Influence of Zn2⁺ Concentration on Ceramic Coatings for Corrosion Protection of Magnesium-Lithium Alloys
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of PEO Coatings
2.2. Microstructural Characterizations
2.3. Corrosion Test
3. Results and Discussion
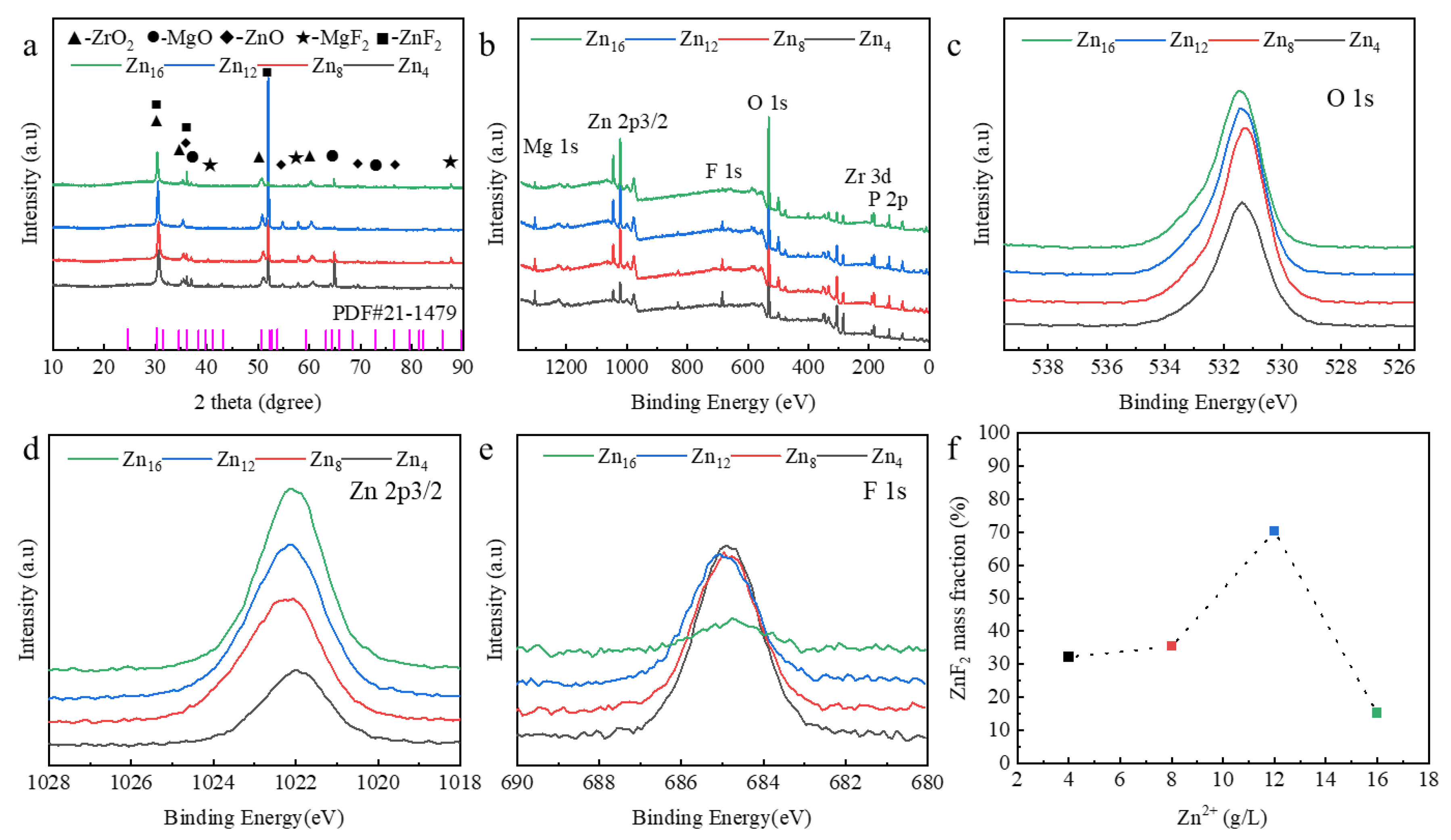
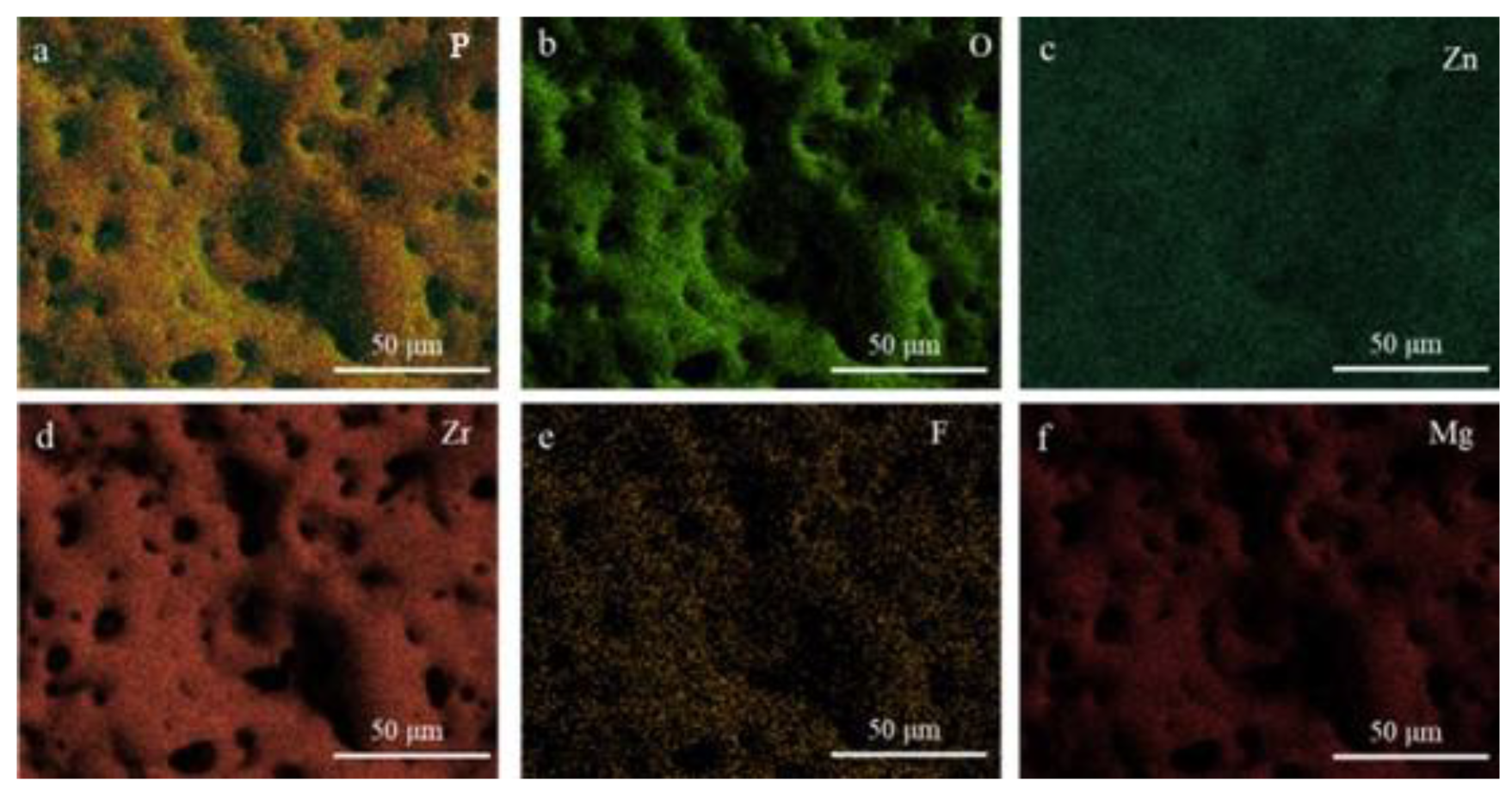
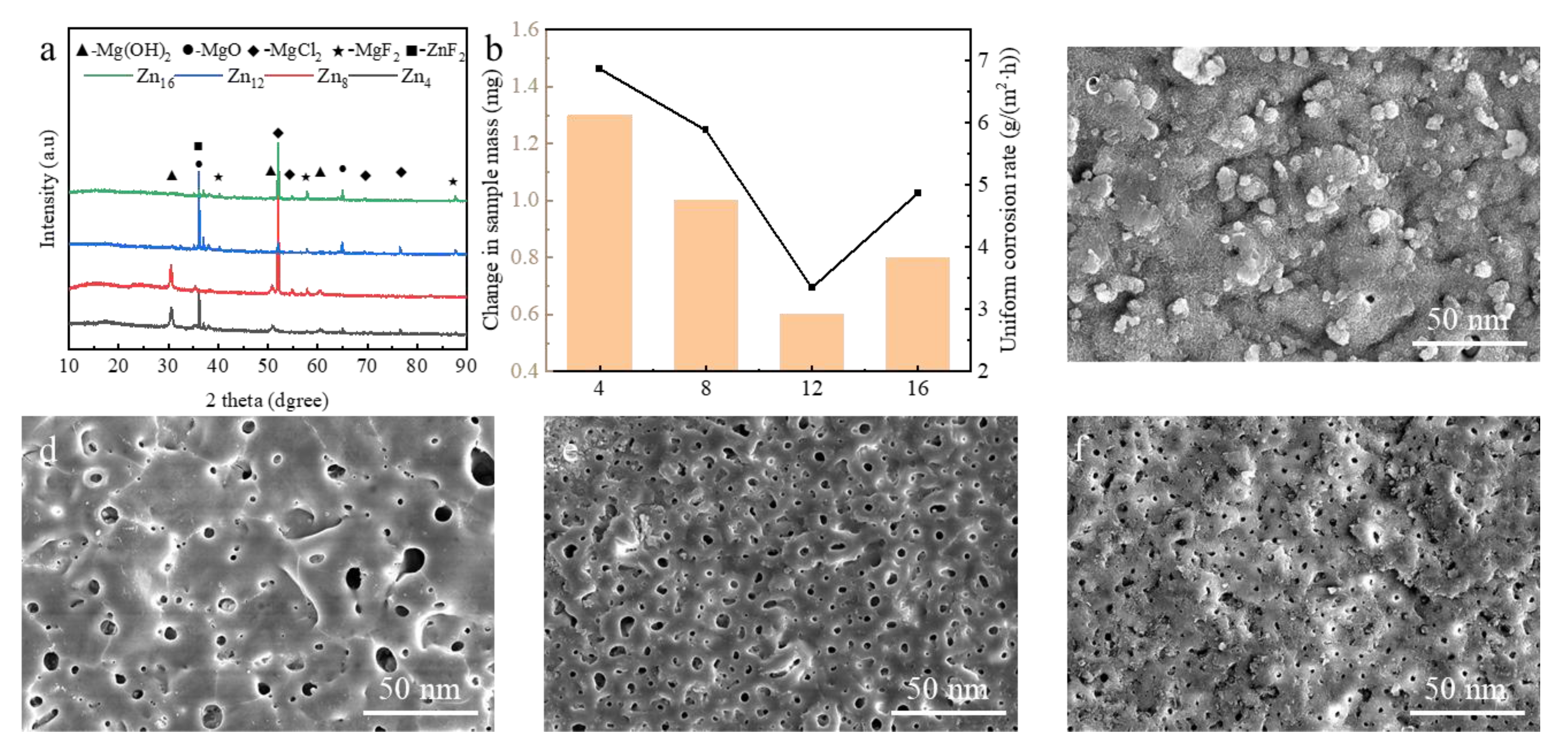
3.1. In Situ Synthesis of ZnF2 Nanoparticles
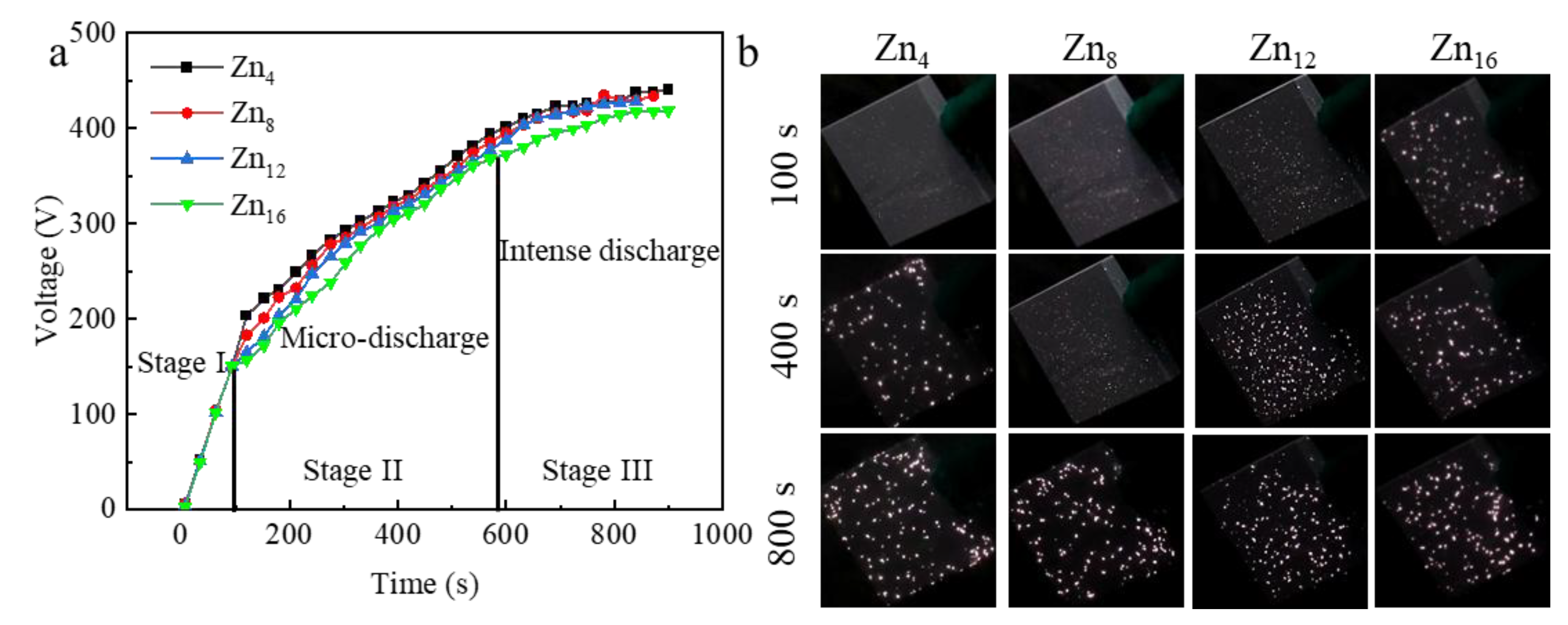
3.2. Voltage-Time Response During the PEO Process
