Fabrication of Antibacterial and Ultraviolet Protective Wool Fabric Using Multi-Walled Carbon Nanotubes Functionalized with Guanidinylated Hyperbranched Polyethyleneimine Derivative
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
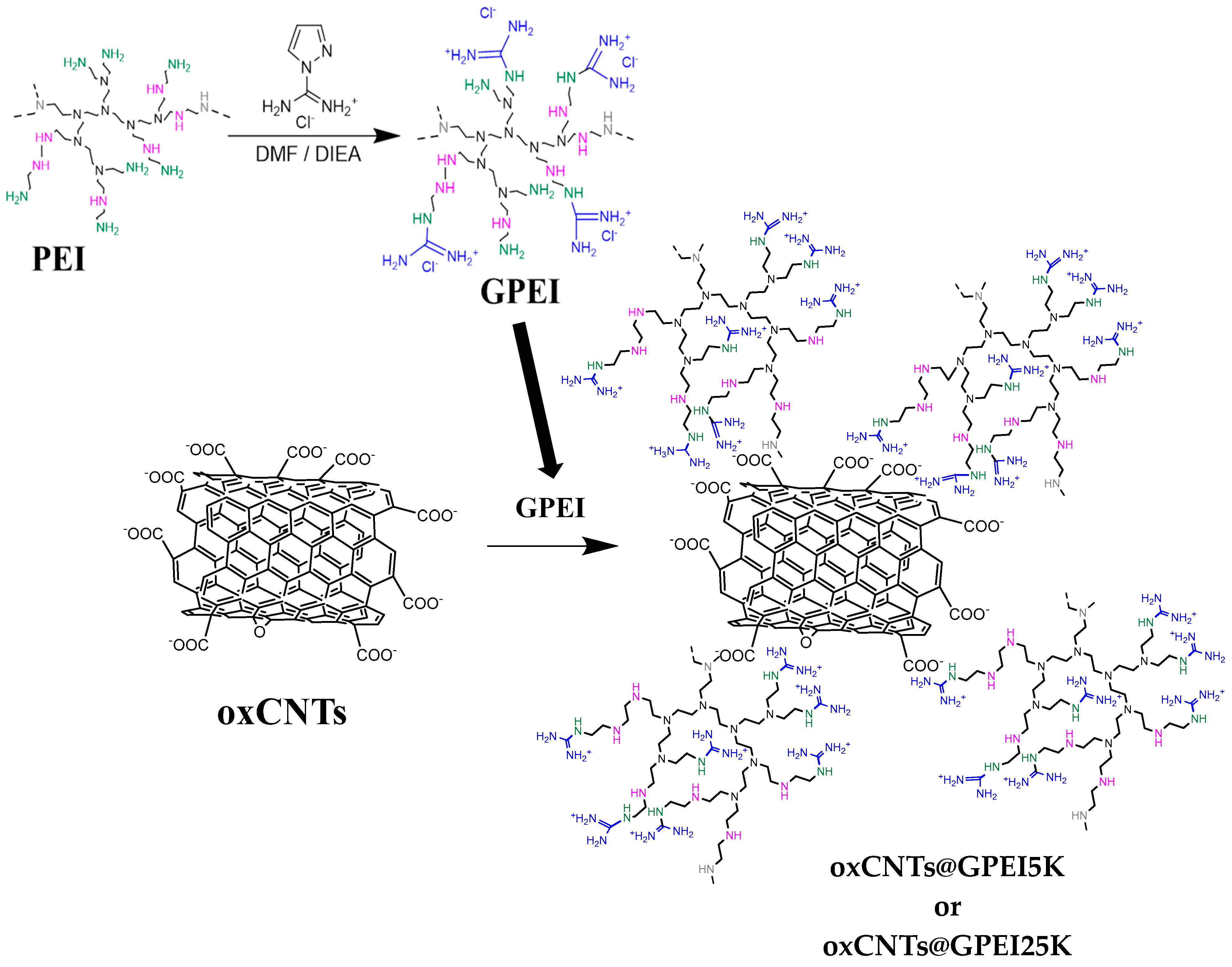
2.2. Preparation of GPEIs Functionalized oxCNTs
2.3. Antibacterial Evaluation of GPEIs Functionalized oxCNTs
2.4. In Vitro Cytotoxicity Studies
2.5. Wool Fabric Finishing Using oxCNTs@GPEI5K Nanohybrid
2.6. Determination of Color Strength and Related Parameters
2.7. Wash Fastness Analysis
2.8. Measurement of Antibacterial Properties of HTs
2.9. Measurement of UV-Blocking Properties
3. Results and Discussion
3.1. Antibacterial and Cytotoxic Evaluation of GPEIs-Functionalized oxCNTs
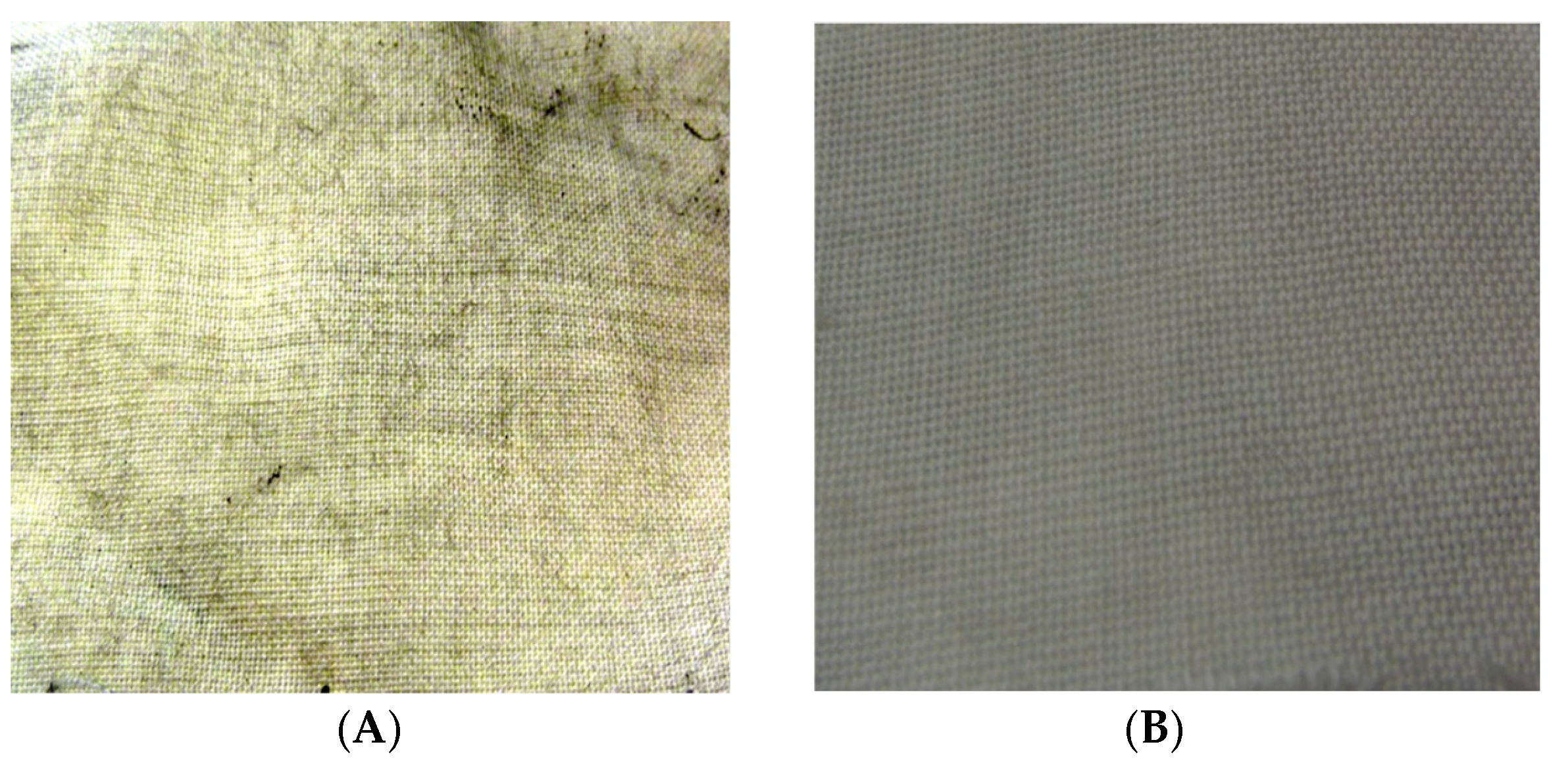
3.2. Finishing of Textiles with oxCNTs@GPEI5K
3.3. Chromatometric Analysis
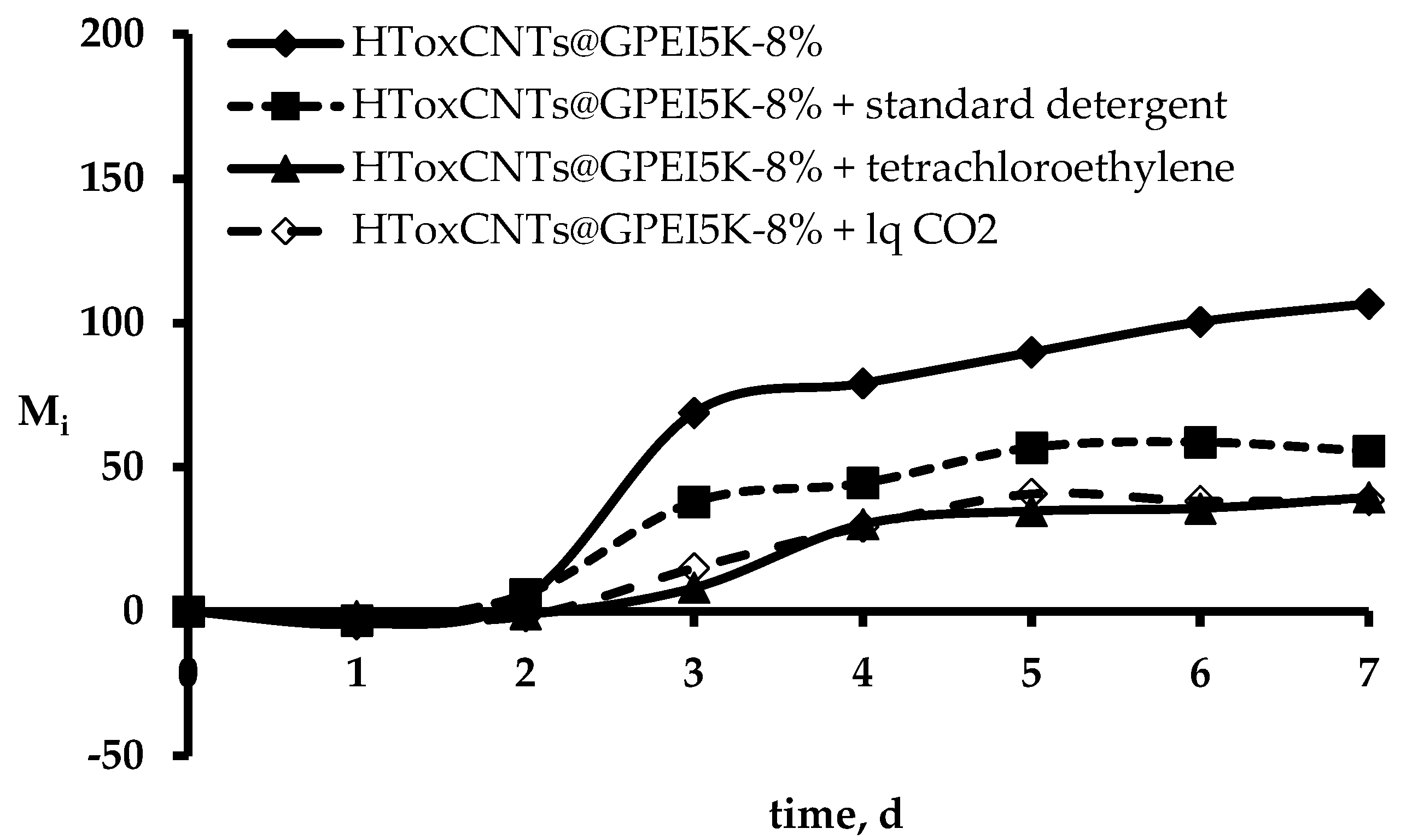
3.4. Determination of Fastness Properties
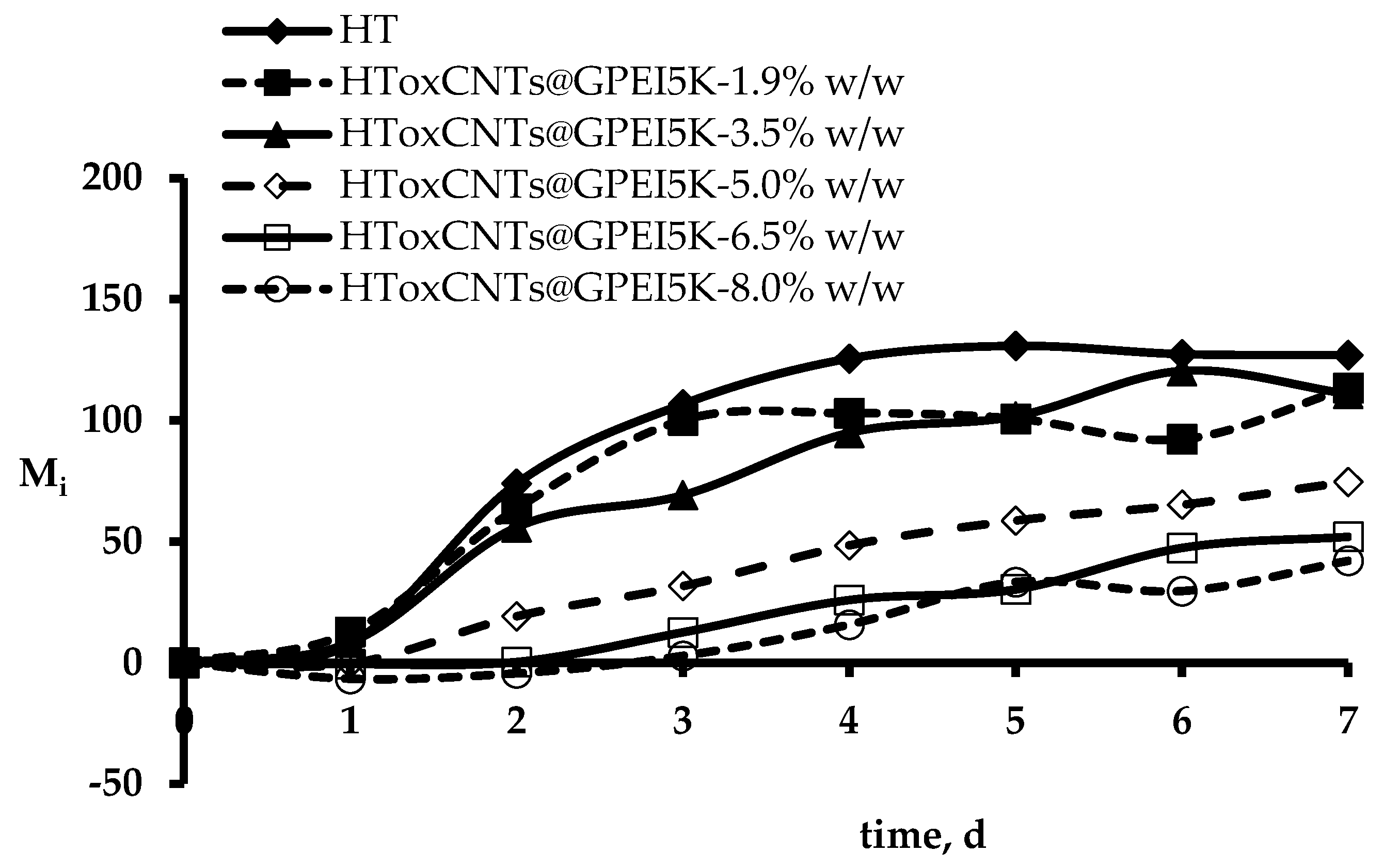
3.5. Evaluation of Antibacterial Properties
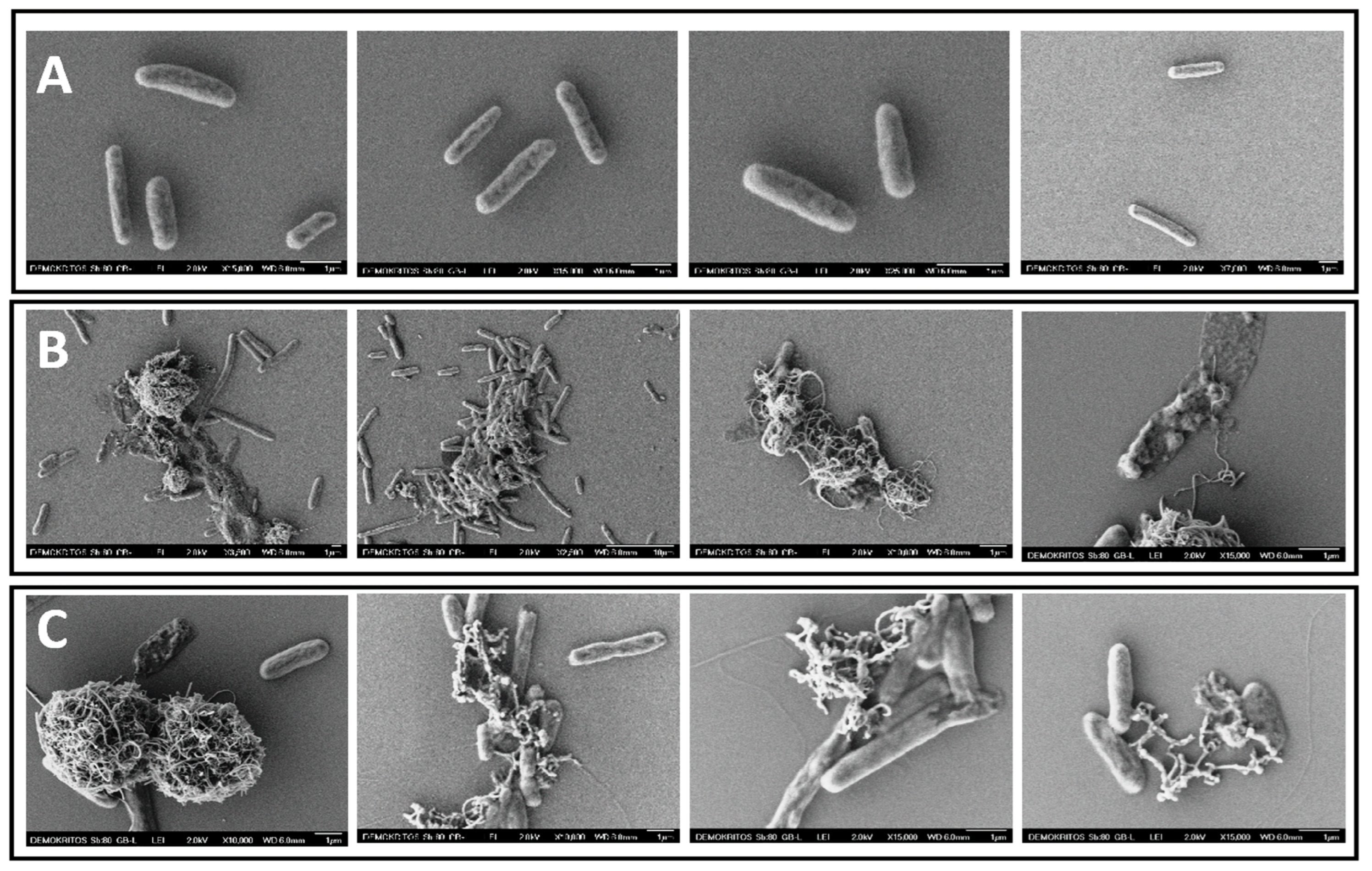
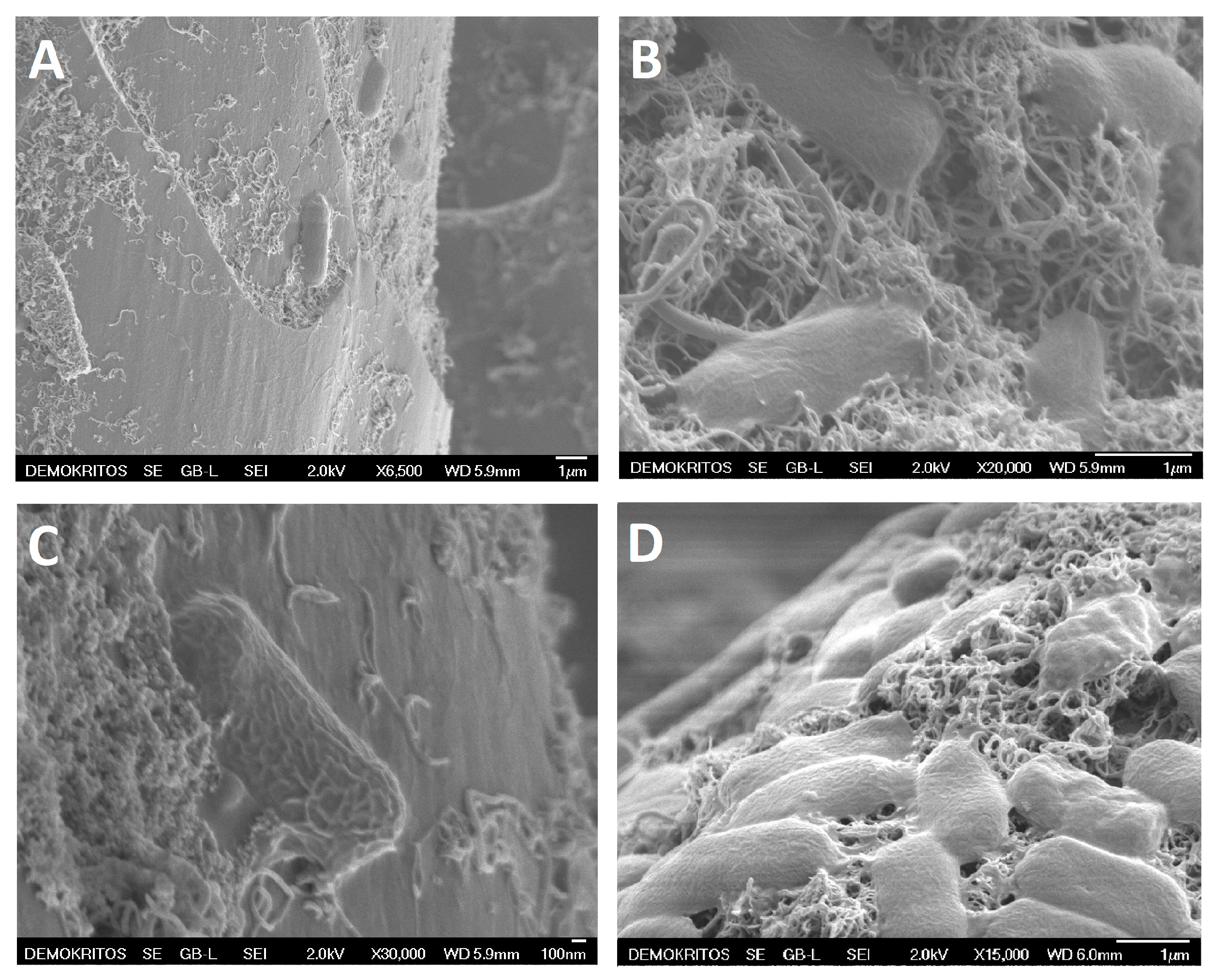
3.6. Bacteria SEM Analysis
3.7. Ultraviolet Radiation Blocking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shirgholami, M.A.; Karimi, L.; Mirjalili, M. Multifunctional modification of wool fabric using graphene/TiO2 nanocomposite. Fibers Polym. 2016, 17, 220–228. [Google Scholar] [CrossRef]
- Ghaheh, F.S.; Mortazavi, S.M.; Alihosseini, F.; Fassihi, A.; Nateri, A.S.; Abedi, D. Assessment of antibacterial activity of wool fabrics dyed with natural dyes. J. Clean. Prod. 2014, 72, 139–145. [Google Scholar] [CrossRef]
- Abou-Taleb, M.; El-Sawy, E.R.; Abdel-Aziz, M.S.; El-Sayed, H. Wool Fabric with an Improved Durable Biological Resistance Using a Coumarin Derivative. ACS Appl. Bio Mater. 2025, 8, 1664–1674. [Google Scholar] [CrossRef]
- Tamilvanan, S.; Ramadoss, A. Nanomaterial coatings on textile structures for antibacterial and antiviral applications. In Antibacterial and Antiviral Functional Materials, 1st ed.; Kalim, D., Chaudhery, M.H., Eds.; ACS Publication: Washington, DC, USA, 2024; Volume 2, Chapter 11; pp. 329–359. [Google Scholar]
- Shahidi, S.; Moazzenchi, B. Carbon nanotube and its applications in textile industry—A review. J. Text. Inst. 2018, 109, 1653–1666. [Google Scholar] [CrossRef]
