Effect of Ti6Al4V Alloy Surface and Porosity on Bone Osseointegration: In Vivo Pilot Study in Rabbits
Abstract
1. Introduction
2. Materials and Methods
2.1. Ti6Al4V Implant Designs
2.2. In Vivo Ti6Al4V Alloy Implant Study
2.3. Evaluation of Ti6Al4V Implant Osseointegration
2.4. Densitometric Analysis with µ-CT and 3D Reconstruction
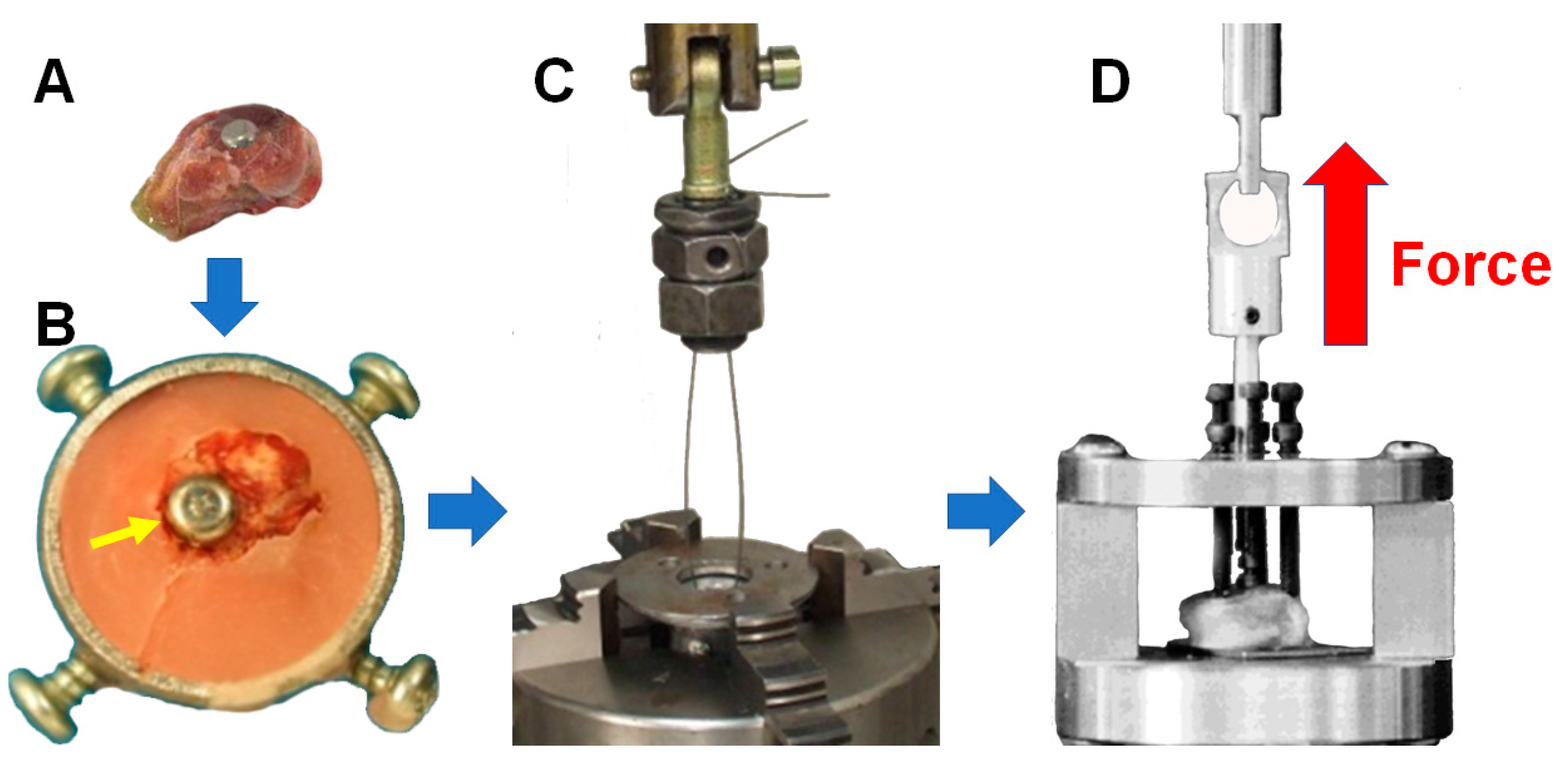
2.5. Pull-Out Test
3. Statistical Analysis
4. Results
4.1. Bone Growth AROUND the Implant
4.2. Bone Growth INSIDE the Implant
4.3. Pull-Out Test Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Cui, X.; Hooper, G.J.; Lim, K.S.; Woodfield, T.B.F. Rational design, bio-functionalization and biological performance of hybrid additive manufactured titanium implants for orthopaedic applications: A review. J. Mech. Behav. Biomed. Mater. 2020, 105, 103671. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Lanzutti, A. Biomedical Applications of Titanium Alloys: A Comprehensive Review. Materials 2023, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Brogini, S.; Sartori, M.; Giavaresi, G.; Cremascoli, P.; Alemani, F.; Bellini, D.; Martini, L.; Maglio, M.; Pagani, S.; Fini, M. Osseointegration of additive manufacturing Ti-6Al-4V and Co-Cr-Mo alloys, with and without surface functionalization with hydroxyapatite and type I collagen. J. Mech. Behav. Biomed. Mater. 2021, 115, 104262. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hady Gepreel, M.; Niinomi, M. Biocompatibility of Ti-alloys for long-term implantation. J. Mech. Behav. Biomed. Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Chaturvedi, T.P. An overview of the corrosion aspect of dental implants (titanium and its alloys). Indian J. Dent. Res. 2009, 20, 91–98. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; Wu, Y.; Zhang, Z.; Jiang, D.; Jia, R.; Wang, X.; Liu, Z. In Vitro and In Vivo Analysis of the Effects of 3D-Printed Porous Titanium Alloy Scaffold Structure on Osteogenic Activity. Biomed. Res. Int. 2022, 2022, 8494431. [Google Scholar] [CrossRef]
- Lautenschlager, E.P.; Monaghan, P. Titanium and titanium alloys as dental materials. Int. Dent. J. 1993, 43, 245–253. [Google Scholar]
- Pobloth, A.-M.; Checa, S.; Razi, H.; Petersen, A.; Weaver, J.C.; Schmidt-Bleek, K.; Windolf, M.; Tatai, A.Á.; Roth, C.P.; Schaser, K.-D.; et al. Mechanobiologically optimized 3D titanium-mesh scaffolds enhance bone regeneration in critical segmental defects in sheep. Sci. Transl. Med. 2018, 10, eaam8828. [Google Scholar] [CrossRef]
- Pan, C.-T.; Hsu, W.-H.; Cheng, Y.-S.; Wen, Z.-H.; Chen, W.-F. A New Design of Porosity Gradient Ti-6Al-4V Encapsulated Hydroxyapatite Dual Materials Composite Scaffold for Bone Defects. Micromachines 2021, 12, 1294. [Google Scholar] [CrossRef]
- Raffa, M.L.; Nguyen, V.-H.; Hernigou, P.; Flouzat-Lachaniette, C.-H.; Haiat, G. Stress shielding at the bone-implant interface: Influence of surface roughness and of the bone-implant contact ratio. J. Orthop. Res. 2021, 39, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, J.; Liu, L.; Xu, D.; Liu, Y.; Li, S.; Hou, W.; Wang, J.; Chen, X.; Sheng, L.; et al. Improving osteoinduction and osteogenesis of Ti6Al4V alloy porous scaffold by regulating the pore structure. Front. Chem. 2023, 11, 1190630. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.-K.; Hsu, H.-C.; Wu, S.-C.; Ho, W.-F. A Review: Design from Beta Titanium Alloys to Medium-Entropy Alloys for Biomedical Applications. Materials 2023, 16, 7046. [Google Scholar] [CrossRef]
- Bistolfi, A.; Cimino, A.; Lee, G.-C.; Ferracini, R.; Maina, G.; Berchialla, P.; Massazza, G.; Massè, A. Does metal porosity affect metal ion release in blood and urine following total hip arthroplasty? A short term study. HIP Int. 2018, 28, 522–530. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Saha, R.; Saha, B. Toxicity of inorganic vanadium compounds. Res. Chem. Intermed. 2015, 41, 4873–4897. [Google Scholar] [CrossRef]
- Leban, M.B.; Kosec, T.; Finšgar, M. Corrosion characterization and ion release in SLM-manufactured and wrought Ti6Al4V alloy in an oral environment. Corros. Sci. 2022, 209, 110716. [Google Scholar] [CrossRef]
- Ng, S.L.; Das, S.; Ting, Y.-P.; Wong, R.C.W.; Chanchareonsook, N. Benefits and Biosafety of Use of 3D-Printing Technology for Titanium Biomedical Implants: A Pilot Study in the Rabbit Model. Int. J. Mol. Sci. 2021, 22, 8480. [Google Scholar] [CrossRef]
- Calazans Neto, J.V.; Celles, C.A.S.; de Andrade, C.S.A.F.; Afonso, C.R.M.; Nagay, B.E.; Barão, V.A.R. Recent Advances and Prospects in β-type Titanium Alloys for Dental Implants Applications. ACS Biomater. Sci. Eng. 2024, 10, 6029–6060. [Google Scholar] [CrossRef]
- Witkowska, J.; Sobiecki, J.; Wierzchoń, T. Advancements in Surface Modification of NiTi Alloys for Orthopedic Implants: Focus on Low-Temperature Glow Discharge Plasma Oxidation Techniques. Int. J. Mol. Sci. 2025, 26, 1132. [Google Scholar] [CrossRef]
- Kapat, K.; Srivas, P.K.; Rameshbabu, A.P.; Maity, P.P.; Jana, S.; Dutta, J.; Majumdar, P.; Chakrabarti, D.; Dhara, S. Influence of Porosity and Pore-Size Distribution in Ti6Al4 V Foam on Physicomechanical Properties, Osteogenesis, and Quantitative Validation of Bone Ingrowth by Micro-Computed Tomography. ACS Appl. Mater. Interfaces 2017, 9, 39235–39248. [Google Scholar] [CrossRef]
