2.1. 3D Imaging Using Absorption Contrast
Figure 1 shows equiaxed morphologies for powders of (a) LiBH
4, (b) MgH
2, and (c) a 1:1 mixture of LiBH
4:MgH
2. Similar morphologies were found for the 1:3 and 2:1 mixtures.
Figure 2 shows a single slice (in x-z plane) from the 1:1 mixture of LiBH
4:MgH
2. Here, it is clear to see that the phase contrast between LiBH
4 (above 90% transmission at 15 keV) and MgH
2 (18% transmission at 15 keV) gives rise to LiBH
4 particles which appear lighter than the darker MgH
2 ones. The LiBH
4 particles are ~50 μm–100 μm in size and the MgH
2 particles are slightly larger at ~100 μm–150 μm. Because of high transmission for LiBH
4, sample to detector distances were adjusted to increase edge contrast of the LiBH
4 phase.
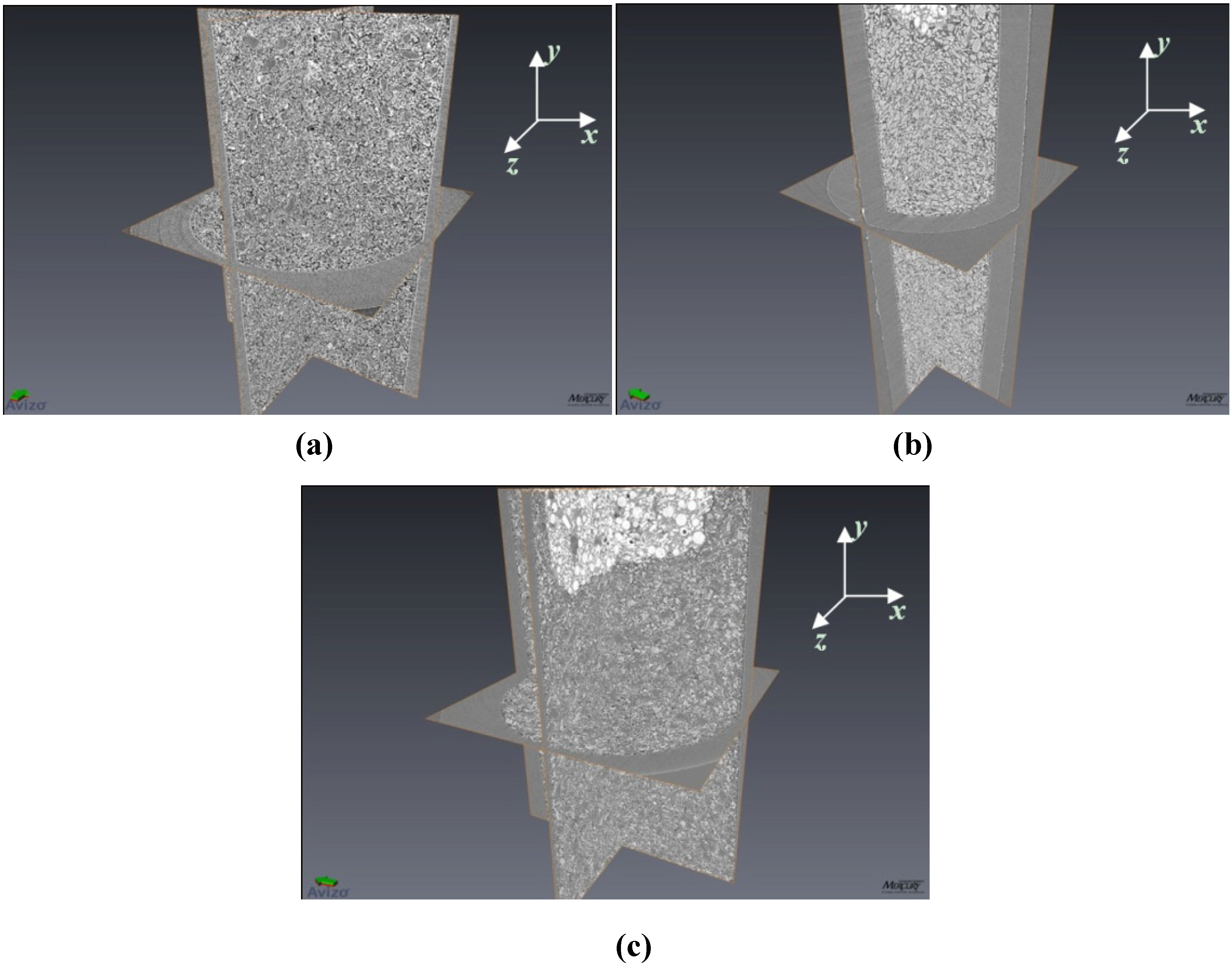
Figure 1.
Three dimensional images of (a) 4 mol % TiCl3 catalyzed LiBH4; (b) 4 mol % TiCl3 catalyzed MgH2, and (c) 4 mol % TiCl3 catalyzed LiBH4:MgH2 in 1:1 molar ratio. Here only the x-y, y-z, and x-z planes are rendered in the images. For reference, the inner diameter of the tube (seen in the x-z plane) is 1.87 mm. In (b) and (c) the bright/white areas are tomographic images of the epoxy putty used to seal the samples within the holders (and are not a part of the sample itself). In subsequent sections, image analysis data performed on all individual x-z planar slices is reported.
Figure 1.
Three dimensional images of (a) 4 mol % TiCl3 catalyzed LiBH4; (b) 4 mol % TiCl3 catalyzed MgH2, and (c) 4 mol % TiCl3 catalyzed LiBH4:MgH2 in 1:1 molar ratio. Here only the x-y, y-z, and x-z planes are rendered in the images. For reference, the inner diameter of the tube (seen in the x-z plane) is 1.87 mm. In (b) and (c) the bright/white areas are tomographic images of the epoxy putty used to seal the samples within the holders (and are not a part of the sample itself). In subsequent sections, image analysis data performed on all individual x-z planar slices is reported.