- Jonathan, E.M.; Agbini, O.A. Exploring Carbon Nanotubes as Antimicrobial Agents: Efficacy, Toxicity, Challenges, and Future Prospects. J. Appl. Sci. Environ. Manag. 2024, 28, 2601–2613. [Google Scholar] [CrossRef]
- Alavi, M.; Jabari, E.; Jabbari, E. Functionalized carbon-based nanomaterials and quantum dots with antibacterial activity: A review. Expert Rev. Anti-Infect. Ther. 2021, 19, 35–44. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial Activity of Carbon-Based Nanoparticles. Adv. Pharm. Bull. 2015, 5, 19–23. [Google Scholar] [CrossRef]
- Maas, M. Carbon Nanomaterials as Antibacterial Colloids. Materials 2016, 9, 617. [Google Scholar] [CrossRef]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef]
- Sabet, M. Advanced Functionalization Strategies for Carbon Nanotube Polymer Composites: Achieving Superior Dispersion and Compatibility. Polym.-Plast. Technol. Mater. 2025, 64, 465–494. [Google Scholar] [CrossRef]
- Gao, C.; Guo, M.; Liu, Y.; Zhang, D.; Gao, F.; Sun, L.; Li, J.; Chen, X.; Terrones, M.; Wang, Y. Surface modification methods and mechanisms in carbon nanotubes dispersion. Carbon 2023, 212, 118133. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Chemical Functionalization of Carbon Nanotubes with Polymers: A Brief Overview. Macromol 2021, 1, 64–83. [Google Scholar] [CrossRef]
- Manilo, M.V.; Lebovka, N.I.; Barany, S. A short review on regulation of stability of aqueous suspensions of carbon nanotubes. Chem. Phys. Technol. Surf./Khimiya Fiz. Ta Tekhnologiya Poverhni 2020, 11, 144–159. [Google Scholar] [CrossRef]
- Heliopoulos, N.S.; Kythreoti, G.; Lyra, K.M.; Panagiotaki, K.N.; Papavasiliou, A.; Sakellis, E.; Papageorgiou, S.; Kouloumpis, A.; Gournis, D.; Katsaros, F.K.; et al. Cytotoxicity Effects of Water-Soluble Multi-Walled Carbon Nanotubes Decorated with Quaternized Hyperbranched Poly(ethyleneimine) Derivatives on Autotrophic and Heterotrophic Gram-Negative Bacteria. Pharmaceuticals 2020, 13, 293. [Google Scholar] [CrossRef]
- Kassem, A.; Ayoub, G.M.; Malaeb, L. Antibacterial activity of chitosan nano-composites and carbon nanotubes: A review. Sci. Total. Environ. 2019, 668, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Lavagna, L.; Nisticò, R.; Musso, S.; Pavese, M. Functionalization as a way to enhance dispersion of carbon nanotubes in matrices: A review. Mater. Today Chem. 2021, 20, 100477. [Google Scholar] [CrossRef]
