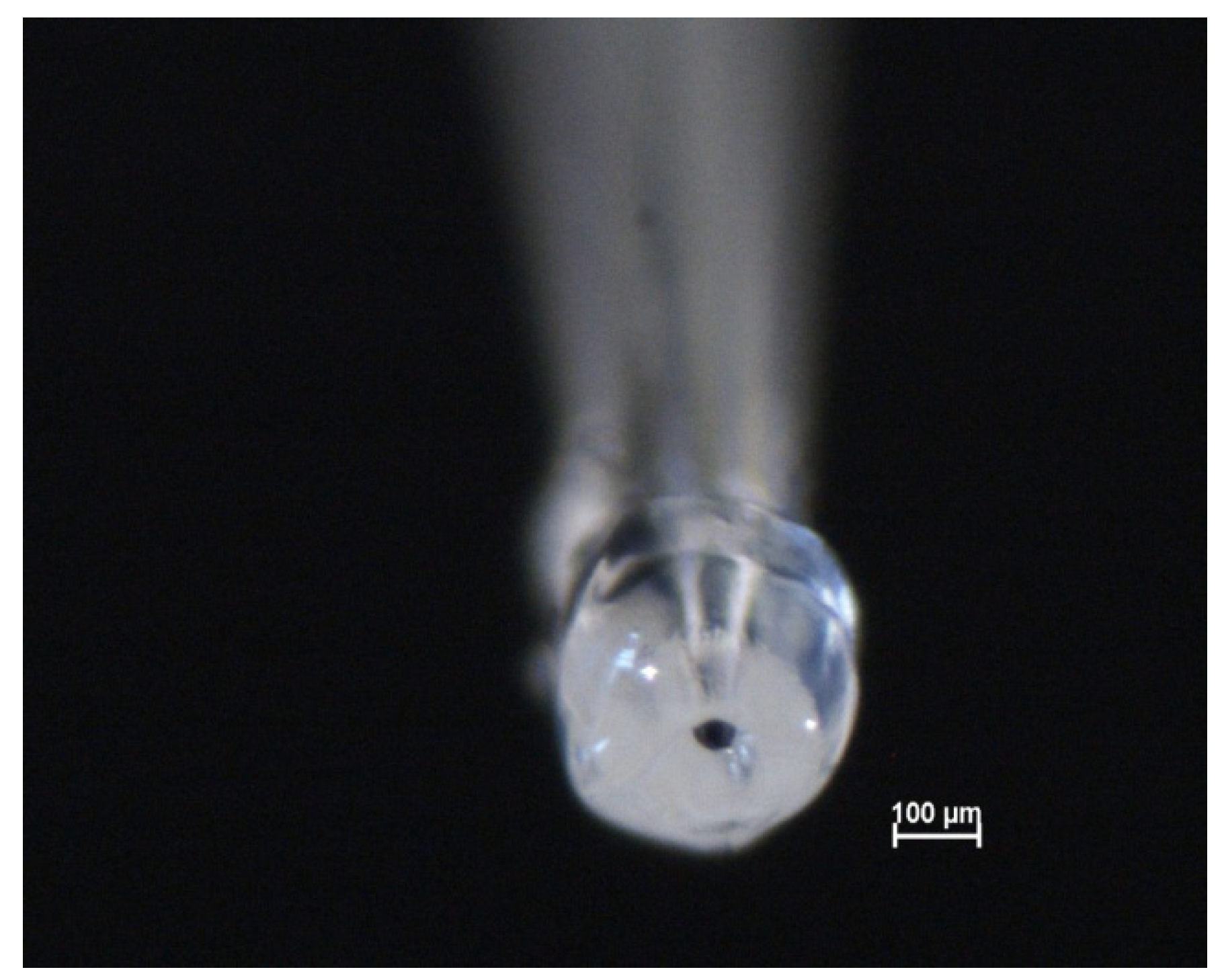
3.3. Preparation of the Microcapillaries with a Silicone Gasket
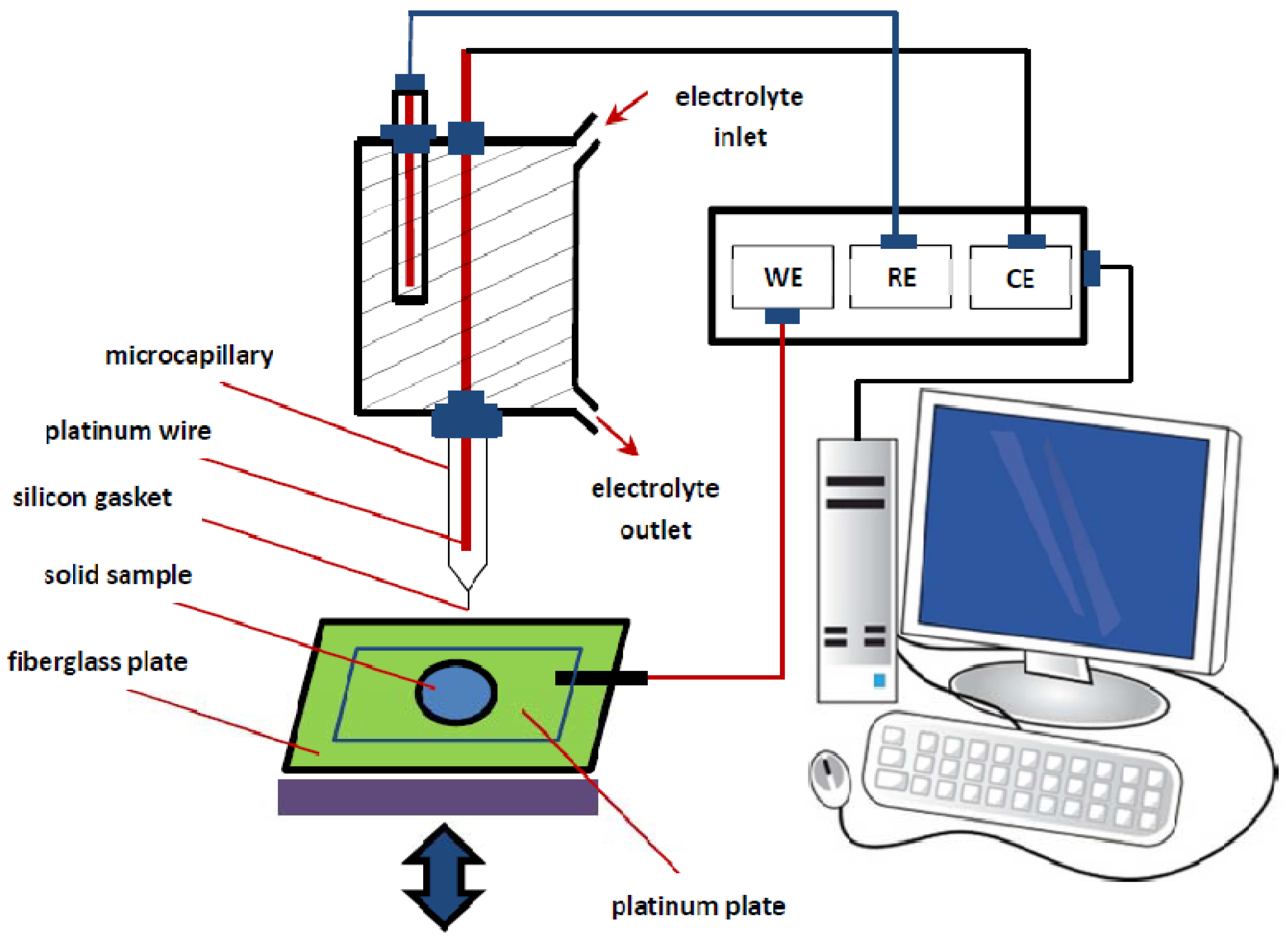
3.4. Electrochemical Set-Up
3.5. Experimental Design
4. Conclusions
Acknowledgments
References
- Kear, G.; Walsh, F.C.; Barker, D.B.; Stokes, K.S. Electrochemical corrosion characteristics of copper in filtered and artificial seawater as a function of mass transfer conditions. In EuroCorr. 2000; Institute of Corrosion: Leighton Buzzard, UK, 2000. [Google Scholar]
- Mathiyarasu, J.; Palaniswamy, N.; Muralidharan, V.S. Electrochemical behaviour of copper-nickel alloy in chloride solution. J. Chem. Sci. 1999, 111, 377–386. [Google Scholar]
- Georgiadou, M.; Alkire, R. Modelling of copper etching in aerated chloride solutions. J. Appl. Electrochem. 1998, 28, 127–134. [Google Scholar] [CrossRef]
- Wagner, D.; Peimemann, H.; Siedlarek, H.; Campbell, S.A.; Campbell, N.; Walsh, F.C. Development in Marine Corrosion; The Royal Society of Chemistry: Cambridge, UK, 1998; pp. 103–118. [Google Scholar]
- King, F.; Quin, M.J.; Litke, C.D. Oxygen reduction on copper in neutral NaCl solution. J. Electroanal. Chem. 1995, 385, 45–55. [Google Scholar] [CrossRef]
- Mansfeld, F.B.; Liu, G.; Xiao, H.; Tsai, C.H.; Little, B.J. The corrosion behavior of copper alloys, stainless steels and titanium in seawater. Corros. Sci. 1994, 36, 2063–2095. [Google Scholar] [CrossRef]
- Wood, R.J.K.; Fry, S.A. Corrosion of pure copper in flowing seawater under cavitating and noncavitating flow conditions. J. Fluids. Eng. 1990, 112, 218–224. [Google Scholar] [CrossRef]
- Deslouis, C.; Tribollet, B.; Mengoli, G.; Musiani, M. Electrochemical behaviour of copper in neutral aerated chloride solution. I. Steady-state investigation. J. Appl. Electrochem. 1988, 18, 374–383. [Google Scholar] [CrossRef]
- Lee, H.P.; Nobe, K. Kinetics and Mechanisms of Cu Electrodissolution in Chloride Media. J. Electrochem. Soc. 1986, 133, 2035–2043. [Google Scholar] [CrossRef]
- Smyrl, W.H. Digital Impedance for Faradaic Analysis: II. Electrodissolution of Cu in HCl. J. Electrochem. Soc. 1985, 132, 1555–1562. [Google Scholar] [CrossRef]
