Phase-Dependent Electrochemical Performance of CoxSy (x = 1,9; y = 2,8) for Symmetric Supercapacitor Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
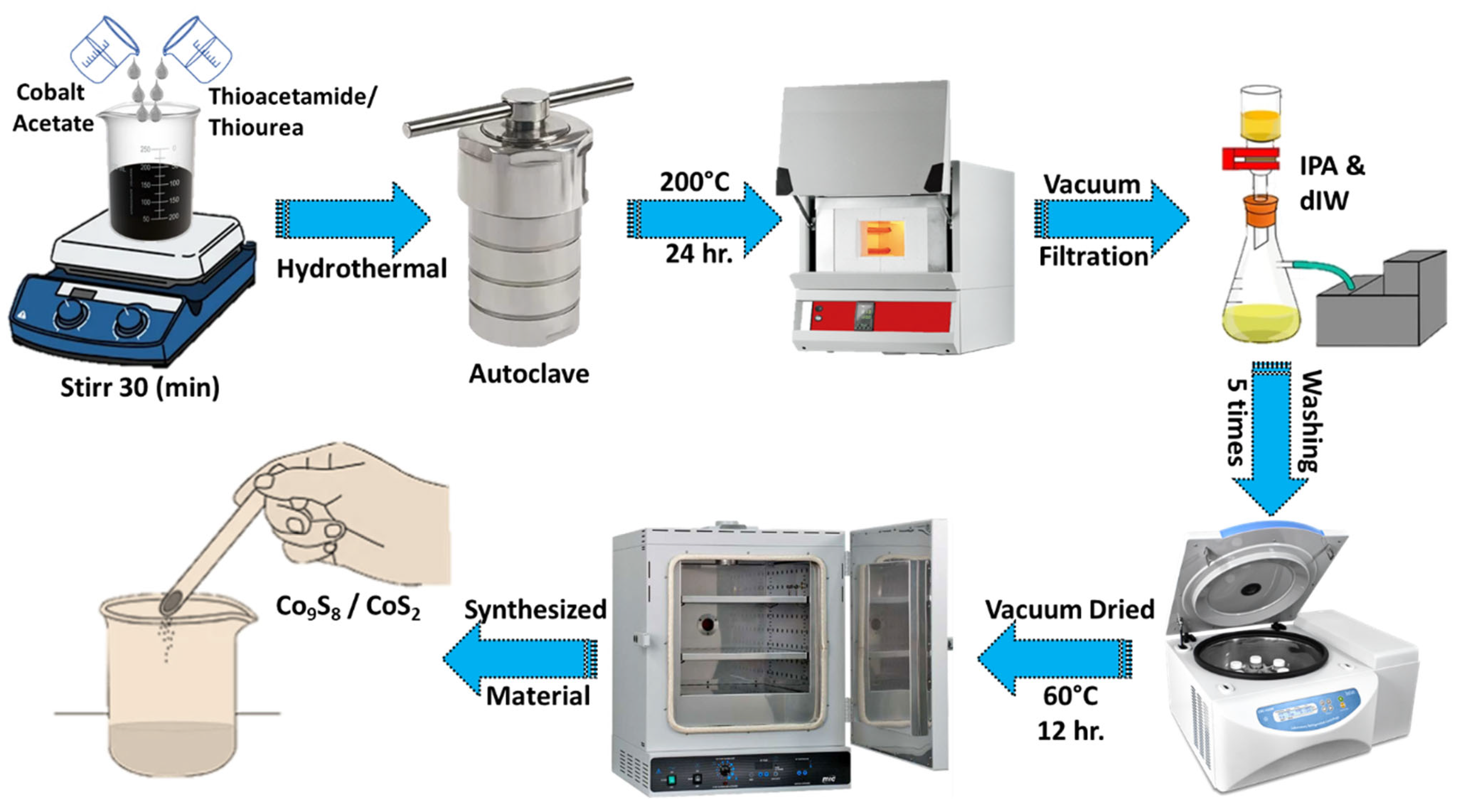
2.2. Synthesis of CoxSy
2.2.1. Synthesis of CoS2
2.2.2. Synthesis of Co9S8
2.3. Material Characterization
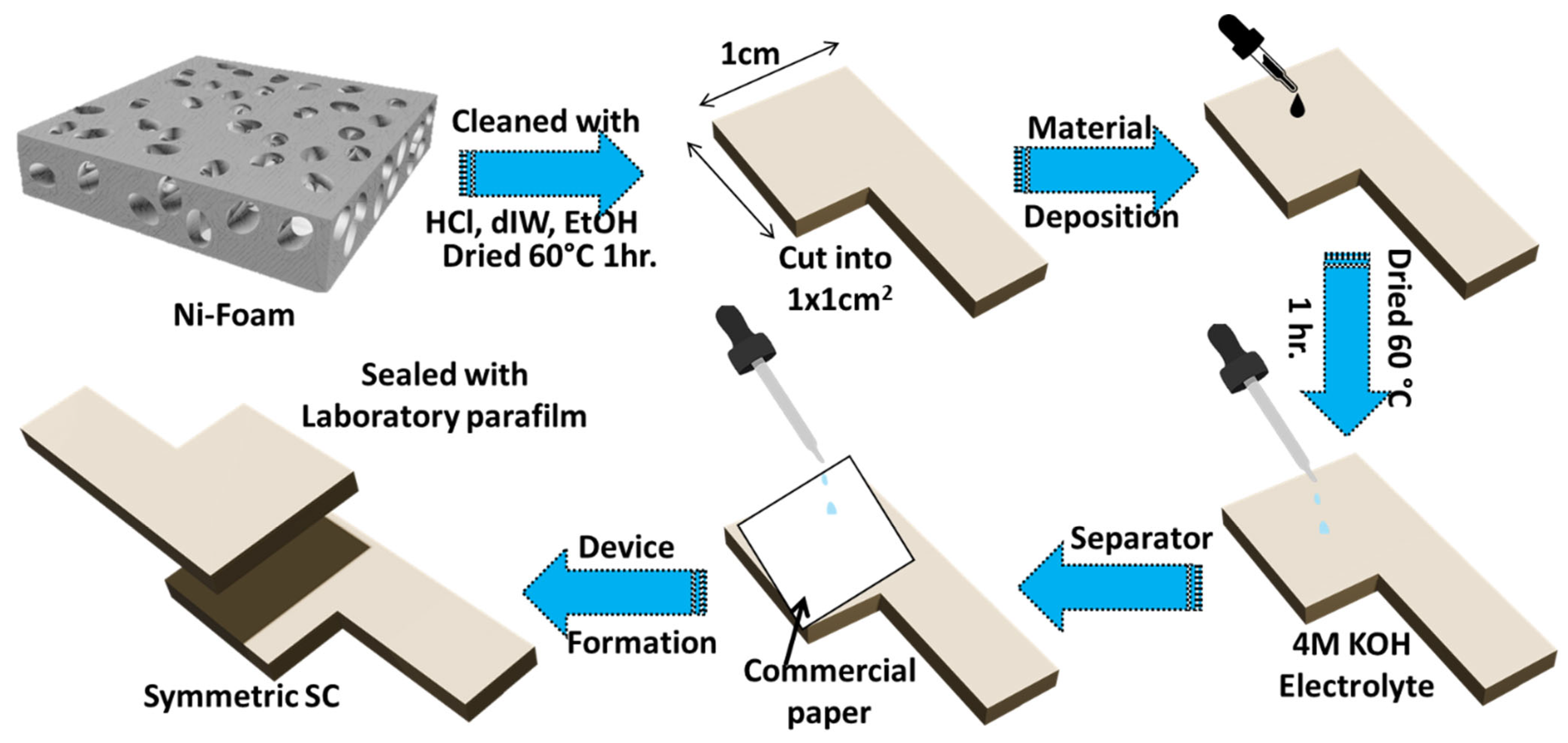
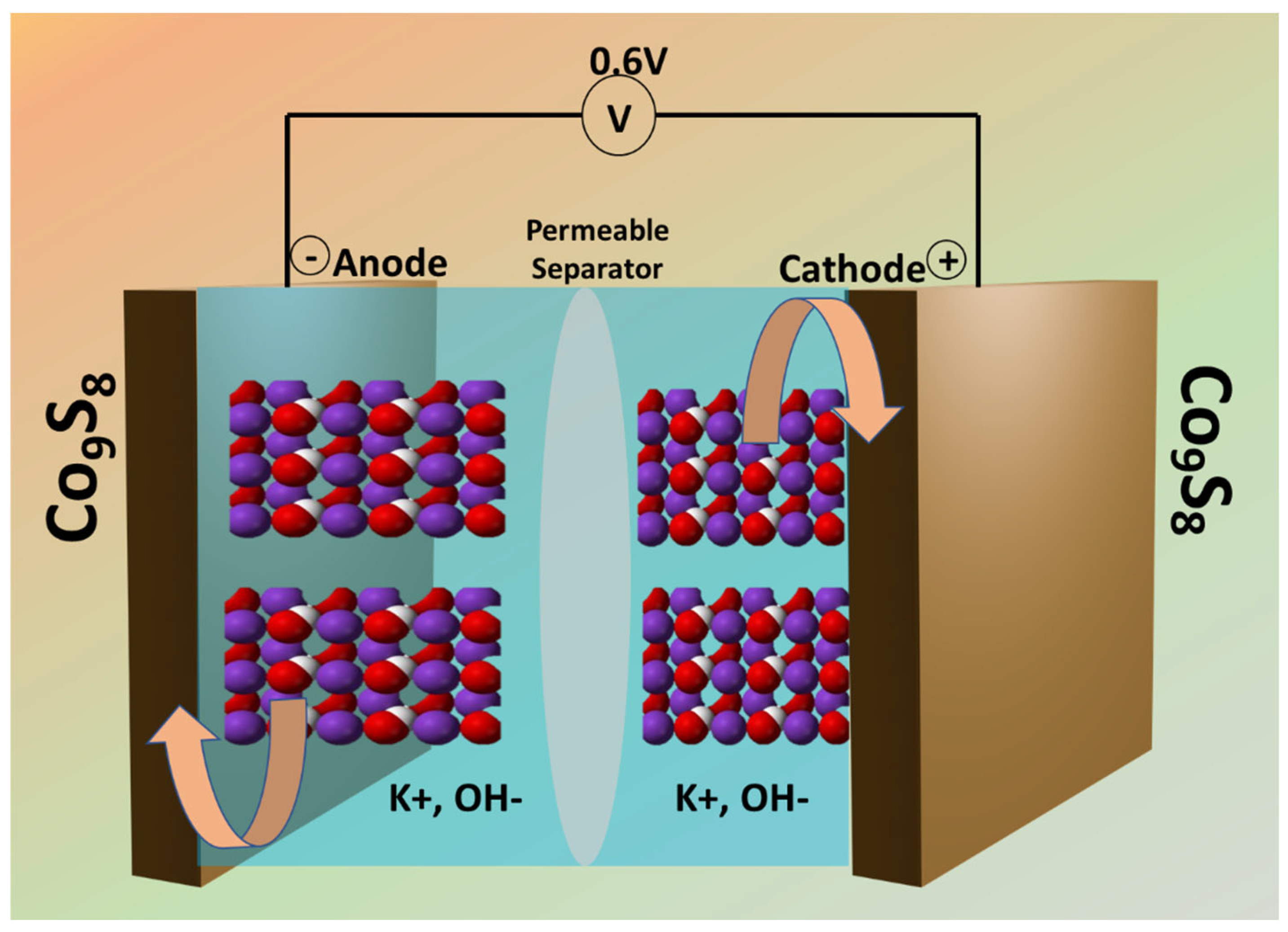
2.4. Assembly of Symmetric Supercapacitor
2.5. Electrochemical Measurement
3. Results
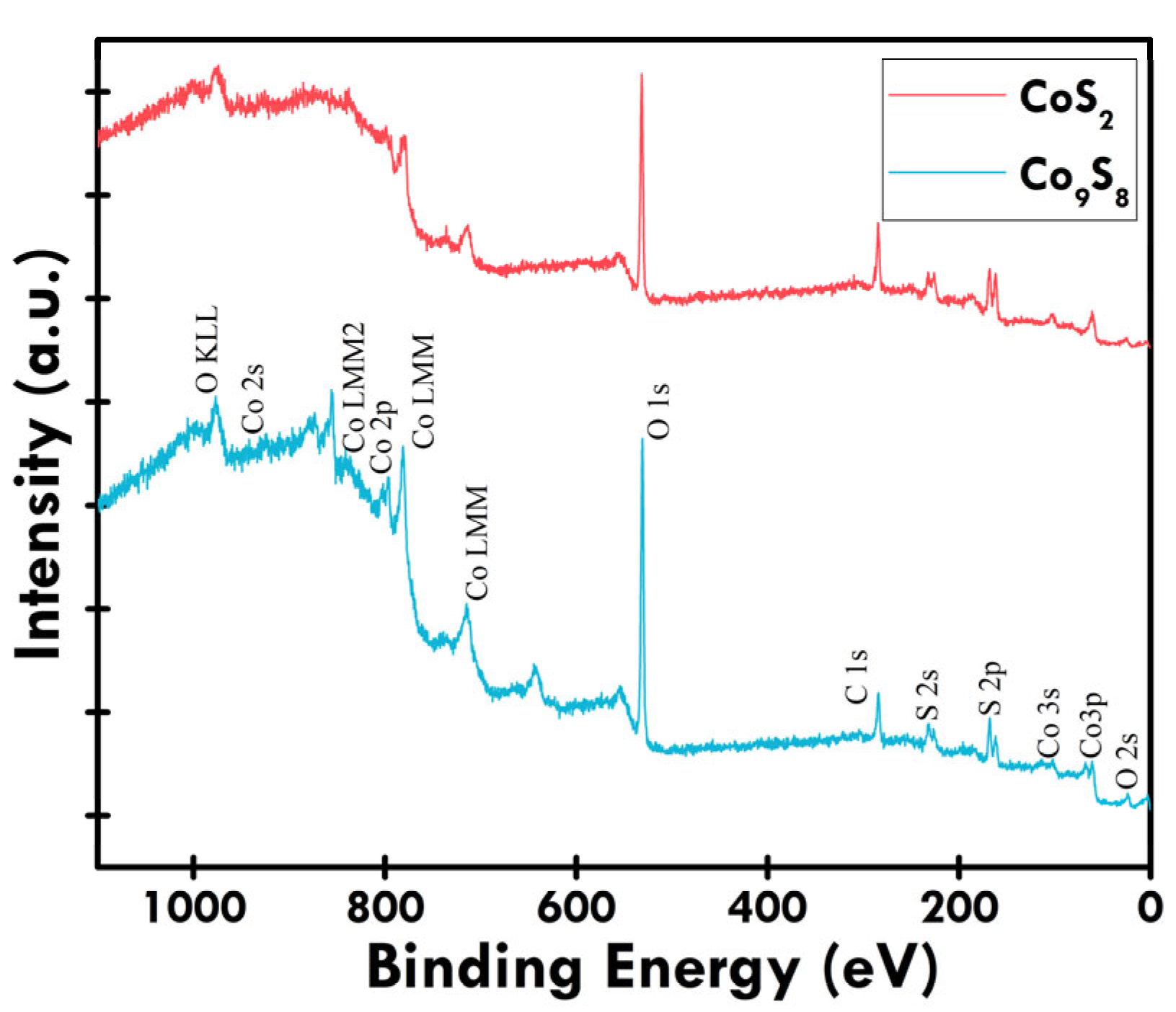
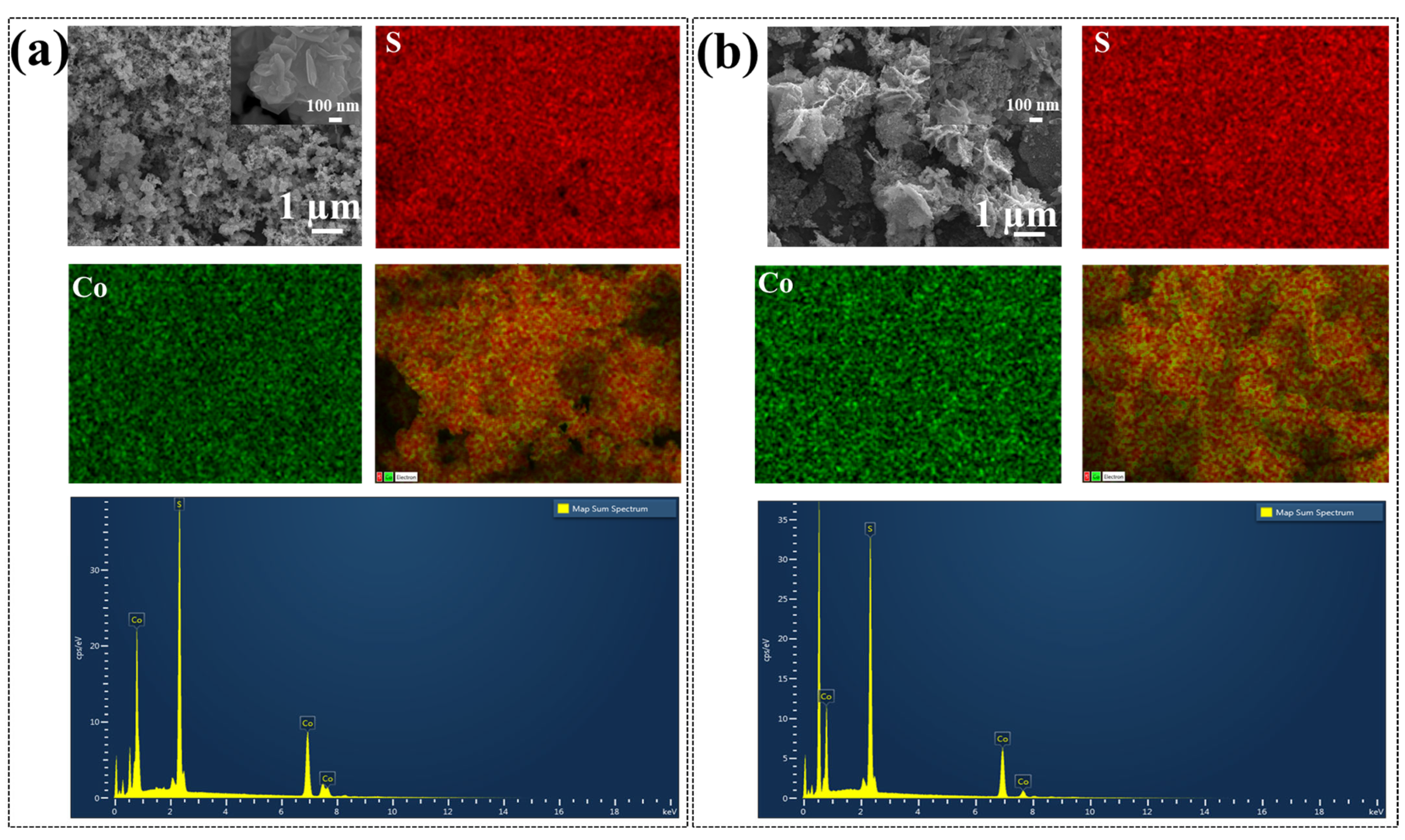
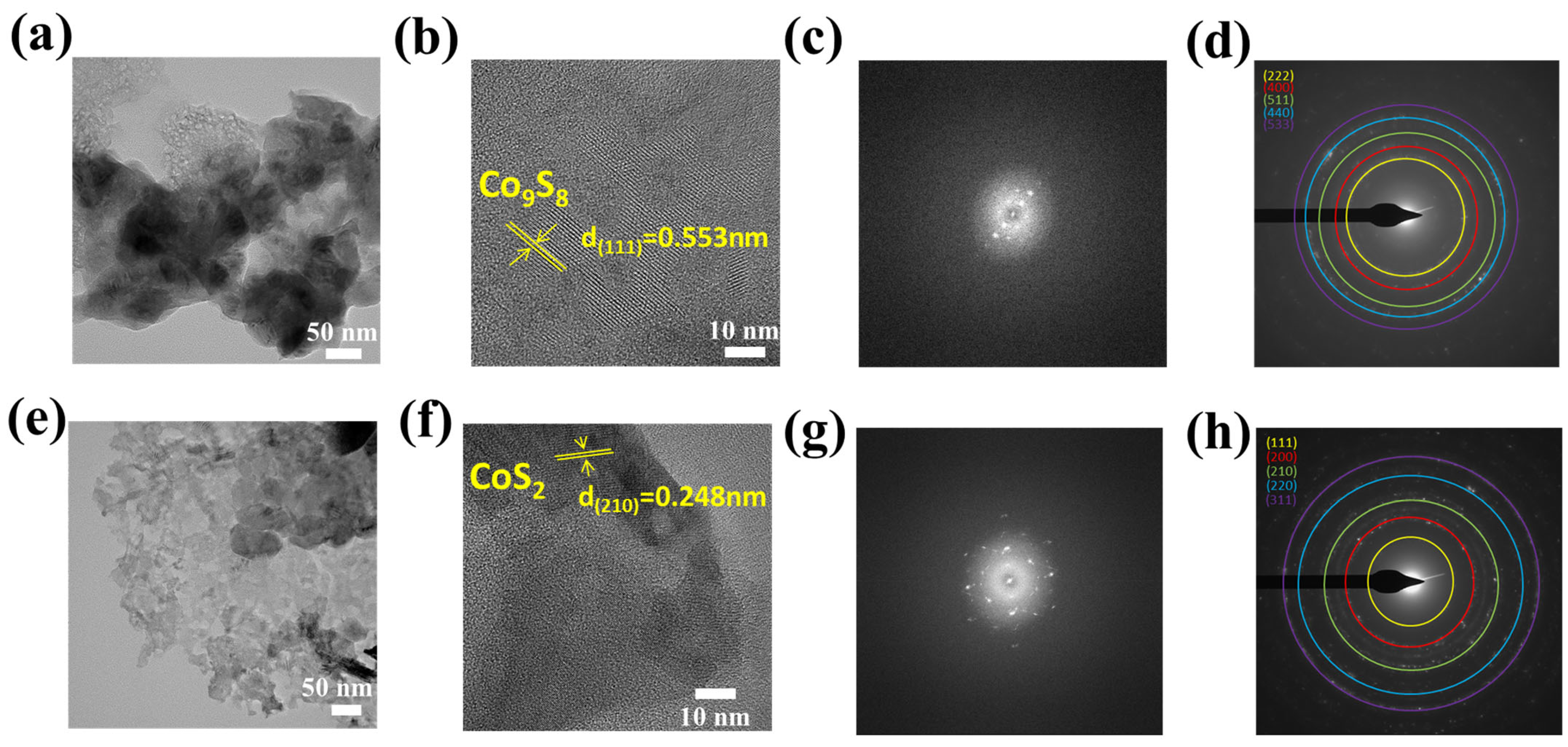
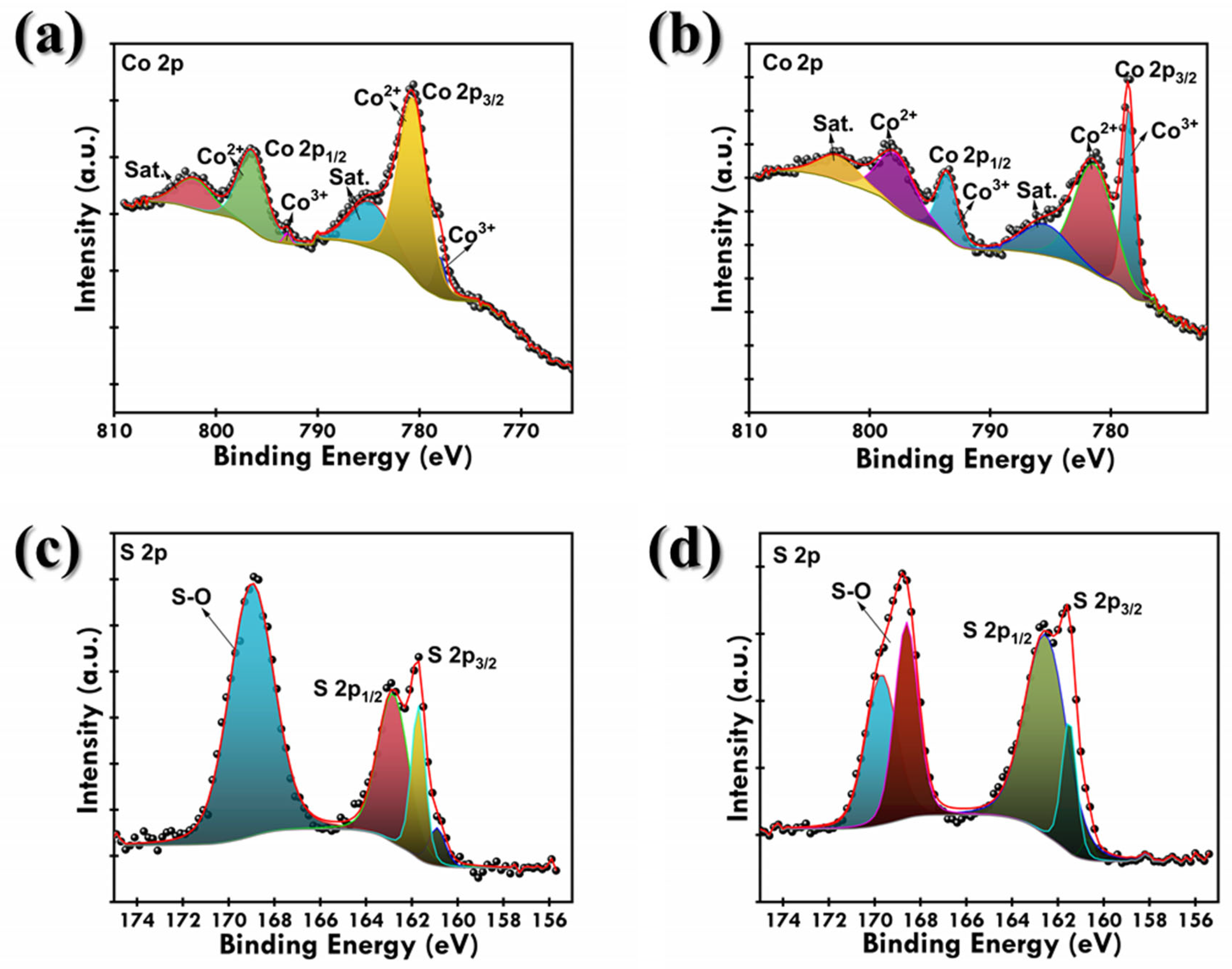
3.1. Physiochemical Examination
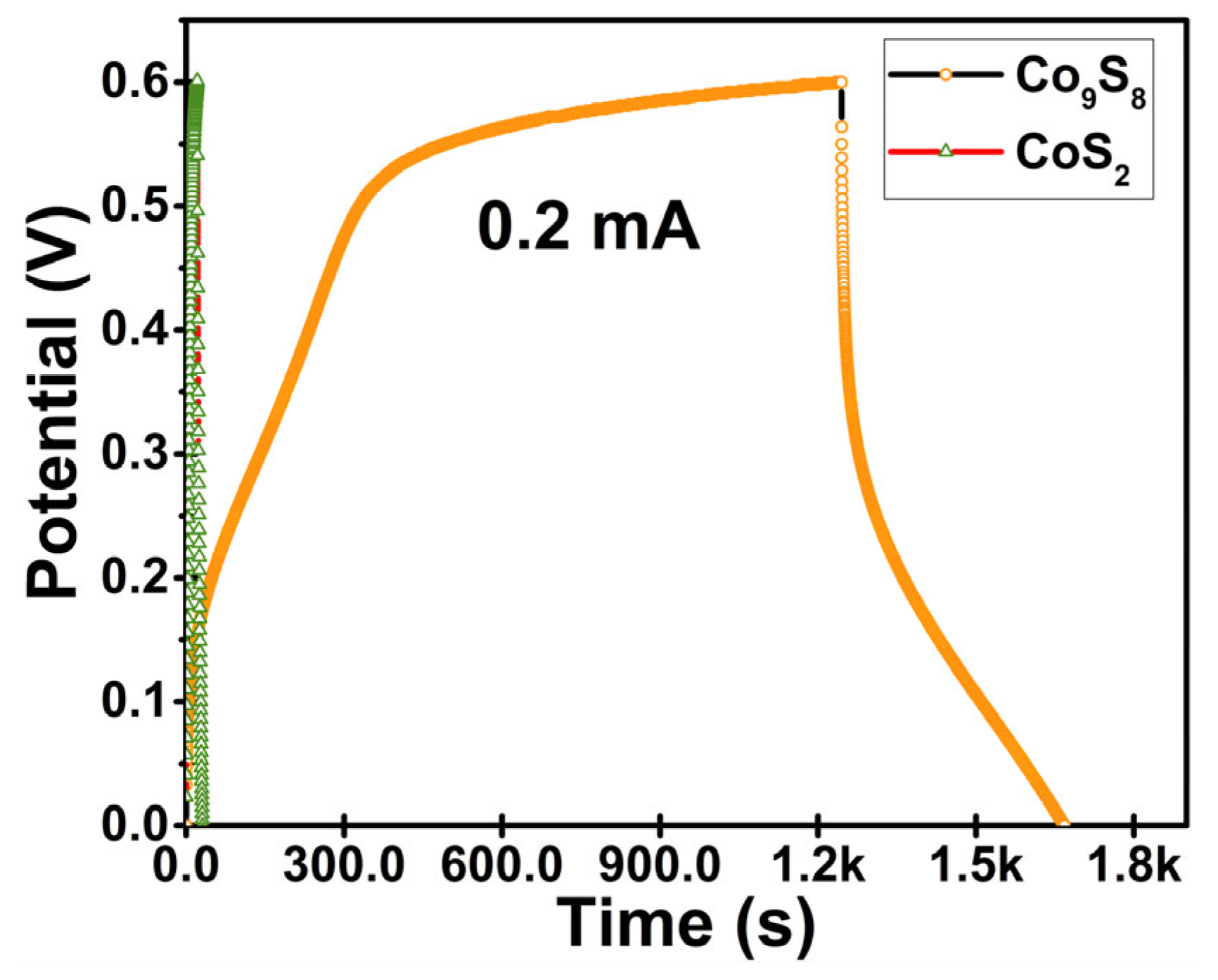
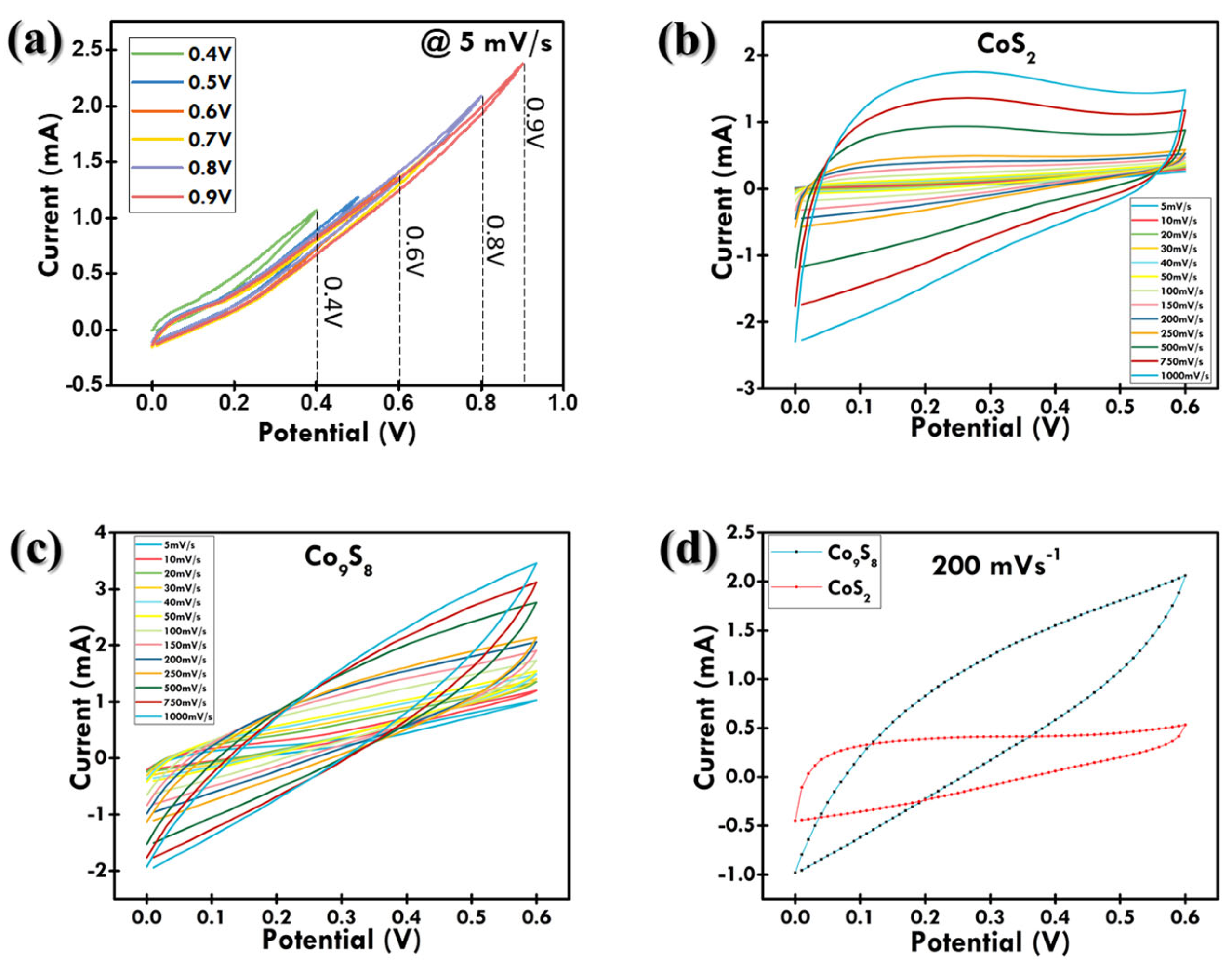
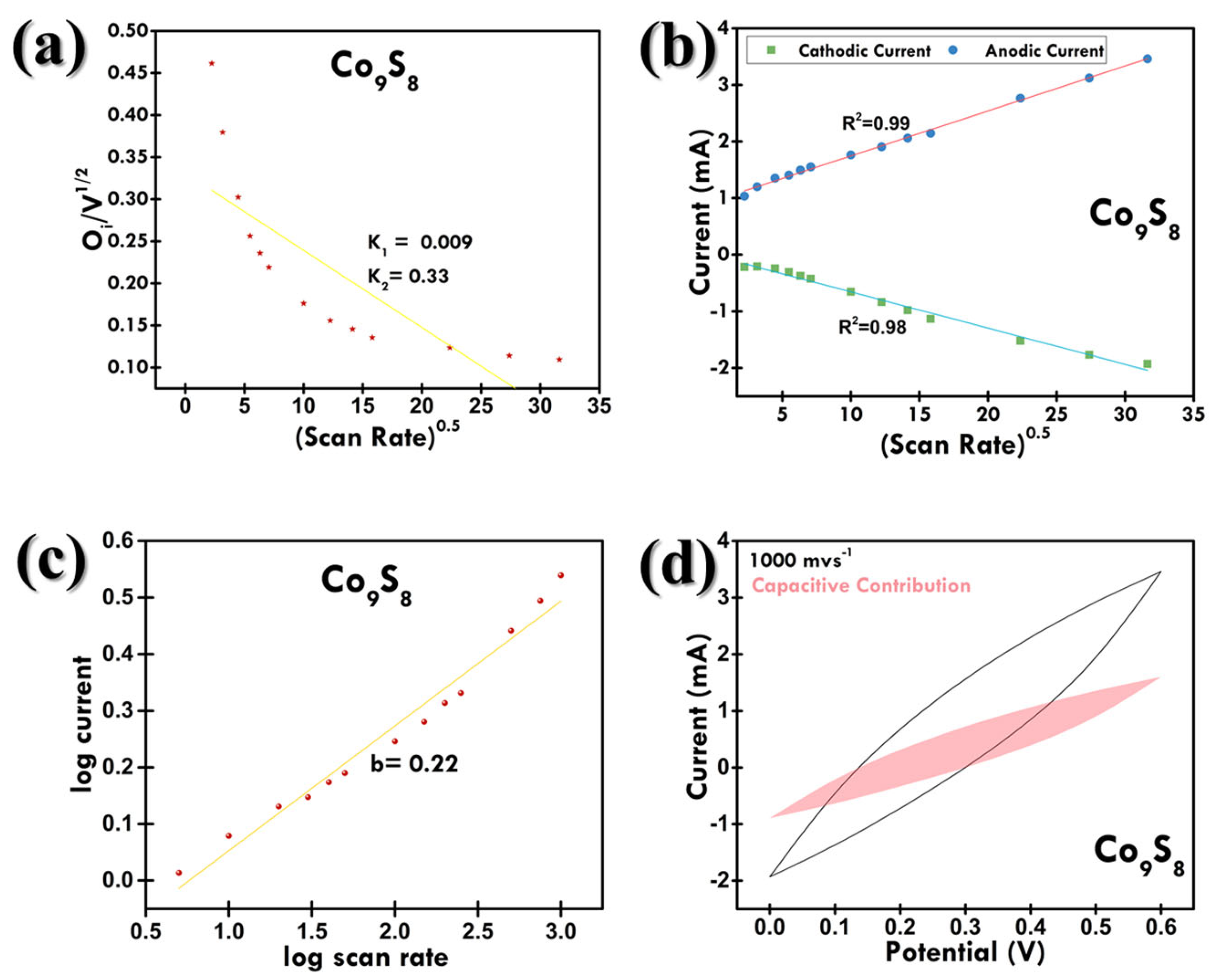
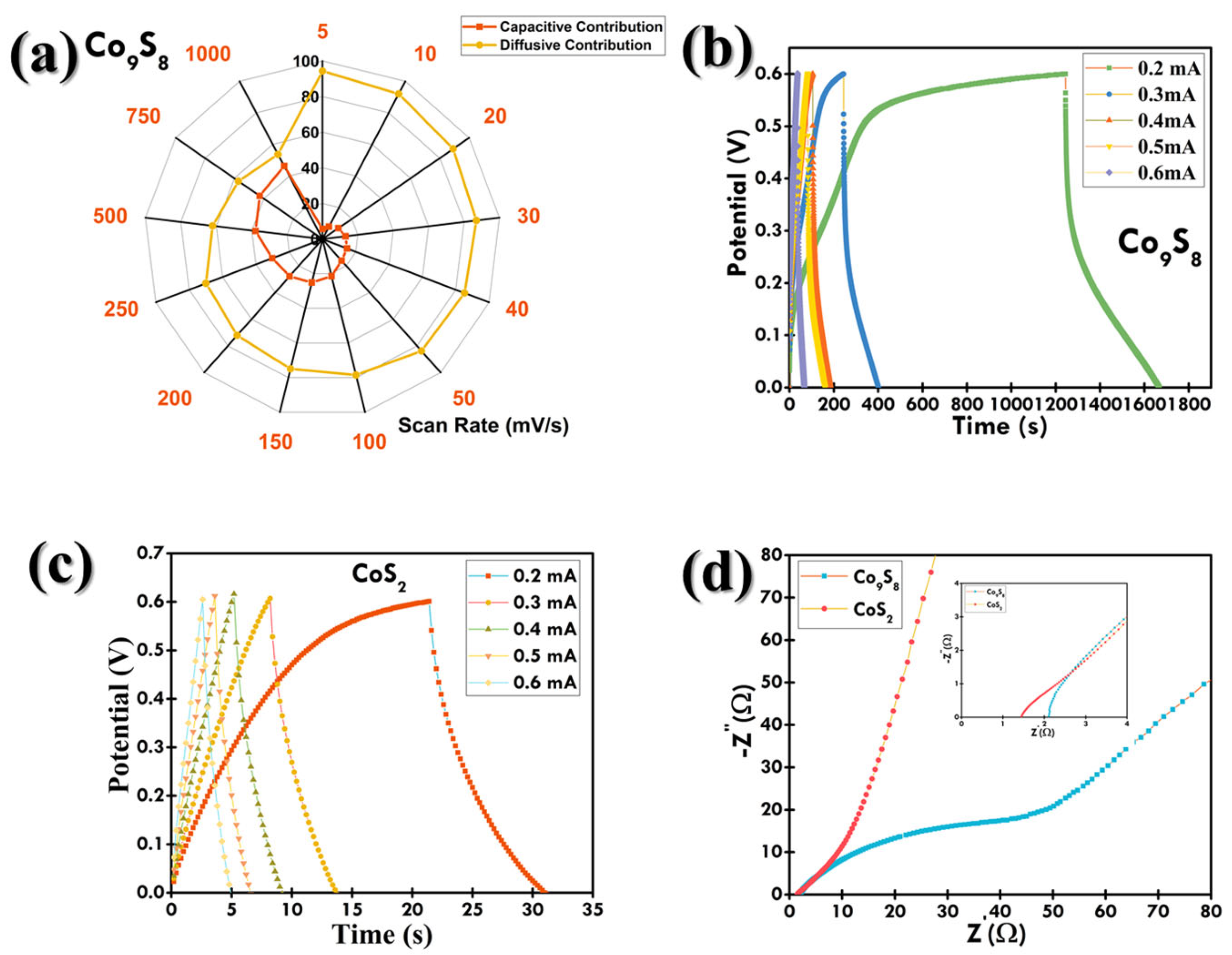
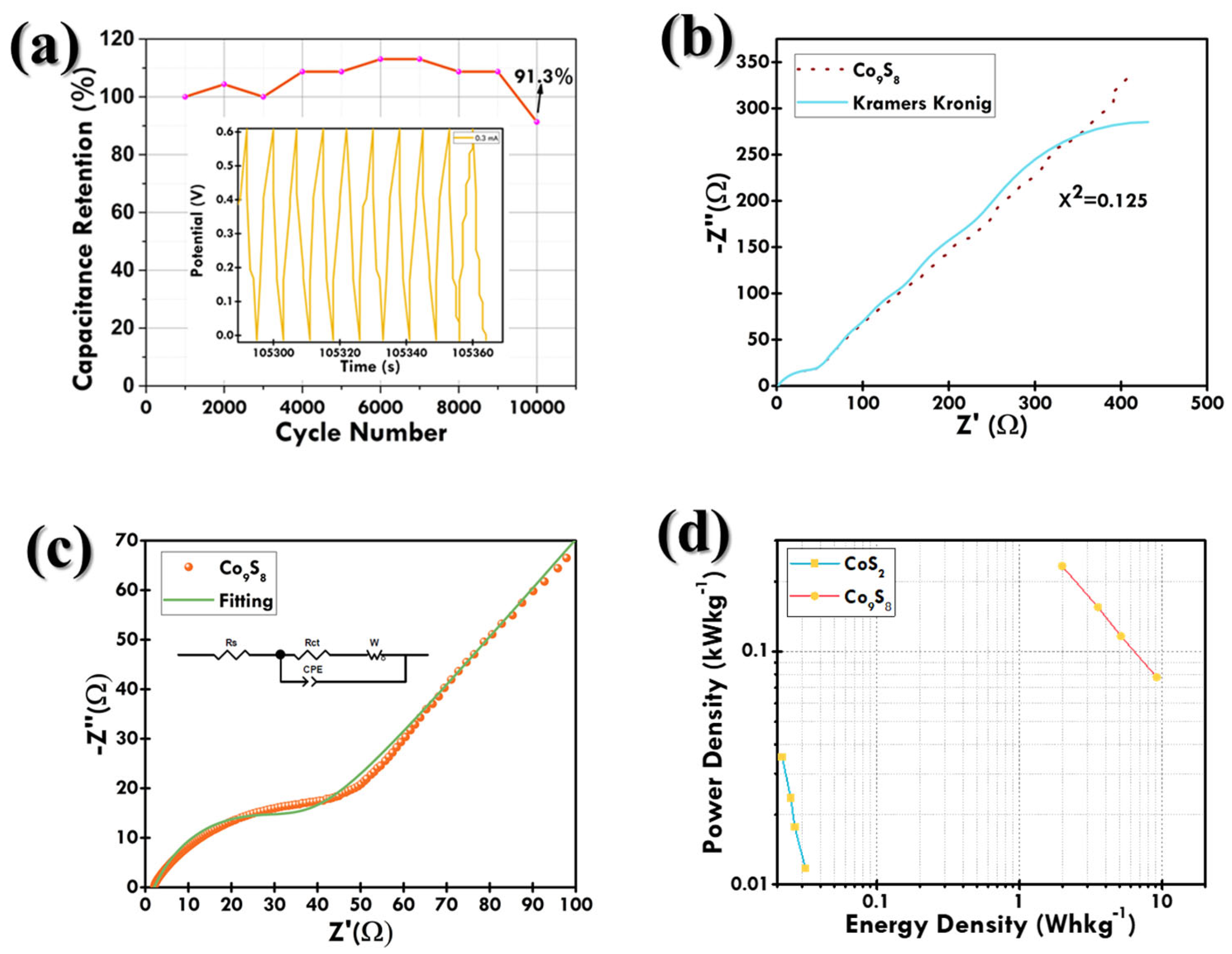
3.2. Electrochemical Examination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
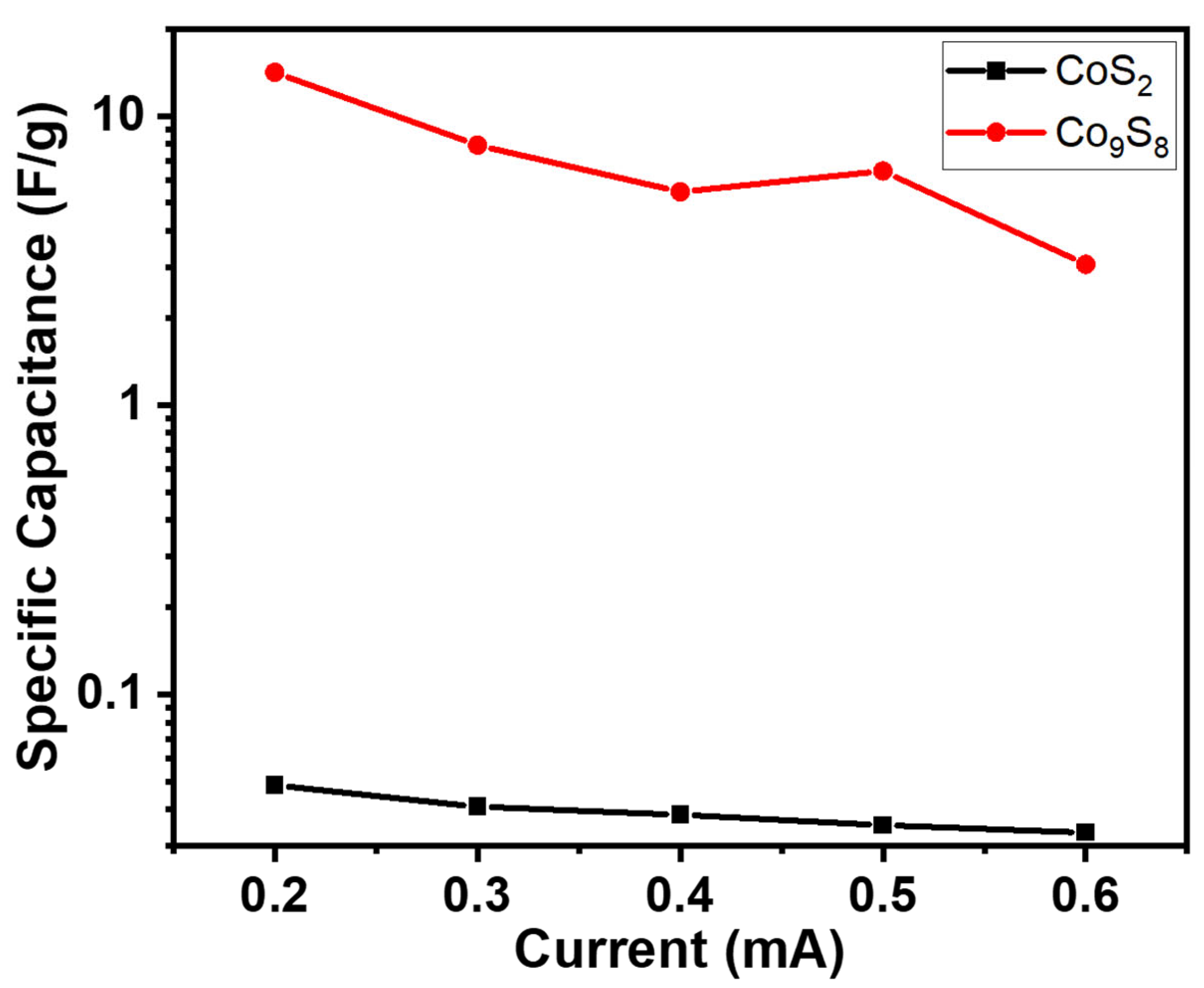
| Current (mA) | CoS2 | Co9S8 |
|---|---|---|
| Ccell (F/g) | Ccell (F/g) | |
| 0.2 | 0.048 | 14.12 |
| 0.3 | 0.040 | 7.91 |
| 0.4 | 0.038 | 5.45 |
| 0.5 | 0.035 | 6.43 |
| 0.6 | 0.033 | 3.06 |
| Current (mA) | CoS2 | Co9S8 | ||
|---|---|---|---|---|
| Specific Energy Density (Wh/kg) | Specific Power Density (W/kg) | Specific Energy Density (Wh/kg) | Specific Power Density (kW/kg) | |
| 0.2 | 0.031 | 0.011 | 9.14976 | 0.07776 |
| 0.3 | 0.02 | 0.017 | 5.12568 | 0.11664 |
| 0.4 | 0.024 | 0.023 | 3.53376 | 0.15552 |
| 0.5 | 0.022 | 0.029 | 4.1688 | 0.1944 |
| 0.6 | 0.021 | 0.035 | 1.98288 | 0.23328 |
References
- Balasubramaniam, S.; Mohanty, A.; Balasingam, S.K.; Kim, S.J.; Ramadoss, A. Comprehensive insight into the mechanism, material selection and performance evaluation of supercapatteries. Nano-Micro Lett. 2020, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Bakay, M.S.; Ağbulut, Ü. Electricity production based forecasting of greenhouse gas emissions in Turkey with deep learning, support vector machine and artificial neural network algorithms. J. Clean. Prod. 2021, 285, 125324. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Owusu, P.A.; Asumadu-Sarkodie, S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng. 2016, 3, 1167990. [Google Scholar] [CrossRef]
- Shinde, P.A.; Abbas, Q.; Chodankar, N.R.; Ariga, K.; Abdelkareem, M.A.; Olabi, A.G. Strengths, weaknesses, opportunities, and threats (SWOT) analysis of supercapacitors: A review. J. Energy Chem. 2023, 79, 611–638. [Google Scholar] [CrossRef]