- Soleyman, R.; Hirbod, S.; Adeli, M. Advances in the biomedical application of polymer-functionalized carbon nanotubes. Biomater. Sci. 2015, 3, 695–711. [Google Scholar] [CrossRef]
- Bai, Y.; Park, I.S.; Lee, S.J.; Bae, T.S.; Watari, F.; Uo, M.; Lee, M.H. Aqueous dispersion of surfactant-modified multiwalled carbon nanotubes and their application as an antibacterial agent. Carbon 2011, 49, 3663–3671. [Google Scholar] [CrossRef]
- Bai, Y.; Park, I.S.; Lee, S.J.; Wen, P.S.; Bae, T.S.; Lee, M.H. Effect of AOT-assisted multi-walled carbon nanotubes on antibacterial activity. Colloids Surf. B Biointerfaces 2012, 89, 101–107. [Google Scholar] [CrossRef]
- Akca, C.; Gürgen, N.M.; Ateş, M.; Demiç, Ş. Synthesis of imidazole derivatives and their immobilization to wool fabric to impart antibacterial properties. Chem. Pap. 2024, 78, 3297–3314. [Google Scholar] [CrossRef]
- Meng, X.; Nan, Q.; Zeng, Q.; Zhang, J.; Liu, S.; Tan, L.; Zheng, Z.; Wang, X.; Li, G. Antibacterial and mothproofing wool fabrics modified by M-Arg/PHMG and ZnO. Appl. Surf. Sci. 2025, 680, 161341. [Google Scholar] [CrossRef]
- Sherlin, H.S.N.; Korumilli, T.; Rao, K.J. Non-leaching nanoparticle functionalized natural fabrics: A review on durability, environmental impacts, and applications. Nanotechnol. Environ. Eng. 2025, 10, 7. [Google Scholar] [CrossRef]
- Ghosh, J.; Rupanty, N.S.; Khan, F.; Noor, T.; Jahangir, R.; Mirmohammadsadeghi, S.; Islam, T. Grafting modification for textile functionalization: Innovations and applications. Discov. Appl. Sci. 2025, 7, 1–29. [Google Scholar] [CrossRef]
- El-Hady, M.M.A.; Farouk, A.; Sharaf, S. Multiwalled-Carbon-Nanotubes (MWCNTs)–GPTMS/Tannic-Acid-Nanocomposite-Coated Cotton Fabric for Sustainable Antibacterial Properties and Electrical Conductivity. Coatings 2022, 12, 178. [Google Scholar] [CrossRef]
- Ragab, M.M.; Mosaad, M.M. Carbon nanotubes: Structure, synthesis, functionalization, characteristics, and textile applications (a review). J. Text. Color. Polym. Sci. 2024, 21, 333–353. [Google Scholar] [CrossRef]
- Alimohammadi, F.; Gashti, M.P.; Mozaffari, A. Polyvinylpyrrolidone/Carbon Nanotube/Cotton Functional Nanocomposite: Preparation and Characterization of Properties. Fibers Polym. 2018, 19, 1940–1947. [Google Scholar] [CrossRef]
