Synthesis and Hydrolytic Degradation of Substituted Poly(DL-Lactic Acid)s
Abstract
:1. Introduction
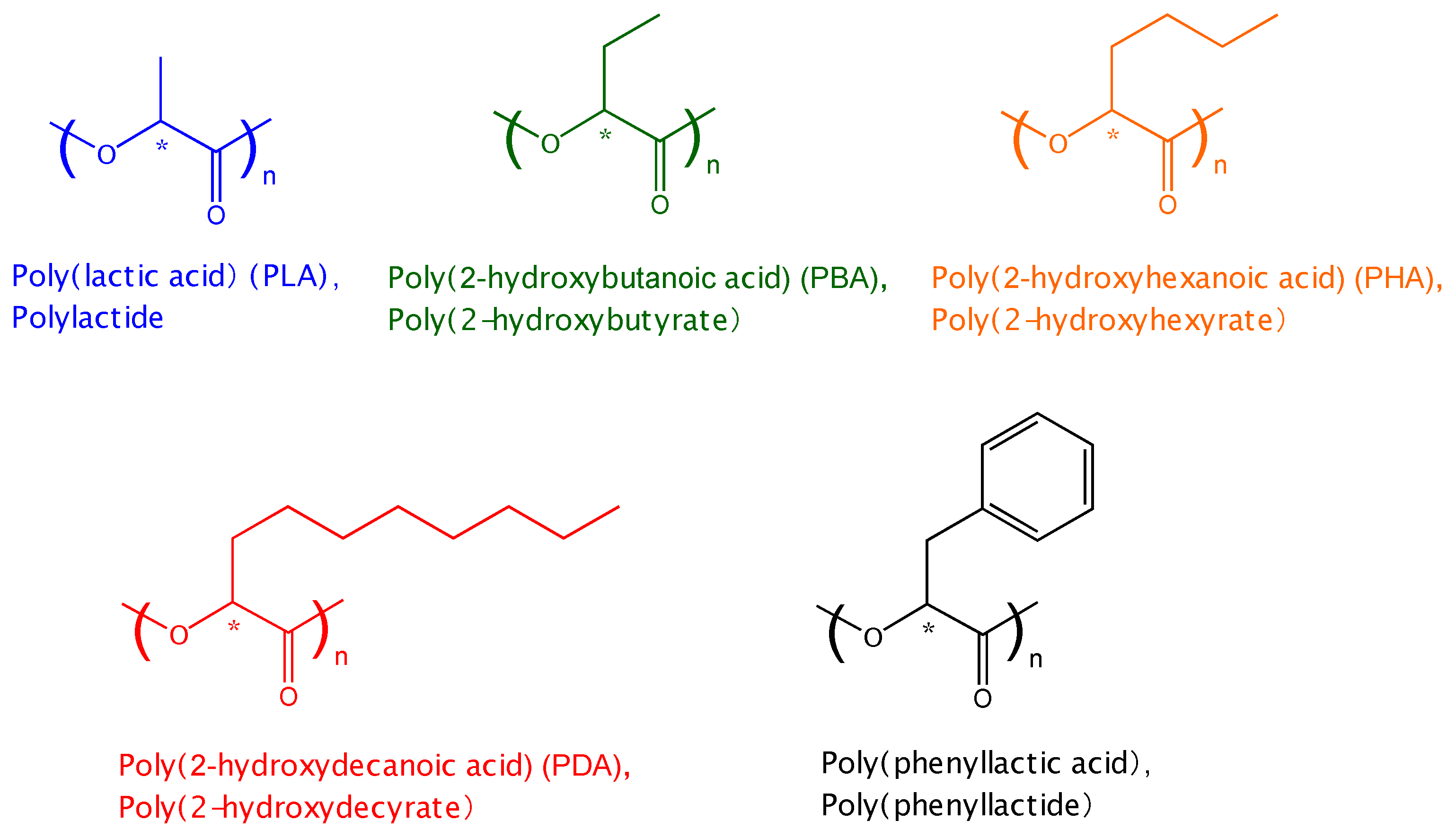
2. Experimental Section
2.1. Materials
2.2. Hydrolytic Degradation
2.3. Measurements
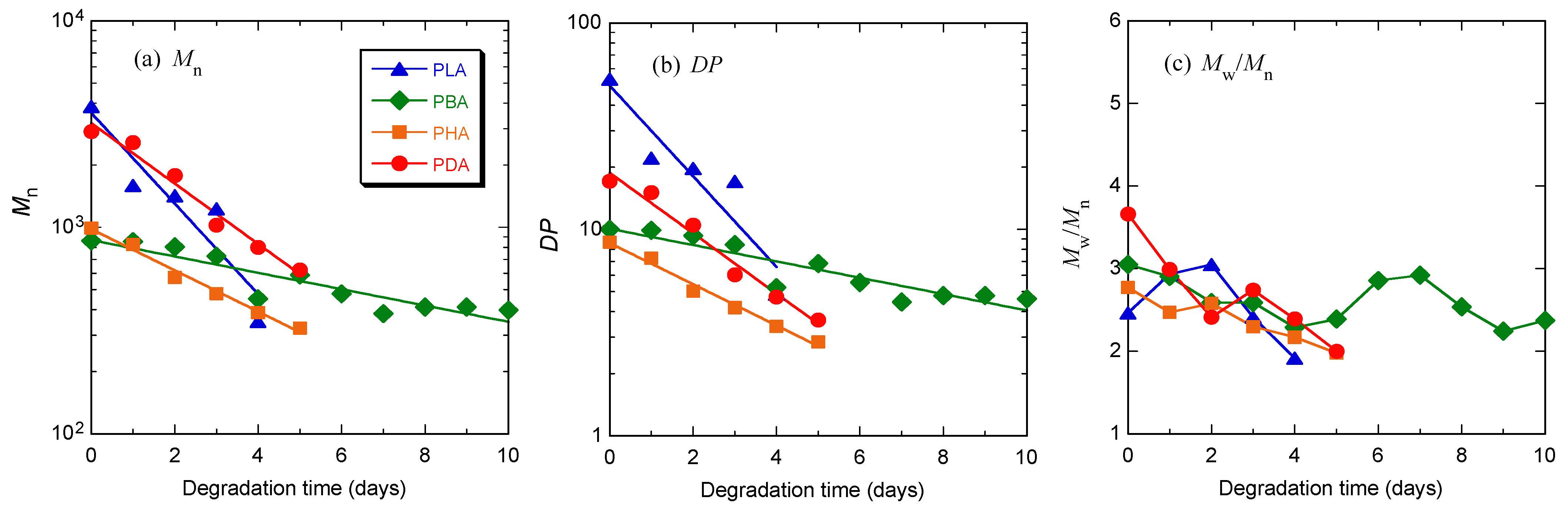
3. Results
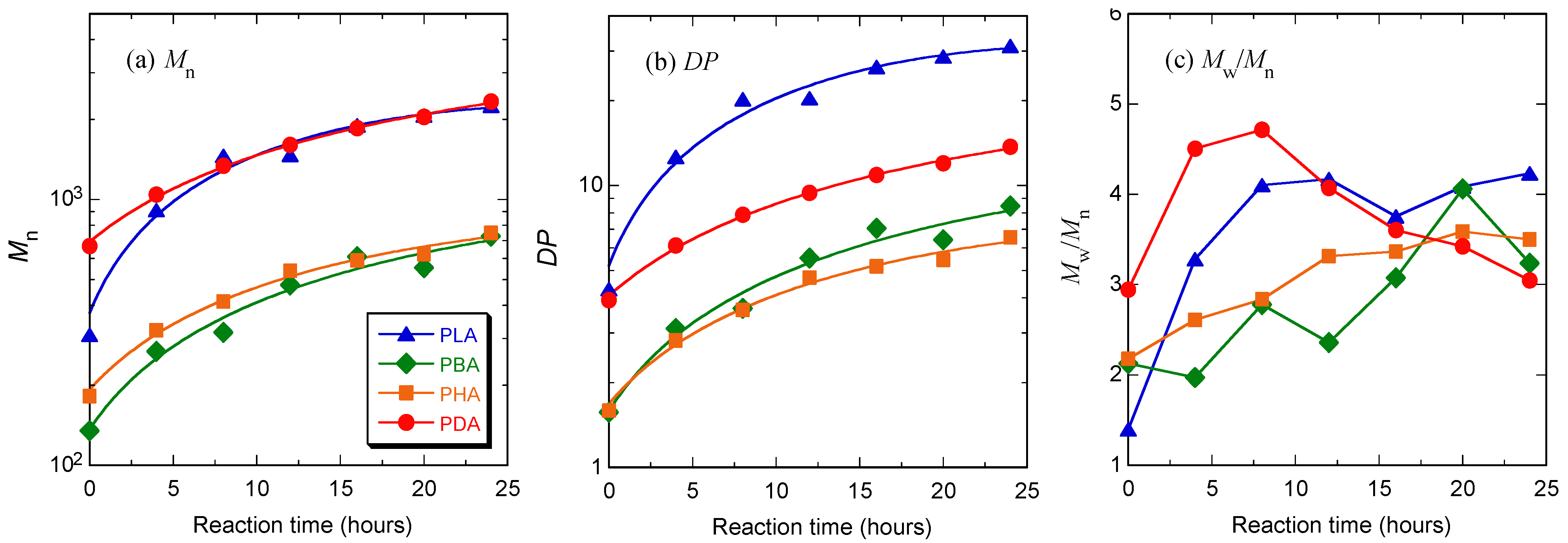
3.1. Synthesis
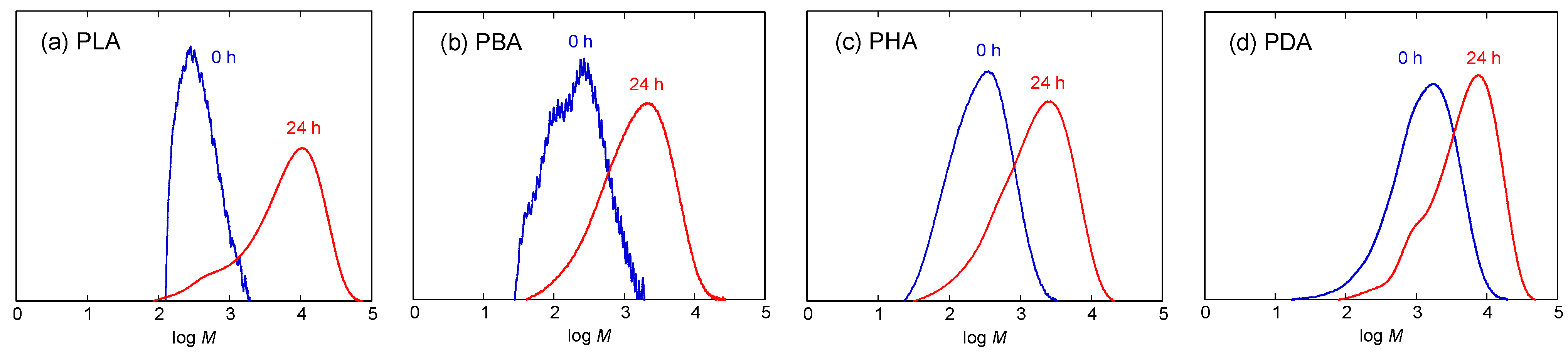
| Code | After first polymerization step at atmospheric pressure for 5 hours | After second polymerization step under reduced pressure for 24 hours | |||||||
