Evolution of Phase Transformations in the Mg-Ni-Ce System After Mechanical Synthesis and Spark Plasma Sintering
Abstract
1. Introduction
2. Materials and Methods
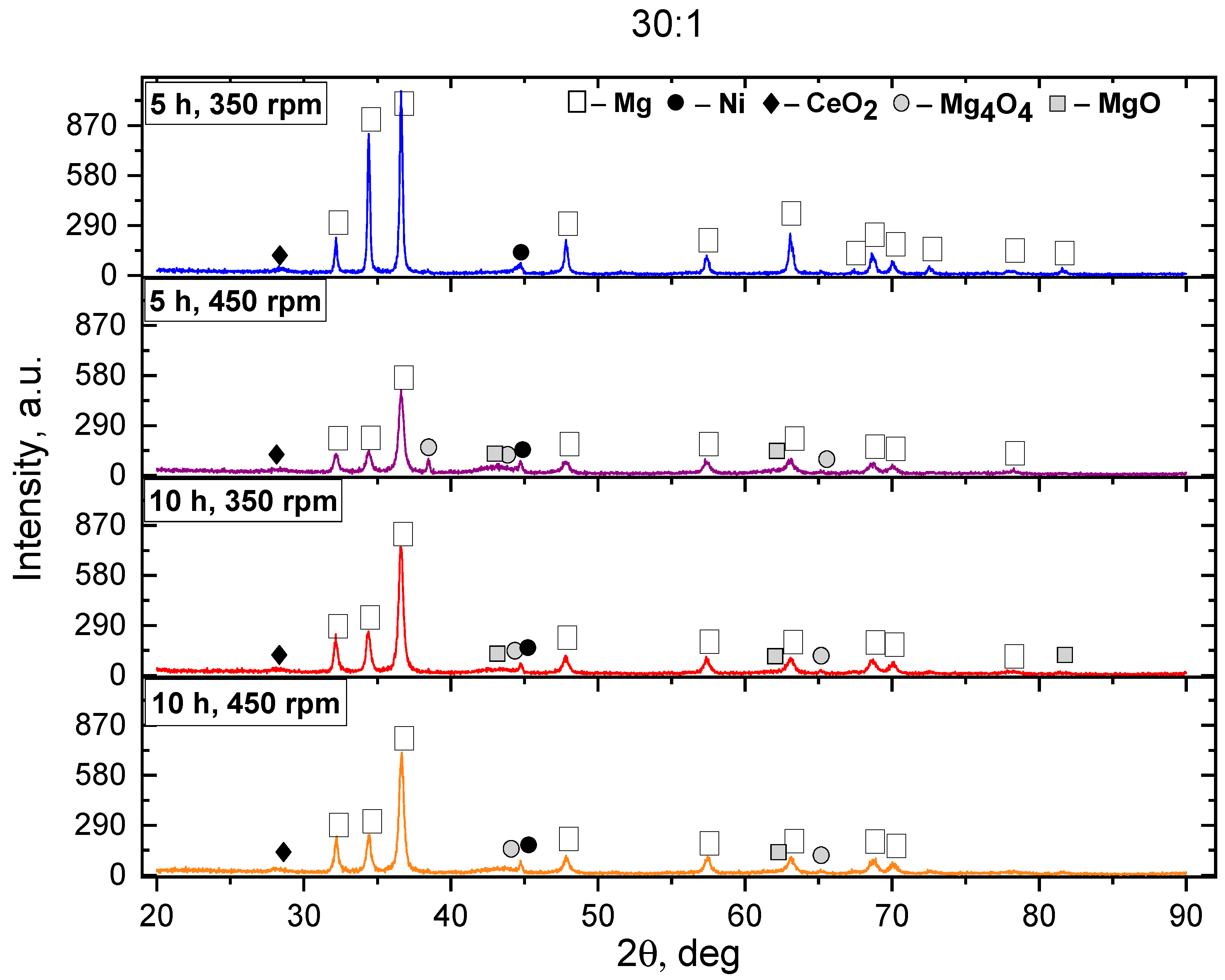
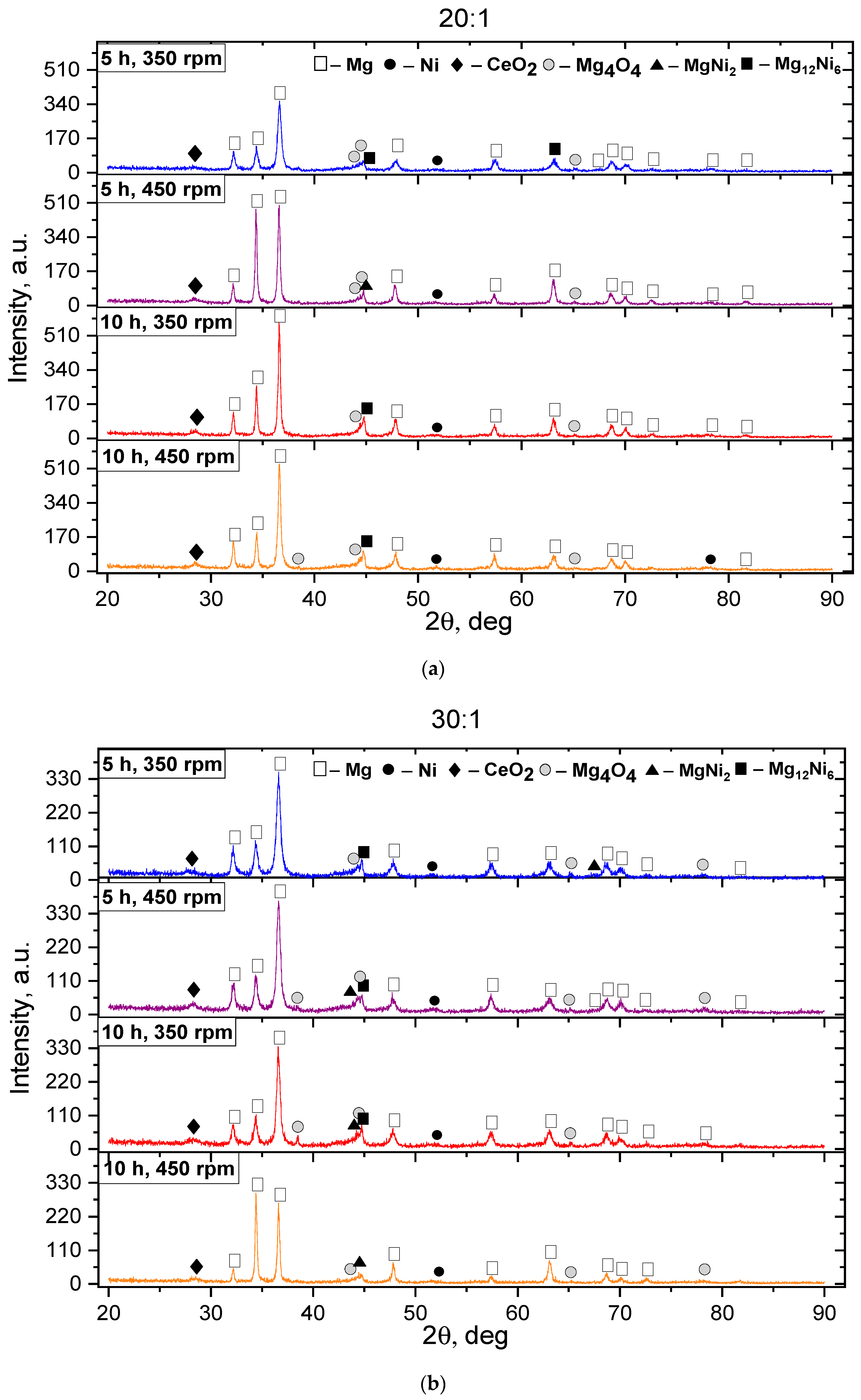