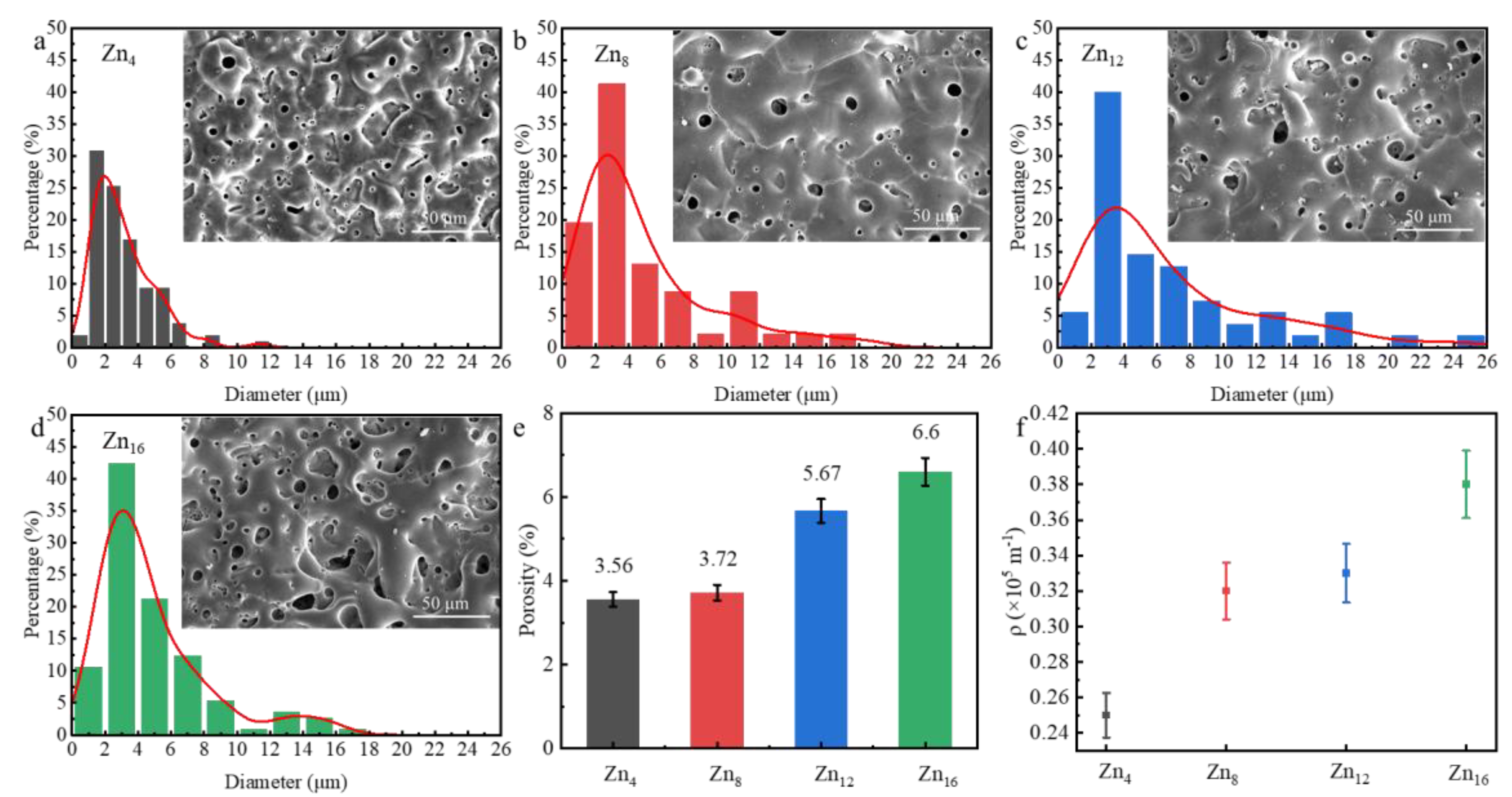
3.3. Effect of Zn2+ Concentration on Coating Microscopic Morphology
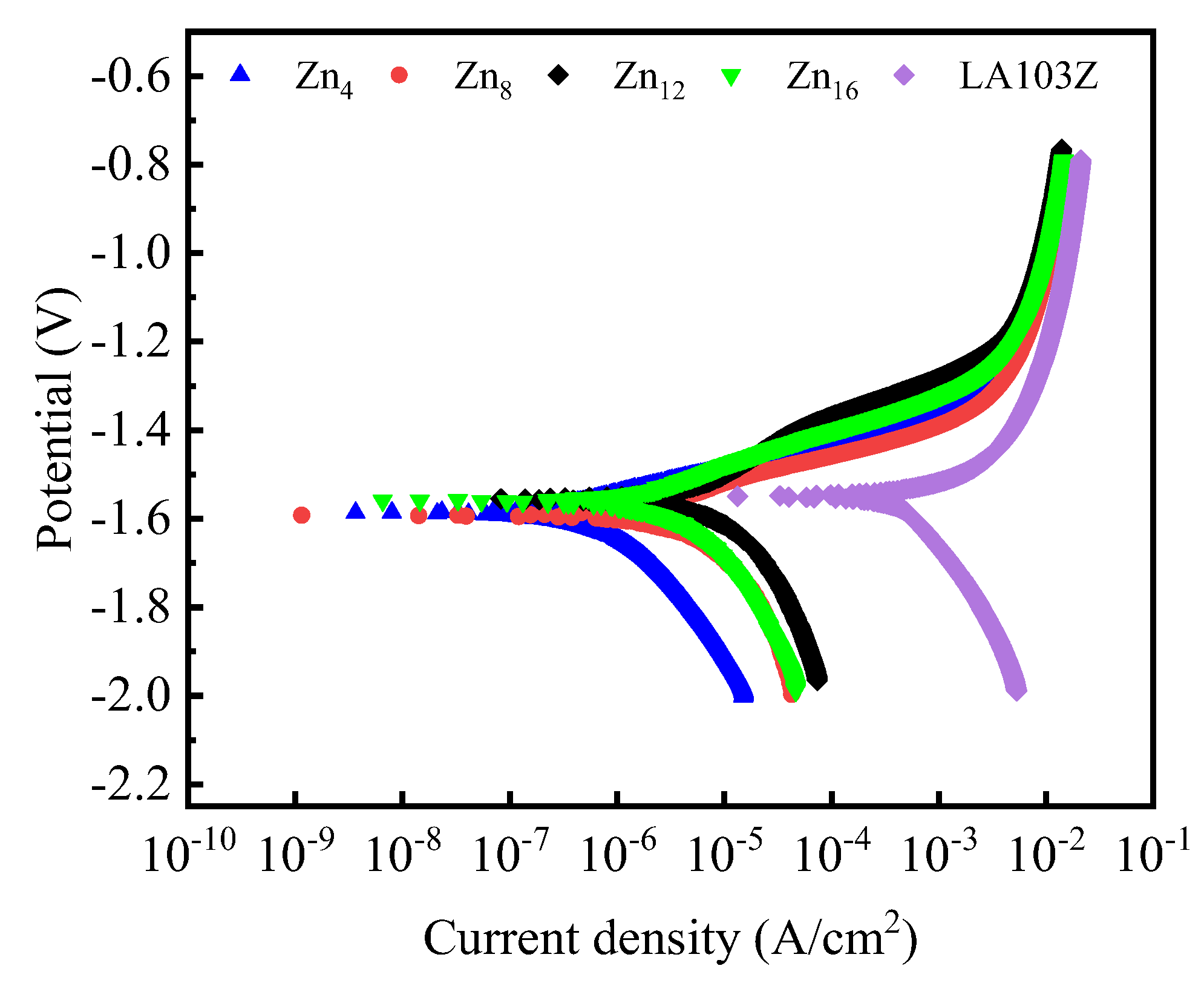
3.4. Corrosion Resistance
4. Conclusions
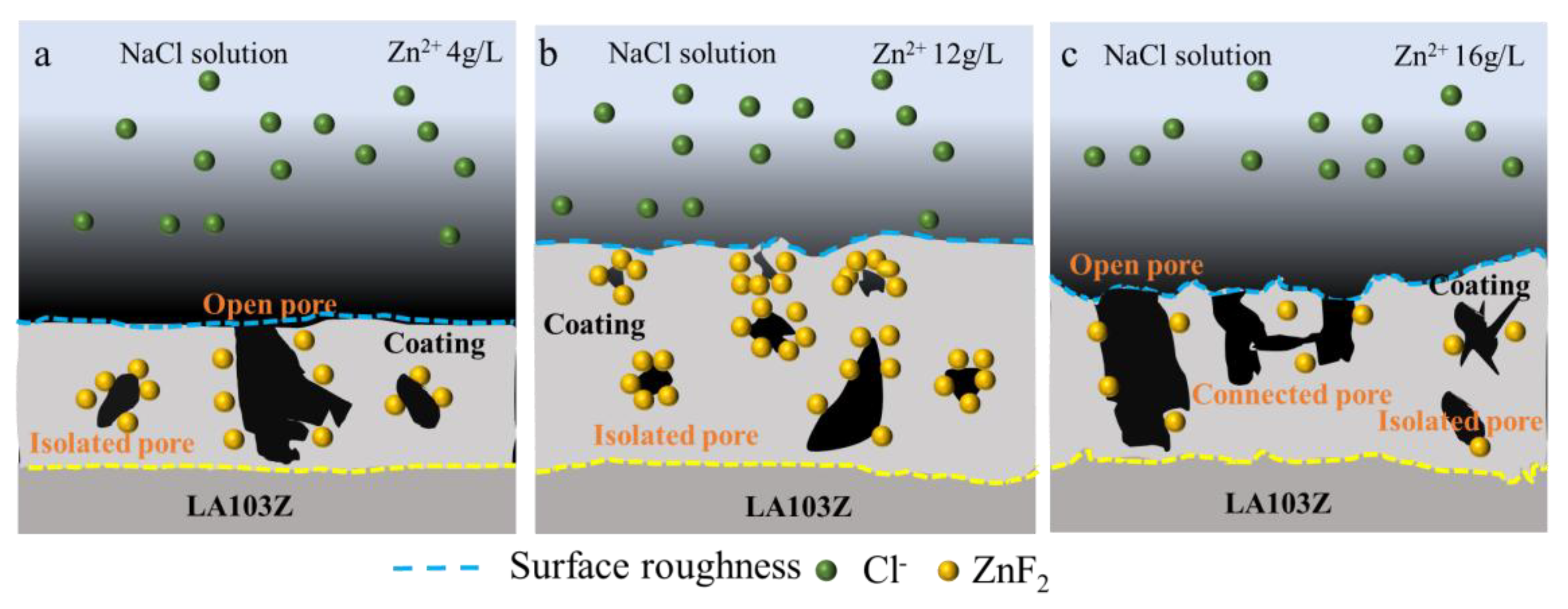
- Zn2⁺ concentration critically governs ZnF2 content via in situ synthesis. At 12 g/L Zn2⁺, ZnF2 peaks, preferentially filling pores/cracks to form localized passivation layers. Excessive Zn2⁺ (>12 g/L) promotes ZnO formation, reducing ZnF2 content and weakening passivation.
