The Effect of B Coating in Enhancing Properties of Al/Diamond Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
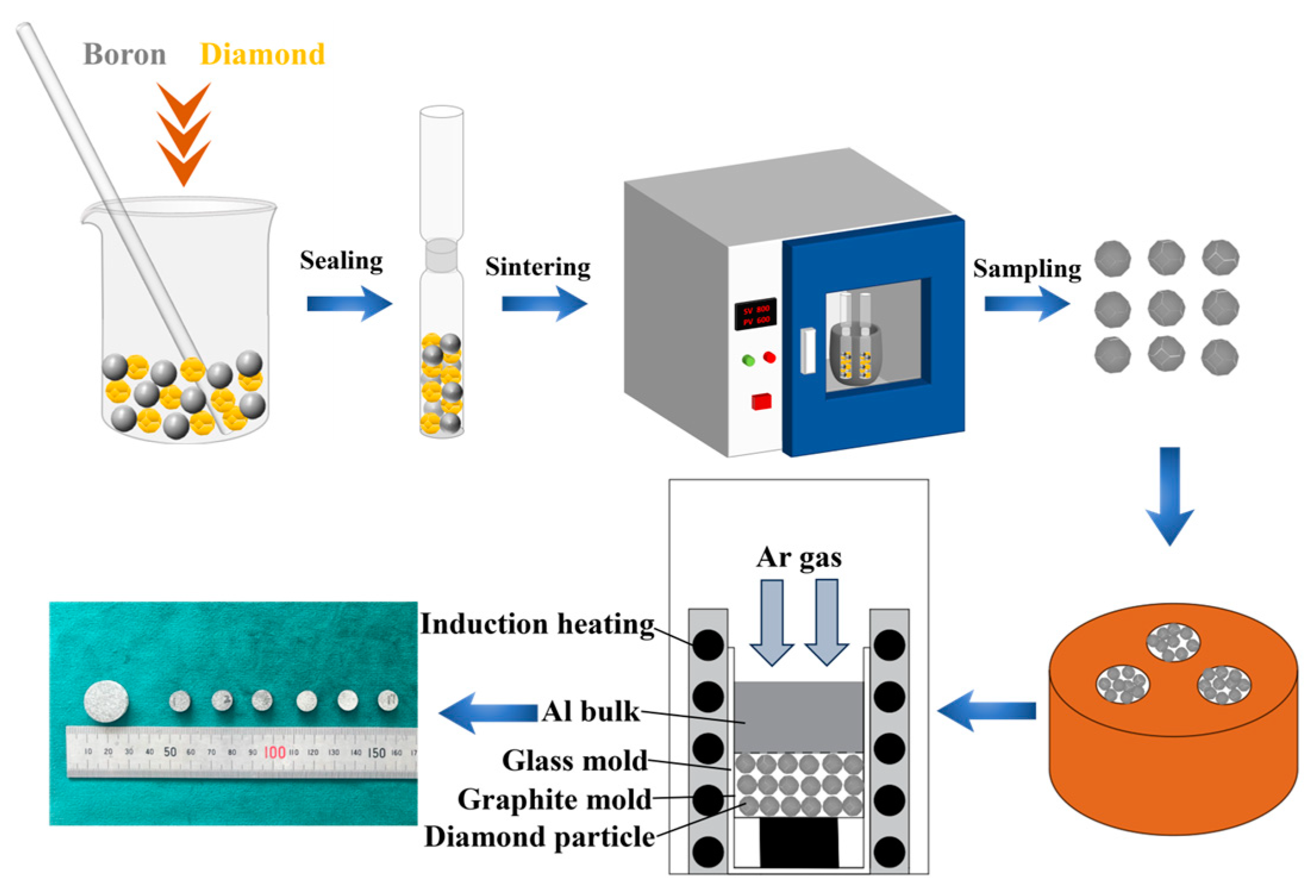
2.2. Preparation of Al/Diamond Composites
2.3. Characterization and Testing
3. Results and Discussion