- Sanchez, M.; Gamby, J.; Perrot, H.; Rose, D.; Vivier, V. Microelectrochemistry of copper in NaCl solution: Comparison between conventional microelectrode and microelectrochemical cell. Electrochem. Commun. 2010, 12, 1230–1232. [Google Scholar] [CrossRef]
- Pilaski, M.; Hamelmann, T.; Moehring, A.; Lohrengel, M.M. Impedance spectroscopy in micro systems. Electrochim. Acta 2002, 47, 2127–2134. [Google Scholar] [CrossRef]
- Lohrengel, M.M.; Heiroth, S.; Kluger, K.; Pilaski, M.; Walther, B. Microimpedance—Localized material analysis. Electrochim. Acta 2006, 51, 1431–1436. [Google Scholar] [CrossRef]
- Dubuisson, E.; Lavie, P.; Dalard, F.; Caire, J.P.; Szunerits, S. Study of the atmospheric corrosion of galvanised steel in a micrometric electrolytic droplet. Electrochem. Commun. 2006, 8, 911–915. [Google Scholar] [CrossRef]
- Jorcin, J.B.; Krawiec, H.; Pebere, N.; Vignal, V. Comparison of local electrochemical impedance measurements derived from bi-electrode and microcapillary techniques. Electrochim. Acta 2009, 54, 5775–5781. [Google Scholar] [CrossRef]
- Birbilis, N.; Padgett, B.N.; Buchheit, R.G. Limitations in microelectrochemical capillary cell testing and transformation of electrochemical transients for acquisition of microcell impedance data. Electrochim. Acta 2005, 50, 3536–3544. [Google Scholar] [CrossRef]
- Dong, C.F.; Fu, A.Q.; Li, X.G.; Cheng, Y.F. Localized EIS characterization of corrosion of steel at coating defect under cathodic protection. Electrochim. Acta 2008, 54, 628–633. [Google Scholar] [CrossRef]
- Zhang, G.A.; Cheng, Y.F. Micro-electrochemical characterization and Mott–Schottky analysis of corrosion of welded X70 pipeline steel in carbonate/bicarbonate solution. Electrochim. Acta 2009, 55, 316–324. [Google Scholar] [CrossRef]
- Tang, X.; Cheng, Y.F. Micro-electrochemical characterization of the effect of applied stress on local anodic dissolution behavior of pipeline steel under near-neutral pH condition. Electrochim. Acta 2009, 54, 1499–1505. [Google Scholar] [CrossRef]
