Tunable Surfactant-Assisted WO3 Nanogranules as High-Performance Electrocatalysts for the Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
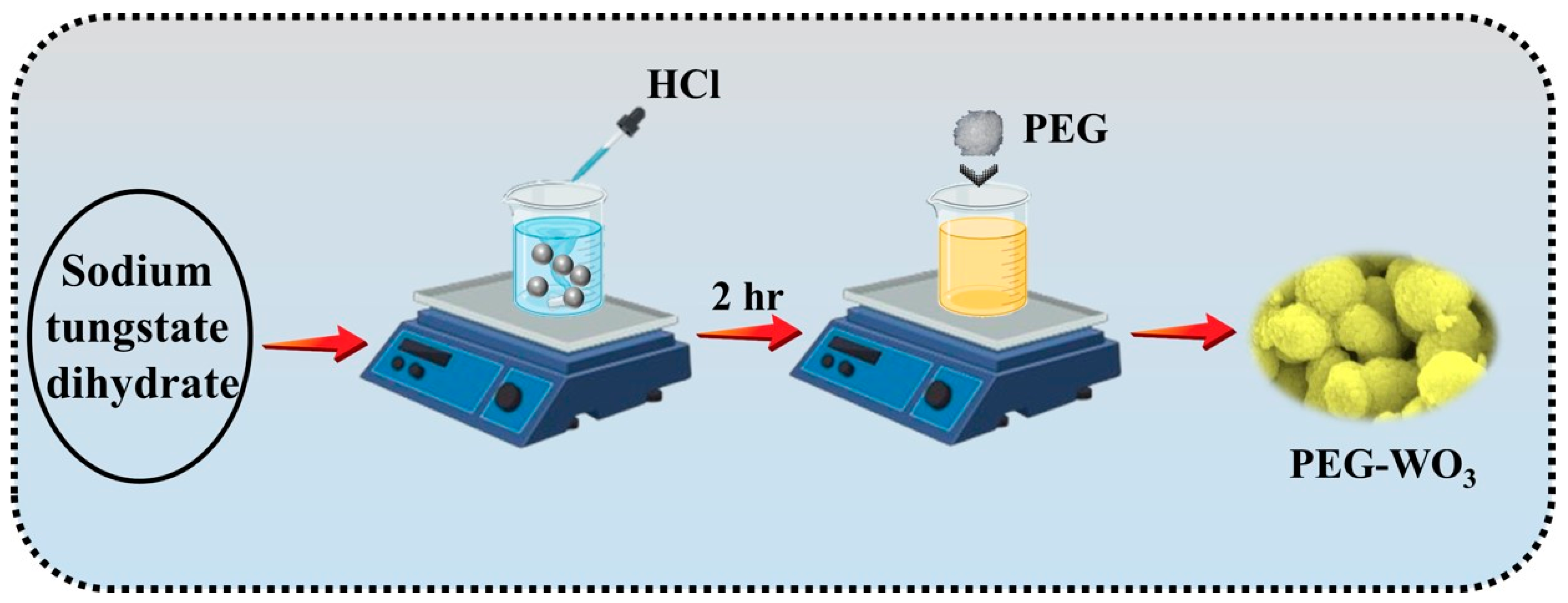
2.2. Preparation of Pure and WO3 and PEG Assisted WO3
2.3. Material Characterization
2.4. Electrochemical Analysis
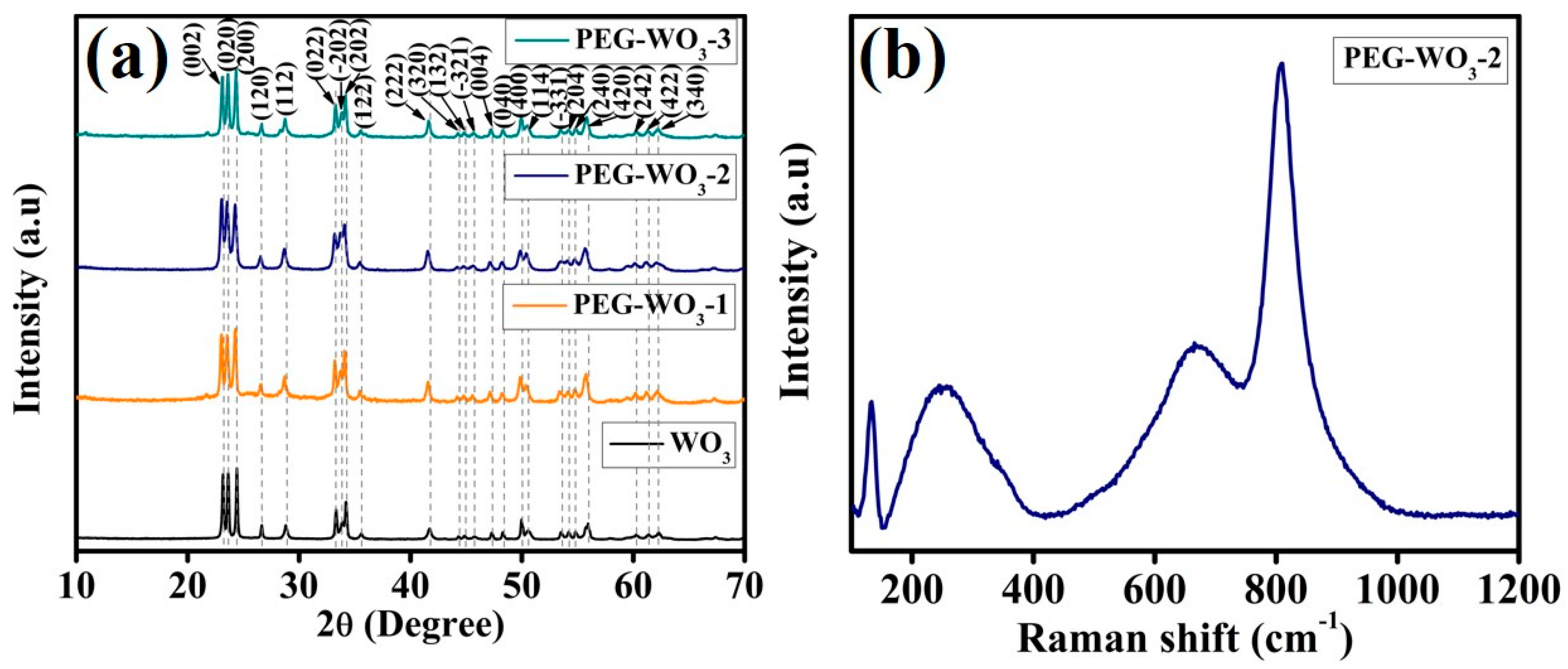
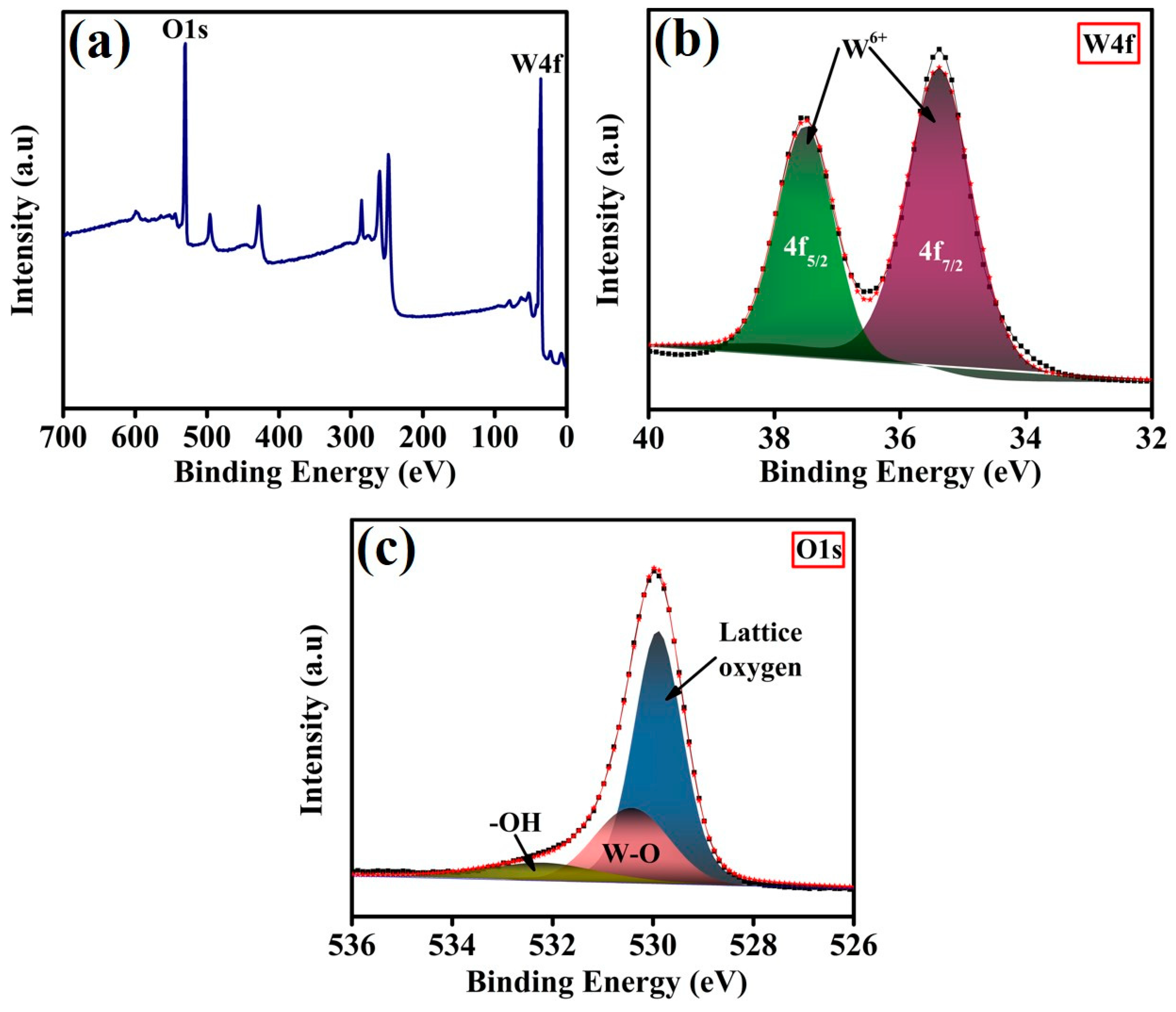
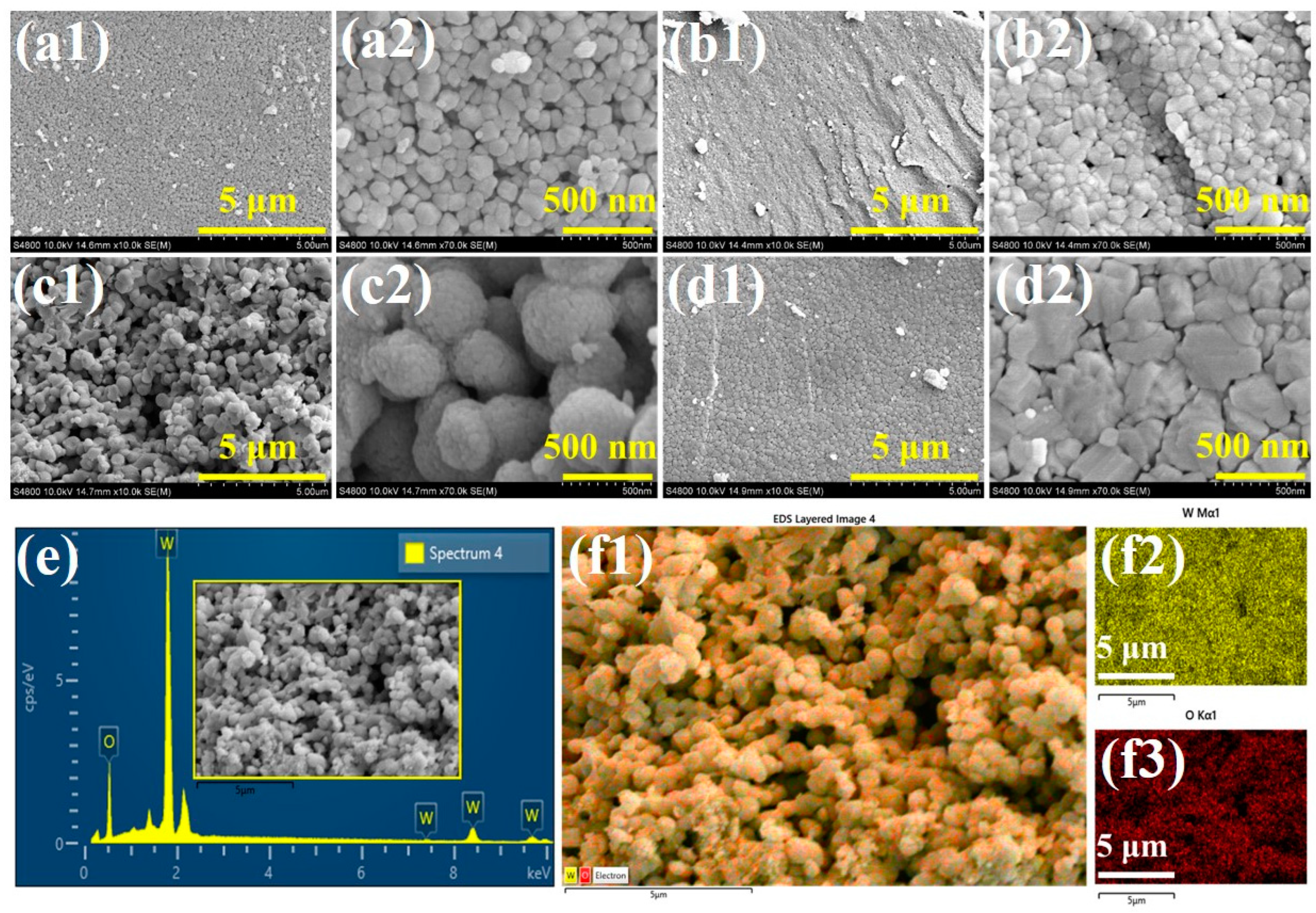
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Zheng, Y.; Ma, T.; Yang, C.; Peng, Y.; Zhou, Z.; Zhou, M.; Li, S.; Wang, Y.; Cheng, C. Designing MOF nanoarchitectures for electrochemical water splitting. Adv. Mater. 2021, 33, 2006042. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, F.; Guo, X.; Wang, S.; Feng, L. Crystal phase dependent hydrogen spillover effect in Ru/WO3 for hydrogen evolution electrocatalysis. J. Energy Chem. 2025, 105, 170–177. [Google Scholar] [CrossRef]
- Wang, C.; Yu, L.; Yang, F.; Feng, L. MoS2 nanoflowers coupled with ultrafine Ir nanoparticles for efficient acid overall water splitting reaction. J. Energy Chem. 2023, 87, 144–152. [Google Scholar] [CrossRef]
- Li, L.; Wang, P.; Shao, Q.; Huang, X. Metallic nanostructures with low dimensionality for electrochemical water splitting. Chem. Soc. Rev. 2020, 49, 3072–3106. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Sohail, M.; Ali, H.; Taha, T.; Qazi, H.; Ur Rahman, N.; Ajmal, Z.; Kalam, A.; Al-Sehemi, A.G.; Wageh, S. Recent advances and future perspectives of metal-based electrocatalysts for overall electrochemical water splitting. Chem. Rec. 2023, 23, e202200149. [Google Scholar] [CrossRef]
- Gaikwad, M.A.; Burungale, V.V.; Malavekar, D.B.; Ghorpade, U.V.; Suryawanshi, U.P.; Jang, S.; Guo, X.; Shin, S.W.; Ha, J.S.; Suryawanshi, M.P. Self-supported Fe-based nanostructured electrocatalysts for water splitting and selective oxidation reactions: Past, present, and future. Adv. Energy Mater. 2024, 14, 2303730. [Google Scholar] [CrossRef]