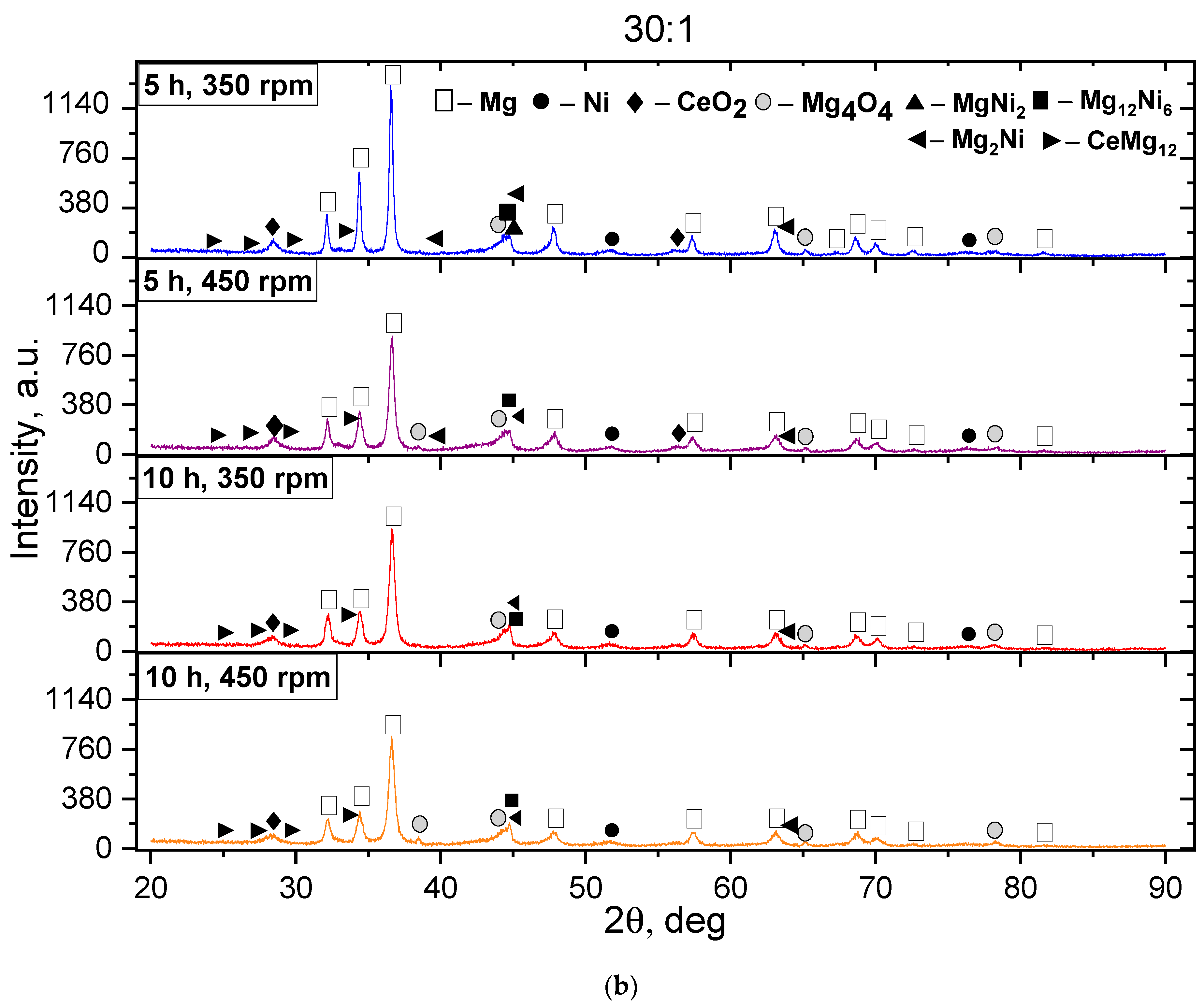
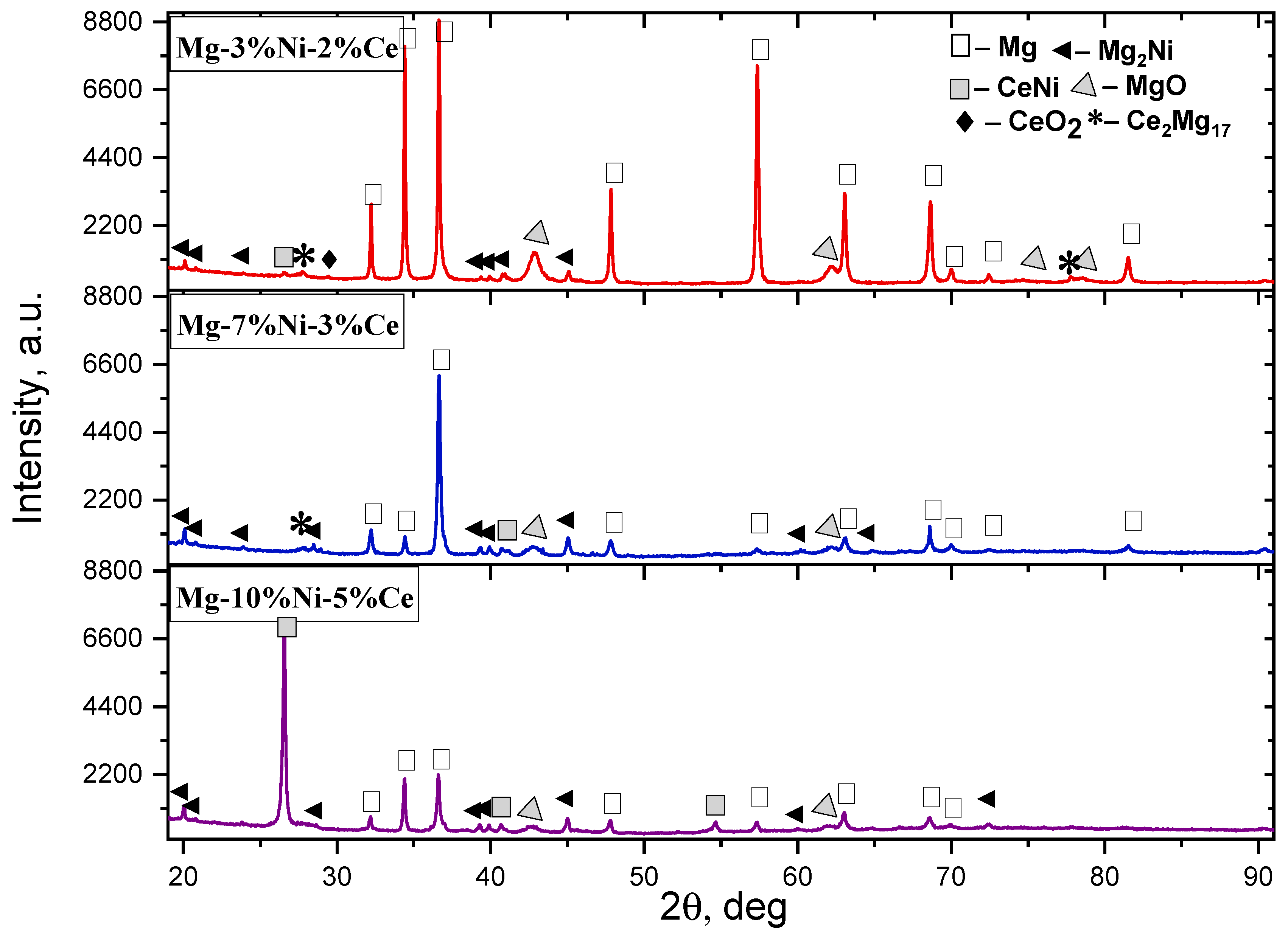
3. Results and Discussion
4. Conclusions
- The successive use of MS and SPS allows for targeted changes in the phase composition and structure of the material.
- An increase in the BPR, machining duration, and rotation speed accelerates the formation of the intermetallic compounds Mg2Ni, Mg12Ni6, and CeMg3.
- At the final stage of SPS, the microstructure of the alloy is stabilized. Intermetallic phases are distributed uniformly, and the residual oxide content decreases.
- For the Mg2Ni phase, the effective crystal size for storing hydrogen is 20–50 nm. In the case under study, after MS, the size of the Mg2Ni phase was 38.84 nm, and after SPS, it was 55.64 nm, which corresponds to the boundary of the optimal range.