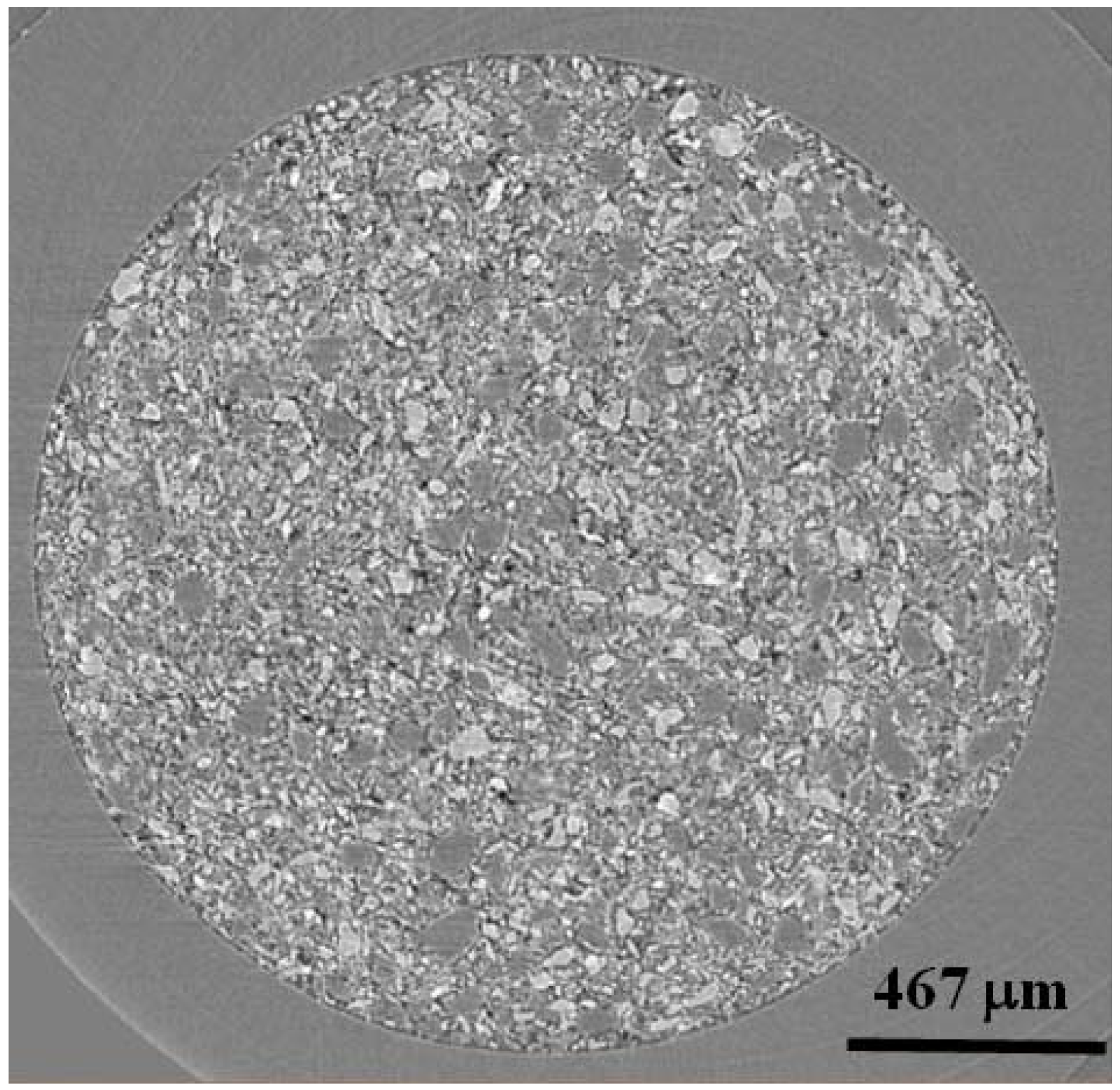
Figure 2.
A single x-z oriented slice from 4 mol % TiCl3 catalyzed LiBH4:MgH2 in 1:1 molar ratio. Light contrasting particles are LiBH4 and dark contrasting particles are MgH2. The black areas are air or void space.
Figure 2.
A single x-z oriented slice from 4 mol % TiCl3 catalyzed LiBH4:MgH2 in 1:1 molar ratio. Light contrasting particles are LiBH4 and dark contrasting particles are MgH2. The black areas are air or void space.
2.2. Using Microtomography to Determine Relative Amounts of LiBH4 and MgH2
Figure 3 shows an example of image analysis thresholds used. Here the 1:3 LiBH
4:MgH
2 (uncatalyzed) sample is shown. The thresholded images were derived from histograms of the grey-scale images wherein a narrow peak (corresponding to the lightest grey) denotes the LiBH
4 phase—whilst a second, broader peak-like feature in the histogram corresponds to the MgH
2 phase. These two peak-like features showed a slight overlap. For LiBH
4 thresholding, the levels were selected to capture most of the “narrow” peak (omitting overlap regions). For both LiBH
4 and MgH
2 thresholding, the levels were selected to capture both the “narrow” peak and the broadened peak-like features—using the difference in the volumes of those levels computed by Avizio
TM software as the MgH
2 volume. In all cases, images were inspected after the first threshold selection (of the narrow peak) to ensure all highlighted (red) pixels are comprised of LiBH
4. Images were also inspected upon the second threshold selection (corresponding to both the narrow and broader peaks) to ensure that highlighted pixels correspond to material (not void space).