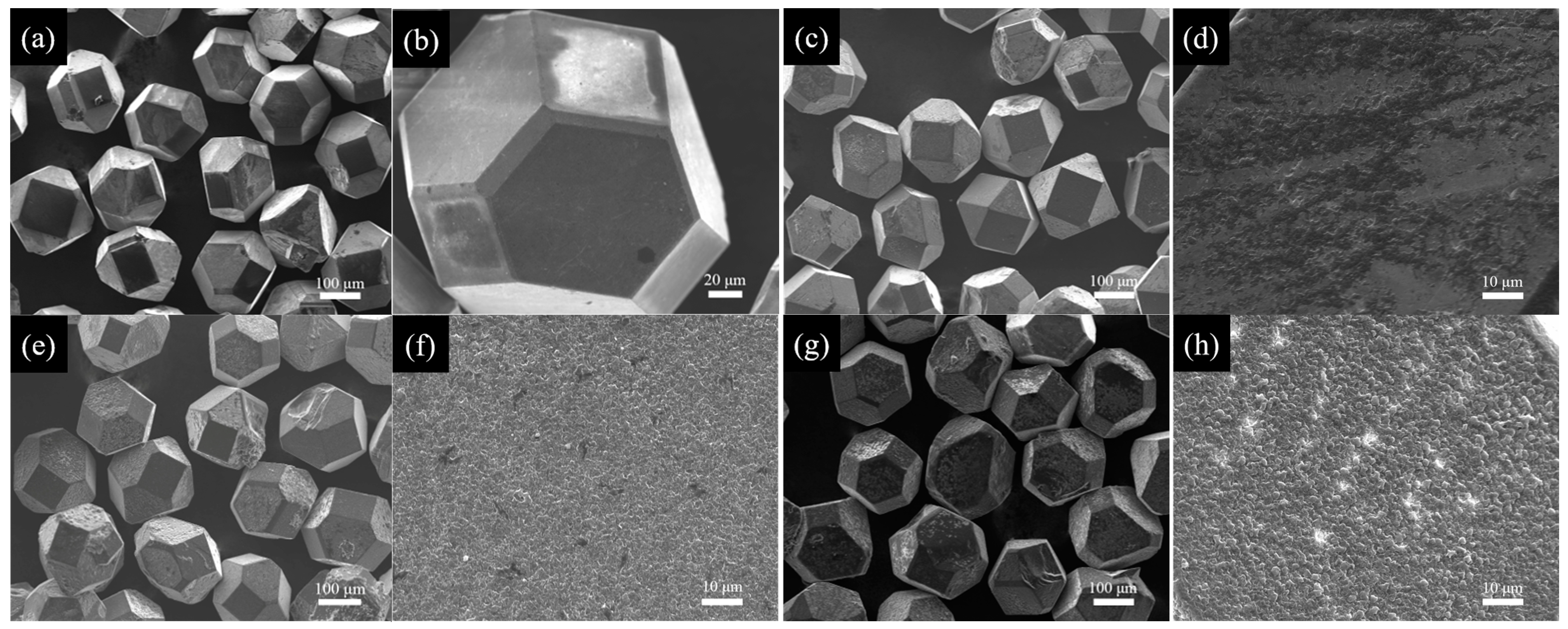
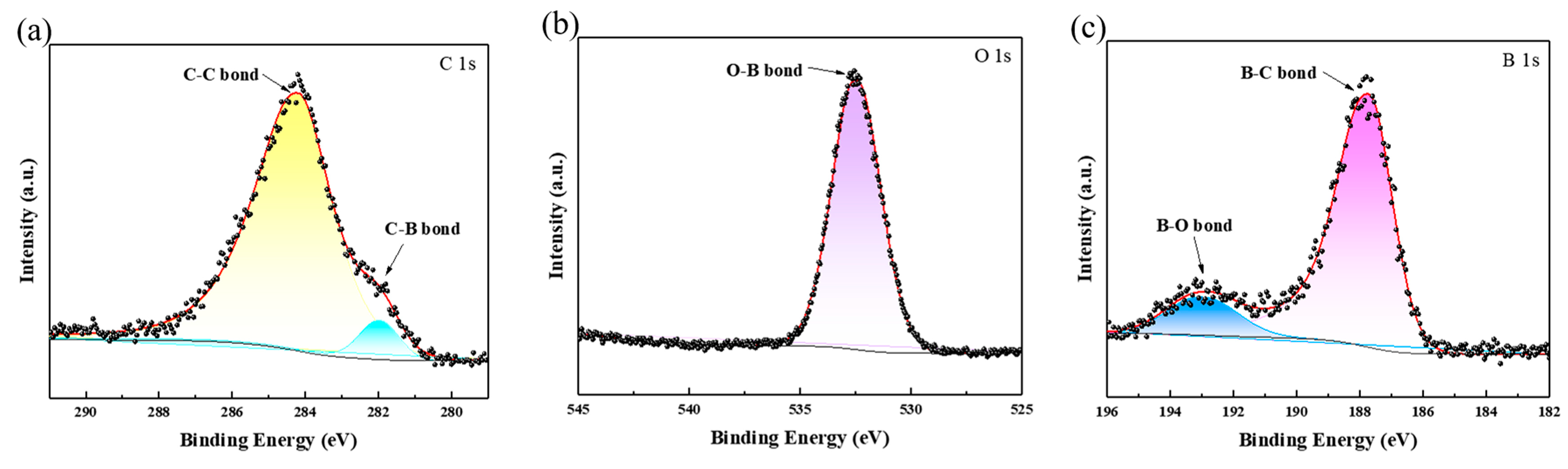
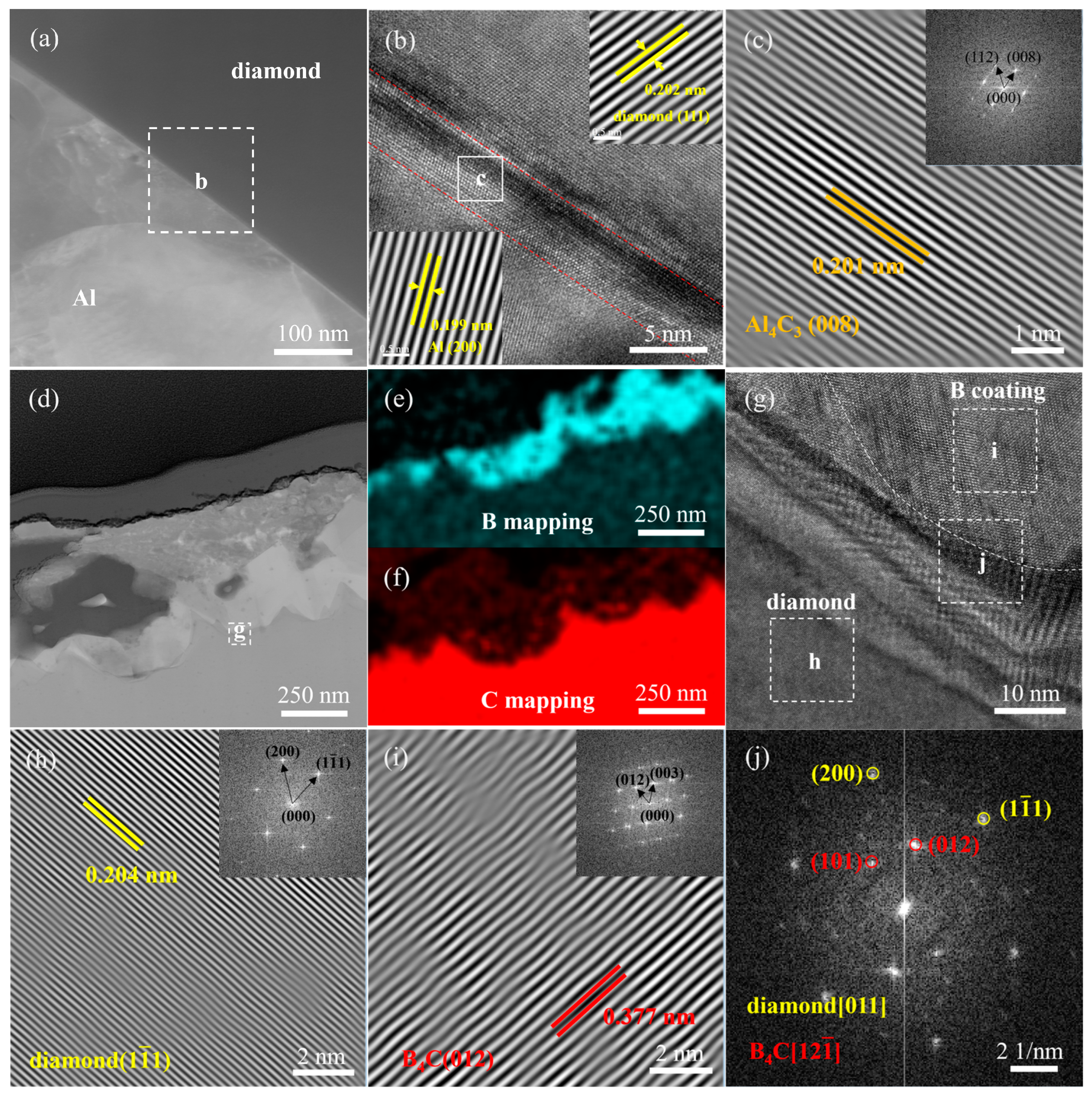
3.1. Characterization of B-Coated Diamond Particles
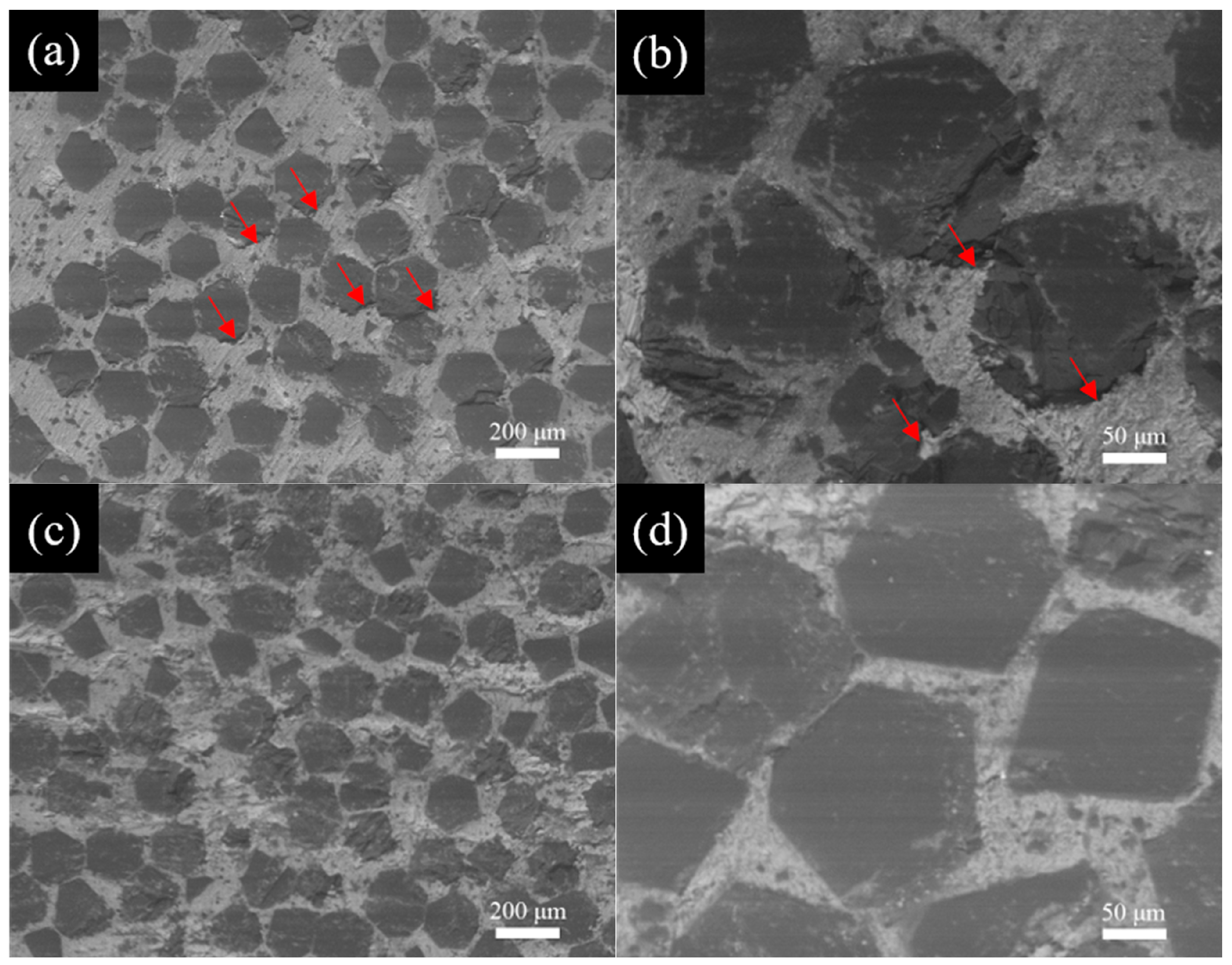
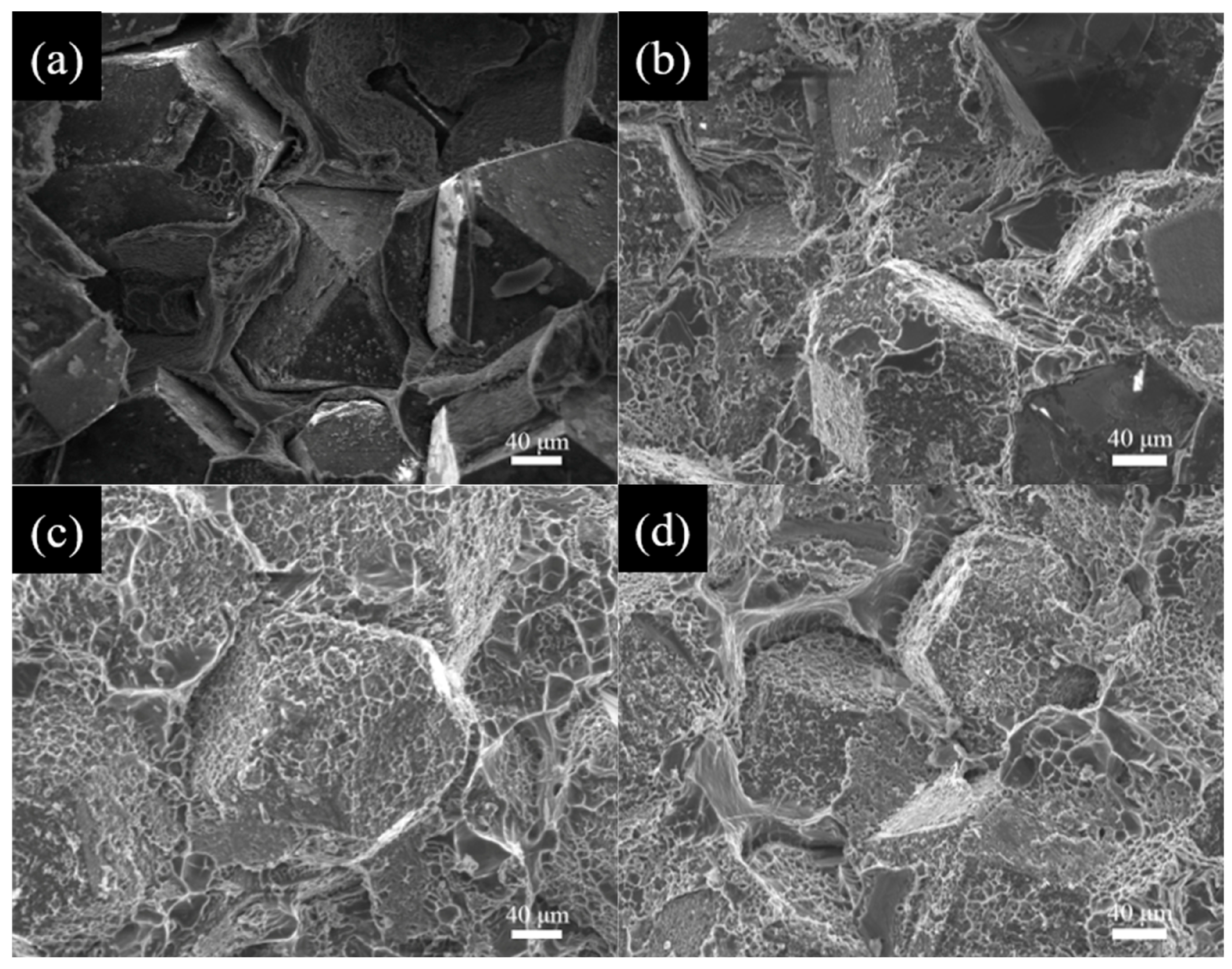