- Liu, X.; Gong, M.; Deng, S.; Zhao, T.; Shen, T.; Zhang, J.; Wang, D. Transforming damage into benefit: Corrosion engineering enabled electrocatalysts for water splitting. Adv. Funct. Mater. 2021, 31, 2009032. [Google Scholar] [CrossRef]
- Solanki, R.; Patra, I.; Ahmad, N.; Kumar, N.B.; Parra, R.M.R.; Zaidi, M.; Yasin, G.; Kumar, T.C.A.; Hussein, H.A.; Sivaraman, R. Investigation of recent progress in metal-based materials as catalysts toward electrochemical water splitting. J. Environ. Chem. Eng. 2022, 10, 108207. [Google Scholar] [CrossRef]
- Chen, N.; Du, X.; Zhang, X. Controlled synthesis of MnS/ZnS hybrid material with different morphology as efficient water and urea electrolysis catalyst. Renew. Energy 2022, 193, 715–724. [Google Scholar] [CrossRef]
- Urhan, B.K. Hierarchical Ni/Co-hydroxides on NiCo2O4 for boosting the electrocatalytic activity of the oxygen evolution reaction. Int. J. Hydrogen Energy 2023, 48, 17097–17105. [Google Scholar] [CrossRef]
- Khalate, S.A.; Kadam, S.A.; Ma, Y.-R.; Pujari, S.S.; Patil, U.M. Cobalt doped iron phosphate thin film: An effective catalyst for electrochemical water splitting. J. Alloys Compd. 2021, 885, 160914. [Google Scholar] [CrossRef]
- Sanati, S.; Morsali, A.; García, H. First-row transition metal-based materials derived from bimetallic metal–organic frameworks as highly efficient electrocatalysts for electrochemical water splitting. Energy Environ. Sci. 2022, 15, 3119–3151. [Google Scholar] [CrossRef]
- Deng, Y.; Lu, Y.; Dai, R.; Xiang, M.; Zhang, Z.; Zhang, X.; Zhou, Q.; Gu, H.; Bai, J. Designing hierarchical iron doped nickel-vanadium hydroxide microsphere as an efficient electrocatalyst for oxygen evolution reaction. J. Colloid Interface Sci. 2022, 627, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhu, Y.; Guan, J.; Zou, Q.; Geng, M.; Guo, B.; Wang, L.; Zhang, M. N-doped hollow nanofibers loaded with RuCoNi ternary alloy as an efficient catalyst for hydrogen evolution reaction. J. Alloys Compd. 2024, 1003, 175352. [Google Scholar] [CrossRef]