- Despite increasing surface porosity and crack density (0.35 × 105 m−1) with Zn2⁺ concentration, Zn12 exhibited optimal corrosion resistance. This arises from its isolated pore structure, which elongates Cl− diffusion paths, and enhanced inner-layer densification (Rᵢ = 3.01 × 104 Ω·cm2), offsetting surface defects.
- Zn12 maintained structural integrity after 168 h immersion, with minimal mass loss (0.6 mg), while Zn4 and Zn16 suffered severe localized corrosion due to interconnected pores. This work proposes an in situ ZnF2 synthesis strategy for Mg-Li alloy PEO coatings, resolving the paradox between porosity and corrosion resistance by emphasizing pore isolation and ZnF2 passivation. It provides a novel framework for optimizing high-reactivity Mg alloys in corrosive environments.
- We propose replacement of toxic Zn(H2PO4)2 with organic zinc salts in future experiments to reduce the ecological risks associated with fluorine. This includes combining nanoparticle doping (e.g., ZrO2) or post-sealing treatments to further reduce the porosity and improve the homogeneity of ZnF2 and degradation of the coatings under cyclic stress, high temperatures, or acidic conditions for industrial validation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Gusieva, K.; Davies, C.H.J.; Scully, J.R.; Birbilis, N. Corrosion of magnesium alloys: The role of alloying. Int. Mater. Rev. 2014, 60, 169–194. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, R.; Liu, X.; Liu, Q.; Liu, J.; Yu, J.; Liu, P.; Gao, L.; Wang, J. Anticorrosion study of phytic acid ligand binding with exceptional self-sealing functionality. J. Alloys Compd. 2020, 818, 152875. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.; Coy, A.; Arrabal, R.; Viejo, F.; Matykina, E. Corrosion behaviour of magnesium/aluminium alloys in 3.5wt.% NaCl. Corros. Sci. 2008, 50, 823–834. [Google Scholar] [CrossRef]
- Gray, J.E.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, T.; Sun, J.; Wu, R.; Zhang, M. Influence of rolling directions on microstructure, mechanical properties and anisotropy of Mg-5Li-1Al-0.5Y alloy. J. Magnes. Alloys 2015, 3, 345–351. [Google Scholar] [CrossRef]
- Xu, D.K.; Han, E.H. Effect of quasicrystalline phase on improving the corrosion resistance of a duplex structured Mg–Li alloy. Scr. Mater. 2014, 71, 21–24. [Google Scholar] [CrossRef]
- Yang, H.; Wu, L.; Jiang, B.; Liu, W.; Song, J.; Huang, G.; Zhang, D.; Pan, F. Clarifying the roles of grain boundary and grain orientation on the corrosion and discharge processes of α-Mg based Mg-Li alloys for primary Mg-air batteries. J. Mater. Sci. Technol. 2021, 62, 128–138. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, X.; Zhang, M.; Gao, L.; Wu, R. Electrochemical characterization of the corrosion of a Mg–Li Alloy. Mater. Lett. 2008, 62, 2177–2180. [Google Scholar] [CrossRef]
- Li, H.; Feng, X.; Peng, Y.; Zeng, R. Durable lubricant-infused coating on a magnesium alloy substrate with anti-biofouling and anti-corrosion properties and excellent thermally assisted healing ability. Nanoscale 2020, 12, 7700–7711. [Google Scholar] [CrossRef]