| Mn a) | Mw/Mn a) | DP | DPa − DPb b) | Mn a) | Mw/Mn a) | DP | DPa − DPb b) | ||
| PLA | 309 | 1.40 | 4.29 | 3.29 (4.29–1) | 2250 | 4.23 | 31.2 | 26.9 (31.2–4.3) | |
| PBA | 135 | 2.13 | 1.56 | 0.56 (1.56–1) | 728 | 3.24 | 8.46 | 6.90 (8.46–1.56) | |
| PHA | 182 | 2.18 | 1.59 | 0.59 (1.59–1) | 748 | 3.50 | 6.55 | 4.96 (6.55–1.59) | |
| PDA | 668 | 2.95 | 3.92 | 2.92 (2.92–1) | 2340 | 3.05 | 13.7 | 9.8 (13.7–3.9) | |
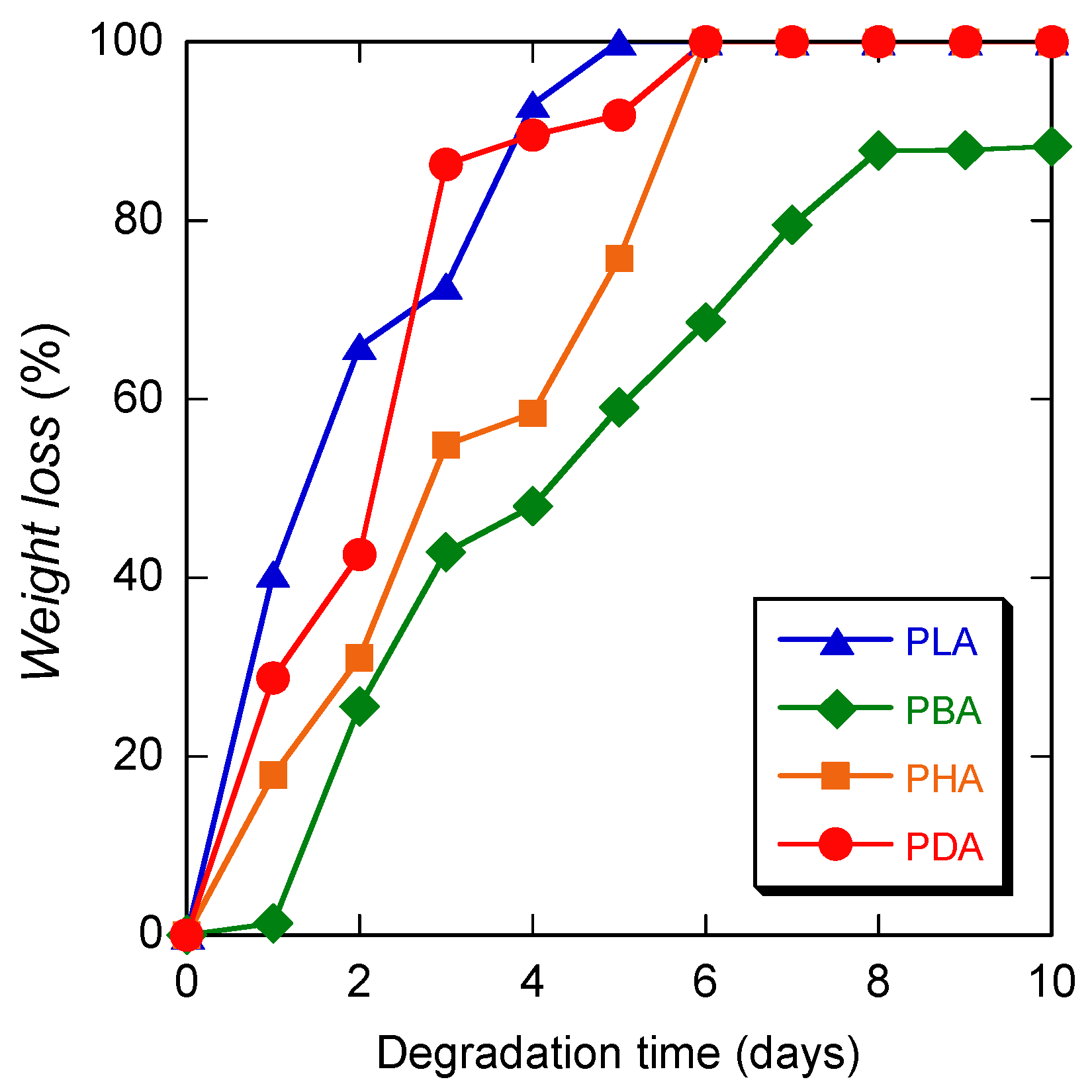
3.2. Hydrolytic Degradation at 80 °C