- The combination of MS and SPS helps to reduce the size of the crystal lattice. Preliminary MS ensures compositional homogeneity and activates diffusion processes, which are necessary for the formation of full-fledged intermetallic phases, whereas the absence of a mechanical treatment stage can lead to incomplete sintering and the formation of heterogeneous structures. Future studies may be aimed at a detailed assessment of the sorption characteristics of the alloys and their behavior in cyclic hydrogenation and dehydrogenation processes. Thus, the method of processing Mg-Ni-Ce alloys provides new prospects for the development of effective hydrogen absorption materials with high structural stability. Further studies may be aimed at a detailed assessment of the sorption characteristics of the obtained alloys and their behavior in cyclic hydrogenation and dehydrogenation processes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brito, C.; Bertelli, F.; Castanho, M.A.P.; Goulart, P.R.; Cheung, N.; Spinelli, J.E.; Garcia, A. Upward and downward unsteady-state directional solidification of a hypoeutectic Al-3wt.%Mg alloy. Ciência Tecnol. Dos Mater. 2017, 29, e65–e70. [Google Scholar] [CrossRef]
- Haque, M.S.; Nomani, M.; Akter, A.; Ovi, I.A. Synergistic effect of Mg addition on the enhancement of the mechanical properties and evaluation of corrosion behaviors in 3.5 wt.% NaCl of Aluminum Alloys. Heliyon 2024, in press. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Li, Y.; Ding, Z. Research Progress and Application Prospects of Solid-State Hydrogen Storage Technology. Molecules 2024, 29, 1767. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, Q.; Ding, Z.; Jiang, H.; Yang, H.; Du, W.; Zheng, Y.; Huo, K.; Shaw, L.L. MOFs-Based Materials for Solid-State Hydrogen Storage: Strategies and Perspectives. Chem. Eng. J. 2024, 485, 149665. [Google Scholar] [CrossRef]
- Ionchică, A.; Munteanu, C.; Istrate, B.; Lupu, F.C. Modern Methods of Surface Deposition for Improving Material Properties. Bull. Inst. Politech. Iași Mech. Eng. 2024, 70, 87–96. [Google Scholar] [CrossRef]
- Stern, A.G. A new sustainable hydrogen clean energy paradigm. Int. J. Hydrog. Energy 2018, 43, 4244–4255. [Google Scholar] [CrossRef]
- Graetz, J.; Vajo, J.J. Controlled hydrogen release from metastable hydrides. J. Alloys Compd. 2018, 743, 691–696. [Google Scholar] [CrossRef]
- Lebrouhi, B.E.; Djoupo, J.J.; Lamrani, B.; Benabdelaziz, K.; Kousksou, T. Global hydrogen development—A technological and geopolitical overview. Int. J. Hydrog. Energy 2022, 47, 7016–7048. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrog. Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Barthelemy, H.; Weber, M.; Barbier, F. Hydrogen storage: Recent improvements and industrial perspectives. Int. J. Hydrog. Energy 2017, 42, 7254–7262. [Google Scholar] [CrossRef]
- Huang, C.; Feng, Y.; Wang, J.; Liu, C.; Li, D. Effects of noble metal modification on the performance of LaNiO3 catalyst for hydrogen production from ethanol steam reforming. Int. J. Hydrog. Energy 2019, 44, 11326–11337. [Google Scholar]
- Lin, H.J.; Cai, W.T.; Ouyang, L.Z.; Zhu, M. Tuning kinetics and thermodynamics of hydrogen storage in light metal element-based systems. J. Alloys Compd. 2016, 658, 280–300. [Google Scholar] [CrossRef]
- Ding, Z.; Li, Y.; Yang, H.; Lu, Y.; Tan, J.; Li, J.; Li, Q.; Chen, Y.A.; Shaw, L.L.; Pan, F. Tailoring MgH2 for hydrogen storage through nanoengineering and catalysis. J. Magnes. Alloys 2022, 10, 2946–2967. [Google Scholar] [CrossRef]