- Gu, C.D.; Yan, W.; Zhang, J.L.; Tu, J. Corrosion resistance of AZ31B magnesium alloy with a conversion coating produced from a choline chloride—Urea based deep eutectic solvent. Corros. Sci. 2016, 106, 108–116. [Google Scholar] [CrossRef]
- Liu, C.; Xu, T.; Shao, Q.; Huang, S.; Jiang, B.; Liang, J.; Li, H. Effects of beta phase on the growth behavior of plasma electrolytic oxidation coating formed on magnesium alloys. J. Alloys Compd. 2019, 784, 414–421. [Google Scholar] [CrossRef]
- Liu, W.-Y.; Liu, Y.; Blawert, C.; Zheludkevich, M.-L.; Fan, C.-L.; Talha, M.; Lin, Y.-H. Microstructure, wear and corrosion performance of plasma electrolytic oxidation coatings formed on D16T Al alloy. Rare Met. 2020, 39, 1425–1439. [Google Scholar] [CrossRef]
- An, L.-Y.; Ma, Y.; Yan, X.-X.; Wang, S.; Wang, Z.-Y. Effects of electrical parameters and their interactions on plasma electrolytic oxidation coatings on aluminum substrates. Trans. Nonferrous Met. Soc. China 2020, 30, 883–895. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M. Review of electrochemical properties of hybrid coating systems on Mg with plasma electrolytic oxidation process as pretreatment. Surf. Interfaces 2019, 14, 262–295. [Google Scholar] [CrossRef]
- Tuo, Y.; Yang, Z.; Guo, Z.; Chen, Y.; Hao, J.; Zhao, Q.; Kang, Y.; Zhang, Y.; Zhao, Y. Pore structure optimization of MoS2/Al2O3 self-lubricating ceramic coating for improving corrosion resistance. Vacuum 2022, 207, 111687. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Guo, Z.; Yang, Z.; Qian, W.; Chen, Y.; Li, H.; Zhao, Q.; Xing, Y.; Zhao, Y. Improved corrosion resistance of ZrO2/MgO coating for magnesium alloys by manipulating the pore structure. J. Mater. Res. Technol. 2023, 24, 2403–2415. [Google Scholar] [CrossRef]
- He, B.; Xin, C.; Chen, Y.; Xu, Y.; Zhao, Q.; Hou, Z.; Tang, Y.; Liu, H.; Su, X.; Zhao, Y. Biological performance and tribocorrosion behavior of in-situ synthesized CuxO/TiO2 coatings. Appl. Surf. Sci. 2022, 600, 154096. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z.; Chen, Y.; Zhao, Q.; Xu, Y.; Zhang, F.; Zhan, H.; Wang, S.; Li, H.; Hao, J.; et al. Controllable in situ fabrication of self-lubricating nanocomposite coating for light alloys. Scr. Mater. 2022, 211, 114493. [Google Scholar] [CrossRef]
- Mu, M.; Liang, J.; Zhou, X.; Xiao, Q. One-step preparation of TiO2/MoS2 composite coating on Ti6Al4V alloy by plasma electrolytic oxidation and its tribological properties. Surf. Coat. Technol. 2013, 214, 124–130. [Google Scholar] [CrossRef]
- Ren, J.; Xue, Y.; Zhang, J.; Liu, B.; Luo, S.; Xu, S.; Xing, F.; Liu, M. Pre-treatment of reclaimed concrete slurry waste for substituting cementitious materials: Effect of treatment approach and substitution content. J. Clean. Prod. 2022, 380, 134987. [Google Scholar] [CrossRef]
- Yang, Z.; Ning, B.; Chen, Y.; Zhao, Q.; Xu, Y.; Gao, G.; Tang, Y.; Zhao, Y.; Zhan, H. Large lattice mismatch of nanocomposite coating: In-situ establishment of MoS2 by precursor and desulfurization reaction. Appl. Surf. Sci. 2023, 639, 158147. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Z.; Qian, W.; Chen, Y.; Xu, Y.; Xu, X.; Zhao, Q.; Li, H.; Zhao, Y.; Zhan, H. Achieving enhanced toughness of a nanocomposite coating by lattice distortion at the variable metallic oxide interface. Mater. Des. 2022, 224, 111316. [Google Scholar] [CrossRef]