- Azega, R.; Smith, A.D.; Chowdhury, N.R.; Vyas, A.; Li, Q.; Haque, M.; Xun, Q.; Zhang, X.; Thurakkal, S.; Thiringer, T.; et al. Supercapacitors and rechargeable batteries, a tale of two technologies: Past, present and beyond. Sustain. Mater. Technol. 2024, 41, e01111. [Google Scholar] [CrossRef]
- Olabi, A.; Wilberforce, T.; Sayed, E.T.; Abo-Khalil, A.G.; Maghrabie, H.M.; Elsaid, K.; Abdelkareem, M.A. Battery energy storage systems and SWOT (strengths, weakness, opportunities, and threats) analysis of batteries in power transmission. Energy 2022, 254, 123987. [Google Scholar] [CrossRef]
- Olabi, A.; Abdelkareem, M.A.; Wilberforce, T.; Alkhalidi, A.; Salameh, T.; Abo-Khalil, A.G.; Hassan, M.M.; Sayed, E.T. Battery electric vehicles: Progress, power electronic converters, strength (S), weakness (W), opportunity (O), and threats (T). Int. J. Thermofluids 2022, 16, 100212. [Google Scholar] [CrossRef]
- Elalfy, D.A.; Gouda, E.; Kotb, M.F.; Bureš, V.; Sedhom, B.E. Comprehensive review of energy storage systems technologies, objectives, challenges, and future trends. Energy Strategy Rev. 2024, 54, 101482. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Roy, N.; Ramachandran, T.; Hussien, M.; Moniruzzaman, M.; Joo, S.W. Shaping the future of energy: The rise of supercapacitors progress in the last five years. J. Energy Storage 2024, 98, 113040. [Google Scholar] [CrossRef]
- Khan, H.R.; Ahmad, A.L. Supercapacitors: Overcoming current limitations and charting the course for next-generation energy storage. J. Ind. Eng. Chem. 2024, 141, 46–66. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Senthil, R.A.; Nithyadharseni, P.; Lee, S.J.; Durai, G.; Kuppusami, P.; Madhavan, J.; Choi, M.Y. Recent progress and emerging challenges of transition metal sulfides based composite electrodes for electrochemical supercapacitive energy storage. Ceram. Int. 2020, 46, 14317–14345. [Google Scholar] [CrossRef]
- Rehman, J.; Eid, K.; Ali, R.; Fan, X.; Murtaza, G.; Faizan, M.; Laref, A.; Zheng, W.; Varma, R.S. Engineering of transition metal sulfide nanostructures as efficient electrodes for high-performance supercapacitors. ACS Appl. Energy Mater. 2022, 5, 6481–6498. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Jiang, S.; Zhao, Q.; Jiang, T. Design of high-performance transition metal sulfide electrode materials and its application in supercapacitors. J. Power Sources 2024, 606, 234560. [Google Scholar] [CrossRef]
- Li, P.; Luo, S.; Xiong, Z.; Xiao, H.; Wang, X.; Peng, K.; Xie, X.; Zhang, Z.; Deng, G.; Yang, M.; et al. RuxMoS2 interfacial heterojunctions achieve efficient overall water splitting and stability in both alkaline and acidic media under large current density exceeding 100 mA cm−2. Mol. Catal. 2025, 570, 114710. [Google Scholar] [CrossRef]