- Rosace, G.; Trovato, V.; Colleoni, C.; Caldara, M.; Re, V.; Brucale, M.; Piperopoulos, E.; Mastronardo, E.; Milone, C.; De Luca, G.; et al. Structural and morphological characterizations of MWCNTs hybrid coating onto cotton fabric as potential humidity and temperature wearable sensor. Sensors Actuators B Chem. 2017, 252, 428–439. [Google Scholar] [CrossRef]
- Alamer, F.A.; Almalki, G.A. Fabrication of Conductive Fabrics Based on SWCNTs, MWCNTs and Graphene and Their Applications: A Review. Polymers 2022, 14, 5376. [Google Scholar] [CrossRef]
- Nafeie, N.; Montazer, M.; Nejad, N.H.; Harifi, T. Electrical conductivity of different carbon nanotubes on wool fabric: An investigation on the effects of different dispersing agents and pretreatments. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 497, 81–89. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Brzeziński, S.; Makowski, T.; Fortuniak, W. Conductive hydrophobic hybrid textiles modified with carbon nanotubes. Appl. Surf. Sci. 2015, 357, 1007–1014. [Google Scholar] [CrossRef]
- Hassabo, A.G.; Reda, E.M.; Ghazal, H.; Othman, H.A. Synthesis of AgNPs and ZnONPs using tea leaves extract and their utilization to improve dyeability, printability and functionality of cotton and wool fabrics. Inorg. Chem. Commun. 2023, 150, 110525. [Google Scholar] [CrossRef]
- Mowafi, S.; Kafafy, H.; Arafa, A.; Haggag, K.; Rehan, M. Facile and environmental benign in situ synthesis of silver nanoparticles for multifunctionalization of wool fibers. Environ. Sci. Pollut. Res. 2018, 25, 29054–29069. [Google Scholar] [CrossRef]
- Mura, S.; Greppi, G.; Malfatti, L.; Lasio, B.; Sanna, V.; Mura, M.E.; Marceddu, S.; Lugliè, A. Multifunctionalization of wool fabrics through nanoparticles: A chemical route towards smart textiles. J. Colloid Interface Sci. 2015, 456, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, M.; Montazer, M.; Harifi, T. Protein and silver nitrate interaction during finer wool production: Enhancing tensile properties along with synthesis of nano silver. J. Text. Inst. 2017, 108, 78–83. [Google Scholar] [CrossRef]
- Yu, D.; Tian, W.; Sun, B.; Li, Y.; Wang, W.; Tian, W. Preparation of silver-plated wool fabric with antibacterial and anti-mould properties. Mater. Lett. 2015, 151, 1–4. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Hu, B.; Guo, X.; Wang, D.; Mai, Z.; Xing, G. Stretchable Electrodes of Extremely Conductive and Stable Enabled by SWCNTs-Coated Prestretched Wool Yarn. Ind. Eng. Chem. Res. 2022, 61, 15997–16004. [Google Scholar] [CrossRef]