- Li, J.; Zheng, Y.; Yu, Z.; Kankala, R.K.; Lin, Q.; Shi, J.; Chen, C.; Luo, K.; Chen, A.; Zhong, Q. Surface-modified titanium and titanium-based alloys for improved osteogenesis: A critical review. Heliyon 2023, 10, e23779. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ding, T.; Cheng, Y.; Zheng, J.; Fang, X.; Feng, Z. The rational design, biofunctionalization and biological properties of orthopedic porous titanium implants: A review. Front. Bioeng. Biotechnol. 2025, 13, 1548675. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xu, J.; Zou, L.; Luo, S.; Yao, R.; Zheng, B.; Liang, G.; Wu, D.; Li, Y. Long-lasting renewable antibacterial porous polymeric coatings enable titanium biomaterials to prevent and treat peri-implant infection. Nat. Commun. 2021, 12, 3303. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Fei, D.; Zhang, Y.; Wang, Q. Functionalized titanium implant in regulating bacteria and cell response. Int. J. Nanomed. 2019, 14, 1433–1450. [Google Scholar] [CrossRef]
- Kontakis, M.G.; Diez-Escudero, A.; Hariri, H.; Andersson, B.; Järhult, J.D.; Hailer, N.P. Antimicrobial and osteoconductive properties of two different types of titanium silver coating. Eur. Cell Mater. 2021, 41, 694–706. [Google Scholar] [CrossRef]
- Ghilini, F.; Fagali, N.; Pissinis, D.E.; Benítez, G.; Schilardi, P.L. Multifunctional Titanium Surfaces for Orthopedic Implants: Antimicrobial Activity and Enhanced Osseointegration. ACS. Appl. Bio. Mater. 2021, 4, 6451–6461. [Google Scholar] [CrossRef]
- Ding, M.; Shi, J.; Wang, W.; Li, D.; Tian, L. Early osseointegration of micro-arc oxidation coated titanium alloy implants containing Ag: A histomorphometric study. BMC Oral Health 2022, 22, 628. [Google Scholar] [CrossRef]
- He, J.; Feng, W.; Zhao, B.-H.; Zhang, W.; Lin, Z. In Vivo Effect of Titanium Implants with Porous Zinc-Containing Coatings Prepared by Plasma Electrolytic Oxidation Method on Osseointegration in Rabbits. Int. J. Oral. Maxillofac. Implant. 2018, 33, 298–310. [Google Scholar] [CrossRef]
- Wang, N.; Ma, Y.; Shi, H.; Song, Y.; Guo, S.; Yang, S. Mg-, Zn-, and Fe-Based Alloys with Antibacterial Properties as Orthopedic Implant Materials. Front. Bioeng. Biotechnol. 2022, 10, 888084. [Google Scholar] [CrossRef]
- Zhang, E.; Zhao, X.; Hu, J.; Wang, R.; Fu, S.; Qin, G. Antibacterial metals and alloys for potential biomedical implants. Bioact. Mater. 2021, 6, 2569–2612. [Google Scholar] [CrossRef]
- Zhao, Q.; Yi, L.; Jiang, L.; Ma, Y.; Lin, H.; Dong, J. Surface functionalization of titanium with zinc/strontium-doped titanium dioxide microporous coating via microarc oxidation. Nanomedicine 2019, 16, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yi, L.; Jiang, L.; Ma, Y.; Lin, H.; Dong, J. Osteogenic activity and antibacterial ability on titanium surfaces modified with magnesium-doped titanium dioxide coating. Nanomedicine 2019, 14, 1109–1133. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, P.M.; Marcantonio, C.C.; De Oliveira, D.P.; Lopes, M.E.S.; Puetate, J.C.S.; Faria, L.V.; De Carvalho, L.F.; De Molon, R.S.; Garcia, I.R.; Nogueira, A.V.B.; et al. Titanium micro-nano textured surface with strontium incorporation improves osseointegration: An in vivo and in vitro study. J. Appl. Oral Sci. 2024, 32, e20240144. [Google Scholar] [CrossRef]
- Mumith, A.; Cheong, V.S.; Fromme, P.; Coathup, M.J.; Blunn, G.W. The effect of strontium and silicon substituted hydroxyapatite electrochemical coatings on bone ingrowth and osseointegration of selective laser sintered porous metal implants. PLoS ONE 2020, 15, e0227232. [Google Scholar] [CrossRef]
- Taga, T.; Kabata, T.; Kajino, Y.; Inoue, D.; Ohmori, T.; Yamamoto, T.; Takagi, T.; Tsuchiya, H. Comparison with the osteoconductivity and bone-bonding ability of the iodine supported titanium, titanium with porous oxide layer and the titanium alloy in the rabbit model. J. Orthop. Sci. 2018, 23, 585–591. [Google Scholar] [CrossRef]
- Song, W.; Seta, J.; Chen, L.; Bergum, C.; Zhou, Z.; Kanneganti, P.; Kast, R.E.; Auner, G.W.; Shen, M.; Markel, D.C.; et al. Doxycycline-loaded coaxial nanofiber coating of titanium implants enhances osseointegration and inhibits Staphylococcus aureus infection. Biomed. Mater. 2017, 12, 045008. [Google Scholar] [CrossRef]
- Rabbitt, D.; Villapún, V.M.; Carter, L.N.; Man, K.; Lowther, M.; O’Kelly, P.; Knowles, A.J.; Mottura, A.; Tang, Y.T.; Luerti, L.; et al. Rethinking Biomedical Titanium Alloy Design: A Review of Challenges from Biological and Manufacturing Perspectives. Adv. Healthc. Mater. 2025, 14, 2403129. [Google Scholar] [CrossRef]
- Mishchenko, O.; Volchykhina, K.; Maksymov, D.; Manukhina, O.; Pogorielov, M.; Pavlenko, M.; Iatsunskyi, I. Advanced Strategies for Enhancing the Biocompatibility and Antibacterial Properties of Implantable Structures. Materials 2025, 18, 822. [Google Scholar] [CrossRef]
- Berahmani, S.; Janssen, D.; van Kessel, S.; Wolfson, D.; de Waal Malefijt, M.; Buma, P.; Verdonschot, N. An experimental study to investigate biomechanical aspects of the initial stability of press-fit implants. J. Mech. Behav. Biomed. Mater 2015, 42, 177–185. [Google Scholar] [CrossRef]
- Tsuang, F.-Y.; Chen, C.-H.; Wu, L.-C.; Kuo, Y.-J.; Lin, S.-C.; Chiang, C.-J. Biomechanical arrangement of threaded and unthreaded portions providing holding power of transpedicular screw fixation. Clin. Biomech. 2016, 39, 71–76. [Google Scholar] [CrossRef]