- Tang, J.; Guan, D.; Xu, H.; Zhao, L.; Arshad, U.; Fang, Z.; Zhu, T.; Kim, M.; Pao, C.-W.; Hu, Z.; et al. Undoped ruthenium oxide as a stable catalyst for the acidic oxygen evolution reaction. Nat. Commun. 2025, 16, 801. [Google Scholar] [CrossRef]
- Wan, Z.; Yang, K.; Li, P.; Yang, S.; Wang, X.; Gao, R.; Xie, X.; Deng, G.; Yang, M.; Wang, Z. Atomically dispersed Au single sites and nanoengineered structural defects enable a high electrocatalytic activity and durability for hydrogen evolution reaction and overall urea electrolysis. Electrochim. Acta 2024, 499, 144685. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Zhang, S.; Du, X.; Duan, Z.; Sun, X. Hollow Co9S8 cores encapsulated in hierarchical MXene@ Bi2O3 multiple shells for constructing binder-free electrodes of foldable supercapacitors. J. Mater. Sci. Technol. 2023, 147, 112–123. [Google Scholar] [CrossRef]
- Huai, X.; Liu, J.; Wu, X. Cobalt-doped NiMoO4 nanosheet for high-performance flexible supercapacitor. Chin. J. Struct. Chem. 2023, 42, 100158. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, Z.; Chen, J.; Zhang, M.; Zu, H.; Qin, R.; Qu, W.; Yang, J.; Leng, L.; Li, H. Universal pathway towards metal sulfides decorated 3D porous Ti3C2Tx MXene aerogel for vapor-phase mercury removal. Chem. Eng. J. 2023, 476, 146402. [Google Scholar] [CrossRef]
- Xiao, Z.; Xiao, G.; Shi, M.; Zhu, Y. Homogeneously dispersed Co9S8 anchored on nitrogen and sulfur co-doped carbon derived from soybean as bifunctional oxygen electrocatalysts and supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 16436–16448. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Yu, M.; Lu, L.; Feng, H.; Yang, Y.; Chen, Y.; Cui, H.; Xiao, R.; Liu, J. Pseudocapacitive Co9S8/graphene electrode for high-rate hybrid supercapacitors. Carbon 2019, 141, 134–142. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, N.; Sun, L.; Yu, X.; Liu, C.; Yang, L.; Zhang, S.; Wang, Z. Multi-layer-stacked Co9S8 micro/nanostructure directly anchoring on carbon cloth as a flexible electrode in supercapacitors. Nanoscale 2019, 11, 7457–7464. [Google Scholar] [CrossRef]
- Rathinamala, I.; Babu, I.M.; William, J.J.; Muralidharan, G.; Prithivikumaran, N. Extra-durable hybrid supercapacitor based on cobalt sulfide and carbon (MWCNT) matrix electrodes. J. Energy Storage 2021, 34, 102200. [Google Scholar] [CrossRef]
- Sajjad, M.; Khan, Y. Rational design of self-supported Ni3S2 nanoparticles as a battery type electrode material for high-voltage (1.8 V) symmetric supercapacitor applications. CrystEngComm 2021, 23, 2869–2879. [Google Scholar] [CrossRef]