Figure 3a shows images without thresholding and Figures 3b,c show thresholding for the LiBH
4 phase (selection of the narrow peak) and the combined MgH
2/LiBH
4 phases (selection of both peaks), respectively.
After thresholding the images using 2D x-z plane slices, volume fraction data is calculated by Avizo
TM software using voltex volume data.
Table 1 shows volume data (in μm
3) computed using pixel count of microtomographic data.
Table 2 shows molar ratios calculated from volume fraction data. Results show that experimental determination of relative amount of each phase measured by tomographic imaging did not correctly predict the relative amounts of LiBH
4 and MgH
2 used for sample preparation. It is hypothesized that the poor correlations to target compositions prepared at LiBH
4:MgH
2 molar ratios of 1:1, 1:3, and 2:1 may have occurred because the high energy ball milling process resulted in particles sizes which are either below the 2 μm spatial resolution of the tomographic imaging or smaller particles settled to the bottom of the sample vial where they were not included in the measurement.
The molar ratio data shown in
Table 2 indicates that the majority constituent in each two phase mixture is milled to finer sizes than the smaller constituent. High energy ball milling results in reduced particle sizes with increased mill time. The smallest particles in the system may not have been included in the image sets because of gravitational settling or because of milling to sizes below the spatial resolution of the tomography. Some factors which influence the final particle size are material hardness, initial particle size, and ratio of powder-to-milling media (
i.e., stainless steel mill balls). In these experiments, LiBH
4 and MgH
2 phases were milled simultaneously keeping the ratio of powder-to milling media fixed. It is important to note that when molar ratio of the constituent powders changes—the ratio of powder-to-milling media for each phase is also changed. This variable would determine which phase attrition mills at a higher rate and hence reaches to sizes below the spatial resolution of the tomographic instrument or settles to bottom of sample vial. For example, in the 1:3 LiBH
4:MgH
2 (molar ratio) mixed powder system, the stoichiometric starting composition is 0.3 mol LiBH
4 to 1 mol of MgH
2—however, the tomographic imaging data erroneously measures 0.46 mol of LiBH
4 to 1 mol of MgH
2 (