- Gao, J.; Tao, H.; Liu, B. Progress of nonprecious-metal-based electrocatalysts for oxygen evolution in acidic media. Adv. Mater. 2021, 33, 2003786. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, P.; Bai, H.; Ai, H.; Du, X.; Chen, M.; Liu, D.; Ip, W.F.; Lo, K.H.; Kwok, C.T. Development of perovskite oxide-based electrocatalysts for oxygen evolution reaction. Small 2021, 17, 2101605. [Google Scholar] [CrossRef]
- Deeksha; Kour, P.; Ahmed, I.; Sunny; Sharma, S.K.; Yadav, K.; Mishra, Y.K. Transition metal-based perovskite oxides: Emerging electrocatalysts for oxygen evolution reaction. ChemCatChem 2023, 15, e202300040. [Google Scholar] [CrossRef]
- Li, P.; Zeng, H.C. Sandwich-like nanocomposite of CoNiOx/reduced graphene oxide for enhanced electrocatalytic water oxidation. Adv. Funct. Mater. 2017, 27, 1606325. [Google Scholar] [CrossRef]
- Feng, C.; Faheem, M.B.; Fu, J.; Xiao, Y.; Li, C.; Li, Y. Fe-based electrocatalysts for oxygen evolution reaction: Progress and perspectives. Acs Catal. 2020, 10, 4019–4047. [Google Scholar] [CrossRef]
- Du, Z.; Qian, J.; Bai, J.; Li, H.; Wang, M.; Zhao, X.; Xiong, D. Surfactant-modified hydrothermal synthesis of Ca-doped CuCoO2 nanosheets with abundant active sites for enhanced electrocatalytic oxygen evolution. Inorg. Chem. 2020, 59, 9889–9899. [Google Scholar] [CrossRef]
- Yang, M.; Han, N.; Shi, L.; Gao, H.; Liu, X.; Mi, Y.; Zeng, X.; Bai, J.; Xiong, D. Effect of nickel doping on the structure, morphology and oxygen evolution reaction performance of Cu-BTC derived CuCoO2. Dalton Trans. 2022, 51, 8757–8765. [Google Scholar] [CrossRef] [PubMed]