- Ayoob, S.; Gupta, A.K. Fluoride in Drinking Water: A Review on the Status and Stress Effects. Crit. Rev. Environ. Sci. Technol. 2006, 36, 433–487. [Google Scholar] [CrossRef]
- Dou, J.; Chen, Y.; Yu, H.; Chen, C. Research status of magnesium alloys by micro-arc oxidation: A review. Surf. Eng. 2017, 33, 731–738. [Google Scholar] [CrossRef]
- Qi, Y.; Peng, Z.; Wang, L.; Zhou, J.; Wang, P.; Liang, J. Fluoride-dominated coating on Mg alloys fabricated by plasma electrolytic process in ambient non-aqueous electrolyte. Surf. Eng. 2020, 37, 360–364. [Google Scholar] [CrossRef]
- Sun, R.X.; Liu, P.; Zhang, R.X.; Lv, Y.P.; Chen, K.Z. Hydrothermal synthesis of microstructured fluoridated hydroxyapatite coating on magnesium alloy. Surf. Eng. 2016, 32, 879–884. [Google Scholar] [CrossRef]
- Tang, M.; Feng, Z.; Li, G.; Zhang, Z.; Zhang, R. High-corrosion resistance of the microarc oxidation coatings on magnesium alloy obtained in potassium fluotitanate electrolytes. Surf. Coat. Technol. 2015, 264, 105–113. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, F.; Liu, X. Protection of magnesium alloys: From physical barrier coating to smart self-healing coating. J. Alloys Compd. 2021, 853, 157010. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Ai, J.; Bu, S.; Liu, H. Preparation of Coating on the Titanium Surface by Micro-Arc Oxidation to Improve Corrosion Resistance. Coatings 2021, 11, 230. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, S.; Zhao, R.; Lou, J.; Huan, X.; Zhang, Y. Influences of Na2SiO3 and EDTA-ZnNa2 concentration on properties of zinc-containing coatings on WE43 magnesium alloys. Surf. Coat. Technol. 2018, 356, 108–122. [Google Scholar] [CrossRef]
- Wen, C.; Bai, J.; Zhao, K.; Cui, Q.; Jiao, B.; Wang, X.; Zhang, L. Corrosion Initiation Behavior of Thermal Control Oxidation Film of LA103Z Alloy. Front. Mater. 2021, 8, 717663. [Google Scholar] [CrossRef]
- Zhu, C.-Q.; Wang, P.; Wang, X.; Li, Y. Facile Phosphine-Free Synthesis of CdSe/ZnS Core/Shell Nanocrystals Without Precursor Injection. Nanoscale Res. Lett. 2008, 3, 213–220. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Manik, S.; Dutta, H.; Pradhan, S. Microstructure characterization and phase transformation kinetics of polymorphic transformed ball milled a-TiO2–10 mol% m-ZrO2 mixture by Rietveld method. Mater. Chem. Phys. 2003, 82, 848–859. [Google Scholar] [CrossRef]
- Kim, J.Y.; Liu, G.; Ardhi, R.E.A.; Park, J.; Kim, H.; Lee, J.K. Stable Zn Metal Anodes with Limited Zn-Doping in MgF2 Interphase for Fast and Uniformly Ionic Flux. Nano-Micro Lett. 2022, 14, 46. [Google Scholar] [CrossRef]
- Ishii, T.; Furuichi, R.; Hara, Y.; Shimada, S. Effects of fluoride additives, LiF, NaF and CaF2, on the solid-state reactions in the MgO-Cr2O3 and ZnO-Cr2O3 systems. J. Therm. Anal. Calorim. 1979, 15, 261–270. [Google Scholar] [CrossRef]
- Zhang, R.F. Film formation in the second step of micro-arc oxidation on magnesium alloys. Corros. Sci. 2010, 52, 1285–1290. [Google Scholar] [CrossRef]