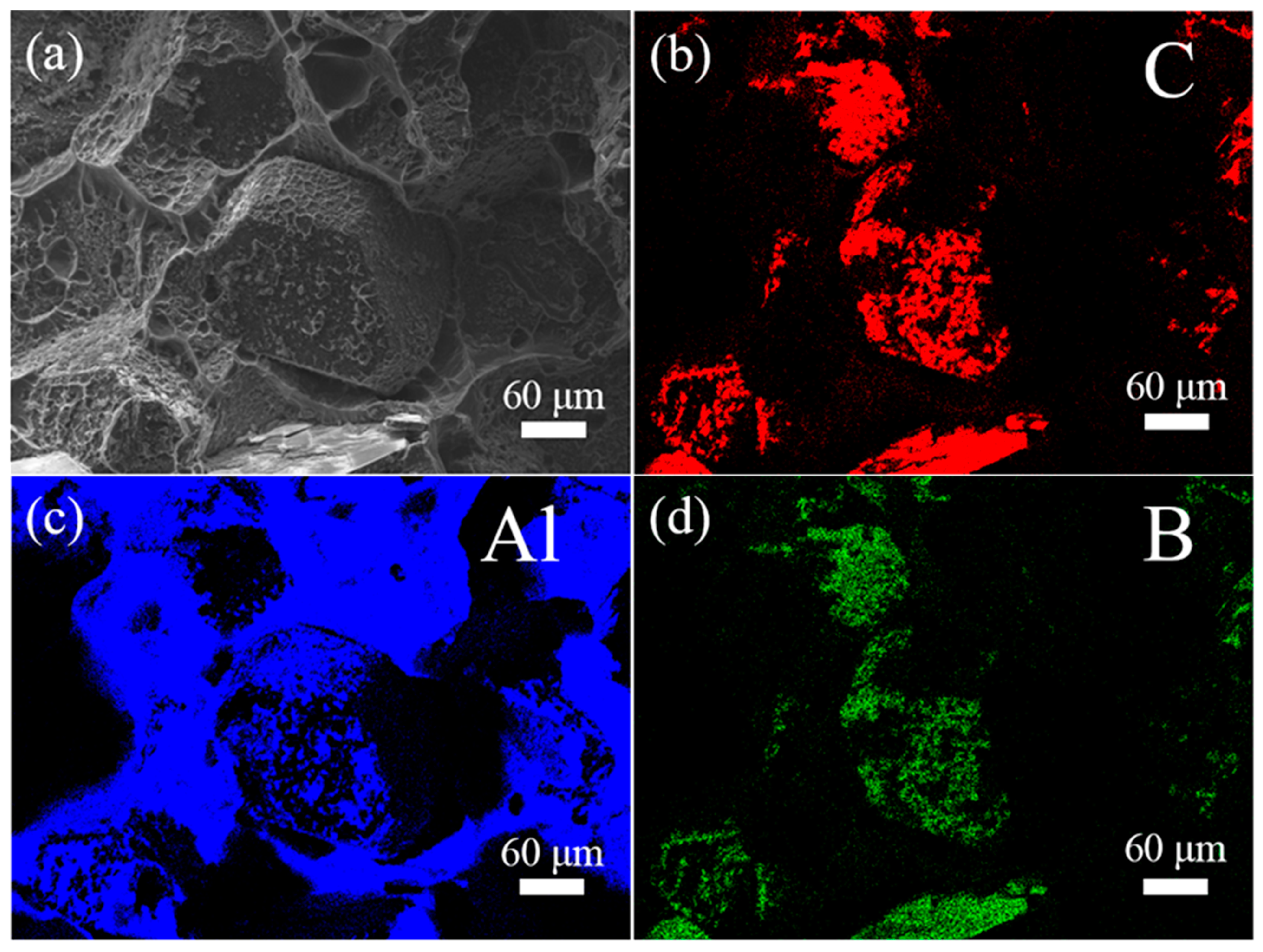
3.2. Microstructure of Al/B-Diamond Composites
3.3. Thermal-Physical Properties of Al/B-Diamond Composites
4. Conclusions
- (1)
- The continuous B4C coating layer on the surface of diamond particles