- Gardner, G.; Al-Sharab, J.; Danilovic, N.; Go, Y.B.; Ayers, K.; Greenblatt, M.; Dismukes, G.C. Structural basis for differing electrocatalytic water oxidation by the cubic, layered and spinel forms of lithium cobalt oxides. Energy Environ. Sci. 2016, 9, 184–192. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, Y.; Zhong, Y.; Miao, J.; Lin, B.; Zhou, W.; Shao, Z. Enabling high and stable electrocatalytic activity of iron-based perovskite oxides for water splitting by combined bulk doping and morphology designing. Adv. Mater. Interfaces 2019, 6, 1801317. [Google Scholar] [CrossRef]
- Novak, T.G.; Kim, J.; DeSario, P.A.; Jeon, S. Synthesis and applications of WO3 nanosheets: The importance of phase, stoichiometry, and aspect ratio. Nanoscale Adv. 2021, 3, 5166–5182. [Google Scholar] [CrossRef]
- Jafari, F.; Gholivand, M. Investigation of the oxygen evolution reaction at the NiSe2/WO3 nanocomposite catalyst. Mater. Today Chem. 2023, 29, 101432. [Google Scholar] [CrossRef]
- Shang, X.; Rao, Y.; Lu, S.-S.; Dong, B.; Zhang, L.-M.; Liu, X.-H.; Li, X.; Liu, Y.-R.; Chai, Y.-M.; Liu, C.-G. Novel WS2/WO3 heterostructured nanosheets as efficient electrocatalyst for hydrogen evolution reaction. Mater. Chem. Phys. 2017, 197, 123–128. [Google Scholar] [CrossRef]
- Wang, D.; Li, H.; Du, N.; Hou, W. Single platinum atoms immobilized on monolayer tungsten trioxide nanosheets as an efficient electrocatalyst for hydrogen evolution reaction. Adv. Funct. Mater. 2021, 31, 2009770. [Google Scholar] [CrossRef]
- Amate, R.U.; Morankar, P.J.; Teli, A.M.; Beknalkar, S.A.; Chavan, G.T.; Ahir, N.A.; Dalavi, D.S.; Jeon, C.-W. Versatile electrochromic energy storage smart window utilizing surfactant-assisted niobium oxide thin films. Chem. Eng. J. 2024, 484, 149556. [Google Scholar] [CrossRef]
- Hariharan, V.; Radhakrishnan, S.; Parthibavarman, M.; Dhilipkumar, R.; Sekar, C. Synthesis of polyethylene glycol (PEG) assisted tungsten oxide (WO3) nanoparticles for l-dopa bio-sensing applications. Talanta 2011, 85, 2166–2174. [Google Scholar] [CrossRef]
- Pan, U.N.; Singh, T.I.; Paudel, D.R.; Gudal, C.C.; Kim, N.H.; Lee, J.H. Covalent doping of Ni and P on 1T-enriched MoS2 bifunctional 2D-nanostructures with active basal planes and expanded interlayers boosts electrocatalytic water splitting. J. Mater. Chem. A 2020, 8, 19654–19664. [Google Scholar] [CrossRef]