- Hao, X.; Wang, J.; Ding, B.; Wang, Y.; Chang, Z.; Dou, H.; Zhang, X. Bacterial-cellulose-derived interconnected meso-microporous carbon nanofiber networks as binder-free electrodes for high-performance supercapacitors. J. Power Sources 2017, 352, 34–41. [Google Scholar] [CrossRef]
- Shao, J.; Ma, F.; Wu, G.; Dai, C.; Geng, W.; Song, S.; Wan, J. In-situ MgO (CaCO3) templating coupled with KOH activation strategy for high yield preparation of various porous carbons as supercapacitor electrode materials. Chem. Eng. J. 2017, 321, 301–313. [Google Scholar] [CrossRef]
- Kuznetsov, V.G.; Sokolova, M.A.; Palkina, K.K.; Popova, Z.V. The cobalt-sulfur system. Inorg. Mater. 1965, 1, 617–632. [Google Scholar]
- Rajamani, V.; Prewitt, C. Refinement of the structure of Co9S8. Can. Mineral. 1975, 13, 75–78. [Google Scholar]
- Voorhees, P.W. The theory of Ostwald ripening. J. Stat. Phys. 1985, 38, 231–252. [Google Scholar] [CrossRef]
- Dong, W.; Wang, X.; Li, B.; Wang, L.; Chen, B.; Li, C.; Li, X.; Zhang, T.; Shi, Z. Hydrothermal synthesis and structure evolution of hierarchical cobalt sulfide nanostructures. Dalton Trans. 2011, 40, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Y.; Wen, H.; Yang, J.; Maouche, C.; Liu, Q.; Wu, Y.; Cheng, C.; Zhu, J.; Cheng, X. N,S-Atom-coordinated Co9S8 trinary dopants within a porous graphene framework as efficient catalysts for oxygen reduction/evolution reactions. Dalton Trans. 2018, 47, 14992–15001. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Wang, Q.S.; Li, J.; Long, Y.; Liu, Y.; Song, S.Y.; Zhang, H.J. Co9S8 Nanoparticles-Embedded N/S-Codoped Carbon Nanofibers Derived from Metal–Organic Framework-Wrapped CdS Nanowires for Efficient Oxygen Evolution Reaction. Small 2018, 14, 1704035. [Google Scholar] [CrossRef]
- Guan, D.; Xu, H.; Huang, Y.C.; Jing, C.; Tsujimoto, Y.; Xu, X.; Lin, Z.; Tang, J.; Wang, Z.; Sun, X. Operando studies redirect spatiotemporal restructuration of model coordinated oxides in electrochemical oxidation. Adv. Mater. 2025, 37, 2413073. [Google Scholar] [CrossRef]
- Ai, G.; Hu, Q.; Zhang, L.; Dai, K.; Wang, J.; Xu, Z.; Huang, Y.; Zhang, B.; Li, D.; Zhang, T.; et al. Investigation of the Nanocrystal CoS2 Embedded in 3D Honeycomb-like Graphitic Carbon with a Synergistic Effect for High-Performance Lithium Sulfur Batteries. ACS Appl. Mater. Interfaces 2019, 11, 33987–33999. [Google Scholar] [CrossRef]
- Feng, X.; Jiao, Q.; Liu, T.; Li, Q.; Yin, M.; Zhao, Y.; Li, H.; Feng, C.; Zhou, W. Facile Synthesis of Co9S8 Hollow Spheres as a High-Performance Electrocatalyst for the Oxygen Evolution Reaction. ACS Sustain. Chem. Eng. 2018, 6, 1863–1871. [Google Scholar] [CrossRef]