was prepared by the vacuum thermal diffusion method at 1250 °C. The B4C coating avoided the formation of Al4C3 at the interface of Al/B-diamond composites.
- (2)
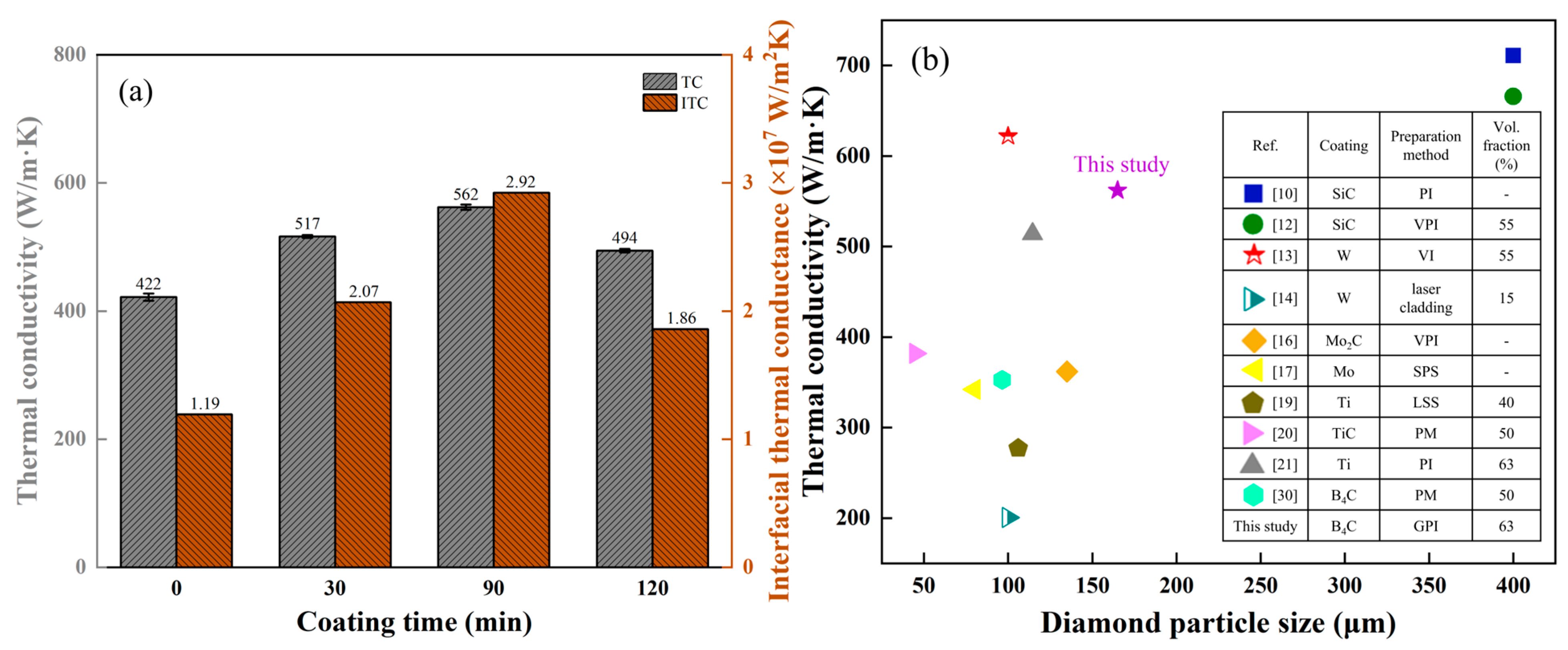
- The B4C coating improved the interfacial bonding and interfacial thermal conductance between the Al matrix and diamond particles. The highest interfacial thermal conductance value of 2.92 × 107 W/m2K was obtained for the Al/B-diamond composite, increasing 145% compared with the Al/diamond composite.