i.e., the imaging data reports too little MgH
2). Since MgH
2 is the most abundant phase in the starting powder—its ratio of powder-to-milling media is higher resulting in a higher probability of contact with milling media and a higher rate of particle attrition leading to size reduction. Indeed, this explanation would account for the poor correlations in the 2:1 LiBH
4:MgH
2 (molar ratio) mixed powders whereby a higher ratio of powder-to-milling media for LiBH
4 yields more rapid reduction in particle size for this phase. As a result, imaging data erroneously measures lower than expected amounts of LiBH
4 (measured at 0.45 mol of LiBH
4 per 1 mol of MgH
2). In the case of the 1:1 sample, the measurements show 0.39 mol of LiBH
4 to 1 mol of MgH
2 for the uncatalyzed and 0.59 mol of LiBH
4 to 1 mol of MgH
2 for the catalyzed system. In both cases (
i.e., catalyzed and uncatalyzed), the amount of LiBH
4 was underestimated by these measurements indicating that LiBH
4 was milled more effectively to smaller sizes. The density of LiBH
4 is approximately half that of MgH
2 and so it can be reasoned that, at equal molar amounts, the volume of LiBH
4 is twice that of the MgH
2 (leading to more opportunity for the LiBH
4 phase to encounter the milling media and hence more particle size reduction).
Table 2 summarizes this data and includes—in parenthesis—notation of the phase which is most abundant and below spatial resolution. Again, the abundant phase in each mixture is underestimated by the imaging data because of gravitation sedimentation which may have caused it to fall to the bottom of the sample vial where it was excluded from measurement or because during attrition milling it achieved sizes smaller than the spatial resolution of the tomographic instrument.
In all cases of measured compositions, the percent difference between measured and target composition is very large. Therefore, to bring into focus the actual compositions of the three samples, x-ray diffraction measurements were made.
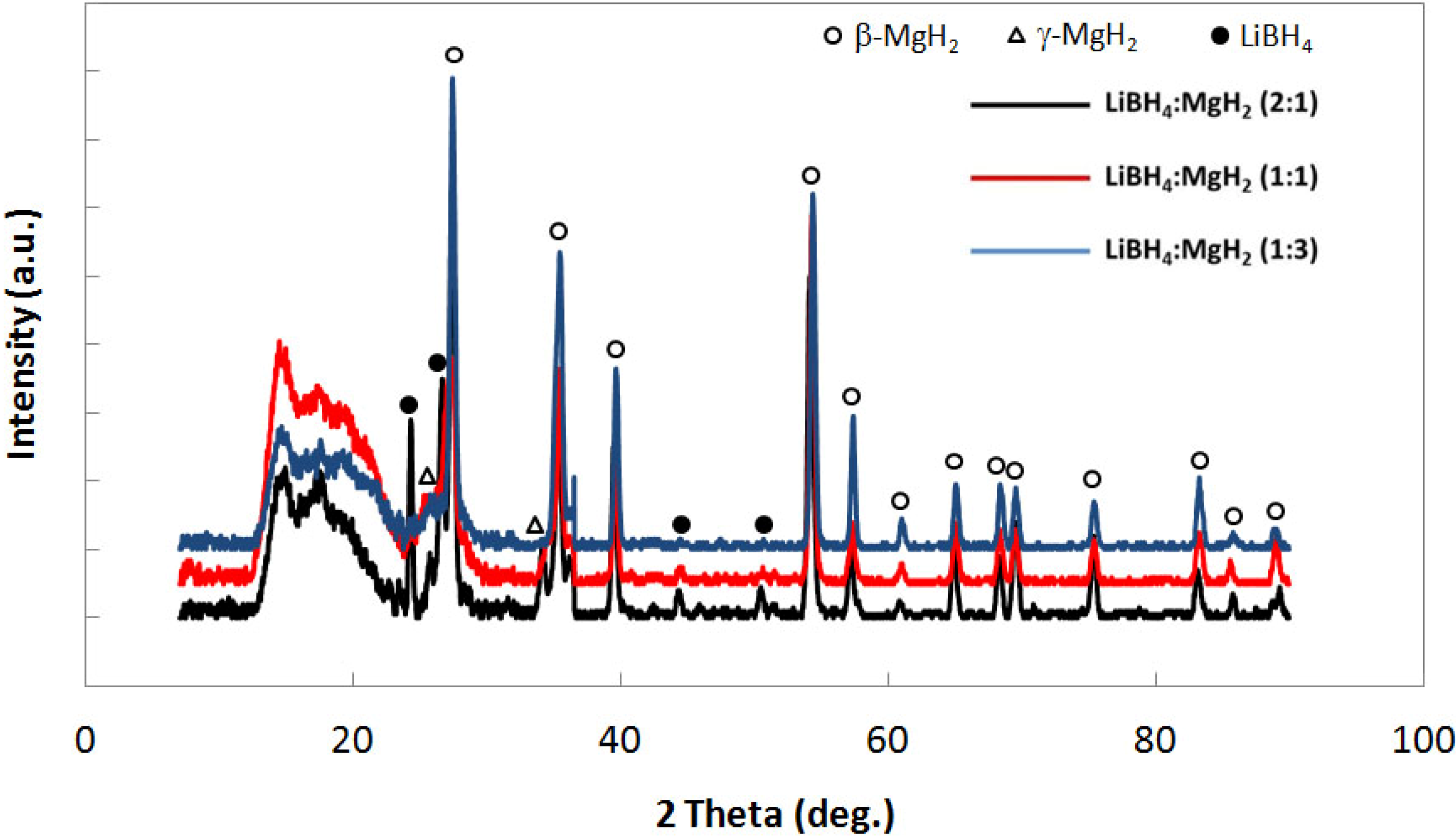
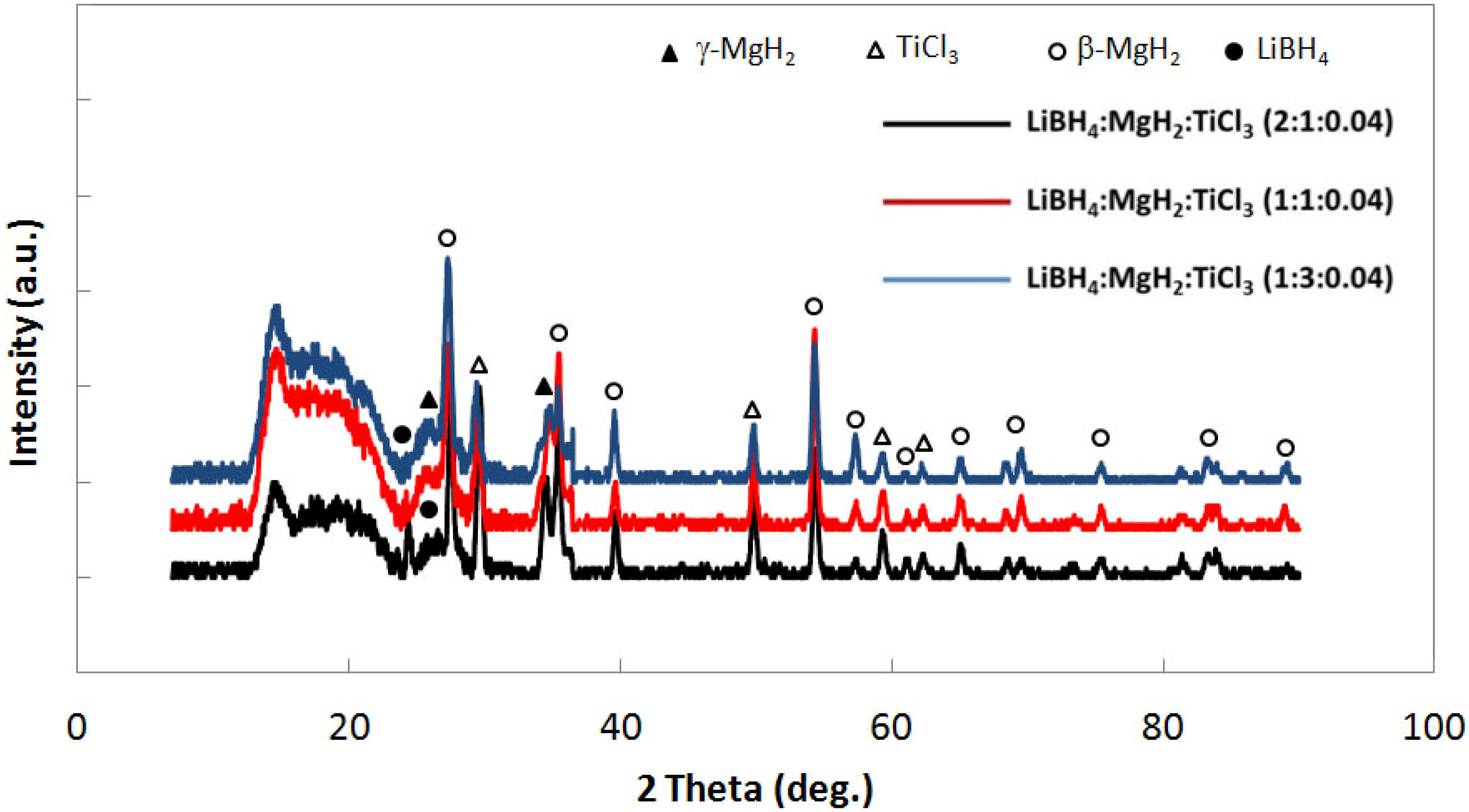
Figure 4 shows x-ray diffraction of the uncatalyzed samples and clearly indicates increasing amounts of LiBH
4 in going from the 1:3, 1:1 and 2:1 (LiBH
4:MgH
2) as indicated by the increasing in peak intensity at 2θ~22° and at 2θ~49.98°. By x-ray diffraction, it is confirmed that no desorption product,
i.e., LiH, is present. LiH diffraction peaks occur at 2θ~38° and 44° [
8].