- Li, Q.; Lu, Y.; Luo, Q.; Yang, X.; Yang, Y.; Tan, J.; Dong, Z.; Dang, J.; Li, J.; Chen, Y.; et al. Thermodynamics and kinetics of hydriding and dehydriding reactions in Mg-based hydrogen storage materials. J. Magnes. Alloys 2021, 9, 1922–1941. [Google Scholar] [CrossRef]
- Shang, C.X.; Guo, Z.X. Structural and desorption characterizations of milled powder mixtures for hydrogen storage. Int. J. Hydrog. Energy 2007, 32, 2920–2925. [Google Scholar] [CrossRef]
- Pluengphon, P.; Bovornratanaraks, T.; Tsuppayakorn-aek, P.; Pinsook, U.; Inceesungvorn, B. High-pressure phases induce H-vacancy diffusion kinetics in TM-doped MgH2. Int. J. Hydrog. Energy 2019, 44, 21948–21954. [Google Scholar] [CrossRef]
- Adams, M.; Grant, D.M.; Stuart, A.; Walker, G.S. Modeling of the kinetic deviation of the magnesium hydrogenation reaction under conditions close to equilibrium. Int. J. Hydrog. Energy 2019, 44, 29123–29131. [Google Scholar] [CrossRef]
- Leiva, D.R.; Jorge, A.M., Jr.; Ishikawa, T.T.; Botta, W.J. Hydrogen Storage in Mg and Mg-Based Alloys and Composites Processed by Severe Plastic Deformation. Mater. Trans. 2019, 60, 1561–1570. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.; Akiba, E.; Albert, R.; Antonov, V.; Ares, J.; Baricco, M.; Bourgeois, N.; Buckley, C.; von Colbe, J.B.; et al. Magnesium based materials for hydrogen based energy storage: Past, present and future. Int. J. Hydrog. Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Kozhakhmetov, Y.; Skakov, M.; Wieleba, W.; Sherzod, K.; Mukhamedova, N. Evolution of intermetallic compounds in Ti-Al-Nb system by the action of mechanoactivation and spark plasma sintering. J. AIMS Mater. Sci. 2020, 7, 182–191. [Google Scholar] [CrossRef]
- Huot, J.; Ravnsbæk, D.; Zhang, J.; Cuevas, F.; Latroche, M.; Jensen, T.R. Mechanochemical synthesis of hydrogen storage materials. Prog. Mater. Sci. 2013, 58, 30–75. [Google Scholar] [CrossRef]
- Peng, C.; Li, Y.; Zhang, Q. Enhanced hydrogen desorption properties of MgH2 by highly dispersed Ni: The role of in-situ hydrogenolysis of nickelocene in ball milling process. J. Alloys Compd. 2022, 900, 163547. [Google Scholar] [CrossRef]
- Xie, L.; Li, J.; Zhang, T.; Kou, H. De/hydrogenation kinetics against air exposure and microstructure evolution during hydrogen absorption/desorption of Mg-Ni-Ce alloys. Renew. Energy 2017, 113, 1399–1407. [Google Scholar] [CrossRef]
- Lin, H.-J.; Zhang, C.; Wang, H.; Ouyang, L.; Zhu, Y.; Li, L.; Wang, W.; Zhu, M. Controlling nanocrystallization and hydrogen storage property of Mg-based amorphous alloy via a gas-solid reaction. J. Alloys Compd. 2016, 685, 272–277. [Google Scholar] [CrossRef]
- Song, M.; Lee, J.Y. Studying hydrogenation kinetics of Mg-(10–20 w/o) LaNi5. Int. J. Hydrog. Energy 1983, 8, 363–367. [Google Scholar] [CrossRef]
- Baraban, A.P.; Chernov, I.; Dmitriev, V.; Elets, D.; Gabis, I.; Kuznetsov, V.; Voyt, A. The Mg2NiH4 film on nickel substrate: Synthesis, properties and kinetics of formation. Thin Solid Film. 2022, 762, 139556. [Google Scholar] [CrossRef]
- Qi, Y.; Sheng, P.; Sun, H.; Li, J.; Zhang, W.; Guo, S.; Zhao, D.; Zhang, Y. Hydrogen storage thermodynamics and kinetics of the as-cast and milled Ce-Mg-Ni-based alloy. Mater. Today 2023, 35, 106217. [Google Scholar] [CrossRef]