- Tavares, M.d.M.; Vitoriano, J.d.O.; da Silva, R.C.; Franco, A.R.; de Souza, G.B.; da Costa, J.A.P.; Alves-Junior, C. Effect of duty cycle and treatment time on electrolytic plasma oxidation of commercially pure Al samples. J. Mater. Res. Technol. 2019, 8, 2141–2147. [Google Scholar] [CrossRef]
- Torres-Ceron, D.A.; Restrepo-Parra, E.; Acosta-Medina, C.D.; Escobar-Rincon, D.; Ospina-Ospina, R. Study of duty cycle influence on the band gap energy of TiO2/P coatings obtained by PEO process. Surf. Coat. Technol. 2019, 375, 221–228. [Google Scholar] [CrossRef]
- Dou, J.; Wang, J.; Li, H.; Lu, Y.; Yu, H.; Chen, C. Enhanced corrosion resistance of magnesium alloy by plasma electrolytic oxidation plus hydrothermal treatment. Surf. Coat. Technol. 2021, 424, 127662. [Google Scholar] [CrossRef]
- Kokalj, A.; Lozinšek, M.; Kapun, B.; Taheri, P.; Neupane, S.; Losada-Pérez, P.; Xie, C.; Stavber, S.; Crespo, D.; Renner, F.U.; et al. Simplistic correlations between molecular electronic properties and inhibition efficiencies: Do they really exist. Corros. Sci. 2021, 179, 108856. [Google Scholar] [CrossRef]
- Sobolev, A.; Bograchev, D.; Zinigrad, M.; Borodianskiy, K. Evolution of corrosion on microstructure of ceramic coating produced by plasma electrolytic oxidation in molten salt. Ceram. Int. 2021, 48, 10990–10998. [Google Scholar] [CrossRef]

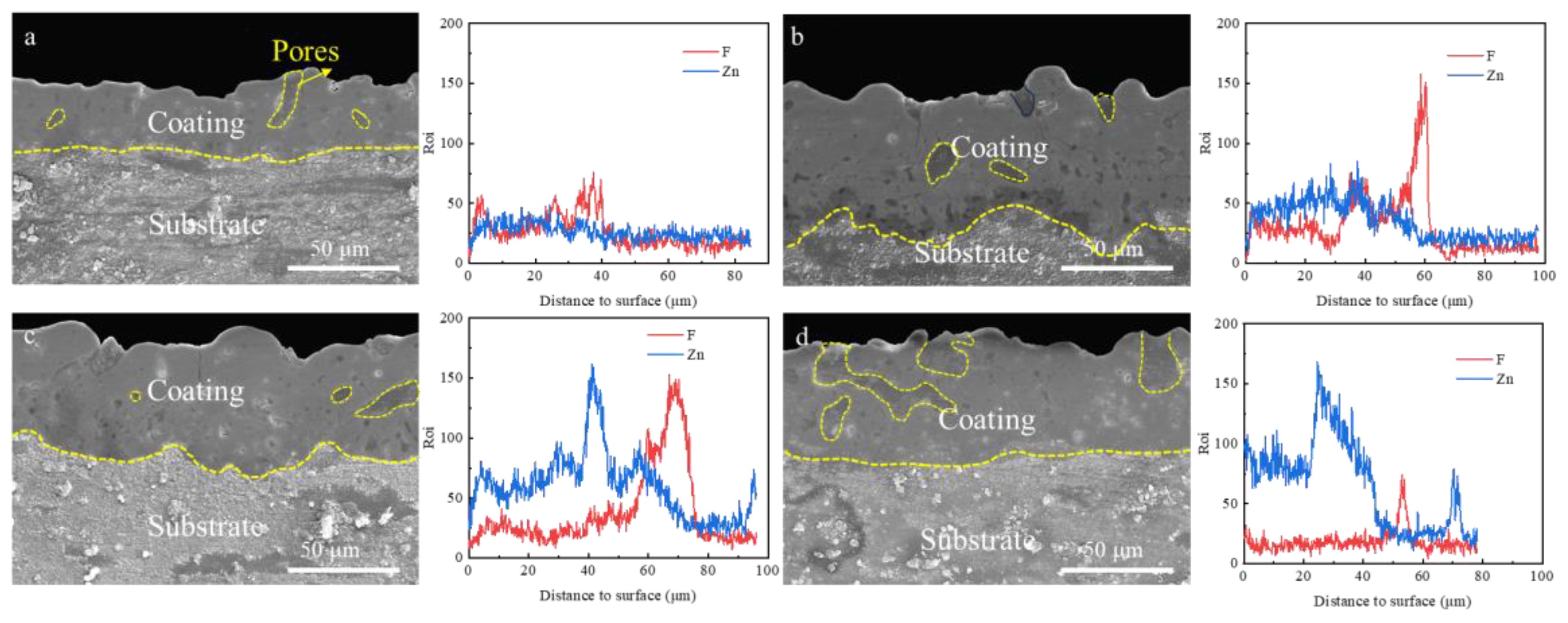
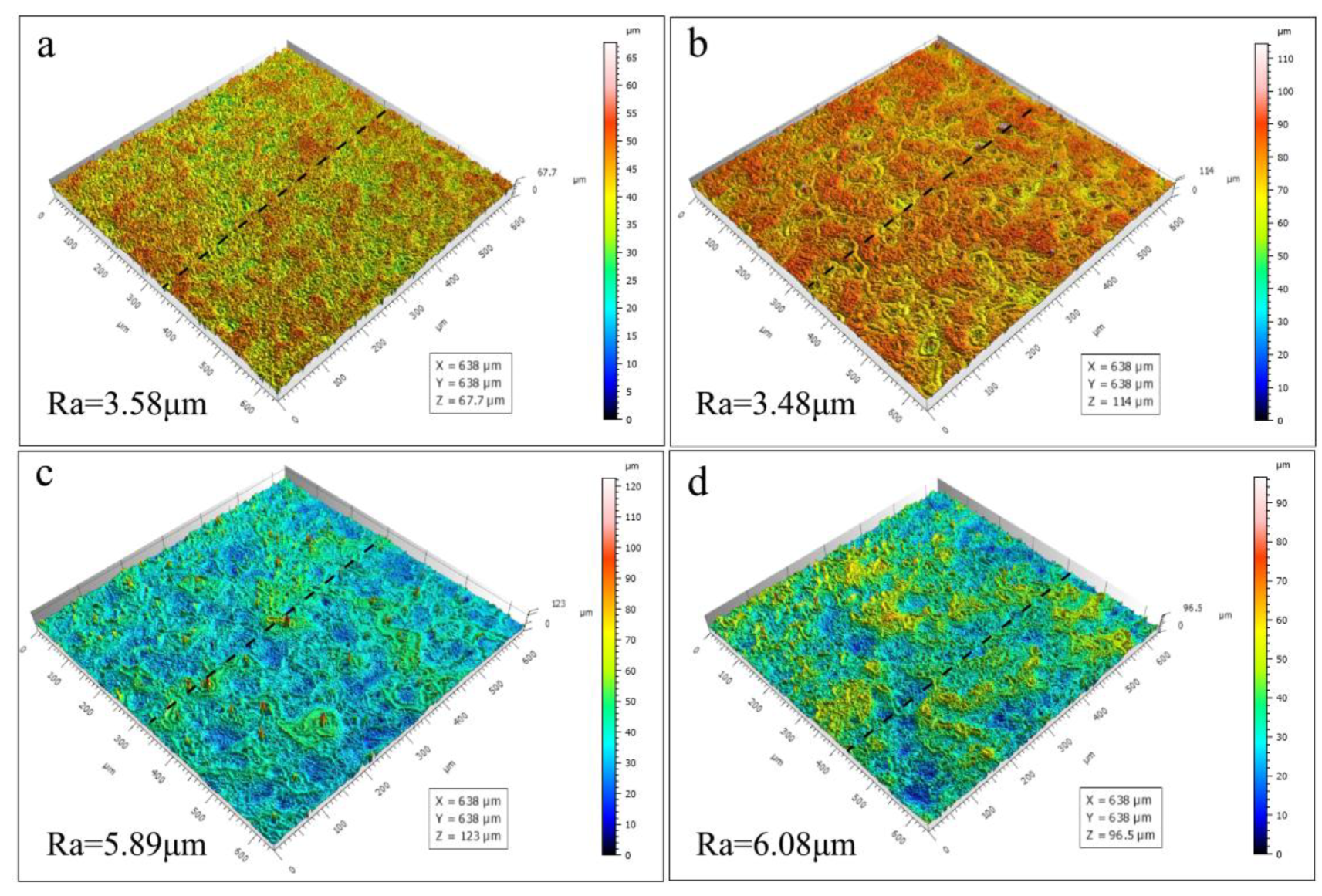

| Element | Li | Al | Zn | Si | Fe | Mg |
|---|---|---|---|---|---|---|
| Content/% | 10.0 | 3.2 | 2.8 | 0.05 | 0.05 | Bal |
| Sample | icorr (A/cm2) | Ecorr (V) | (V/dec) | (V/dec) | (Ω·cm2) | |
|---|---|---|---|---|---|---|
| Zn4 | 1.8 × 10−7 | −1.59 | 0.14 | 0.06 | 2.8 × 105 | 116.5 |
| Zn8 | 1.1 × 10−7 | −1.58 | 0.13 | 0.38 | 3.7 × 105 | 155.3 |
| Zn12 | 4.4 × 10−8 | −1.54 | 0.13 | 0.27 | 8.5 × 105 | 354 |
| Zn16 | 1.2 × 10−7 | −1.55 | 0.13 | 0.05 | 4.1 × 105 | 169.8 |
| LA103Z | 3.9 × 10−5 | −1.55 | 0.62 | 0.33 | 2.4 × 103 |
| Sample | Rs (Ω·cm2) | C1 (Ω−1 Sn·cm2) | n1 | R0 (Ω·cm2) | C2 (Ω−1 Sn·cm2) | n2 | Ri (Ω·cm2) | |
|---|---|---|---|---|---|---|---|---|
| Zn4 | 36.2 | 1.3 × 10−5 | 0.79 | 478 | 4.9 × 10−5 | 0.84 | 7036 | 1.78 × 10−3 |
| Zn8 | 40.1 | 3.8 × 10−5 | 0.86 | 1932 | 2.7 × 10−5 | 0.92 | 20,640 | 9.68 × 10−3 |
| Zn12 | 42.7 | 2.3 × 10−5 | 0.85 | 6515 | 2.4 × 10−6 | 0.91 | 30,130 | 2.71 × 10−2 |
| Zn16 | 36.5 | 7.0 × 10−6 | 0.82 | 356 | 9.3 × 10−5 | 0.84 | 6157 | 7.24 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, C.; Li, H.; Zhang, Z. Influence of Zn2⁺ Concentration on Ceramic Coatings for Corrosion Protection of Magnesium-Lithium Alloys. Materials 2025, 18, 2072. https://doi.org/10.3390/ma18092072
Wang Y, Liu C, Li H, Zhang Z. Influence of Zn2⁺ Concentration on Ceramic Coatings for Corrosion Protection of Magnesium-Lithium Alloys. Materials. 2025; 18(9):2072. https://doi.org/10.3390/ma18092072
Chicago/Turabian StyleWang, Yifei, Chunming Liu, Hongzhan Li, and Zhen Zhang. 2025. "Influence of Zn2⁺ Concentration on Ceramic Coatings for Corrosion Protection of Magnesium-Lithium Alloys" Materials 18, no. 9: 2072. https://doi.org/10.3390/ma18092072
APA StyleWang, Y., Liu, C., Li, H., & Zhang, Z. (2025). Influence of Zn2⁺ Concentration on Ceramic Coatings for Corrosion Protection of Magnesium-Lithium Alloys. Materials, 18(9), 2072. https://doi.org/10.3390/ma18092072