- Guo, J.; Wei, Z.; Wang, K.; Zhang, H. Synergistic coupling of CoFe-layered double hydroxide nanosheet arrays with reduced graphene oxide modified Ni foam for highly efficient oxygen evolution reaction and hydrogen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 27529–27542. [Google Scholar] [CrossRef]
- Wang, P.; Yu, Y.; Yan, Y.; Qin, B.; Ye, Z.; Zhong, W.; Cai, W.; Zheng, X. N plasma assisted Fe doped NiCo nanosheet arrays for alkaline electrocatalytic oxygen evolution. J. Alloys Compd. 2023, 941, 168954. [Google Scholar] [CrossRef]
- Mohan, L.; Avani, A.; Kathirvel, P.; Marnadu, R.; Packiaraj, R.; Joshua, J.R.; Nallamuthu, N.; Shkir, M.; Saravanakumar, S. Investigation on structural, morphological and electrochemical properties of Mn doped WO3 nanoparticles synthesized by co-precipitation method for supercapacitor applications. J. Alloys Compd. 2021, 882, 160670. [Google Scholar] [CrossRef]
- Sekar, S.; Ahmed, A.T.A.; Pawar, S.M.; Lee, Y.; Im, H.; Kim, D.Y.; Lee, S. Enhanced water splitting performance of biomass activated carbon-anchored WO3 nanoflakes. Appl. Surf. Sci. 2020, 508, 145127. [Google Scholar] [CrossRef]
- Morankar, P.J.; Amate, R.U.; Chavan, G.T.; Teli, A.M.; Dalavi, D.S.; Jeon, C.-W. Improved electrochromic performance of potentiostatically electrodeposited nanogranular WO3 thin films. J. Alloys Compd. 2023, 945, 169363. [Google Scholar] [CrossRef]
- Morankar, P.J.; Amate, R.U.; Teli, A.M.; Chavan, G.T.; Beknalkar, S.A.; Dalavi, D.S.; Ahir, N.A.; Jeon, C.-W. Surfactant integrated nanoarchitectonics for controlled morphology and enhanced functionality of tungsten oxide thin films in electrochromic supercapacitors. J. Energy Storage 2023, 73, 109095. [Google Scholar] [CrossRef]