| Code | After Purification (Before hydrolytic degradation) | After hydrolytic degradation at 80 °C for 4 days | After hydrolytic degradation at 37 °C for 28 days | ||||||||
| Mn a) | Mw/Mn a) | DP | Mn a) | Mw/Mn a) | DP | Mn a) | Mw/Mn a) | DP | |||
| PLA | 3850 | 2.46 | 53.5 | 350 | 1.92 | 4.85 | 856 | 6.02 | 11.9 | ||
| PBA | 861 | 3.05 | 10.0 | 450 | 2.28 | 5.23 | 573 | 3.22 | 6.66 | ||
| PHA | 991 | 2.77 | 8.68 | 386 | 2.17 | 3.38 | 657 | 3.30 | 5.75 | ||
| PDA | 2910 | 3.66 | 17.1 | 799 | 2.39 | 4.69 | 1436 | 3.54 | 8.43 | ||
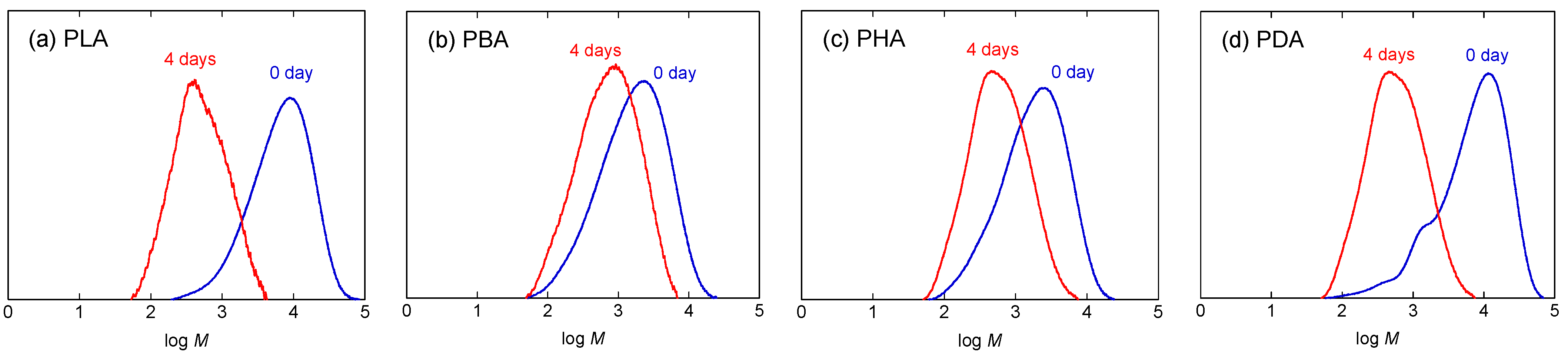
3.3. Hydrolytic degradation at 37 °C
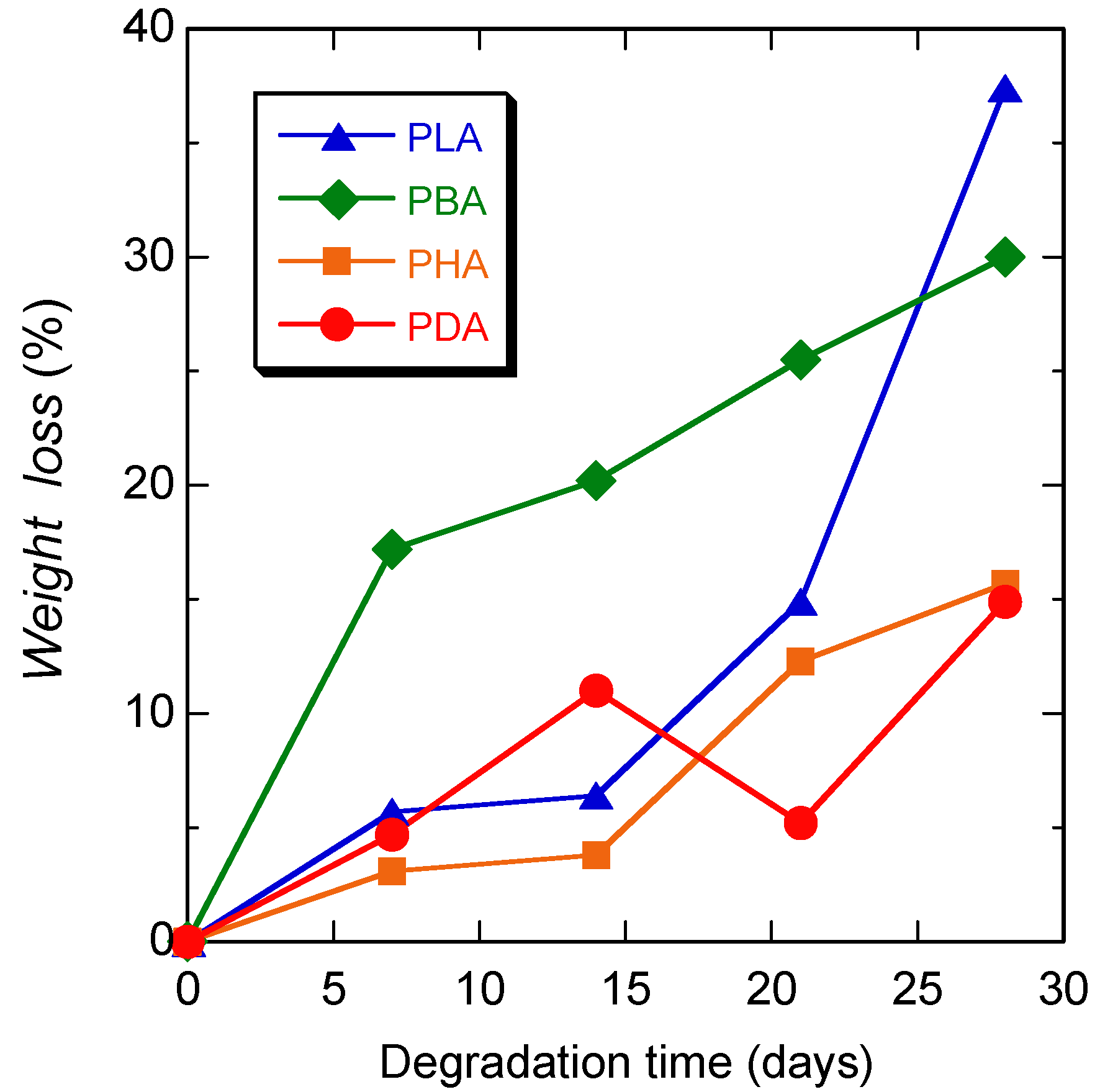
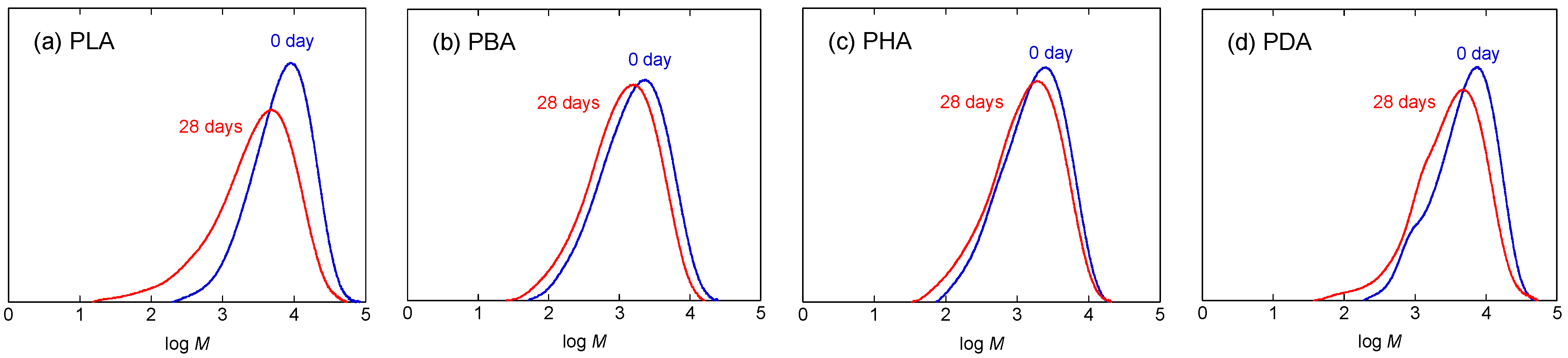

4. Discussion
5. Conclusions
Acknowledgement
References
- Biodegradable Polymers and Plastics; Vert, M.; Feijen, J.; Albertsson, A.-C.; Scott, G.; Chiellini, E. (Eds.) Royal Society of Chemistry: Cambridge, UK, 1992.
- Plastics from Microbes; Mobley, D.P. (Ed.) Hanser Publishers: New York, NY, USA, 1994.
- Handbook of Biodegradable Polymers (Drug Targeting and Delivery); Domb, A.J.; Kost, J.; Wieseman, D.M. (Eds.) Harwood Academic Publishers: Amsterdam, The Netherlands, 1997; Volume 7.
- Biopolymers from Renewable Resources; Kaplan, D.L. (Ed.) Springer: Berlin, Germany, 1998.
- Degradable Aliphatic Polyesters (Advances in Polymer Science); Albertsson, A.-C. (Ed.) Springer: Berlin, Germany, 2002; Volume 157.
- Polyesters I, II, III (Biopolymers); Doi, Y.; Steinbüchel, A. (Eds.) Wiley-VCH: Weinheim, Germany, 2002; Volume 3a, 3b, 4.