- Swami, V.; Vijayaraghavan, V.; Swami, V. Current trends to measure implant stability. J. Indian Prosthodont. Soc. 2016, 16, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Kang, S.-M.; Seo, K.-W.; Nahm, K.-Y.; Chung, K.-R.; Kim, S.-H.; Ahn, J.-P. Nanoscale bonding between human bone and titanium surfaces: Osseohybridization. Biomed. Res. Int. 2015, 2015, 960410. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. 2015, R 87, 1–57. [Google Scholar] [CrossRef]
- Chamrad, J.; Marcián, P.; Cizek, J. Beneficial osseointegration effect of hydroxyapatite coating on cranial implant—FEM investigation. PLoS ONE 2021, 16, e0254837. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lu, X.; Liu, S.; Wu, X.; Xie, Y.; Zheng, X. Boron-incorporated micro/nano-topographical calcium silicate coating dictates osteo/angio-genesis and inflammatory response toward enhanced osseointegration. Biol. Trace Elem. Res. 2021, 199, 3801–3816. [Google Scholar] [CrossRef]
- Takada, S.; Hirata, E.; Sakairi, M.; Miyako, E.; Takano, Y.; Ushijima, N.; Yudasaka, M.; Iijima, S.; Yokoyama, A. Carbon nanohorn coating by electrodeposition accelerate bone formation on titanium implant. Artif. Cells Nanomed. Biotechnol. 2021, 49, 20–29. [Google Scholar] [CrossRef]
- Li, K.; Wang, C.; Yan, J.; Zhang, Q.; Dang, B.; Wang, Z.; Yao, Y.; Lin, K.; Guo, Z.; Bi, L.; et al. Evaluation of the osteogenesis and osseointegration of titanium alloys coated with graphene: An in vivo study. Sci. Rep. 2018, 8, 1843. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, S.-S.; Jiang, N. Effect of micro/nanoscaled Ti phosphate/Ti oxide hybrid coating on the osseointegration of Ti implants. Hua Xi Kou Qiang Yi Xue Za Zhi 2021, 39, 531–539. [Google Scholar] [CrossRef]
- Battocchio, C.; Concolato, S.; De Santis, S.; Fahlman, M.; Iucci, G.; Santi, M.; Sotgiu, G.; Orsini, M. Chitosan functionalization of titanium and Ti6Al4V alloy with chloroacetic acid as linker agent. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1133–1140. [Google Scholar] [CrossRef]
- Florian, F.; Guastaldi, F.P.S.; Cominotte, M.A.; Pires, L.C.; Guastaldi, A.C.; Cirelli, J.A. Behavior of rat bone marrow stem cells on titanium surfaces modified by laser-beam and deposition of calcium phosphate. J. Mater. Sci. Mater. Med. 2021, 32, 57. [Google Scholar] [CrossRef]
- Chen, D.; Bertollo, N.; Lau, A.; Taki, N.; Nishino, T.; Mishima, H.; Kawamura, H.; Walsh, W.R. Osseointegration of porous titanium implants with and without electrochemically deposited DCPD coating in an ovine model. J. Orthop. Surg. Res. 2011, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, K.; Lu, M.; Liu, L.; Yan, Y.; Chu, Z.; Ge, Y.; Wang, T.; Qiu, J.; Bu, S.; et al. Micro/nanostructured calcium phytate coating on titanium fabricated by chemical conversion deposition for biomedical application. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111402. [Google Scholar] [CrossRef] [PubMed]
- Văruţ, R.M.; Melinte, P.R.; Pîrvu, A.S.; Gîngu, O.; Sima, G.; Oancea, C.N.; Teişanu, A.C.; Drăgoi, G.; Biţă, A.; Manolea, H.O.; et al. Calcium fructoborate coating of titanium-hydroxyapatite implants by chemisorption deposition improves implant osseointegration in the femur of New Zealand White rabbit experimental model. Rom. J. Morphol. Embryol. 2020, 61, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-L.; He, R.-Z.; Tu, B.; Cao, X.; He, J.-S.; Xia, H.-S.; Liang, C.; Zou, M.; Wu, S.; Wu, Z.-J.; et al. Enhanced biocompatibility and osseointegration of calcium titanate coating on titanium screws in rabbit femur. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017, 37, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Turker, N.; Özarslan, M.M.; Buyukkaplan, U.S.; Başar, E.K. Effect of Different Surface Treatments Applied to Short Zirconia and Titanium Abutments. Int. J. Oral Maxillofac. Implant. 2020, 35, 948–954. [Google Scholar] [CrossRef]
- Rifai, A.; Tran, N.; Lau, D.W.; Elbourne, A.; Zhan, H.; Stacey, A.D.; Mayes, E.L.H.; Sarker, A.; Ivanova, E.P.; Crawford, R.J.; et al. Polycrystalline Diamond Coating of Additively Manufactured Titanium for Biomedical Applications. ACS Appl. Mater. Interfaces 2018, 10, 8474–8484. [Google Scholar] [CrossRef]
- Nemcakova, I.; Litvinec, A.; Mandys, V.; Potocky, S.; Plencner, M.; Doubkova, M.; Nanka, O.; Olejnickova, V.; Sankova, B.; Bartos, M.; et al. Coating Ti6Al4V implants with nanocrystalline diamond functionalized with BMP-7 promotes extracellular matrix mineralization in vitro and faster osseointegration in vivo. Sci. Rep. 2022, 12, 5264. [Google Scholar] [CrossRef]
- Brama, M.; Rhodes, N.; Hunt, J.; Ricci, A.; Teghil, R.; Migliaccio, S.; Rocca, C.D.; Leccisotti, S.; Lioi, A.; Scandurra, M.; et al. Effect of titanium carbide coating on the osseointegration response in vitro and in vivo. Biomaterials 2007, 28, 595–608. [Google Scholar] [CrossRef]
- Rappe, K.S.; Ortiz-Hernandez, M.; Punset, M.; Molmeneu, M.; Barba, A.; Mas-Moruno, C.; Guillem-Marti, J.; Caparrós, C.; Rupérez, E.; Calero, J.; et al. On-Growth and In-Growth Osseointegration Enhancement in PM Porous Ti-Scaffolds by Two Different Bioactivation Strategies: Alkali Thermochemical Treatment and RGD Peptide Coating. Int. J. Mol. Sci. 2022, 23, 1750. [Google Scholar] [CrossRef]