- (3)
- The thermal conductivity increased from 422 W/m·K for Al/diamond composite to 562 W/m·K for Al/B-diamond composite with 90 min plating time. When the plating time increased to 120 min, the low thermal conductivity was attributed to the thickening of the B4C coating with low thermal conductivity and the graphitization of carbon atoms on the surface of the diamond.
- (4)
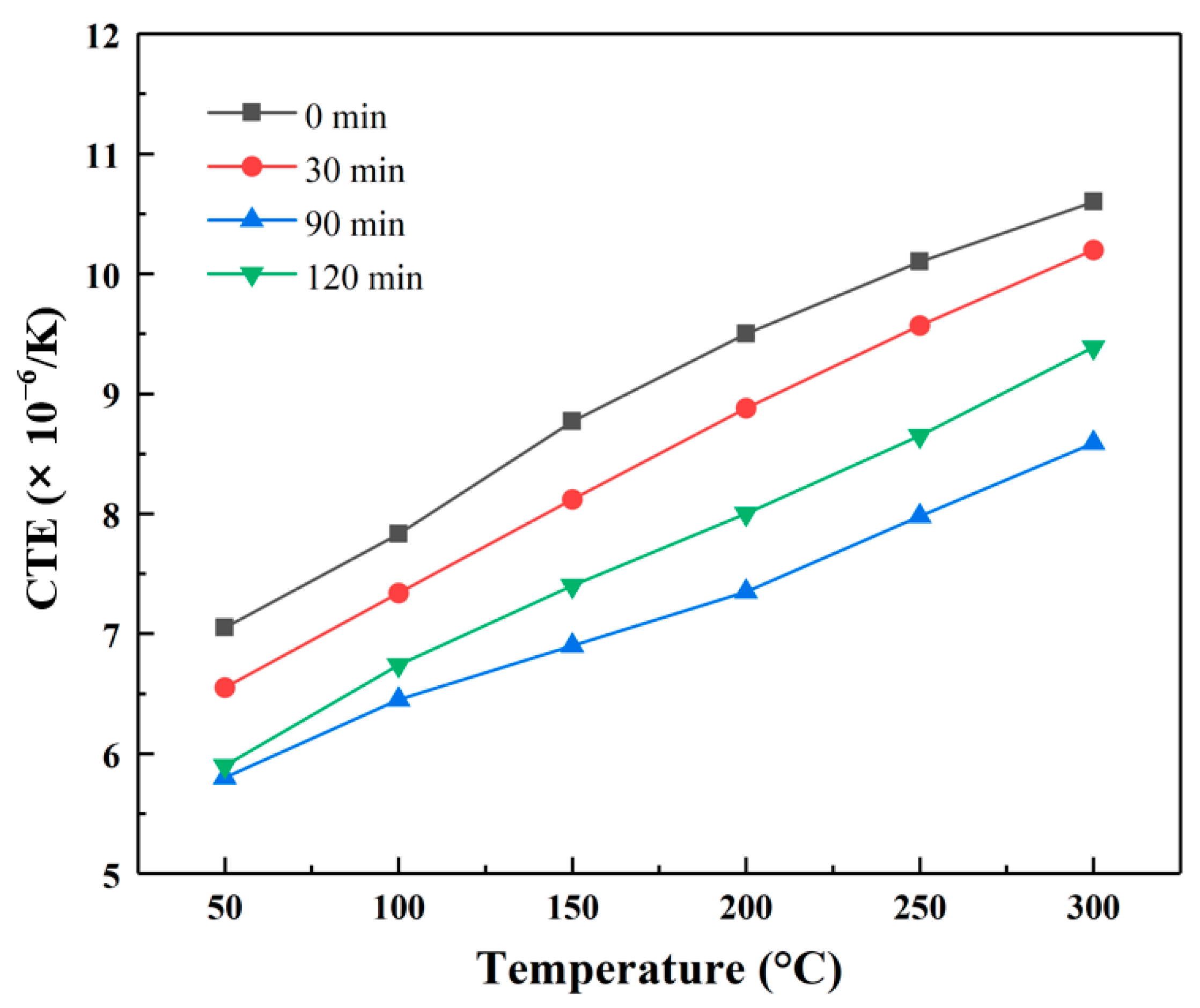
- The coefficient of thermal expansion of the Al/B-diamond composites was reduced and reached a level of 5.8 × 10−6/K–8.5× 10−6/K in the temperature range from 50 °C to 300 °C. The strong interfacial bonding between B-coated diamond particles and Al matrix restricted the expansion of the surrounding Al matrix.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Z.; Yan, Y.; Zhang, Z. Thermal management and temperature uniformity enhancement of electronic devices by micro heat sinks: A review. Energy 2021, 216, 119223. [Google Scholar] [CrossRef]
- Molina, J.M.; Narciso, J.; Weber, L.; Mortensen, A.; Louis, E. Thermal conductivity of Al–SiC composites with monomodal and bimodal particle size distribution. Mater. Sci. Eng. A 2008, 480, 483–488. [Google Scholar] [CrossRef]
- Moore, A.L.; Shi, L. Emerging challenges and materials for thermal management of electronics. Mater. Today 2014, 17, 163–174. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, Q.; Qu, S.; Wang, Z.; Gou, H.; Shil’ko, S.V.; Kobayashi, E.; Wu, G. Effect of interface structure on thermal conductivity and stability of diamond/aluminum composites. Compos. Part A Appl. Sci. Manuf. 2022, 162, 107161. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, Q.; An, J.; Ma, L.; Zhou, K.; Ye, W.; Yu, Z.; Gan, X.; Lin, C.-T.; Luo, J. Construction of 3D interconnected diamond networks in Al-matrix composite for high-efficiency thermal management. Chem. Eng. J. 2020, 380, 122551. [Google Scholar] [CrossRef]
- Yang, W.; Chen, G.; Wang, P.; Qiao, J.; Hu, F.; Liu, S.; Zhang, Q.; Hussain, M.; Dong, R.; Wu, G. Enhanced thermal conductivity in Diamond/Aluminum composites with tungsten coatings on diamond particles prepared by magnetron sputtering method. J. Alloys Compd. 2017, 726, 623–631. [Google Scholar] [CrossRef]
- Che, Z.; Zhang, Y.; Li, J.; Zhang, H.; Wang, X.; Sun, C.; Wang, J.; Kim, M.J. Nucleation and growth mechanisms of interfacial Al4C3 in Al/diamond composites. J. Alloys Compd. 2016, 657, 81–89. [Google Scholar] [CrossRef]
- Rodríguez-Reyes, M.; Pech-Canul, M.I.; Parga-Torres, J.R.; Acevedo-Dávila, J.L.; Sánchez-Araiza, M.; Lopez, H.F. Development of aluminum hydroxides in Al–Mg–Si/SiCp in infiltrated composites exposed to moist air. Ceram. Int. 2011, 37, 2719–2722. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, X.; Zhang, Y.; Wang, J.; Kim, M.J.; Zhang, H. Aluminum carbide hydrolysis induced degradation of thermal conductivity and tensile strength in diamond/aluminum composite. J. Compos. Mater. 2018, 52, 2709–2717. [Google Scholar] [CrossRef]
- Sang, J.; Chen, Q.; Yang, W.; Zhu, J.; Fu, L.; Li, D.; Zhou, L. Architecting micron SiC particles on diamond surface to improve thermal conductivity and stability of Al/diamond composites. Surf. Interfaces 2022, 31, 102019. [Google Scholar] [CrossRef]