- Morankar, P.J.; Amate, R.U.; Teli, A.M.; Beknalkar, S.A.; Jeon, C.-W. Synergistic effects of niobium phosphate/tungsten oxide core-shell nanocomposites for asymmetric supercapacitor. Surf. Interfaces 2025, 56, 105639. [Google Scholar] [CrossRef]
- Tian, H.; Cui, X.; Zeng, L.; Su, L.; Song, Y.; Shi, J. Oxygen vacancy-assisted hydrogen evolution reaction of the Pt/WO3 electrocatalyst. J. Mater. Chem. A 2019, 7, 6285–6293. [Google Scholar] [CrossRef]
- He, W.; Li, W.; Liu, J.; Lou, G.; Zhang, C.; Liu, Y.; Li, J. Controlling Crystallographic Orientation in h-WO3 Films to Maximize Photoelectrochemical Water Splitting. J. Mater. Chem. A 2025, 13, 7284–7294. [Google Scholar] [CrossRef]
- Baby, N.; Murugan, N.; Thangarasu, S.; Kim, Y.A.; Oh, T.-H. Synergistic insights into the electrocatalytic mechanisms of ZIF-derived Co3S4 on 1T-WS2/WO3 for electrochemical water splitting. Int. J. Hydrogen Energy 2024, 94, 1005–1017. [Google Scholar] [CrossRef]
- Morankar, P.J.; Amate, R.U.; Teli, A.M.; Beknalkar, S.A.; Jeon, C.-W. Exploring electrochromic performance via layered deposition of tungsten oxide on niobium oxide composite electrode. J. Power Sources 2024, 613, 234930. [Google Scholar] [CrossRef]
- Masoumi, Z.; Tayebi, M.; Kolaei, M.; Lee, B.-K. Efficient and stable core-shell α–Fe2O3/WS2/WOx photoanode for oxygen evolution reaction to enhance photoelectrochemical water splitting. Appl. Catal. B Environ. 2022, 313, 121447. [Google Scholar] [CrossRef]
- Yourey, J.E.; Bartlett, B.M. Electrochemical deposition and photoelectrochemistry of CuWO4, a promising photoanode for water oxidation. J. Mater. Chem. 2011, 21, 7651–7660. [Google Scholar] [CrossRef]
- Ji, X.; Ma, M.; Ge, R.; Ren, X.; Wang, H.; Liu, J.; Liu, Z.; Asiri, A.M.; Sun, X. WO3 nanoarray: An efficient electrochemical oxygen evolution catalyst electrode operating in alkaline solution. Inorg. Chem. 2017, 56, 14743–14746. [Google Scholar] [CrossRef]
- Deng, Y.; Fu, X.; Zhang, Y.; Zhu, Y.; Wei, Y. Efficient Oxygen Evolution Reaction on Polyethylene Glycol-Modified BiVO4 Photoanode by Speeding up Proton Transfer. Small 2022, 18, 2201410. [Google Scholar] [CrossRef]