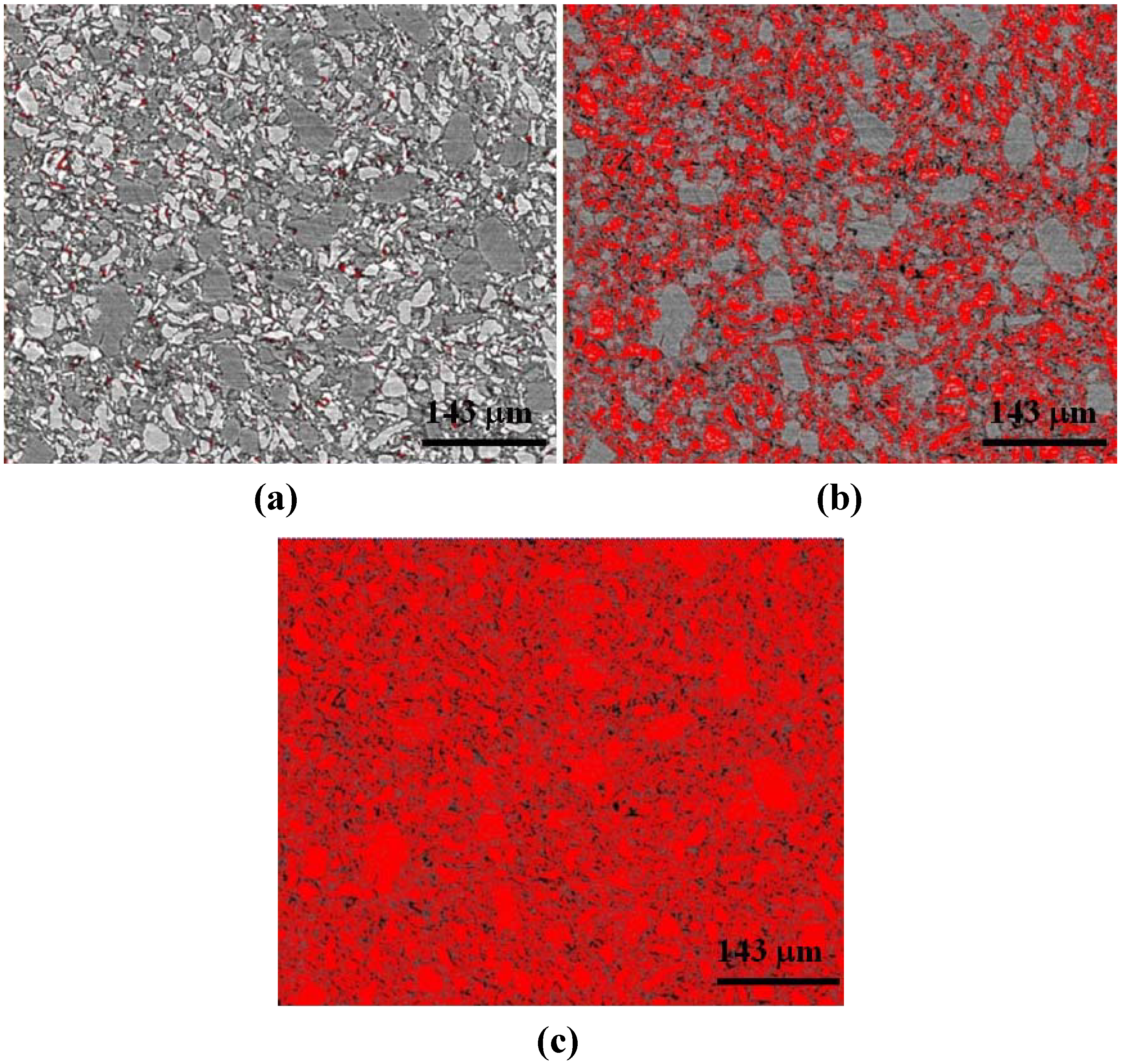
Figure 3.
Two dimensional x-z plane slices were used for thresholding. Here the 1:3 LiBH4:MgH2 (uncatalyzed) sample with (a) no thresholding; (b) red highlighted pixels corresponding to thresholding for LiBH4; and (c) red highlighted pixels corresponding to thresholding for both LiBH4 and MgH2. Taking the difference in volume computed from (c) and (b), we may determine the volume of MgH2. The black areas are air or void space.
Figure 3.
Two dimensional x-z plane slices were used for thresholding. Here the 1:3 LiBH4:MgH2 (uncatalyzed) sample with (a) no thresholding; (b) red highlighted pixels corresponding to thresholding for LiBH4; and (c) red highlighted pixels corresponding to thresholding for both LiBH4 and MgH2. Taking the difference in volume computed from (c) and (b), we may determine the volume of MgH2. The black areas are air or void space.
Table 1.
Total volume of void space, LiBH
4, and MgH
2 (in μm
3) computed from pixel count in computed microtomographic images. These data were used (along with density and molecular weight) to compute molar ratios or molar fractions (reported in
Table 2). In computing molar ratios, the void space volume was attributed equally to LiBH
4 and MgH
2.
Table 1.