- Liu, P.; He, X.; Qu, X. Effect of diamond surface structure on the interfacial reaction and properties of diamond/SiC composites. Diamond Relat. Mater. 2022, 129, 109342. [Google Scholar] [CrossRef]
- Li, X.; Yang, W.; Sang, J.; Zhu, J.; Fu, L.; Li, D.; Zhou, L. Low-temperature synthesizing SiC on diamond surface and its improving effects on thermal conductivity and stability of diamond/Al composites. J. Alloys Compd. 2020, 846, 156258. [Google Scholar] [CrossRef]
- Xin, L.; Tian, X.; Yang, W.; Chen, G.; Qiao, J.; Hu, F.; Zhang, Q.; Wu, G. Enhanced stability of the Diamond/Al composites by W coatings prepared by the magnetron sputtering method. J. Alloys Compd. 2018, 763, 305–313. [Google Scholar] [CrossRef]
- Huang, S.; Zhao, Y.; Xie, H.; Guo, H.; Peng, L.; Zhang, W. Microstructure and Properties of Aluminum Alloy/Diamond Composite Materials Prepared by Laser Cladding. Materials 2024, 17, 5280. [Google Scholar] [CrossRef]
- Chen, G.; Yang, W.; Xin, L.; Wang, P.; Liu, S.; Qiao, J.; Hu, F.; Zhang, Q.; Wu, G. Mechanical properties of Al matrix composite reinforced with diamond particles with W coatings prepared by the magnetron sputtering method. J. Alloys Compd. 2018, 735, 777–786. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, N.; Shi, C.; Liu, E.; He, C.; He, F.; Ma, L. Mo2C coating on diamond: Different effects on thermal conductivity of diamond/Al and diamond/Cu composites. Appl. Surf. Sci. 2017, 402, 372–383. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, W.; Wang, C.; Tian, W.; He, J.; Liao, W. Influence mechanism of magnetron sputtering process parameters on diamond surface preprocessing interface for chip heat sink. Diam. Relat. Mater. 2024, 150, 111695. [Google Scholar] [CrossRef]
- Li, K.; Hu, Z.; Yang, W.; Duan, W.; Ni, X.; Hu, Z.; He, W.; Cai, Z.; Liu, Y.; Zhao, Z.; et al. Selective laser melting and mechanical behavior of Mo-coated diamond particle reinforced metal matrix composites. Diam. Relat. Mater. 2024, 144, 110952. [Google Scholar] [CrossRef]
- Zhou, H.; Jia, Q.; Sun, J.; Li, Y.; He, Y.; Bi, W.; Zheng, W. Improved Bending Strength and Thermal Conductivity of Diamond/Al Composites with Ti Coating Fabricated by Liquid-Solid Separation Method. Materials 2024, 17, 1485. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, W.G.; Wang, D.; Ni, D.R.; Chen, L.Q.; Ma, Z.Y. Effect of nanometer TiC coated diamond on the strength and thermal conductivity of diamond/Al composites. Mater. Chem. Phys. 2016, 182, 256–262. [Google Scholar] [CrossRef]
- Guo, C.Y.; He, X.B.; Ren, S.B.; Qu, X.H. Thermal properties of diamond/Al composites by pressure infiltration: Comparison between methods of coating Ti onto diamond surfaces and adding Si into Al matrix. Rare Metals 2016, 35, 249–255. [Google Scholar] [CrossRef]
- Krstic, Z.; Mecca, W.; Haerle, A.G.; Tumavitch, N.J.; Shaffer, B.C. Nickel Coated Diamond Particles and Method of Making Said Particles. U.S. Patent 0004890A1, 1 January 2015. [Google Scholar]
- Mudholkar, M.S.; Goetz, R.J. Autocatalytic Nickel-Boron Coating Process for Diamond Particles. U.S. Patent 0137229A1, 15 July 2004. [Google Scholar]
- Ras, A.H.; Auret, F.D.; Nel, J.M. Boron carbide coatings on diamond particles. Diam. Relat. Mater. 2010, 19, 1411–1414. [Google Scholar] [CrossRef]