- Tsuji, H. Hydrolytic Degradation, in Poly(lactic acid): Synthesis, Structures, Properties, Processing, and Applications (Wiley Series on Polymer Engineering and Technology); Auras, R., Lin, L.-T., Selke, S.E.M., Tsuji, H., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 345–381. [Google Scholar]
- Fukuzaki, H.; Yoshida, M.; Asano, M.; Kumakura, M. Synthesis of Copoly(D,L-lactic acid) with relatively low molecular weight and in vitro degradation. Eur. Polym. J. 1989, 25, 1019–1026. [Google Scholar] [CrossRef]
- Hyon, S.H.; Jamshidi, K.; Ikada, Y. Effects of residual monomer on the degradation of DL-lactide polymer. Polym. Int. 1998, 46, 196–202. [Google Scholar] [CrossRef]
- Cam, D.; Hyon, S.H.; Ikada, Y. Degradation of high molecular weight poly(-lactide) in alkaline medium. Biomaterials 1995, 16, 833–843. [Google Scholar] [PubMed]
- Tsuji, H.; Miyauchi, S. Enzymatic hydrolysis of poly(lactide)s: Effects of molecular weight, L-lactide content, and enantiomeric and diastereoisomeric polymer blending. Biomacromolecules 2001, 2, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Tsuji, H. Effects of molecular weight and small amounts of D-lactide units on hydrolytic degradation of poly(L-lactic acid)s. Polym. Degrad. Stab. 2006, 91, 1665–1673. [Google Scholar] [CrossRef]
- Tsuji, H.; Tezuka, Y. Alkaline and enzymatic degradation of L-lactide copolymers, 1: Amorphous-made Films of L-lactide copolymers with D-lactide, glycolide, and ε-caprolactone. Macromol Biosci. 2005, 5, 135–148. [Google Scholar] [PubMed]
- Tsuji, H. Autocatalytic Hydrolysis of amorphous-made polylactides: Effects of L-lactide content, tacticity, and enantiomeric polymer blending. Polymer 2002, 43, 1789–1796. [Google Scholar] [CrossRef]
- Reed, A.M.; Gilding, D.K. Biodegradable polymers for use in surgery-poly(glycolic)/poly(lactic acid) homo and copolymers: 2. In vitro degradation. Polymer 1981, 22, 494–498. [Google Scholar]
- Vert, M.; Li, S.M.; Garreau, H. More about the degradation of LA/GA-derived matrices in aqueous media. J. Control. Release 1991, 16, 15–26. [Google Scholar] [CrossRef]
- Vert, M.; Li, S.M.; Garreau, H. New insights on the degradation of bioresorbable polymeric devices based on lactic and glycolic acids. Clin. Mater. 1992, 10, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Vert, M.; Mauduit, J.; Li, S.M. Biodegradation of PLA/GA polymers: Increasing complexity. Biomaterials 1994, 15, 1209–1213. [Google Scholar] [PubMed]
- Li, S.M. Hydrolytic degradation characteristics of aliphatic polyesters derived from lactic and glycolic acids. J. Biomed. Mater. Res. (Appl. Mater.) 1999, 48, 342–353. [Google Scholar] [CrossRef]
- Kishida, A.; Yoshioka, S.; Takeda, Y.; Uchiyama, M. Base-induced polymer hydrolysis in poly(β-hydroxybutyrate/β-hydroxyvalerate) matrices. Chem. Pharm. Bull. 1989, 37, 1954–1956. [Google Scholar] [CrossRef]
- Nakamura, T.; Hitomi, S.; Watanabe, S.; Shimizu, Y.; Jamshidi, K.; Hyon, S.H.; Ikada, Y. Bioabsorption of polylactide with different molecular properties. J. Biome. Mater. Res. 1989, 23, 1115–1130. [Google Scholar]
- Pitt, C.G.; Cha, Y.; Shah, S.S.; Zhu, K.J. Blends of PVA and PLGA: Control of permeability and degradability of hydrogels by blending. J. Control. Release 1992, 19, 189–200. [Google Scholar] [CrossRef]
- Mauduit, J.; Pérouse, E.; Vert, M. Hydrolytic degradation of films prepared from blends of high and low molecular weight poly(DL-lactic acid)s. J. Biomed. Mater. Res. 1996, 30, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Nijenhuis, A.J.; Colstee, E.; Grijpma, D.W.; Pennings, A.J. High molecular weight poly(L-lactide) and poly(ethylene oxide) blends: Thermal characterization and physical properties. Polymer 1996, 37, 5849–5857. [Google Scholar] [CrossRef]
- Shikinami, Y.; Okuno, M. Bioresorbable devices made of forged composites of hydroxyapatite (HA) particles and poly-L-lactide (PLLA): Part II. Practical properties of miniscrews and miniplates. Biomaterials 2001, 22, 3197–3211. [Google Scholar] [CrossRef] [PubMed]
- Ignatius, A.A.; Augat, P.; Claes, L.E. Degradation behavior of composite pins made of tricalcium phosphate and poly(L,DL-lactide). J. Biomater. Sci. Polym. Ed. 2001, 12, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Muramatsu, H. Blends of aliphatic polyesters: V. Non-enzymatic and enzymatic hydrolysis of blends from hydrophobic poly(L-lactide) and hydrophilic poly(vinyl alcohol). Polym. Degrad. Stab. 2001, 71, 403–415. [Google Scholar] [CrossRef]