Total volume of void space, LiBH4, and MgH2 (in μm3) computed from pixel count in computed microtomographic images. These data were used (along with density and molecular weight) to compute molar ratios or molar fractions (reported in Table 2). In computing molar ratios, the void space volume was attributed equally to LiBH4 and MgH2.
| Volume Air | Volume LiBH4 | Volume MgH2 |
|---|
| 1:3 Catalyzed LiBH4:MgH2 |
| 1.60 × 108 | 3.23 × 108 | 2.26 × 108 |
| 1:3 Uncatalyzed LiBH4:MgH2 |
| 1.71 × 108 | 1.76 × 108 | 2.27 × 108 |
| 1:1 Catalyzed LiBH4:MgH2 |
| 1.77 × 108 | 2.78 × 108 | 2.57 × 108 |
| 1:1 Uncatalyzed LiBH4:MgH2 |
| 1.04 × 108 | 7.64 × 107 | 1.16 × 108 |
| 2:1 Catalyzed LiBH4:MgH2 |
| 1.45 × 108 | 1.68 × 108 | 2.49 × 108 |
| 2:1 Uncatalyzed LiBH4:MgH2 |
| 1.46 × 108 | 1.30 × 107 | 3.24 × 107 |
Table 2.
LiBH4:MgH2 molar ratios computed from microtomographic image analysis for catalyzed and uncatalyzed systems. Poor correlations between the target molar ratios of 0.3, 1.0 and 2.0 may be explained if high energy ball milling preferentially reduces particle sizes for the most abundant phase in the mixture.
Table 2.
LiBH4:MgH2 molar ratios computed from microtomographic image analysis for catalyzed and uncatalyzed systems. Poor correlations between the target molar ratios of 0.3, 1.0 and 2.0 may be explained if high energy ball milling preferentially reduces particle sizes for the most abundant phase in the mixture.
| Target LiBH4:MgH2 Composition | LiBH4:MgH2 Molar Fractions for Uncatalyzed Samples | LiBH4:MgH2 Molar Fractions for 4 mol % TiCl3 Catalyzed Samples |
|---|
| 1:3 | 0.46 | 0.73 |
| (0.33 LiBH4 molar fraction) | (more MgH2 below spatial resolution) | (more MgH2 below spatial resolution) |
| 1:1 | 0.39 | 0.59 |
| (1.0 LiBH4 molar fraction) | (more LiBH4 below spatial resolution) | (more LiBH4 below spatial resolution) |
| 2:1 | 0.45 | 0.41 |
| (2.0 LiBH4 molar fraction) | (more LiBH4 below spatial resolution) | (more LiBH4 below spatial resolution) |
Figure 4.
X-ray diffraction for uncatalyzed LiBH4:MgH2 in 2:1, 1:1 and 1:3 molar ratios.
Figure 4.
X-ray diffraction for uncatalyzed LiBH4:MgH2 in 2:1, 1:1 and 1:3 molar ratios.
2.3. Interfacial Volume for Single Phase MgH2 and LiBH4 and Interphase Volume for 3:1, 1:1, and 2:1 Mixtures of LiBH4:MgH2
The tomographic images showed a bright fringe around the edges of the LiBH
4 particles—and so Avizo
TM software could be used to threshold those edges in the mixed powder systems. This may have been possible because of phase contrast yielding brighter edges around LiBH
4 and optimal edge enhancement for that phase. Within the grey-scale histogram, a very low intensity peak appearing to the left of the LiBH
4 (narrow) and MgH
2 (broadened) peaks is present. This peak was selected for generating the edge thresholded images.
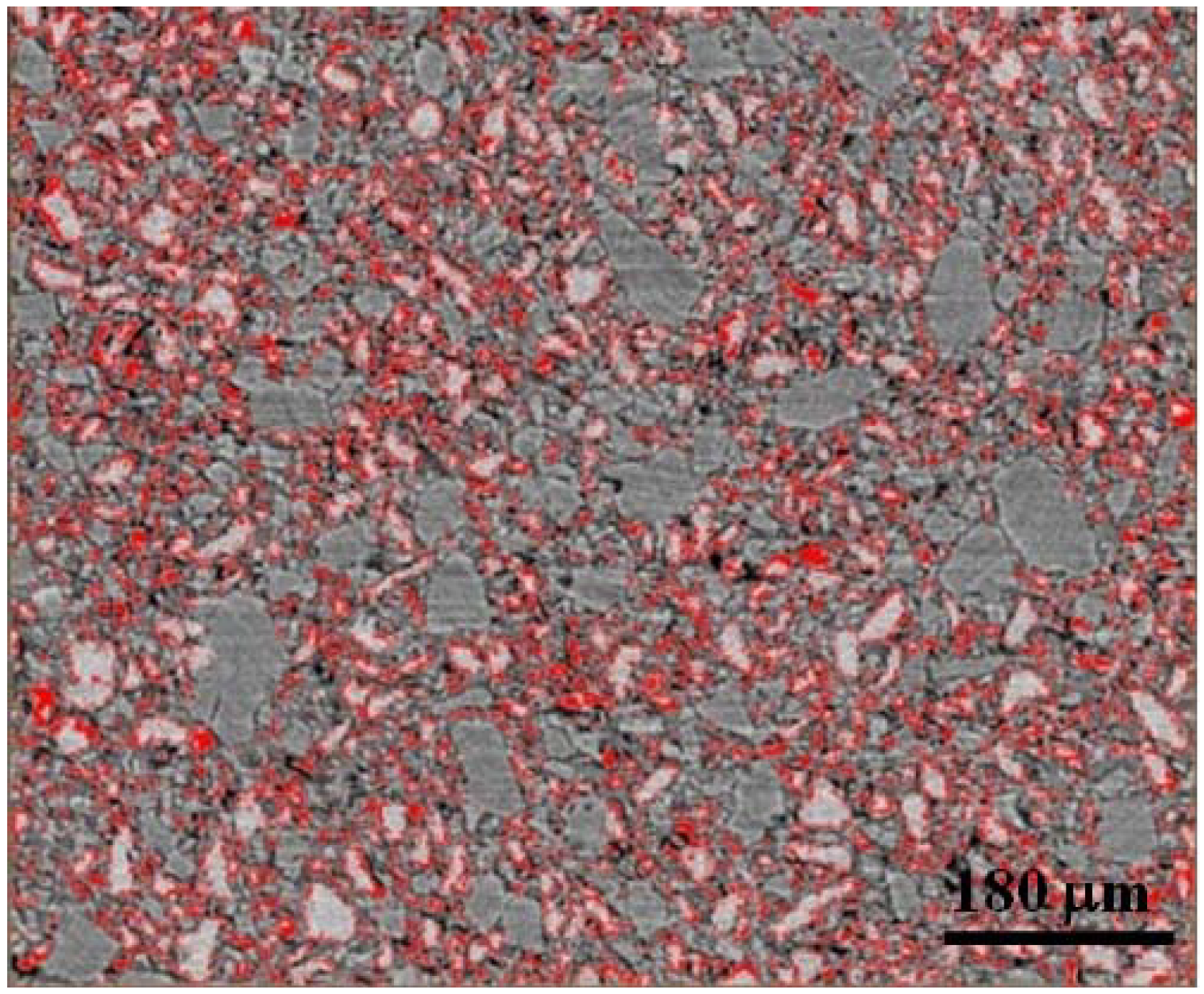
Figure 5 shows an example of the edge thresholding of LiBH
4 particles in a sample comprised of 1:3 LiBH
4:MgH
2 (uncatalyzed).