- Tang, X.; Cheng, Y.F. Localized dissolution electrochemistry at surface irregularities of pipeline steel. Appl. Surf. Sci. 2008, 254, 5199–5205. [Google Scholar] [CrossRef]
- Krawiec, H.; Vignal, V.; Banas, J. Local electrochemical impedance measurements on inclusion-containing stainless steels using microcapillary-based techniques. Electrochim. Acta 2009, 54, 6070–6074. [Google Scholar] [CrossRef]
- Li, M.C.; Cheng, Y.F. Corrosion of the stressed pipe steel in carbonate–bicarbonate solution studied by scanning localized electrochemical impedance spectroscopy. Electrochim. Acta 2008, 53, 2831–2836. [Google Scholar] [CrossRef]
- Zhong, C.; Tang, X.; Cheng, Y.F. Corrosion of steel under the defected coating studied by localized electrochemical impedance spectroscopy. Electrochim. Acta 2008, 53, 4740–4747. [Google Scholar] [CrossRef]
- Krawiec, H.; Vignal, V.; Amar, H.; Peyre, P. Local electrochemical impedance spectroscopy study of the influence of ageing in air and laser shock processing on the micro-electrochemical behaviour of AA2050-T8 aluminium alloy. Electrochim. Acta 2011, 56, 9581–9587. [Google Scholar] [CrossRef]
- Fleischmann, M.; Lasserre, F.; Robinson, J.; Swan, D. The application of microelectrodes to the study of homogeneous processes coupled to electrode reactions: Part I. EC' and CE reactions. J. Electroanal. Chem. 1984, 117, 97–114. [Google Scholar] [CrossRef]
- Galceran, J.; Puy, J.; Salvador, J.; Cecilia, J.; Van Leeuwen, H.P. Voltammetric liability of metal complexes at spherical microelectrodes with various radii. J. Electroanal. Chem. 2001, 505, 85–94. [Google Scholar] [CrossRef]
- Brookes, B.A.; Macfie, G.; Compton, R.G. Simulation of square wave voltammetry at hemispherical electrodes: electrochemically reversible, irreversible and quasi-reversible processes. J. Phys. Chem. B 2000, 104, 5784–5789. [Google Scholar] [CrossRef]
- Van Leeuwen, H.P.; Pinheiro, J.P. Lability criteria for metal complexes in micro-electrode voltammetry. J. Electroanal. Chem. 1999, 471, 55–61. [Google Scholar] [CrossRef]