- Renouf-Glauser, A.C.; Rose, J.; Farrar, D.F.; Cameron, R.E. A degradation study of PLLA containing lauric acid. Biomaterials 2005, 26, 2415–2422. [Google Scholar] [PubMed]
- Renouf-Glauser, A.C.; Rose, J.; Farrar, D.F.; Cameron, R.E. Comparison of the hydrolytic degradation and deformation properties of a PLLLA-lauric acid based family of biomaterials. Biomacromolecules 2006, 7, 612–617. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Lee, S.W.; Youk, J.H.; Min, B. M.; Lee, S.J.; Park, W.H. In vitro degradation behaviour of non-porous ultra-fine poly(glycolic acid)/poly(L-lactic acid) fibres and porous ultra-fine poly(glycolic acid) fibres. Polym. Degrad. Stab. 2005, 90, 441–448. [Google Scholar] [CrossRef]
- Shirahase, T.; Komatsu, Y.; Tominaga, Y.; Asai, S.; Sumita, M. Miscibility and hydrolytic degradation in alkaline solution of poly(L-lactide) and poly(methyl methacrylate) blends. Polymer 2006, 47, 4839–4844. [Google Scholar]
- Li, S. M.; Garreau, H.; Vert, M. Structure-property relationships in the case of the degradation of massive poly(α-hydroxy acids) in aqueous media. Part 3. Influence of the morphology of poly(L-lactic acid). J. Mater. Sci. Mater. Med. 1990, 1, 198–206. [Google Scholar] [CrossRef]
- Tsuji, H.; Miyauchi, S. Poly(L-lactide). VI. Effects of crys-tallinity on enzymatic hydrolysis of poly(L-lactide) without free amorphous region. Polym. Degrad. Stab. 2001, 71, 415–424. [Google Scholar] [CrossRef]
- Tsuji, H.; Mizuno, A.; Ikada, Y. Properties and morphology of poly(L-lactide). 3. Effects of crystallinity on long-term in vitro hydrolysis of poly(L-lactide) film in phosphate-buffered solution. J. Appl. Polym. Sci. 2000, 77, 1452–1464. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Properties and Morphology of Poly(L-lactide). II. Hydrolysis in Alkaline Solution. Polym. Sci. Part A Polym. Chem. 1998, 36, 59–66. [Google Scholar] [CrossRef]
- Tsuji, H.; Miyauchi, S. Poly(L-lactide): 7. Enzymatic hydrolysis of free and restricted amorphous regions in poly(L-lactide) Films with different crystallinities and a fixed crystalline thickness. Polymer 2001, 42, 4463–4467. [Google Scholar]
- Tsuji, H.; Ikada, Y. Properties and morphology of poly(L-lactide). 4. Effects of structural parameters on long-term in vitro hydrolysis of poly(L-lactide) in phosphate-buffered solution. Polym. Degrad. Stab. 2000, 67, 179–189. [Google Scholar]
- Tsuji, H.; Nakahara, K. Poly(L-lactide): 8. high-temperature hydrolysis of poly(L-lactide) films with different crystallinities and crystalline thicknesses in phosphate-buffered solution. Macromol. Mater. Eng. 2001, 286, 398–406. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikarash, K.; Fukuda, N. Poly(L-lactide): XII. Formation, growth, and morphology of crystalline residues as extended-chain crystallites through hydrolysis of poly(L-lactide) films in phosphate-buffered solution. Polym. Degrad. Stab. 2004, 84, 515–523. [Google Scholar] [CrossRef]
- Hyon, S. H.; Jamshidi, K.; Ikada, Y. Melt spinning of poly-L-lactide and hydrolysis of the fiber in vitro. In Polymers as Biomaterials; Shalaby, S.W., Hoffman, A.S., Ratner, B.D., Horbett, T.A., Eds.; Plenum Press: New York, NY, USA, 1984; pp. 51–65. [Google Scholar]
- Jamshidi, K.; Hyon, S.H.; Nakamura, T.; Ikada, Y.; Shimidu, Y.; Teramatsu, T. In vitro and in vivo degradation of poly-L-lactide fibers. In Biological and Biomechanical Performance of Biomaterials; Christel, P., Meunier, A., Lee, A.J.C., Eds.; Elsevier Science Publisher B.V.: Amsterdam, The Netherlands, 1985; pp. 227–232. [Google Scholar]
- Hyon, S.H.; Jin, F.; Jamshidi, K.; Tsutsumi, S.; Kanamoto, T. Biodegradable ultra high strength poly(L-lactide) rods for bone fixation. Macromol. Symp. 2003, 197, 355–368. [Google Scholar] [CrossRef]
- Tsuji, H.; Kidokoro, Y.; Mochizuki, M. Enzymatic degradation of poly(L-lactic acid) fibers: effects of small drawing. J. Appl. Polym. Sci. 2007, 103, 2064–2071. [Google Scholar] [CrossRef]
- Tsuji, H.; Ogiwara, M.; Saha, S.K.; Sakaki, T. Enzymatic, alkaline, and autocatalytic degradation of poly(L-lactic acid): Effects of biaxial orientation. Biomacromolecules 2006, 7, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Grizzi, I.; Garreau, H.; Li, S.; Vert, M. Hydrolytic degradation of devices based on poly(L-lactic acid) size-dependence. Biomaterials 1995, 16, 305–311. [Google Scholar]