- Lu, R.-J.; Wang, X.; He, H.-X.; E, L.-L.; Li, Y.; Zhang, G.-L.; Li, C.-J.; Ning, C.-Y.; Liu, H.-C. Tantalum-incorporated hydroxyapatite coating on titanium implants: Its mechanical and in vitro osteogenic properties. J. Mater. Sci. Mater. Med. 2019, 30, 111. [Google Scholar] [CrossRef]
- Otsuka, Y.; Kojima, D.; Mutoh, Y. Prediction of cyclic delamination lives of plasma-sprayed hydroxyapatite coating on Ti-6Al-4V substrates with considering wear and dissolutions. J. Mech. Behav. Biomed. Mater. 2016, 64, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lü, J.; Chen, F.; Li, L.; Tan, F.; Liu, J. Effect of Co-Cr coating by EB-PVD on the bonding strength between titanium and porcelain. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2020, 45, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kang, H.; Lu, J.; Dai, Y.; Wang, F. Experimental study of the effects of hypoxia simulator on osteointegration of titanium prosthesis in osteoporotic rats. BMC. Musculoskelet. Disord. 2021, 22, 944. [Google Scholar] [CrossRef] [PubMed]
- Savvidis, M.; Papavasiliou, K.; Taitzoglou, I.; Giannakopoulou, A.; Kitridis, D.; Galanis, N.; Vrabas, I.; Tsiridis, E. Postoperative Administration of Alpha-tocopherol Enhances Osseointegration of Stainless Steel Implants: An In Vivo Rat Model. Clin. Orthop. Relat. Res. 2020, 478, 406–419. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Song, G.; Liu, X.; Hu, J. Additive effects of estrogen replacement therapy and bisphosphonates on osseointegration of hydroxyapatite-coated titanium screws in ovariectomized rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 700–705. [Google Scholar] [CrossRef]
- Tao, Z.-S.; Wu, X.-J.; Yang, M.; Xu, H.-G. Local administration with silymarin could increase osseointegration of hydroxyapatite-coated titanium implants in ovariectomized rats. J. Biomater. Appl. 2019, 34, 664–672. [Google Scholar] [CrossRef]
- Barik, A.; Ray, S.K.; Byram, P.K.; Sinha, R.; Chakravorty, N. Extensive early mineralization of pre-osteoblasts, inhibition of osteoclastogenesis and faster peri-implant bone healing in osteoporotic rat model: Principle effectiveness of bone-specific delivery of Tibolone as evaluated in vitro and in vivo. Biomed. Mater. 2020, 15, 064102. [Google Scholar] [CrossRef]
- Albano, C.S.; Moreira Gomes, A.; da Silva Feltran, G.; da Costa Fernandes, C.J.; Trino, L.D.; Zambuzzi, W.F.; Lisboa-Filho, P.N. Biofunctionalization of titanium surfaces with alendronate and albumin modulates osteoblast performance. Heliyon 2020, 6, e04455. [Google Scholar] [CrossRef]
- He, Y.; Bao, W.; Wu, X.-D.; Huang, W.; Chen, H.; Li, Z. Effects of Systemic or Local Administration of Zoledronate on Implant Osseointegration: A Preclinical Meta-Analysis. Biomed. Res. Int. 2019, 2019, 9541485. [Google Scholar] [CrossRef]
- Apostu, D.; Lucaciu, O.; Mester, A.; Oltean-Dan, D.; Gheban, D.; Rares Ciprian Benea, H. Tibolone, alendronate, and simvastatin enhance implant osseointegration in a preclinical in vivo model. Clin. Oral Implants Res. 2020, 31, 655–668. [Google Scholar] [CrossRef]
- Sheng, X.; Wang, A.; Wang, Z.; Liu, H.; Wang, J.; Li, C. Advanced Surface Modification for 3D-Printed Titanium Alloy Implant Interface Functionalization. Front. Bioeng. Biotechnol. 2022, 10, 850110. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-T.; Lin, H.-I.; Chung, C.-J.; Tang, C.-H.; He, J.-L. Osseointegrating and phase-oriented micro-arc-oxidized titanium dioxide bone implants. J. Appl. Biomater. Funct. Mater 2021, 19, 22808000211006878. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, P.; Malmström, J.; Emanuelsson, L.; René, M.; Snis, A. Electron beam-melted, free-form-fabricated titanium alloy implants: Material surface characterization and early bone response in rabbits. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Im, C.; Park, J.-H.; Jeon, Y.-M.; Kim, J.-G.; Jang, Y.-S.; Lee, M.-H.; Jeon, W.-Y.; Kim, J.-M.; Bae, T.-S. Improvement of osseointegration of Ti–6Al–4V ELI alloy orthodontic mini-screws through anodization, cyclic pre-calcification, and heat treatments. Prog. Orthod. 2022, 23, 11. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Wu, R.; Wei, J. Construction of functional surfaces for dental implants to enhance osseointegration. Front. Bioeng. Biotechnol. 2023, 11, 1320307. [Google Scholar] [CrossRef]
- Bai, X.; Li, J.; Zhao, Z.; Wang, Q.; Lv, N.; Wang, Y.; Gao, H.; Guo, Z.; Li, Z. In vivo evaluation of osseointegration ability of sintered bionic trabecular porous titanium alloy as artificial hip prosthesis. Front. Bioeng. Biotechnol. 2022, 10, 928216. [Google Scholar] [CrossRef]
- Ibn-Mohammed, T.; Randall, C.A.; Mustapha, K.B.; Guo, J.; Walker, J.; Berbano, S.; Koh, S.C.L.; Wang, D.; Sinclair, D.C.; Reaney, I.M. Decarbonising ceramic manufacturing: A techno-economic analysis of energy efficient sintering technologies in the functional materials sector. J. Eur. Ceram. Soc. 2019, 39, 5213–5235. [Google Scholar] [CrossRef]
- Bencharit, S.; Byrd, W.C.; Altarawneh, S.; Hosseini, B.; Leong, A.; Reside, G.; Morelli, T.; Offenbacher, S. Development and applications of porous tantalum trabecular metal-enhanced titanium dental implants. Clin. Implant Dent. Relat. Res. 2014, 16, 817–826. [Google Scholar] [CrossRef]