- Motaghi, Z.; Shahidi, S. Effect of Single Wall and Carboxylated Single Wall Carbon Nanotube on Conduction Properties of Wool Fabrics. J. Nat. Fibers 2015, 12, 388–398. [Google Scholar] [CrossRef]
- Ifijen, I.H.; Omonmhenle, S.I. Antimicrobial Properties of Carbon Nanotube: A Succinct Assessment. Biomed. Mater. Devices 2024, 2, 113–120. [Google Scholar] [CrossRef]
- Aggarwal, M.; Husain, S.; Kumar, B. Role of functionalized carbon nanotubes in antimicrobial activity: A review. In Functionalized Carbon Nanotubes for Biomedical Applications, 1st ed.; Aslam, J., Hussain, C.M., Aslam, R., Eds.; Wiley: Hoboken, NJ, USA, 2023; Chapter 15; pp. 377–411. [Google Scholar]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef]
- Mocan, T.; Matea, C.T.; Pop, T.; Mosteanu, O.; Buzoianu, A.D.; Suciu, S.; Puia, C.; Zdrehus, C.; Iancu, C.; Mocan, L. Carbon nanotubes as anti-bacterial agents. Cell. Mol. Life Sci. 2017, 74, 3467–3479. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial effects of carbon nanotubes: Size does matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Mamouni, J.; Tang, Y.; Yang, L. Antimicrobial Activity of Single-Walled Carbon Nanotubes: Length Effect. Langmuir 2010, 26, 16013–16019. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.R.; Yang, L. Inactivation of Bacterial Pathogens by Carbon Nanotubes in Suspensions. Langmuir 2009, 25, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Mauter, M.S.; Elimelech, M. Physicochemical Determinants of Multiwalled Carbon Nanotube Bacterial Cytotoxicity. Environ. Sci. Technol. 2008, 42, 7528–7534. [Google Scholar] [CrossRef]
- Baek, S.; Joo, S.H.; Su, C.; Toborek, M. Antibacterial effects of graphene- and carbon-nanotube-based nanohybrids on Escherichia coli: Implications for treating multidrug-resistant bacteria. J. Environ. Manag. 2019, 247, 214–223. [Google Scholar] [CrossRef]
- Sharmeen, S.; Rahman, A.M.; Lubna, M.M.; Salem, K.S.; Islam, R.; Khan, M.A. Polyethylene glycol functionalized carbon nanotubes/gelatin-chitosan nanocomposite: An approach for significant drug release. Bioact. Mater. 2018, 3, 236–244. [Google Scholar] [CrossRef]