- Han, X.; Chen, Q.; Zhang, H.; Ni, Y.; Zhang, L. Template synthesis of NiCo2S4/Co9S8 hollow spheres for high-performance asymmetric supercapacitors. Chem. Eng. J. 2019, 368, 513–524. [Google Scholar] [CrossRef]
- Li, L.; Ding, Y.; Huang, H.; Yu, D.; Zhang, S.; Chen, H.-Y.; Ramakrishna, S.; Peng, S. Controlled synthesis of unique Co9S8 nanostructures with carbon coating as advanced electrode for solid-state asymmetric supercapacitors. J. Colloid Interface Sci. 2019, 540, 389–397. [Google Scholar] [CrossRef]
- Mundinamani, S.; Rabinal, M. Cyclic voltammetric studies on the role of electrode, electrode surface modification and electrolyte solution of an electrochemical cell. J. Appl. Chem 2014, 7, 45–52. [Google Scholar] [CrossRef]
- Lefrou, C.; Fabry, P.; Poignet, J.-C. Electrochemistry: The Basics, with Examples; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Milović, M.; Vujković, M.; Stephan, A.M.; Ivanović, M.; Jugović, D. Cathode performance of novel γ-LixV2O5/carbon composite in organic and aqueous electrolyte. Electrochim. Acta 2024, 499, 144693. [Google Scholar] [CrossRef]
- Muungani, G.; Pillay, M.N.; van Zyl, W.E. The mineral manaksite, KNaMnSi4O10, as a supercapattery-type electrochemical energy storage material. RSC Adv. 2023, 13, 26732–26743. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Yang, X.; Liu, Z.; Jiang, W.; Wan, J.; Liu, Y.; Ma, F. Construction of CoNi2S4/Co9S8@Co4S3 nanocubes derived from Ni-Co prussian blue analogues@cobalt carbonate hydroxide core–shell heterostructure for asymmetric supercapacitor. J. Colloid Interface Sci. 2024, 661, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Begum, B.; Bilal, S.; Shah, A.u.H.A.; Roese, P. Physical, chemical, and electrochemical properties of redox-responsive polybenzopyrrole as electrode material for faradaic energy storage. Polymers 2021, 13, 2883. [Google Scholar] [CrossRef]
- Cheng, B.; Cheng, R.; Tan, F.; Liu, X.; Huo, J.; Yue, G. Highly efficient quasi-solid-state asymmetric supercapacitors based on MoS2/MWCNT and PANI/MWCNT composite electrodes. Nanoscale Res. Lett. 2019, 14, 66. [Google Scholar] [CrossRef]
- Qin, S.; Liu, P.; Wang, J.; Liu, C.; Wang, Q.; Chen, X.; Zhang, S.; Tian, Y.; Zhang, F.; Wang, L.; et al. In situ N, O co-doped porous carbon derived from antibiotic fermentation residues as electrode material for high-performance supercapacitors. RSC Adv. 2023, 13, 24140–24149. [Google Scholar] [CrossRef]