- Cai, Q.; Shi, G.; Bei, J.; Wang, S. Enzymatic degradation behavior and mechanism of Poly(lactide-co-glycolide) foams by trypsin. Biomaterials 2003, 24, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Garcia, C. A.; Mikos, A.K. In vitro degradation of thin poly(DL-lactic-co-glycolic acid) films. J. Biomed. Mater. Res. 1999, 46, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Baker, G.L. Preparation and characterization of substituted polylactides. Macromolecules 1999, 32, 7711–7718. [Google Scholar] [CrossRef]
- Jing, F.; Smith, M.R., III; Baker, G.L. Cyclohexyl-substituted polyglycolides with high glass transition temperatures. Macromolecules 2007, 40, 9304–9312. [Google Scholar] [CrossRef]
- Liu, T.; Simmons, T.L.; Bohnsack, D.A.; Mackay, M.E.; Smith, M.R., III; Baker, G.L. Synthesis of polymandelide: A degradable polylactide derivative with polystyrene-like properties. Macromolecules 2007, 40, 6040–6047. [Google Scholar] [CrossRef]
- Tsuji, H.; Matsuoka, H.; Itsuno, S. Synthesis, physical properties, and crystallization of optically active poly(L-phenyllactic acid) and poly(L-phenyllactic acid-co-L-lactic acid). J. Appl. Polym. Sci. 2008, 110, 3954–3962. [Google Scholar]
- Tsuji, H.; Okumura, A. Stereocomplex formation between enantiomeric substituted poly(lactide)s: blends of poly[(S)-2-hydroxybutyrate] and poly[(R)-2-hydroxybutyrate]. Macromolecules 2009, 42, 7263–7266. [Google Scholar] [CrossRef]
- Tsuji, H.; Okumura, A. Crystallization and hydrolytic/thermal degradation of a novel stereocomplexationable blend of poly(L-2-hydroxybutyrate) and poly(D-2-hydroxybutyrate). Polym. J. 2011, 43, 317–324. [Google Scholar]
- Tsuji, H.; Yamamoto, S.; Okumura, A.; Sugiura, Y. Hetero-stereocomplexation between biodegradable and optically active polyesters as a versatile preparation method for biodegradable materials. Biomacromolecules 2010, 11, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Matsuoka, H. Stereoselective interaction between isotactic and optically active poly(lactic acid) and phenyl-substituted poly(lactic acid). Macromol. Rapid Commun. 2008, 29, 1372–1377. [Google Scholar] [CrossRef]
- Fukuzaki, H.; Yoshida, M.; Asano, M.; Kumakura, M.; Imasaka, K.; Nagai, T.; Mashimo, T.; Yuasa, H.; Imai, K.; Yamanaka, H. Synthesis of biodegradable copoly(L-lactic acid/aromatic hydroxy acids) with relatively low molecular weight. Eur. Polym. J. 1990, 26, 1273–1277. [Google Scholar] [CrossRef]
- Fukuzaki, H.; Yoshida, M.; Asano, M.; Kumakura, M.; Mashimo, T.; Yuasa, H.; Imai, K.; Yamanaka, H. In vivo characteristics of low molecular weight copolymers composed of L-lactic acid and various DL-hydroxy acids as biodegradablec arriers for drug delivery systems. Biomaterials 1990, 11, 441–446. [Google Scholar]
- Moon, S.-I.; Urayama, H.; Kimura, Y. Structural characterization and degradability of poly(L-lactic acid)s incorporating phenyl-substituted α-hydroxy acids as comonomers. Macromol. Biosci. 2003, 3, 301–309. [Google Scholar] [CrossRef]
- Pitt, C.G.; Zhong-wei, G. Modification of the rates of chain cleavage of poly(ε-caprolactone) and related polyesters in the solid state. J. Control. Release 1987, 4, 283–292. [Google Scholar] [CrossRef]
- Vert, M. Glycolide and Copolyesters with Lactide, in Polyesters III (Biopolymers); Doi, Y., Steinbüchel, A., Eds.; Wiley-VCH: Weinheim, Germany, 2002; Volume 4, pp. 179–202. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tsuji, H.; Eto, T.; Sakamoto, Y. Synthesis and Hydrolytic Degradation of Substituted Poly(DL-Lactic Acid)s. Materials 2011, 4, 1384-1398. https://doi.org/10.3390/ma4081384
Tsuji H, Eto T, Sakamoto Y. Synthesis and Hydrolytic Degradation of Substituted Poly(DL-Lactic Acid)s. Materials. 2011; 4(8):1384-1398. https://doi.org/10.3390/ma4081384
Chicago/Turabian StyleTsuji, Hideto, Takehiko Eto, and Yuzuru Sakamoto. 2011. "Synthesis and Hydrolytic Degradation of Substituted Poly(DL-Lactic Acid)s" Materials 4, no. 8: 1384-1398. https://doi.org/10.3390/ma4081384
APA StyleTsuji, H., Eto, T., & Sakamoto, Y. (2011). Synthesis and Hydrolytic Degradation of Substituted Poly(DL-Lactic Acid)s. Materials, 4(8), 1384-1398. https://doi.org/10.3390/ma4081384