Figure 6 shows the interfacial volume calculated for each of the single phase powders,
i.e., LiBH
4 and MgH
2, in their catalyzed and uncatalyzed state. No significant trend in interfacial volume is noted. The standard deviation for uncatalyzed and catalyzed LiBH
4 is 0.04 and the standard deviation for uncatalyzed and catalyzed MgH
2 is 0.02. The data plotted in
Figure 6 is a ratio of the interfacial boundary volume to bulk volume (and is unitless). The standard deviation is +/−0.04 for uncatalyzed and catalyzed LiBH
4. The +/−0.04 standard deviation is used for the analysis of trends in the interphase volume ratios for the mixed LiBH
4 and MgH
2 systems (shown as error bars in
Figure 7).
Figure 5.
Two dimensional x-z slice used for thresholding edges of the LiBH4 phase in the 1:3 LiBH4:MgH2 (uncatalyzed) sample. Similar threshold levels were selected for computing interfacial boundary volume between LiBH4 and MgH2 for all samples data.
Figure 5.
Two dimensional x-z slice used for thresholding edges of the LiBH4 phase in the 1:3 LiBH4:MgH2 (uncatalyzed) sample. Similar threshold levels were selected for computing interfacial boundary volume between LiBH4 and MgH2 for all samples data.
Figure 6.
Comparison of interfacial boundary volume for LiBH4 (uncatalyzed), LiBH4 (catalyzed), MgH2 (uncatalyzed), and MgH2 (catalyzed). The LiBH4 phase contains smaller particles (i.e. higher interface volume to bulk volume ratios) than the MgH2 phase. No significant trend in interfacial volume for catalyzed and uncatalyzed samples is noted in either the case of pure LiBH4 or MgH2.
Figure 6.
Comparison of interfacial boundary volume for LiBH4 (uncatalyzed), LiBH4 (catalyzed), MgH2 (uncatalyzed), and MgH2 (catalyzed). The LiBH4 phase contains smaller particles (i.e. higher interface volume to bulk volume ratios) than the MgH2 phase. No significant trend in interfacial volume for catalyzed and uncatalyzed samples is noted in either the case of pure LiBH4 or MgH2.
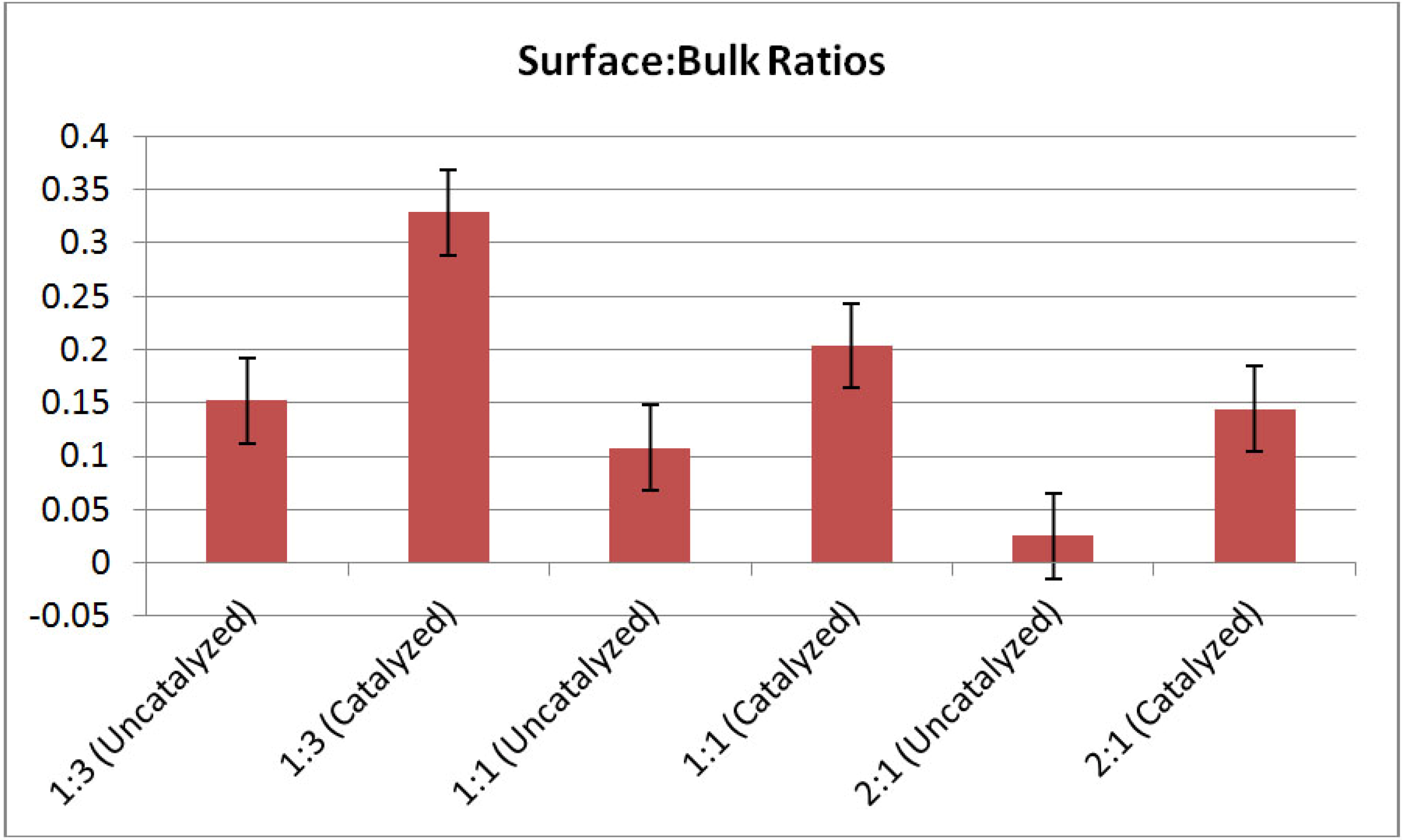
Figure 7 shows interphase boundary to bulk volume ratios for the mixtures. Both uncatalyzed and catalyzed samples are represented. In all cases, catalyzed systems have higher interfacial volumes relative to the uncatalyzed systems for all molar ratios. These data suggest that the catalyst contributes to reduction in particle size in the mixed powder systems. The reason for this enhanced reduction in particle size is yet unknown, however, it may be attributed to particle embrittlement associated with diffusion Ti
3+ cations into individual LiBH
4 or MgH
2 particles. Still, a different explanation for the increased interfacial boundary to bulk volume ratio within the catalyzed samples might be that TiCl
3 remained as a separate powder phase in the system. Although comprising only 4 mol. percent of the powder sample, X-ray diffraction data for the catalyzed samples (shown in
Figure 8) reveals a peak at 2θ~49.84° which could correspond to either TiCl
3 or to LiBH
4 (having a peak at 2θ~49.98°). It is, however, unlikely that the inclusion of TiCl