- Jiao, J.; Hong, Q.; Zhang, D.; Wang, M.; Tang, H.; Yang, J.; Qu, X.; Yue, B. Influence of porosity on osteogenesis, bone growth and osteointegration in trabecular tantalum scaffolds fabricated by additive manufacturing. Front. Bioeng. Biotechnol. 2023, 11, 1117954. [Google Scholar] [CrossRef]
- Dewaele, A.; Mezouar, M.; Guignot, N.; Loubeyre, P. High melting points of tantalum in a laser-heated diamond anvil cell. Phys. Rev. Lett 2010, 104, 255701. [Google Scholar] [CrossRef]
- Cezairliyan, A.; Miiller, A.P. Melting Point, Normal Spectral Emittance (at the Melting Point), and Electrical Resistivity (above 1900 K) of Titanium by a Pulse Heating Method. J. Res. Natl. Bur. Stand. 1977, 82, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.F.; Guo, Z.X.; Yang, R. Fabrication of porous titanium scaffold materials by a fugitive filler method. J. Mater. Sci. Mater. Med. 2008, 19, 3489–3495. [Google Scholar] [CrossRef] [PubMed]
- Maksoud, F.J.; Paz, M.F.V.d.l.; Hann, A.J.; Thanarak, J.; Reilly, G.C.; Claeyssens, F.; Green, N.H.; Zhang, Y.S. Porous biomaterials for tissue engineering: A review. J. Mater. Chem. B 2022, 10, 8111–8165. [Google Scholar] [CrossRef] [PubMed]
- Phuoc, H.D.; Hoang, P.N.; Yang, S.; Fraser, D.; Nguyen, V.T. Osseointegrability of 3D-printed porous titanium alloy implant on tibial shaft bone defect in rabbit model. PLoS ONE 2023, 18, e0282457. [Google Scholar] [CrossRef]
- Losic, D. Advancing of titanium medical implants by surface engineering: Recent progress and challenges. Expert Opin. Drug Deliv. 2021, 18, 1355–1378. [Google Scholar] [CrossRef]
- Kou, X.Y.; Tan, S.T. A simple and effective geometric representation for irregular porous structure modeling. Comput. Aided Des. 2010, 42, 930–941. [Google Scholar] [CrossRef]
- Weber, F.E. Reconsidering Osteoconduction in the Era of Additive Manufacturing. Tissue Eng. Part B Rev. 2019, 25, 375–386. [Google Scholar] [CrossRef]
- Doi, K.; Kobatake, R.; Makihara, Y.; Oki, Y.; Umehara, H.; Kubo, T.; Tsuga, K. Osseointegration Aspects of Implants at the Bone Reconstruction Site by a Novel Porous Titanium Scaffold. J. Oral Maxillofac. Res. 2021, 12, e4. [Google Scholar] [CrossRef]
- Ouyang, P.; Dong, H.; He, X.; Cai, X.; Wang, Y.; Li, J.; Li, H.; Jin, Z. Hydromechanical mechanism behind the effect of pore size of porous titanium scaffolds on osteoblast response and bone ingrowth. Mater. Des. 2019, 183, 108151. [Google Scholar] [CrossRef]
- Barba, D.; Alabort, E.; Reed, R.C. Synthetic bone: Design by additive manufacturing. Acta Biomater. 2019, 97, 637–656. [Google Scholar] [CrossRef]
- Ran, Q.; Yang, W.; Hu, Y.; Shen, X.; Yu, Y.; Xiang, Y.; Cai, K. Osteogenesis of 3D printed porous Ti6Al4V implants with different pore sizes. J. Mech. Behav. Biomed. Mater. 2018, 84, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Wang, H.L.; Li, S.J.; Wang, S.G.; Wang, W.J.; Hou, W.T.; Hao, Y.L.; Yang, R.; Zhang, L.C. Compressive and fatigue behavior of beta-type titanium porous structures fabricated by electron beam melting. Acta Mater. 2017, 126, 58–66. [Google Scholar] [CrossRef]
- Zhao, S.; Li, S.J.; Wang, S.G.; Hou, W.T.; Li, Y.; Zhang, L.C.; Hao, Y.L.; Yang, R.; Misra, R.D.K.; Murr, L.E. Compressive and fatigue behavior of functionally graded Ti-6Al-4V meshes fabricated by electron beam melting. Acta Mater. 2018, 150, 1–15. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.-B.; Yun, J.; Rhyu, I.-C.; Lee, Y.-M.; Lee, S.-M.; Lee, M.-K.; Kim, B.; Kim, P.; Koo, K.-T. The impact of surface treatment in 3-dimensional printed implants for early osseointegration: A comparison study of three different surfaces. Sci. Rep. 2021, 11, 10453. [Google Scholar] [CrossRef]
- Scarano, A.; Crocetta, E.; Quaranta, A.; Lorusso, F. Influence of the Thermal Treatment to Address a Better Osseointegration of Ti6Al4V Dental Implants: Histological and Histomorphometrical Study in a Rabbit Model. Biomed. Res. Int. 2018, 2018, 2349698. [Google Scholar] [CrossRef]
- Zhao, H.; Shen, S.; Zhao, L.; Xu, Y.; Li, Y.; Zhuo, N. 3D printing of dual-cell delivery titanium alloy scaffolds for improving osseointegration through enhancing angiogenesis and osteogenesis. BMC Musculoskelet. Disord. 2021, 22, 734. [Google Scholar] [CrossRef]
- Reig, L.; Tojal, C.; Busquets, D.J.; Amigó, V. Microstructure and Mechanical Behavior of Porous Ti-6Al-4V Processed by Spherical Powder Sintering. Materials 2013, 6, 4868–4878. [Google Scholar] [CrossRef]
- 14:00–17:00 ISO 10993-6:2007. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/04/47/44789.html (accessed on 6 January 2022).
- 14:00–17:00 ISO 10993-11:2006. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/03/59/35977.html (accessed on 6 January 2022).
- Standard Practice for Short-Term Screening of Implant Materials. Available online: https://www.astm.org/f0763-04.html (accessed on 6 January 2022).
- Ministerio de la Presidencia Real Decreto 1201/2005, de 10 de Octubre, Sobre Protección de los Animales Utilizados para Experimentación y Otros Fines Científicos. 2005, Volume BOE-A-2005-17344, pp. 34367–34391. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2005-17344 (accessed on 14 December 2024).
- Ley 4/1994, de 8 de Julio, de la Generalidad Valenciana, Sobre Protección de los Animales de Compañía; Agencia Estatal Boletin Oficial del Estado: Madrid, Spain, 2014; Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-1994-18881 (accessed on 14 December 2024).