- Rizzi, A.; García-Fernández, M.; Rodríguez, M.Á.; De Bona, E.; Moreno, R.; Biesuz, M. Spark Plasma Sintering of TiC with TiAlY as Sintering Aid: Mechanisms and Microstructures. Open Ceram. 2024, 19, 100661. [Google Scholar] [CrossRef]
- Kozhakhmetov, Y.; Tabiyeva, Y.; Mukhamedova, N.; Urkunbay, A.; Aidarova, M.; Kizatov, A.; Sagymbekova, E. A Study of the Sorption Properties and Changes in the Structure and State of the Ti-25Al-25Nb (at.%) Alloy System Under Thermocyclic Loading. Crystals 2025, 15, 173. [Google Scholar] [CrossRef]
- Skakov, M.; Kozhakhmetov, Y.; Mukhamedova, N.; Miniyazov, A.; Sokolov, I.; Urkunbay, A.; Zhanbolatova, G.; Tulenbergenov, T. Effect of a High-Temperature Treatment on Structural-Phase State and Mechanical Properties of IMC of the Ti-25Al-25Nbat.% System. Materials 2022, 15, 5560. [Google Scholar] [CrossRef]
- Floriano, R.; Leiva, D.; Deledda, S.; Hauback, B.; Botta, W. Cold rolling of MgH2 powders containing different additives. Int. J. Hydrog. Energy 2013, 38, 16193–16198. [Google Scholar] [CrossRef]
- Rather, S.U.; Taimoor, A.A.; Muhammad, A.; Alhamed, Y.A.; Zaman, S.F.; Ali, A.M. Kinetics of hydrogen adsorption on MgH2/CNT composite. Mater. Res. Bull. 2016, 77, 23–28. [Google Scholar] [CrossRef]
- Révész, Á.; Fátay, D.; Spassov, T. Hydriding kinetics of ball-milled nanocrystalline MgH2 powders. J. Mater. Res. 2007, 22, 3144–3151. [Google Scholar] [CrossRef]
- ullah Rather, S. Synthesis, characterization and studies of hydrogen absorption by magnesium nanoparticles by solution reduction method. Mater. Res. Bull. 2014, 60, 556–561. [Google Scholar] [CrossRef]
- Shang, Y.; Pistidda, C.; Gizer, G.; Klassen, T.; Dornheim, M. Mg-based materials for hydrogen storage. J. Magnes. Alloys 2021, 9, 1837–1860. [Google Scholar] [CrossRef]
- Cermak, J.; David, B. Catalytic effect of Ni, Mg2Ni and Mg2NiH4 upon hydrogen desorption from MgH2. Int. J. Hydrog. Energy 2011, 36, 13614–13620. [Google Scholar] [CrossRef]
- Varin, R.A.; Zbroniec, L.; Polanski, M.; Bystrzycki, J. A review of recent advances on the effects of microstructural refinement and nano-catalytic additives on the hydrogen storage properties of metal and complex hydrides. Energies 2011, 4, 1–25. [Google Scholar] [CrossRef]
- Varin, R.A.; Czujko, T.; Wronski, Z.S. Nanomaterials for Hydrogen Storage; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Chen, X.; Xie, F.Q.; Ma, T.J.; Li, W.Y.; Wu, X.Q. Microstructure evolution and mechanical properties of linear friction welded Ti2AlNb alloy. J. Alloys Compd. 2015, 646, 490–496. [Google Scholar] [CrossRef]
- Zhou, C.; Peng, Y.; Zhang, Q. Growth kinetics of MgH2 nanocrystallites prepared by ball milling. J. Mater. Sci. Technol. 2020, 50, 178–183. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Li, Y.; Jia, J.; Liu, Y.; Ding, Z. Transition Metal-Engineered Magnesium-Based Materials for Advanced Hydrogen Storage: From Multifaceted Mechanisms to State-of-the-Art Systems. J. Energy Chem. 2024, 13, 115109. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, J.; Lee, K.J.; Stott, N.E.; Kim, D. Large-scale synthesis of copper nanoparticles by chemically controlled reduction for applications of inkjet-printed electronics. Nanotechnology 2008, 19, 415–604. [Google Scholar] [CrossRef]
- Munir, Z.A.; Anselmi-Tamburini, U.; Ohyanagi, M. The effect of electric field and pressure on the synthesis and consolidation of materials: A review of the spark plasma sintering method. J. Mater. Sci. 2006, 41, 763–777. [Google Scholar] [CrossRef]