- Hua, Z.; Wang, K.; Li, W.; Chen, Z. Theoretical Strategy for Interface Design and Thermal Performance Prediction in Diamond/Aluminum Composite Based on Scattering-Mediated Acoustic Mismatch Model. Materials 2023, 16, 4208. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Tan, Z.; Fan, G.; Xiong, D.-B.; Guo, Q.; Guo, C.; Li, Z.; Zhang, D. Theoretical modelling for interface design and thermal conductivity prediction in diamond/Cu composites. Diam. Relat. Mater. 2018, 81, 38–44. [Google Scholar] [CrossRef]
- Bai, G.; Li, N.; Wang, X.; Wang, J.; Kim, M.J.; Zhang, H. High thermal conductivity of Cu-B/diamond composites prepared by gas pressure infiltration. J. Alloys Compd. 2018, 735, 1648–1653. [Google Scholar] [CrossRef]
- Hu, H.; Kong, J. Improved Thermal Performance of Diamond-Copper Composites with Boron Carbide Coating. J. Mater. Eng. Perform. 2013, 23, 651–657. [Google Scholar] [CrossRef]
- Guo, R.-F.; Chen, S.-M.; Shen, P. Influence of Si, Ti, and Cu as alloying elements on the wettability and reactivity of an Al/B4C system. J. Mater. Res. Technol. 2023, 27, 6104–6116. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, C.; He, L.; Meng, Q.; Liu, B.C.; Gao, K.; Wu, J. Enhanced bending strength and thermal conductivity in diamond/Al composites with B4C coating. Sci. Rep. 2018, 8, 11104. [Google Scholar] [CrossRef]
- Yan, X.; Wei, J.; An, K.; Liu, J.; Chen, L.; Zheng, Y.; Zhang, X.; Li, C. High temperature surface graphitization of CVD diamond films and analysis of the kinetics mechanism. Diam. Relat. Mater. 2021, 120, 108647. [Google Scholar] [CrossRef]
- Every, A.G.; Tzou, Y.; Hasselman, D.P.H.; Raj, R. The effect of particle size on the thermal conductivity of ZnS/diamond composites. Acta Metall. Mater. 1992, 40, 123–129. [Google Scholar] [CrossRef]
- Bruggeman, D.A.G. Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen. I. Dielektrizitätskonstanten und Leitfähigkeiten der Mischkörper aus isotropen Substanzen. Ann. Phys. 1935, 416, 665–679. [Google Scholar] [CrossRef]
- Tsagareishvili, G.V.; Nakashidze, T.G.; Jobava, J.S.; Lomidze, G.P.; Khulelidze, D.E.; Tsagareishvili, D.S.; Tsagareishvili, O.A. Thermal expansion of boron and boron carbide. J. Less Common. Met. 1986, 117, 159–161. [Google Scholar] [CrossRef]

| Materials | Density (g/cm3) | TC (W/m·K) | CTE (×10−6/K) |
|---|---|---|---|
| Diamond | 3.52 | 1415.0 | 1.0 |
| Al | 2.70 | 230.0 | 23.0 |
| B | 2.34 | 27.4 | 5.7 |
| B4C | 2.52 | 67.0 | 4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Hou, M.; Chen, H.; Yu, H.; Zhou, J.; Wu, J.; Wang, X. The Effect of B Coating in Enhancing Properties of Al/Diamond Composites. Materials 2025, 18, 2117. https://doi.org/10.3390/ma18092117
Li J, Hou M, Chen H, Yu H, Zhou J, Wu J, Wang X. The Effect of B Coating in Enhancing Properties of Al/Diamond Composites. Materials. 2025; 18(9):2117. https://doi.org/10.3390/ma18092117
Chicago/Turabian StyleLi, Jiaxiong, Muqing Hou, Haiyuan Chen, Huan Yu, Jixue Zhou, Jianhua Wu, and Xitao Wang. 2025. "The Effect of B Coating in Enhancing Properties of Al/Diamond Composites" Materials 18, no. 9: 2117. https://doi.org/10.3390/ma18092117
APA StyleLi, J., Hou, M., Chen, H., Yu, H., Zhou, J., Wu, J., & Wang, X. (2025). The Effect of B Coating in Enhancing Properties of Al/Diamond Composites. Materials, 18(9), 2117. https://doi.org/10.3390/ma18092117