- Munir, M.S.; Alvi, M.I.; Saeed, M.A.; Khan, A.; Shareef, A.; Khan, M. Functionalization and unlocking the potential of silver-decorated multiwall carbon nanotubes for optoelectronic devices and anti-bacterial applications. Mater. Chem. Phys. Sustain. Energy 2025, 2, 100007. [Google Scholar] [CrossRef]
- Lyra, K.-M.; Kaminari, A.; Panagiotaki, K.N.; Spyrou, K.; Papageorgiou, S.; Sakellis, E.; Katsaros, F.K.; Sideratou, Z. Multi-Walled Carbon Nanotubes Decorated with Guanidinylated Dendritic Molecular Transporters: An Efficient Platform for the Selective Anticancer Activity of Doxorubicin. Pharmaceutics 2021, 13, 858. [Google Scholar] [CrossRef]
- Lyra, K.-M.; Tournis, I.; Subrati, M.; Spyrou, K.; Papavasiliou, A.; Athanasekou, C.; Papageorgiou, S.; Sakellis, E.; Karakassides, M.A.; Sideratou, Z. Carbon Nanodisks Decorated with Guanidinylated Hyperbranched Polyethyleneimine Derivatives as Efficient Antibacterial Agents. Nanomaterials 2024, 14, 677. [Google Scholar] [CrossRef]
- Tziveleka, L.-A.; Psarra, A.-M.G.; Tsiourvas, D.; Paleos, C.M. Synthesis and characterization of guanidinylated poly(propylene imine) dendrimers as gene transfection agents. J. Control. Release 2007, 117, 137–146. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. In Approved Standard, 9th ed.; CLSI Document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- CLSI. Methods for Determining Bactericidal Activity of Antimicrobial Agents. In Approved Guideline; CLSI Document M26-A; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 1998. [Google Scholar]
- Subrati, M.; Lyra, K.; Spyrou, K.; Toliou, I.M.; Petrou, G.; Manganiaris, P.; Papavasiliou, A.; Sakellis, E.; Athanasekou, C.P.; Glisenti, A.; et al. Valorization of Industrial Waste Graphite Fines into Graphene Oxide-Based Nanohybrids. Chempluschem 2024, 90, e202400692. [Google Scholar] [CrossRef] [PubMed]
- Panagiotaki, K.N.; Lyra, K.; Papavasiliou, A.; Stamatakis, K.; Sideratou, Z. Synthesis of N-Sulfopropylated Hyperbranched Polyethyleneimine with Enhanced Biocompatibility and Antimicrobial Activity. Chempluschem 2025, 90, e202400454. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. Ein Beitrag zür Optik der Farbanstriche. Z. Für Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Baumann, W.; Groebel, B.T.; Krayer, M.; Oesch, H.P.; Brossman, R.; Kleinemefer, N.; Leaver, A.T. Determination of relative colour strength and residual colour difference by means of reflectance measurements. J. Soc. Dyers Colour. 1987, 103, 100–105. [Google Scholar]