- Kumar, M.; Yun, J.-H.; Bhatt, V.; Singh, B.; Kim, J.; Kim, J.-S.; Kim, B.S.; Lee, C.Y. Role of Ce3+ valence state and surface oxygen vacancies on enhanced electrochemical performance of single step solvothermally synthesized CeO2 nanoparticles. Electrochim. Acta 2018, 284, 709–720. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Parveen, N.; Nandi, D.K.; Ramesh, R.; Ansari, S.A.; Cheon, T.; Kim, S.-H. Enhanced activity of highly conformal and layered tin sulfide (SnSx) prepared by atomic layer deposition (ALD) on 3D metal scaffold towards high performance supercapacitor electrode. Sci. Rep. 2019, 9, 10225. [Google Scholar] [CrossRef]
- Gul, H.; Shah, A.-u.-H.A.; Bilal, S. Achieving ultrahigh cycling stability and extended potential window for supercapacitors through asymmetric combination of conductive polymer nanocomposite and activated carbon. Polymers 2019, 11, 1678. [Google Scholar] [CrossRef]
- Ismail, R.; Azman, N.H.N.; Mohanadas, D.; Mustafa, M.N.; Mohd Abdah, M.A.A.; Raman, V.; Abdullah, J.; Sulaiman, Y. Facile ultrasonication synthesis of MXene/HKUST-1 composite as positive electrode for supercapattery. J. Energy Storage 2024, 94, 112461. [Google Scholar] [CrossRef]
- Wu, Q.; He, T.; Zhang, Y.; Zhang, J.; Wang, Z.; Liu, Y.; Zhao, L.; Wu, Y.; Ran, F. Cyclic stability of supercapacitors: Materials, energy storage mechanism, test methods, and device. J. Mater. Chem. A 2021, 9, 24094–24147. [Google Scholar] [CrossRef]
- Shi, M.; Huang, Z.; Liu, H.; He, J.; Zeng, W.; Wu, Q.; Zhao, Y.; Tian, M.; Mu, S. Ultralow nitrogen-doped carbon coupled carbon-doped Co3O4 microrods with tunable electron configurations for advanced Li-storage properties. Electrochim. Acta 2019, 327, 135059. [Google Scholar] [CrossRef]
- Darowicki, K.; Wysmułek, S.; Karólkowska, A.; Gaweł, Ł. Validation of dynamic electrochemical impedance spectrograms using autocorrelation function. J. Electroanal. Chem. 2024, 962, 118255. [Google Scholar] [CrossRef]
- Agarwal, P.; Orazem, M.E.; Garcia-Rubio, L.H. Measurement models for electrochemical impedance spectroscopy: I. Demonstration of applicability. J. Electrochem. Soc. 1992, 139, 1917. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Urquidi-Macdonald, M. Application of Kramers-Kronig Transforms in the Analysis of Electrochemical Systems: I. Polarization Resistance. J. Electrochem. Soc. 1985, 132, 2316. [Google Scholar] [CrossRef]
- Surender, G.; Omar, F.S.; Bashir, S.; Pershaanaa, M.; Ramesh, S.; Ramesh, K. Growth of nanostructured cobalt sulfide-based nanocomposite as faradaic binder-free electrode for supercapattery. J. Energy Storage 2021, 39, 102599. [Google Scholar] [CrossRef]