- Ogiwara, N.; Tomoda, M.; Miyazaki, S.; Weng, Z.; Takatsu, H.; Kageyama, H.; Misawa, T.; Ito, T.; Uchida, S. Integrating molecular design and crystal engineering approaches in non-humidified intermediate-temperature proton conductors based on a Dawson-type polyoxometalate and poly (ethylene glycol) derivatives. Nanoscale 2021, 13, 8049–8057. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, B.; Fan, L.; Liu, H.; Valvo, M.; Edström, K.; Cuartero, M.; De Marco, R.; Crespo, G.A.; Sun, L. Efficient BiVO4 photoanodes by postsynthetic treatment: Remarkable improvements in photoelectrochemical performance from facile borate modification. Angew. Chem. 2019, 131, 19203–19209. [Google Scholar] [CrossRef]
- Moghaddam, S.K.; Haghighi, B.; Ahmadian, S.M.S.; Rezvani, Z. Carbon paste electrode modified with AgFeO2 as an electrocatalyst with excellent activity for water reduction and oxidation. J. Electroanal. Chem. 2019, 836, 158–164. [Google Scholar] [CrossRef]
- Deng, Y.; Xiong, D.; Gao, H.; Wu, J.; Verma, S.K.; Liu, B.; Zhao, X. Hydrothermal synthesis of delafossite CuScO2 hexagonal plates as an electrocatalyst for the alkaline oxygen evolution reaction. Dalton Trans. 2020, 49, 3519–3524. [Google Scholar] [CrossRef]
- Maheskumar, V.; Saravanakumar, K.; Yea, Y.; Yoon, Y.; Park, C.M. Construction of heterostructure interface with FeNi2S4 and CoFe nanowires as an efficient bifunctional electrocatalyst for overall water splitting and urea electrolysis. Int. J. Hydrogen Energy 2023, 48, 5080–5094. [Google Scholar] [CrossRef]
- Sun, P.; Zheng, X.; Chen, A.; Zheng, G.; Wu, Y.; Long, M.; Zhang, Q.; Chen, Y. Constructing Amorphous-Crystalline Interfacial Bifunctional Site Island-Sea Synergy by Morphology Engineering Boosts Alkaline Seawater Hydrogen Evolution. Adv. Sci. 2024, 11, 2309927. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.-G.; Hidalgo, S.D.S.; Chiang, C.