- ISO 105-J02; Textiles—Tests for Colour Fastness—Part J02: Instrumental Assessment of Relative Whiteness. International Organization for Standardization: Geneva, Switzerland, 1997.
- BS 6923; Method for Calculation of Small Colour Differences. British Standards Institution: London, UK, 1988.
- ISO 105-C10; Textiles—Tests for Colour Fastness—Part C10: Colour Fastness to Washing with Soap or Soap and Soda. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 105-D01; Textiles—Tests for Colour Fastness—Part D01: Colour Fastness to Dry Cleaning Using Perchloroethylene Solvent. International Organization for Standardization: Geneva, Switzerland, 2010.
- ISO 105-A02; Textiles—Tests for Colour Fastness—Part A02: Grey Scale for Assessing Change in Colour. International Organization for Standardization: Geneva, Switzerland, 1993.
- Heliopoulos, N.S.; Galeou, A.; Papageorgiou, S.K.; Favvas, E.P.; Katsaros, F.K.; Stamatakis, K. An in situ antimicrobial susceptibility testing method based on in vivo measurements of chlorophyll α fluorescence. J. Microbiol. Methods 2015, 112, 49–54. [Google Scholar] [CrossRef]
- Heliopoulos, N.S.; Galeou, A.; Papageorgiou, S.K.; Favvas, E.P.; Katsaros, F.K.; Stamatakis, K. Modified in situ antimicrobial susceptibility testing method based on cyanobacteria chlorophyll a fluorescence. J. Microbiol. Methods 2016, 121, 1–4. [Google Scholar] [CrossRef]
- Moran, R. Formulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef]
- Tonis, E.; Frousiou, E.; Heliopoulos, N.S.; Kagkoura, A.; Stangel, C.; Siamidis, D.; Galeou, A.; Prombona, A.; Boukos, K.N.; Tagmatarchis, N.; et al. VAR Fabric Modification: Inducing Antibacterial Properties, Altering Wettability/Water Repellence, and Understanding Reactivity at the Molecular Level. ACS Omega 2023, 8, 44708–44716. [Google Scholar] [CrossRef]
- Heliopoulos, N.S.; Kouzilos, G.N.; Giarmenitis, A.I.; Papageorgiou, S.K.; Stamatakis, K.; Katsaros, F.K. Viscose Fabric Functionalized with Copper and Copper Alginate Treatment Toward Antibacterial and UV Blocking Properties. Fibers Polym. 2020, 21, 1238–1250. [Google Scholar] [CrossRef]
- AATCC Test Method 183; Transmittance or Blocking of Erythemally Weighted Ultraviolet Radiation Through Fabrics. American Association of Textile Chemists and Colorists: Durham, NC, USA, 2004.