3 (as a separate powder phase) is solely responsible for the variation in interphase to bulk volume ratio observed because it was added in only 4 mol % amounts.
Figure 7.
Interphase boundary to bulk volume ratios for LiBH4:MgH2 mixtures in 1:3, 1:1, and 2:1 molar ratios. Error bars shown are +/−0.04. In all cases, the interphase volume is increased after 4 mol % TiCl3 catalyst additions. Again, the interphase volume is computed using the edge thresholding for the LiBH4 phase—and so naming these edges as “interphase volume” makes the assumption that each LiBH4 particle is surrounded by MgH2 (not air or other LiBH4 particles).
Figure 7.
Interphase boundary to bulk volume ratios for LiBH4:MgH2 mixtures in 1:3, 1:1, and 2:1 molar ratios. Error bars shown are +/−0.04. In all cases, the interphase volume is increased after 4 mol % TiCl3 catalyst additions. Again, the interphase volume is computed using the edge thresholding for the LiBH4 phase—and so naming these edges as “interphase volume” makes the assumption that each LiBH4 particle is surrounded by MgH2 (not air or other LiBH4 particles).
Figure 8.
X-ray diffraction for 4 mol % TiCl3 catalyzed LiBH4:MgH2 in 2:1, 1:1 and 1:3 molar ratios.
Figure 8.
X-ray diffraction for 4 mol % TiCl3 catalyzed LiBH4:MgH2 in 2:1, 1:1 and 1:3 molar ratios.
The parameter of interfacial boundary volume-to-bulk volume is an important parameter for any solid-solid phase transformation. The interphase boundary volume would indicate the available volume for solid-solid reactions to occur. Here, the pixels found along the edges of the LiBH4 particles are highlighted and counted via image analysis. However, this edge count is limited to the light grey LiBH4 phase and in no way quantitatively determines the amount of interphase between LiBH4 and MgH2. For example, the method used for processing this image data does not exclude the possibility that a LiBH4 particle is in contact with void space or with another LiBH4 particle. Here, it can only be assumed that each LiBH4 particle is surrounded by MgH2 particles. At low molar fraction of LiBH4 in the powders (i.e., molar fractions below the percolation threshold where LiBH4 particle-to-particle contact occurs)—this is a believed to be a valid assumption.
Other studies have been undertaken to use microtomography for understanding solid-to-solid microstructural and phase transformations. Many studies focus on sintering and neck formation in heterogenous systems [
9,
10]. Fewer studies have undertaken, as this one does, a comprehensive analysis of interfacial volume driven reactions in heterogenous systems [
11].Furthermore, the tomographic imaging allowed (with relative ease) the preparation of samples without exposure to air and moisture known to oxidize or decompose many complex metal hydrides. A detailed image analysis and high spatial resolution of the type presented here would not be possible using a scanning electron microscope unless that microscope is equipped with an environmental sample loading chamber.