- Barrère, F.; van der Valk, C.M.; Meijer, G.; Dalmeijer, R.A.J.; de Groot, K.; Layrolle, P. Osteointegration of biomimetic apatite coating applied onto dense and porous metal implants in femurs of goats. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 67, 655–665. [Google Scholar] [CrossRef]
- Eriksson, A.R.; Albrektsson, T. Temperature threshold levels for heat-induced bone tissue injury: A vital-microscopic study in the rabbit. J. Prosthet. Dent. 1983, 50, 101–107. [Google Scholar] [CrossRef]
- Frosch, S.; Nüsse, V.; Frosch, K.-H.; Lehmann, W.; Buchhorn, G. Osseointegration of 3D porous and solid Ti–6Al–4V implants—Narrow gap push-out testing and experimental setup considerations. J. Mech. Behav. Biomed. Mater. 2021, 115, 104282. [Google Scholar] [CrossRef]
- Diefenbeck, M.; Mückley, T.; Zankovych, S.; Bossert, J.; Jandt, K.D.; Schrader, C.; Schmidt, J.; Finger, U.; Faucon, M. Freezing of Rat Tibiae at −20 °C Does Not Affect the Mechanical Properties of Intramedullary Bone/Implant-Interface: Brief Report. Open Orthop. J. 2011, 5, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, B.; Guo, S.; Yu, S.; Li, H. Manufacture of titanium alloy materials with bioactive sandblasted surfaces and evaluation of osseointegration properties. Front. Bioeng. Biotechnol. 2023, 11, 1251947. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Ortega, E.; Ortiz-Garcia, I.; Jiménez-Guerra, A.; Núñez-Márquez, E.; Moreno-Muñoz, J.; Rondón-Romero, J.L.; Cabanillas-Balsera, D.; Gil, J.; Muñoz-Guzón, F.; Monsalve-Guil, L. Osseointegration of Sandblasted and Acid-Etched Implant Surfaces. A Histological and Histomorphometric Study in the Rabbit. Int. J. Mol. Sci. 2021, 22, 8507. [Google Scholar] [CrossRef]
- Botticelli, D.; Lang, N.P. Dynamics of osseointegration in various human and animal models—A comparative analysis. Clin. Oral. Implants Res. 2017, 28, 742–748. [Google Scholar] [CrossRef]
- Scarano, A.; Khater, A.G.A.; Gehrke, S.A.; Inchingolo, F.; Tari, S.R. Animal Models for Investigating Osseointegration: An Overview of Implant Research over the Last Three Decades. J. Funct. Biomater. 2024, 15, 83. [Google Scholar] [CrossRef]
- Babuska, V.; Moztarzadeh, O.; Kubikova, T.; Moztarzadeh, A.; Hrusak, D.; Tonar, Z. Evaluating the osseointegration of nanostructured titanium implants in animal models: Current experimental methods and perspectives (Review). Biointerphases 2016, 11, 030801. [Google Scholar] [CrossRef]
- Frosch, S.; Buchhorn, G.H. Considerations on the animal model and the biomechanical test arrangements for assessing the osseous integration of orthopedic and dental implants. MethodsX 2021, 8, 101352. [Google Scholar] [CrossRef]
- Kaweblum, M.; Aguilar, M.C.; Blancas, E.; Kaweblum, J.; Lehman, W.B.; Grant, A.D.; Strongwater, A.M. Histological and radiographic determination of the age of physeal closure of the distal femur, proximal tibia, and proximal fibula of the New Zealand white rabbit. J. Orthop. Res. 1994, 12, 747–749. [Google Scholar] [CrossRef]
- Widmer, S.; Steiner, R.P.; Morscher, M.A.; Shasti, M.; Weiner, D.S.; Adamczyk, M.J.; Childs, R.D.; Landis, W.J. An investigation to validate the equivalence of physes obtained from different anatomic regions in a single animal species: Implications for choosing experimental controls in clinical studies. Bone Rep. 2019, 10, 100209. [Google Scholar] [CrossRef]
- Huang, C.-C.; Li, M.-J.; Tsai, P.-I.; Kung, P.-C.; Chen, S.-Y.; Sun, J.-S.; Tsou, N.-T. Novel design of additive manufactured hollow porous implants. Dent. Mater. 2020, 36, 1437–1451. [Google Scholar] [CrossRef]
- Mello, A.S.D.S.; Dos Santos, P.L.; Marquesi, A.; Queiroz, T.P.; Margonar, R.; De Souza Faloni, A.P. Some aspects of bone remodeling around dental implants. Rev. Clín. Periodon. Implantol. Rehabil. Oral, 2016; in press. [Google Scholar] [CrossRef]