- Molina, A.; Serna, C.; Martinez-Ortiz, F. Square wave voltammetry for a pseudo-first-order catalytic process at spherical electrodes. J. Electroanal. Chem. 2000, 486, 9–15. [Google Scholar] [CrossRef]
- Komrosky-Lovrić, S.; Lovrić, M.; Bond, A. Square-wave voltammetry at spherical and disk microelectrodes as a function of electrode radius and frequency. Electroanalysis 1993, 5, 29–40. [Google Scholar] [CrossRef]
- Schultz, J.W.; Bressel, A. Principles of electrochemical micro- and nano-system technologies. Electrochim. Acta 2001, 47, 3–21. [Google Scholar] [CrossRef]
- Bohni, H.; Suter, T.; Schreyer, A. Micro- and nanotechniques to study localized corrosion. Electrochim. Acta 1995, 40, 1361–1368. [Google Scholar] [CrossRef]
- Arjmand, F.; Adriaens, A. Investigation of 304L stainless steel in a NaCl solution using a microcapillary electrochemical droplet cell: Comparison with conventional electrochemical techniques. Electrochim. Acta 2012, 59, 222–227. [Google Scholar] [CrossRef]
- Alfantazi, A.M.; Ahmed, T.M.; Tromans, D. Corrosion behavior of copper alloys in chloride media. Mater. Design 2009, 30, 2425–2430. [Google Scholar] [CrossRef]
- Arjmand, F.; Adriaens, A. Electrochemical quantification of copper-based alloys using voltammetry of microparticles: optimization of the experimental conditions. J. Solid State Electrochem. 2012, 16, 535–543. [Google Scholar] [CrossRef]
- Bonora, P.L.; Deflorian, F.; Fedrizzi, L. Electrochemical impedance spectroscopy as a tool for investigating underpaint corrosion. Electrochim. Acta 1996, 41, 1073–1082. [Google Scholar] [CrossRef]
- Ruan, C.; Yang, L.; Li, Y. Immunobiosensor chips for detection of escherichia coli O157:H7 using electrochemical impedance spectroscopy. Anal. Chem. 2002, 74, 4814–4820. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bi, Q.; Leyland, A.; Matthews, A. An electrochemical impedance spectroscopy study of the corrosion behaviour of PVD coated steels in 0.5 N NaCl aqueous solution: Part I. Establishment of equivalent circuits for EIS data modelling. Corros. Sci. 2003, 45, 1243–1256. [Google Scholar] [CrossRef]