-Y. Controllable electrodeposition of binary metal films from deep eutectic solvent as an efficient and durable catalyst for the oxygen evolution reaction. Dalton Trans. 2019, 48, 14748–14757. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, M.; Murugan, N.; Kim, Y.A.; Thangarasu, S.; Oh, T.H. Interface Engineering of Network-Like 1D/2D (NHCNT/Ni─MOF) Hybrid Nanoarchitecture for Electrocatalytic Water Splitting. Small Methods 2024, 9, 2401492. [Google Scholar] [CrossRef] [PubMed]

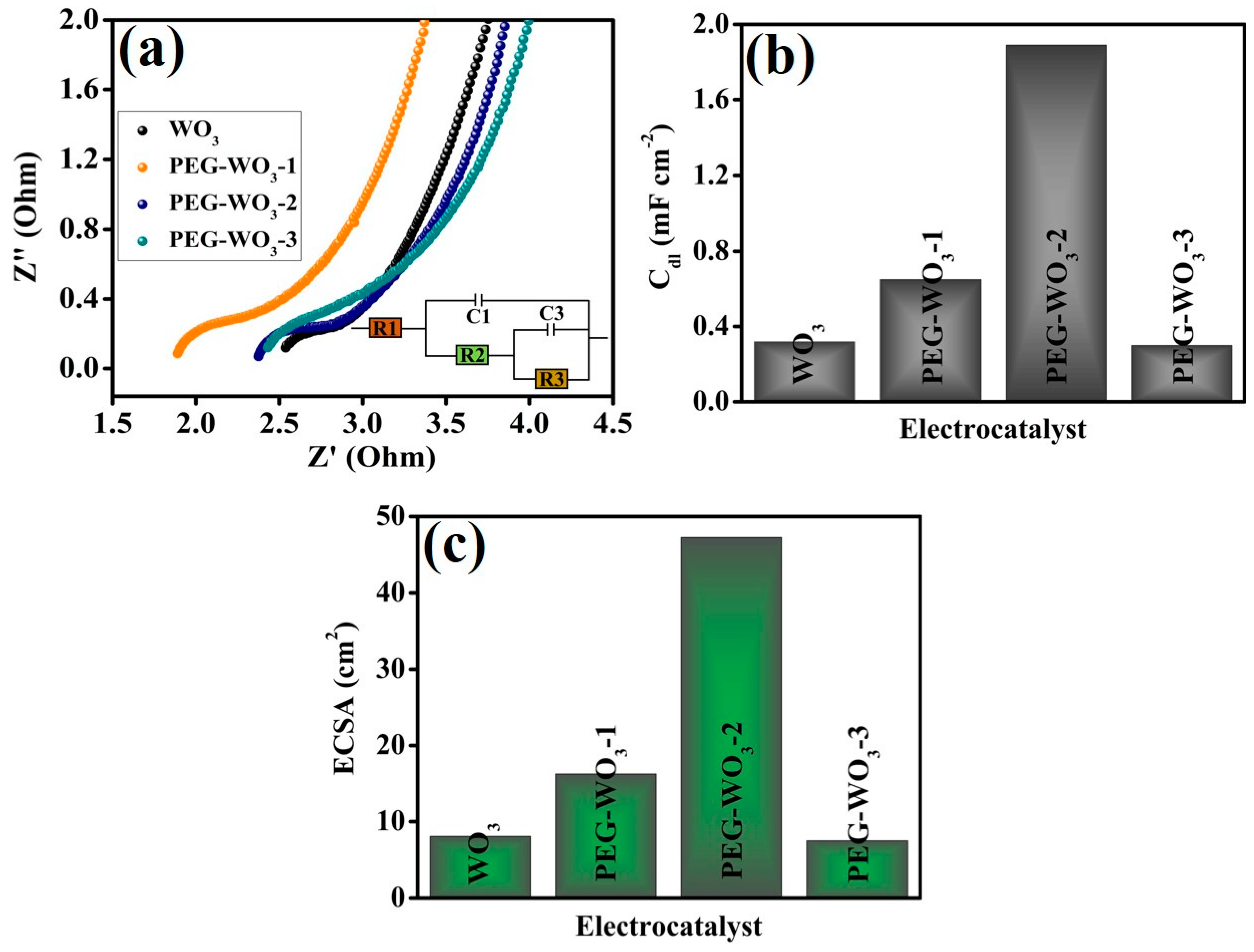
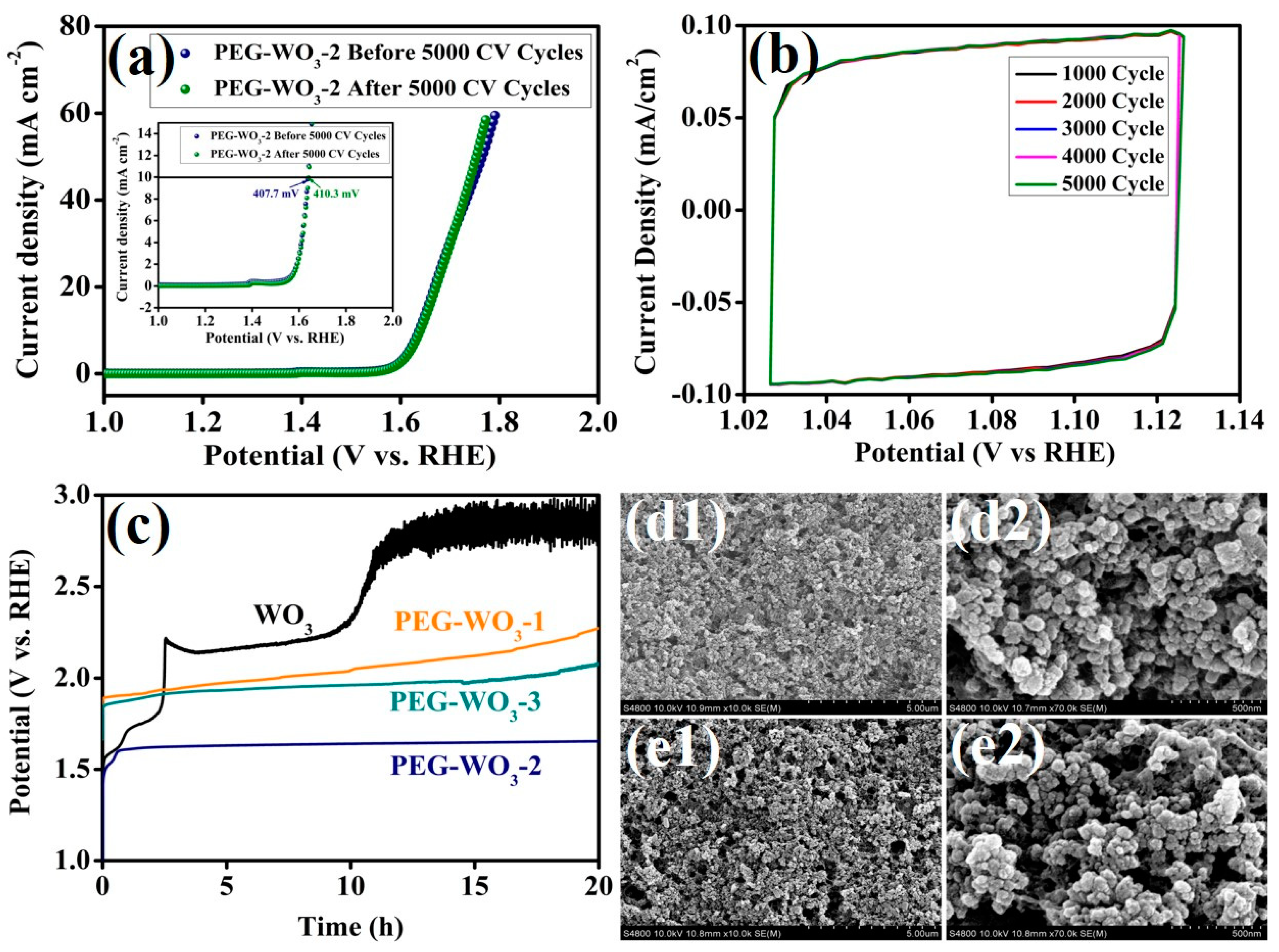
| Electrocatalyst | Electrolyte | Over Potential@10 mA cm−2 (mV) | Ref. |
|---|---|---|---|
| Ca-doped CuCoO2 | 1 M KOH | 470 | [20] |
| Ni doped CuCoO2 | 1 M KOH | 409 | [21] |
| LiCoO2 | 1 M KOH | ∼420 | [22] |
| LaFeO3 | 1 M KOH | 420 | [23] |
| AgFeO2 | 1 M KOH | 400 | [48] |
| CuScO2 | 1 M KOH | 490 | [49] |
| PEG-WO3-2 | 1 M KOH | 407.7 | [Present work] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhosale, M.; Morankar, P.J.; Amate, R.U.; Jeon, C.-W. Tunable Surfactant-Assisted WO3 Nanogranules as High-Performance Electrocatalysts for the Oxygen Evolution Reaction. Materials 2025, 18, 2129. https://doi.org/10.3390/ma18092129
Bhosale M, Morankar PJ, Amate RU, Jeon C-W. Tunable Surfactant-Assisted WO3 Nanogranules as High-Performance Electrocatalysts for the Oxygen Evolution Reaction. Materials. 2025; 18(9):2129. https://doi.org/10.3390/ma18092129
Chicago/Turabian StyleBhosale, Mrunal, Pritam J. Morankar, Rutuja U. Amate, and Chan-Wook Jeon. 2025. "Tunable Surfactant-Assisted WO3 Nanogranules as High-Performance Electrocatalysts for the Oxygen Evolution Reaction" Materials 18, no. 9: 2129. https://doi.org/10.3390/ma18092129
APA StyleBhosale, M., Morankar, P. J., Amate, R. U., & Jeon, C.-W. (2025). Tunable Surfactant-Assisted WO3 Nanogranules as High-Performance Electrocatalysts for the Oxygen Evolution Reaction. Materials, 18(9), 2129. https://doi.org/10.3390/ma18092129