- Cerea, M.; Dolcini, G.A. Custom-Made Direct Metal Laser Sintering Titanium Subperiosteal Implants: A Retrospective Clinical Study on 70 Patients. Biomed. Res. Int. 2018, 2018, 5420391. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.J.; Cheng, A.; Sahingur, K.; Clohessy, R.M.; Hopkins, L.B.; Boyan, B.D.; Schwartz, Z. Performance of laser sintered Ti-6Al-4V implants with bone-inspired porosity and micro/nanoscale surface roughness in the rabbit femur. Biomed. Mater. 2017, 12, 025021. [Google Scholar] [CrossRef] [PubMed]
- Calazans Neto, J.V.; Reis, A.C.d.; Valente, M.L.d.C. Osseointegration in additive-manufactured titanium implants: A systematic review of animal studies on the need for surface treatment. Heliyon 2023, 9, e17105. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.J.; Jung, A.; Ban, H.Y.; Kwak, T.Y.; Shin, E.J.; Gweon, B.; Lim, D.; Wang, J.H. Enhanced osseointegration through direct energy deposition porous coating for cementless orthopedic implant fixation. Sci. Rep. 2021, 11, 22317. [Google Scholar] [CrossRef]
- Tsai, P.-I.; Chen, C.-Y.; Huang, S.-W.; Yang, K.-Y.; Lin, T.-H.; Chen, S.-Y.; Sun, J.-S. Improvement of bone-tendon fixation by porous titanium interference screw: A rabbit animal model. J. Orthop. Res. 2018, 36, 2633–2640. [Google Scholar] [CrossRef]
- Wang, R.; Ni, S.; Ma, L.; Li, M. Porous construction and surface modification of titanium-based materials for osteogenesis: A review. Front. Bioeng. Biotechnol. 2022, 10, 973297. [Google Scholar] [CrossRef]
- Tammas-Williams, S.; Withers, P.J.; Todd, I.; Prangnell, P.B. The Influence of Porosity on Fatigue Crack Initiation in Additively Manufactured Titanium Components. Sci. Rep. 2017, 7, 7308. [Google Scholar] [CrossRef]
- Martinez-Marquez, D.; Delmar, Y.; Sun, S.; Stewart, R.A. Exploring Macroporosity of Additively Manufactured Titanium Metamaterials for Bone Regeneration with Quality by Design: A Systematic Literature Review. Materials 2020, 13, 4794. [Google Scholar] [CrossRef]
- Li, H.; Yao, B.; Li, Z.; Peng, Y.; Fan, H. Compressive properties and deformation mechanism of selective laser melting of Ti6Al4V porous femoral implants based on topological optimization. Compos. Struct. 2023, 321, 117326. [Google Scholar] [CrossRef]
- Yang, L.J.; Zhang, J.; Wang, Z.; Yan, C.C. Finite element analysis of micro-porous structure design and mechanical properties of load-bearing bone scaffolds. Mech. Des. Manuf. 2017, 7, 157–160. [Google Scholar]
- Kanczler, J.M.; Oreffo, R.O.C. Osteogenesis and angiogenesis: The potential for engineering bone. Eur. Cell Mater. 2008, 15, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Biemond, J.E.; Aquarius, R.; Verdonschot, N.; Buma, P. Frictional and bone ingrowth properties of engineered surface topographies produced by electron beam technology. Arch. Orthop. Trauma Surg. 2011, 131, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 690–701. [Google Scholar] [CrossRef]
- Biemond, J.E.; Hannink, G.; Verdonschot, N.; Buma, P. Bone ingrowth potential of electron beam and selective laser melting produced trabecular-like implant surfaces with and without a biomimetic coating. J. Mater. Sci. Mater. Med. 2013, 24, 745–753. [Google Scholar] [CrossRef]
- Korytkin, A.A.; Orlinskaya, N.Y.; Novikova, Y.S.; Gerasimov, S.A.; Davydenko, D.V.; Kulakova, K.V.; Tverdokhlebov, S.I.; Bolbasov, E.N. Biocompatibility and Osseointegration of Calcium Phosphate-Coated and Non-Coated Titanium Implants with Various Porosities. Sovrem Tekhnol. Med. 2021, 13, 52–57. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Park, J.-I.; Chae, J.S.; Yeo, I.-S.L. Comparison of micro-computed tomography and histomorphometry in the measurement of bone-implant contact ratios. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, 87–95. [Google Scholar] [CrossRef]
- Lyu, H.-Z.; Lee, J.H. Correlation between two-dimensional micro-CT and histomorphometry for assessment of the implant osseointegration in rabbit tibia model. Biomater. Res. 2021, 25, 11. [Google Scholar] [CrossRef]
- He, T.; Cao, C.; Xu, Z.; Li, G.; Cao, H.; Liu, X.; Zhang, C.; Dong, Y. A comparison of micro-CT and histomorphometry for evaluation of osseointegration of PEO-coated titanium implants in a rat model. Sci. Rep. 2017, 7, 16270. [Google Scholar] [CrossRef]