- Ralls, A.M.; Daroonparvar, M.; Menezes, P.L. Spark Plasma Sintering of Magnesium-Based Alloys: Microstructure, Mechanical Properties, Corrosion Behavior, and Tribological Characteristics. Magnes. Alloys 2024, 12, 405–442. [Google Scholar] [CrossRef]
- Takamura, H.J.; Miyashita, T.; Kamegawa, A.; Okada, M. Grain size refinement in Mg–Al-based alloy by hydrogen treatment. Mater. Sci. Eng. A 2003, 343, 147–153. [Google Scholar] [CrossRef]
- Hwang, S.; Nicimura, K.; McCormick, P.G. Mechanical grinding of magnesium powder. Mater. Sci. Eng. A 2001, 318, 22–33. [Google Scholar]
- Sutapa, I.W.; Wahid Wahab, A.; Taba, P.; Nafie, N.L. Dislocation, size distribution of crystallites, and lattice deformation of magnesium oxide nanoparticles. J. Phys. Conf. Ser. 2018, 979, 012021. [Google Scholar] [CrossRef]
- Shang, H.; Zhang, W.; Wei, X.; Li, Y.; Yuan, Z.; Li, J.; Zhang, Y. Improving hydrogen storage thermodynamics and kinetics of Ce-Mg-Ni-based alloy by mechanical milling with TiF3. J. Magnes. Alloys 2024, 12, 1593–1607. [Google Scholar] [CrossRef]
- Jain, P.; Dixit, V.; Jain, A.; Srivastava, O.N.; Huot, J. Effect of magnesium fluoride on hydrogenation properties of magnesium hydride. Energies 2015, 8, 12546–12556. [Google Scholar] [CrossRef]
- Pozzo, M.; Alfè, D. Structural properties and formation enthalpy of magnesium hydride from quantum Monte Carlo calculations. Int. J. Hydrog. Energy 2013, 38, 12518–12526. [Google Scholar] [CrossRef]


| Batch | Alloy Composition | Number of Samples | BPR Groups |
| I | Mg-3%Ni-2%Ce | 12 | 10:1, 20:1, 30:1 |
| II | Mg-7%Ni-3%Ce | 12 | 10:1, 20:1, 30:1 |
| III | Mg-10%Ni-5%Ce | 12 | 10:1, 20:1, 30:1 |
| Phase | 2θ (Degree) | FWHM (Degree) | Crystallite Size (nm) |
|---|---|---|---|
| Mg | 36.2 | 0.28 | 29.85 |
| Mg2Ni | 43.2 | 0.22 | 38.84 |
| Mg12Ni6 | 47.8 | 0.19 | 45.73 |
| CeMg12 | 32.5 | 0.30 | 27.58 |
| MgNi2 | 38.1 | 0.25 | 33.62 |
| Sample | Main Phases | Average Size of Crystals (nm) | Mg Content (at.%) | Ni Content (at.%) | Ce Content (at.%) |
|---|---|---|---|---|---|
| Mg-3%Ni-2%Ce | Mg·Mg2Ni·CeMg12 | 350 | 91.84 | 5.24 | 2.92 |
| Mg-7%Ni-3%Ce | Mg·Mg2Ni·CeMg3 | 220 | 88.9 | 8.18 | 2.93 |
| Mg-10%Ni-5%Ce | Mg·Mg2Ni·Ce2Mg17 | 60 | 69.95 | 23.18 | 6.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhamedova, N.M.; Miniyazov, A.Z.; Zhanbolatova, G.K.; Ospanova, Z.N.; Sabyrtayeva, A.A.; Shaikieva, K.S. Evolution of Phase Transformations in the Mg-Ni-Ce System After Mechanical Synthesis and Spark Plasma Sintering. Materials 2025, 18, 2131. https://doi.org/10.3390/ma18092131
Mukhamedova NM, Miniyazov AZ, Zhanbolatova GK, Ospanova ZN, Sabyrtayeva AA, Shaikieva KS. Evolution of Phase Transformations in the Mg-Ni-Ce System After Mechanical Synthesis and Spark Plasma Sintering. Materials. 2025; 18(9):2131. https://doi.org/10.3390/ma18092131
Chicago/Turabian StyleMukhamedova, Nuriya Meiramkanovna, Arman Zhanarbekovich Miniyazov, Gainiya Kaiyrdykyzy Zhanbolatova, Zhanna Nurbolatovna Ospanova, Aisara Askhatkyzy Sabyrtayeva, and Karina Serikkyzy Shaikieva. 2025. "Evolution of Phase Transformations in the Mg-Ni-Ce System After Mechanical Synthesis and Spark Plasma Sintering" Materials 18, no. 9: 2131. https://doi.org/10.3390/ma18092131
APA StyleMukhamedova, N. M., Miniyazov, A. Z., Zhanbolatova, G. K., Ospanova, Z. N., Sabyrtayeva, A. A., & Shaikieva, K. S. (2025). Evolution of Phase Transformations in the Mg-Ni-Ce System After Mechanical Synthesis and Spark Plasma Sintering. Materials, 18(9), 2131. https://doi.org/10.3390/ma18092131