- Hébert, S.; Barbier, T.; Berthebaud, D.; Lebedev, O.I.; Pralong, V.; Maignan, A. Transport and Thermoelectric Coefficients of the Co9S8 Metal: A Comparison with the Spin Polarized CoS2. J. Phys. Chem. C 2021, 125, 5386–5391. [Google Scholar] [CrossRef]
- Kumar, N.; Raman, N.; Sundaresan, A. Synthesis and properties of cobalt sulfide phases: CoS2 and Co9S8. Z. Anorg. Allg. Chem. 2014, 640, 1069–1074. [Google Scholar] [CrossRef]
- Feng, M.; Du, Q.; Su, L.; Zhang, G.; Wang, G.; Ma, Z.; Gao, W.; Qin, X.; Shao, G. Manganese oxide electrode with excellent electrochemical performance for sodium ion batteries by pre-intercalation of K and Na ions. Sci. Rep. 2017, 7, 2219. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, W.; Yang, T.; Wang, E.; Du, Z.; Hou, X.; Liang, T.; Wang, H. In situ reduced MXene/AuNPs composite toward enhanced charging/discharging and specific capacitance. J. Adv. Ceram. 2021, 10, 1061–1071. [Google Scholar] [CrossRef]
- Sun, X.; Wang, H.; Wang, W.; Li, Y.; Wang, K.; Zhang, Q.; Zhang, W.; Wang, J. Cobalt-copolymer-derived Co9S8 superstructure as electrode materials for advanced symmetric supercapacitor. J. Energy Storage 2024, 99, 113296. [Google Scholar] [CrossRef]
- Cui, Z.; Chen, M.; Wang, X.; Guan, C.; Liu, J.; Gao, N.; Yang, M.; Li, H. CoNi2S4/Co9S8 nanorods as advanced electrode material for supercapacitors. J. Energy Storage 2024, 94, 112446. [Google Scholar] [CrossRef]
- Venkatesan, D.; Annamalai, T.; Ramkumar, S.; Kanagajothi, D.; Karthik, P.S. Carbon-supported Co9S8 hollow spheres assembled from ultrathin nanosheets for high-performance supercapacitors. J. Mater. Sci. Mater. Electron. 2024, 35, 1051. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, T.; Su, Z.; Lin, S.; Shang, Y. Fabrication NiCo2S4/Co9S8 composites as a promising electrode material for supercapacitors. J. Solid State Chem. 2024, 332, 124578. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, C.; Ye, W.; Wang, H.; Wabaidur, S.M.; Zhong, Y.; Yan, R.; Ning, J.; Hu, Y. Electroactivated Modulation of Highly Aligned Manganese-Doped Cobalt Sulfide Nanoplate Arrays for High-Performance Hybrid Supercapacitors. ACS Appl. Energy Mater. 2024, 7, 11533–11542. [Google Scholar] [CrossRef]
- Liu, S.; Wu, J.; Wang, X.; He, S.; Yang, J. Fabrication of nickel-cobalt sulfide heterostructures with stable thermodynamic interfaces and enhanced electrochemical performance. Chem. Eng. Sci. 2024, 293, 120065. [Google Scholar] [CrossRef]
- Yu, J.; Wan, H.; Jiang, J.; Ruan, Y.; Miao, L.; Zhang, L.; Xia, D.; Xu, K. Activation mechanism study of dandelion-like Co9S8 nanotubes in supercapacitors. J. Electrochem. Soc. 2014, 161, A996. [Google Scholar] [CrossRef]