- Jones, C.F.; Quarrington, R.D.; Tsangari, H.; Starczak, Y.; Mulaibrahimovic, A.; Burzava, A.L.S.; Christou, C.; Barker, A.J.; Morel, J.; Bright, R.; et al. A Novel Nanostructured Surface on Titanium Implants Increases Osseointegration in a Sheep Model. Clin. Orthop. Relat. Res. 2022, 480, 2232–2250. [Google Scholar] [CrossRef]



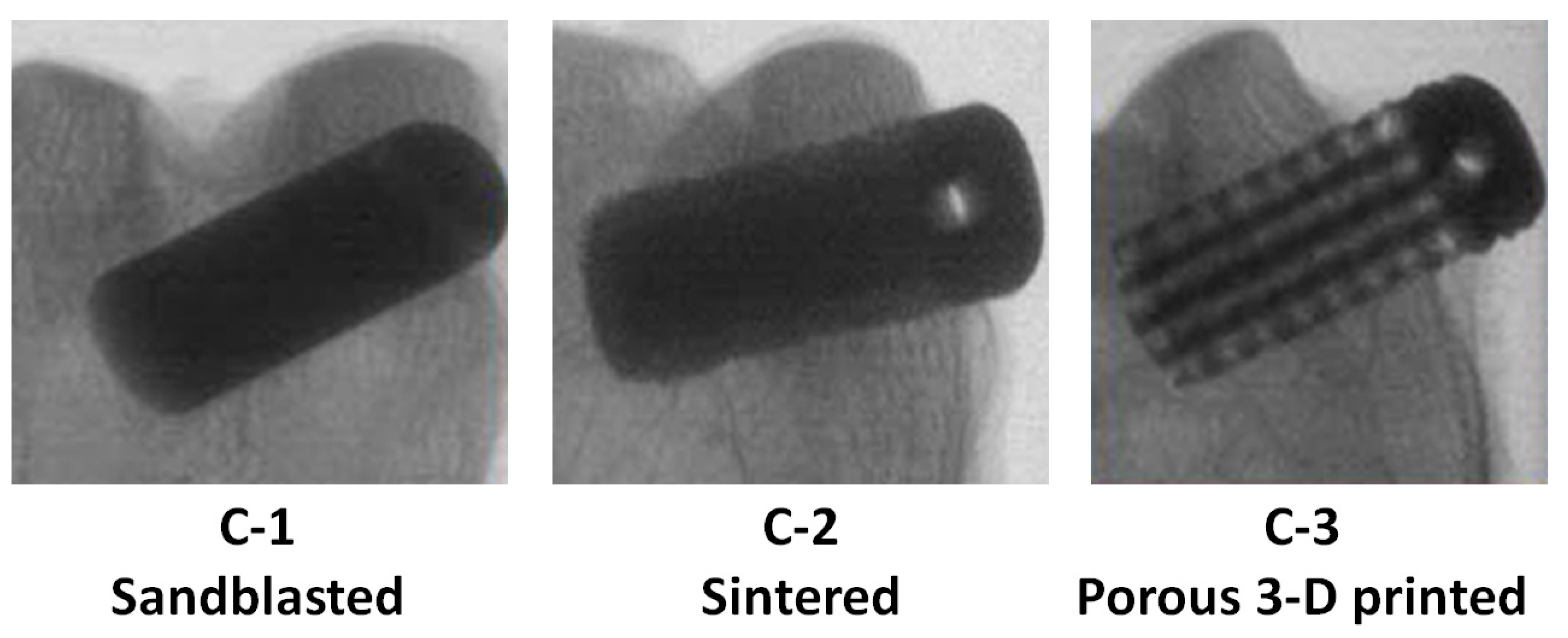

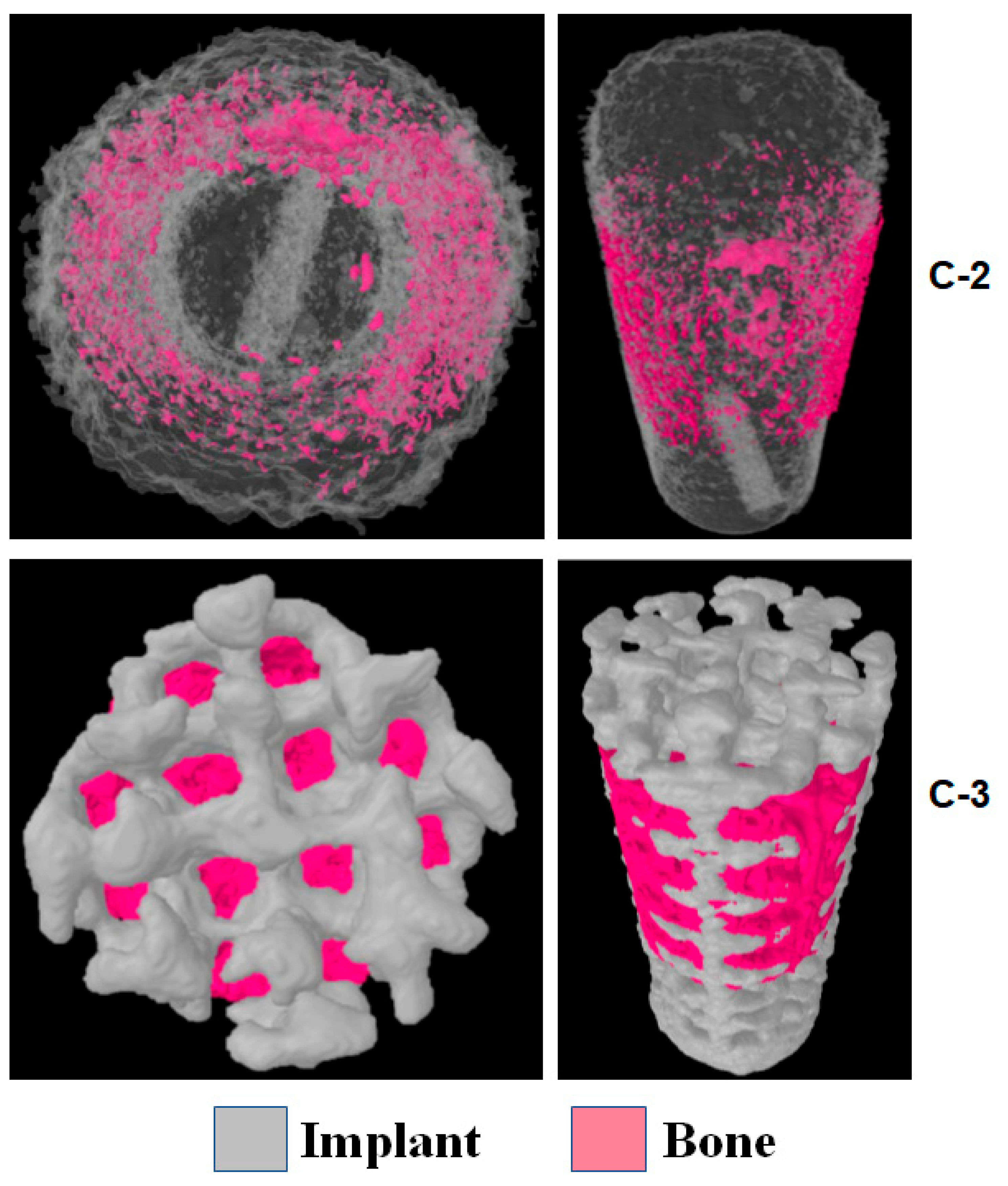
| Pixels | Distance (mm) | IMPLANT TYPE | BV/TV (%) | BS/BV (mm3) | BS/TV (mm3) | Tb.Pf (mm3) | DA | LS/TS (%) |
|---|---|---|---|---|---|---|---|---|
| 2–12 | 0.0703–0.4216 | C-1 | 63.00 ± 5.00 | 21.00 ± 2.00 | 13.00 ± 1.00 | −1.50 ± 0.01 | 0.70 ± 0.06 | 69.00 ± 6.00 |
| C-2 | 42.00 ± 3.00 | 28.00 ± 2.00 | 12.00 ± 1.00 | 0.80 ± 0.03 | 0.50 ± 0.03 | 33.00 ± 3.00 | ||
| C-3 | 31.00 ± 3.00 | 24.00 ± 2.00 | 7.00 ± 1.00 | 7.00 ± 0.04 | 0.30 ± 0.01 | 26.00 ± 6.00 | ||
| 12–22 | 0.4216–0.7729 | C-1 | 49.00 ± 4.00 | 22.00 ± 2.00 | 11.00 ± 1.00 | 4.00 ± 0.03 | 0.70 ± 0.05 | 47.00 ± 3.00 |
| C-2 | 40.00 ± 3.00 | 25.00 ± 2.00 | 10.00 ± 1.00 | −0.80 ± 0.05 | 0.50 ± 0.03 | 28.00 ± 2.00 | ||
| C-3 | 25.00 ± 2.00 | 24.00 ± 2.00 | 6.00 ± 0.60 | 8.00 ± 0.07 | 0.40 ± 0.02 | 19.00 ± 2.00 | ||
| 22–32 | 0.7729–1.1242 | C-1 | 42.00 ± 3.00 | 23.00 ± 2.00 | 10.00 ± 0.80 | 5.00 ± 0.37 | 0.70 ± 0.05 | 38.00 ± 2.00 |
| C-2 | 38.00 ± 3.00 | 24.00 ± 2.00 | 9.00 ± 0.80 | 0.10 ± 0.01 | 0.50 ± 0.04 | 27.00 ± 2.00 | ||
| C-3 | 24.00 ± 2.00 | 23.00 ± 2.00 | 6.00 ± 0.50 | 8.00 ± 0.73 | 0.40 ± 0.02 | 18.00 ± 2.00 | ||
| 32–42 | 1.1242–1.4255 | C-1 | 37.00 ± 2.00 | 24.00 ± 2.00 | 9.00 ± 0.70 | 5.00 ± 0.47 | 0.70 ± 0.05 | 32.00 ± 2.00 |
| C-2 | 35.00 ± 2.00 | 24.00 ± 2.00 | 8.00 ± 0.70 | 1.00 ± 0.01 | 0.50 ± 0.03 | 25.00 ± 2.00 | ||
| C-3 | 23.00 ± 2.00 | 22.00 ± 2.00 | 5.00 ± 0.50 | 9.00 ± 0.72 | 0.40 ± 0.02 | 17.00 ± 1.00 | ||
| 42–52 | 1.4755–1.8269 | C-1 | 29.00 ± 2.00 | 26.00 ± 2.00 | 8.00 ± 0.70 | 8.00 ± 0.67 | 0.70 ± 0.05 | 24.00 ± 2.00 |
| C-2 | 30.00 ± 3.00 | 24.00 ± 2.00 | 7.00 ± 0.60 | 2.00 ± 0.02 | 0.60 ± 0.03 | 21.00 ± 2.00 | ||
| C-3 | 21.00 ± 2.00 | 22.00 ± 2.00 | 5.00 ± 0.40 | 9.00 ± 0.71 | 0.50 ± 0.02 | 16.00 ± 1.00 |
| C-2 | C-3 | |
|---|---|---|
| ROI total volume (mm3) | 110.00 ± 90 | 104.00 ± 8.00 |
| Implant occupied ROI volume (mm3) | 109.00 ± 8.00 | 41.00 ± 4.00 |
| Implant occupied ROI volume % | 99.00 ± 9.00 | 40.00 ± 3.00 |
| Implant NOT occupied ROI volume (mm3) | 1.00 ± 0.04 | 62.00 ± 4.00 |
| Implant NOT occupied ROI volume % | 1.00 ± 0.03 | 60.00 ± 4.00 |
| ROI volume occupied by bone (mm3) | 1.00 ± 0.05 | 54.00 ± 5.00 |
| Bone NOT occupied ROI volume % | 94.00 ± 7.00 | 86.00 ± 7.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanaclocha, A.; Vanaclocha, V.; Atienza, C.M.; Jordá-Gómez, P.; Primo-Capella, V.; Blasco, J.R.; Portolés, L.; Saiz-Sapena, N.; Vanaclocha, L. Effect of Ti6Al4V Alloy Surface and Porosity on Bone Osseointegration: In Vivo Pilot Study in Rabbits. Materials 2025, 18, 2141. https://doi.org/10.3390/ma18092141
Vanaclocha A, Vanaclocha V, Atienza CM, Jordá-Gómez P, Primo-Capella V, Blasco JR, Portolés L, Saiz-Sapena N, Vanaclocha L. Effect of Ti6Al4V Alloy Surface and Porosity on Bone Osseointegration: In Vivo Pilot Study in Rabbits. Materials. 2025; 18(9):2141. https://doi.org/10.3390/ma18092141
Chicago/Turabian StyleVanaclocha, Amparo, Vicente Vanaclocha, Carlos M. Atienza, Pablo Jordá-Gómez, Víctor Primo-Capella, Jose R. Blasco, Luis Portolés, Nieves Saiz-Sapena, and Leyre Vanaclocha. 2025. "Effect of Ti6Al4V Alloy Surface and Porosity on Bone Osseointegration: In Vivo Pilot Study in Rabbits" Materials 18, no. 9: 2141. https://doi.org/10.3390/ma18092141
APA StyleVanaclocha, A., Vanaclocha, V., Atienza, C. M., Jordá-Gómez, P., Primo-Capella, V., Blasco, J. R., Portolés, L., Saiz-Sapena, N., & Vanaclocha, L. (2025). Effect of Ti6Al4V Alloy Surface and Porosity on Bone Osseointegration: In Vivo Pilot Study in Rabbits. Materials, 18(9), 2141. https://doi.org/10.3390/ma18092141