- Andreatta, F.; Lohrengel, M.M.; Terryn, H.; de Wit, J.H.W. Electrochemical characterisation of aluminium AA7075-T6 and solution heat treated AA7075 using a micro-capillary cell. Electrochim. Acta 2003, 48, 3239–3247. [Google Scholar] [CrossRef]
- Box, G.E.P.; Hunter, W.G.; Hunter, J.S. Statistics for Experimenters: An Introduction to Design, Data Analysis and Model Building; John Wiley and Sons: New York, NY, USA, 1978. [Google Scholar]
- Stephan, D.D.; Werner, J.; Yeater, R.P. Essential regression and experimental design for chemist and engineers, MS Excel add in software package, 1998–2001. Available online: http://www.jowerner.homepage.t-online.de/ERPref.html (accessed on 20 November 2012).
- Bulacov, I.; Jirkovsky, J.; Muller, M.; Heimann, R.B. Induction plasma-sprayed photocatalytically active titania coatings and their characterisation by micro-Raman spectroscopy. Surf. Coat. Technol. 2006, 201, 255–264. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Arjmand, F.; Adriaens, A. Influence of pH and Chloride Concentration on the Corrosion Behavior of Unalloyed Copper in NaCl Solution: A Comparative Study Between the Micro and Macro Scales. Materials 2012, 5, 2439-2464. https://doi.org/10.3390/ma5122439
Arjmand F, Adriaens A. Influence of pH and Chloride Concentration on the Corrosion Behavior of Unalloyed Copper in NaCl Solution: A Comparative Study Between the Micro and Macro Scales. Materials. 2012; 5(12):2439-2464. https://doi.org/10.3390/ma5122439
Chicago/Turabian StyleArjmand, Farzin, and Annemie Adriaens. 2012. "Influence of pH and Chloride Concentration on the Corrosion Behavior of Unalloyed Copper in NaCl Solution: A Comparative Study Between the Micro and Macro Scales" Materials 5, no. 12: 2439-2464. https://doi.org/10.3390/ma5122439
APA StyleArjmand, F., & Adriaens, A. (2012). Influence of pH and Chloride Concentration on the Corrosion Behavior of Unalloyed Copper in NaCl Solution: A Comparative Study Between the Micro and Macro Scales. Materials, 5(12), 2439-2464. https://doi.org/10.3390/ma5122439