- Jia, H.; Wang, J.; Fu, W.; Hu, J.; Liu, Y. In-situ MOFs-derived hollow Co9S8 polyhedron welding on the top of MnCo2S4 nanoneedles for high performance hybrid supercapacitors. Chem. Eng. J. 2020, 391, 123541. [Google Scholar] [CrossRef]
- Chang, Y.; Sui, Y.; Qi, J.; Jiang, L.; He, Y.; Wei, F.; Meng, Q.; Jin, Y. Facile synthesis of Ni3S2 and Co9S8 double-size nanoparticles decorated on rGO for high-performance supercapacitor electrode materials. Electrochim. Acta 2017, 226, 69–78. [Google Scholar] [CrossRef]

| Electrode Material | Method | Capacitance (Fg−1) | Electrolyte | Substrate | Cycle Life | Ref |
|---|---|---|---|---|---|---|
| Co9S8/CHS | In situ carbonization | 113.02 | 6 M KOH | Ni-F | 90% (2000) | [61] |
| CoNi2S4/Co9S8 | Hydrothermal | 1546.5 | 6 M KOH | - | 87% (10,000) | [62] |
| Co9S8@RGO | Hydrothermal | 3255 | PVA/KOH | Ni-F | 80.61 (10,000) | [63] |
| NiCo2S4/Co9S8 | Hydrothermal | 2532.5 | 3 M KOH | Ni-F | 94.7% (10,000) | [64] |
| Mn-Co9S8 | Self-templating sulfurization | 234.6 | PVA/KOH | Ni-F | 98.2% (5000) | [65] |
| NiCo2S4/[Ni, Co]9S8 | Hydrothermal | 1789 | 6 M KOH | Ni-F | 70% (3000) | [66] |
| Co9S8 nanotube | Hydrothermal | 285.3 | 6 M KOH | Ni-F | 90.4% (1000) | [67] |
| MnCo2S4/Co9S8 | Hydrothermal | 1100.5 | 6 M KOH | Ni-F | 94.8% (5000) | [68] |
| rGO/Ni3S2/Co9S8 | Solution-based method | 1929.1 | 2 M KOH | Ni-F | 92.8% (1000) | [69] |
| Co9S8 | Hydrothermal | 14.12 at 0.2 mA | 4M KOH | Ni-F | 91.3% (10,000) | Present Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Cho, Y.-B.; Tran, T.B.; Kim, S.J.; Park, D.I.; Kim, T.; Bhatt, V.; Kumar, M.; Yun, J.-H. Phase-Dependent Electrochemical Performance of CoxSy (x = 1,9; y = 2,8) for Symmetric Supercapacitor Application. Materials 2025, 18, 2101. https://doi.org/10.3390/ma18092101
Sharma A, Cho Y-B, Tran TB, Kim SJ, Park DI, Kim T, Bhatt V, Kumar M, Yun J-H. Phase-Dependent Electrochemical Performance of CoxSy (x = 1,9; y = 2,8) for Symmetric Supercapacitor Application. Materials. 2025; 18(9):2101. https://doi.org/10.3390/ma18092101
Chicago/Turabian StyleSharma, Ankush, Young-Bin Cho, Tung Bach Tran, Sung Jin Kim, Dong In Park, Taehoon Kim, Vishwa Bhatt, Manjeet Kumar, and Ju-Hyung Yun. 2025. "Phase-Dependent Electrochemical Performance of CoxSy (x = 1,9; y = 2,8) for Symmetric Supercapacitor Application" Materials 18, no. 9: 2101. https://doi.org/10.3390/ma18092101
APA StyleSharma, A., Cho, Y.-B., Tran, T. B., Kim, S. J., Park, D. I., Kim, T., Bhatt, V., Kumar, M., & Yun, J.-H. (2025). Phase-Dependent Electrochemical Performance of CoxSy (x = 1,9; y = 2,8) for Symmetric Supercapacitor Application. Materials, 18(9), 2101. https://doi.org/10.3390/ma18092101