- Li, P.; Sun, S.; Dong, A.; Hao, Y.; Shi, S.; Sun, Z.; Gao, G.; Chen, Y. Developing of a novel antibacterial agent by functionalization of graphene oxide with guanidine polymer with enhanced antibacterial activity. Appl. Surf. Sci. 2015, 355, 446–452. [Google Scholar] [CrossRef]
- Li, P.; Gao, Y.; Sun, Z.; Chang, D.; Gao, G.; Dong, A. Synthesis, Characterization, and Bactericidal Evaluation of Chitosan/Guanidine Functionalized Graphene Oxide Composites. Molecules 2016, 22, 12. [Google Scholar] [CrossRef] [PubMed]
- Zamperini, C.; Maccari, G.; Deodato, D.; Pasero, C.; D’agostino, I.; Orofino, F.; De Luca, F.; Dreassi, E.; Docquier, J.D.; Botta, M. Identification, synthesis and biological activity of alkyl-guanidine oligomers as potent antibacterial agents. Sci. Rep. 2017, 7, 8251. [Google Scholar] [CrossRef]
- Wang, J.; Lu, C.; Shi, Y.; Feng, X.; Wu, B.; Zhou, G.; Quan, G.; Pan, X.; Cai, J.; Wu, C. Structural superiority of guanidinium-rich, four-armed copolypeptides: Role of multiple peptide–membrane interactions in enhancing bacterial membrane perturbation and permeability. ACS Appl. Mater. Interfaces 2020, 12, 18363–18374. [Google Scholar] [CrossRef]
- Zardini, H.Z.; Amiri, A.; Shanbedi, M.; Maghrebi, M.; Baniadam, M. Enhanced antibacterial activity of amino acids-functionalized multi walled carbon nanotubes by a simple method. Colloids Surf. B Biointerfaces 2012, 92, 196–202. [Google Scholar] [CrossRef]
- Zardini, H.Z.; Davarpanah, M.; Shanbedi, M.; Amiri, A.; Maghrebi, M.; Ebrahimi, L. Microbial toxicity of ethanolamines—Multiwalled carbon nanotubes. J. Biomed. Mater. Res. Part A 2014, 102, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Degorce-Dumas, J.-R.; Yang, H.; Guibal, E.; Li, A.; Cheng, R. Flocculation of Escherichia coli Using a Quaternary Ammonium Salt Grafted Carboxymethyl Chitosan Flocculant. Environ. Sci. Technol. 2014, 48, 6867–6873. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Hampel, S.; Cirillo, G.; Mauro, M.V.; Vittorio, O.; Cavalcanti, P.; Giraldi, C.; Curcio, M.; Picci, N.; Iemma, F. Functional Gelatin-Carbon Nanotubes Nanohybrids With Enhanced Antibacterial Activity. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 439–447. [Google Scholar] [CrossRef]
- Zhou, J.; Qi, X. Multi-walled carbon nanotubes/epilson-polylysine nanocomposite with enhanced antibacterial activity. Lett. Appl. Microbiol. 2011, 52, 76–83. [Google Scholar] [CrossRef]
- Qian, L.; Xiao, H.; Zhao, G.; He, B. Synthesis of Modified Guanidine-Based Polymers and their Antimicrobial Activities Revealed by AFM and CLSM. ACS Appl. Mater. Interfaces 2011, 3, 1895–1901. [Google Scholar] [CrossRef]
- Heredero-Bermejo, I.; Hernández-Ros, J.M.; Sánchez-García, L.; Maly, M.; Verdú-Expósito, C.; Soliveri, J.; de la Mata, F.J.; Copa-Patiño, J.L.; Pérez-Serrano, J.; Sánchez-Nieves, J.; et al. Ammonium and guanidine carbosilane dendrimers and dendrons as microbicides. Eur. Polym. J. 2018, 101, 159–168. [Google Scholar] [CrossRef]
- Lin, L.; Chi, J.; Yan, Y.; Luo, R.; Feng, X.; Zheng, Y.; Xian, D.; Li, X.; Quan, G.; Liu, D.; et al. Membrane-disruptive peptides/peptidomimetics-based therapeutics: Promising systems to combat bacteria and cancer in the drug-resistant era. Acta Pharm. Sin. B 2021, 11, 2609–2644. [Google Scholar] [CrossRef] [PubMed]
- AS 4399; Sun Protective Clothing-Evaluation and Classification. Standards Australian: Sydney, Australia, 2020.
- Kim, Y.K. Ultraviolet protection finishes for textiles. In Functional Finishes for Textiles; Roshan, P., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2015; Chapter 15; pp. 463–485. [Google Scholar]
- Mahmoudifard, M.; Safi, M. Novel study of carbon nanotubes as UV absorbers for the modification of cotton fabric. J. Text. Inst. 2011, 103, 893–899. [Google Scholar] [CrossRef]
- Nasouri, K.; Shoushtari, A.M.; Mirzaei, J.; Merati, A.A. Synthesis of carbon nanotubes composite nanofibers for ultrahigh performance UV protection and microwave absorption applications. Diam. Relat. Mater. 2020, 107, 107896. [Google Scholar] [CrossRef]


| Samples | Gram (-) E. coli Bacteria | Gram (+) S. aureus Bacteria | ||||
|---|---|---|---|---|---|---|
| IC50 (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | IC50 (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | |
| oxCNTs | 400 | >600 | N/A | >600 | 600 | N/A |
| oxCNTs@GPEI5K | 50 | 100 | 200 | 10 | 80 | 150 |
| oxCNTs@GPEI25K | 100 | 200 | 300 | 20 | 150 | 250 |
| Sample | % wt. Dry Wool | ||||
|---|---|---|---|---|---|
| HTGPEI5K | 0.05 | 0.08 | 0.11 | 0.13 | |
| HToxCNTs | 1.9 | 3.5 | 5 | 6.5 | 8 |
| HToxCNTs@GPEI5K | 1.9 | 3.5 | 5 | 6.5 | 8 |
| oxCNTs@GPEI5K % wt. Dry Wool | K/S | RCS (%) | L* | α* | b* | ΔE* |
| 0 (raw wool) | 0.323 | 100.0 | 85.56 | −0.35 | 12.06 | - |
| 1.9 | 0.569 | 176.1 | 70.62 | −0.51 | 6.62 | 15.91 |
| 3.5 | 0.979 | 303.2 | 64.81 | −0.36 | 5.40 | 21.79 |
| 5 | 2.923 | 904.9 | 44.97 | 0.10 | 2.34 | 41.75 |
| 6.5 | 5.290 | 1637.8 | 35.92 | 0.21 | 2.09 | 50.64 |
| 8 | 5.722 | 1771.5 | 34.51 | 0.12 | 1.75 | 52.09 |
| K/S | RCS (%) | L* | α* | b* | ΔE* | Wash Fastness Rate | |
|---|---|---|---|---|---|---|---|
| HToxCNTs@GPEI5K-8% | 5.722 | 100.0 | - | - | - | - | - |
| HToxCNTs@GPEI5K-8% after washing with ISO 105-C10 (B2) | 5.010 | 87.6 | 1.98 | −0.20 | −0.11 | 1.99 | 4 |
| HToxCNTs@GPEI5K-8% after washing with ISO 105-D01 | 5.692 | 99.5 | 0.19 | −0.01 | 0.08 | 0.21 | 5 |
| HToxCNTs@GPEI5K-8% after washing with liquid CO2 | 5.711 | 99.8 | 0.12 | −0.01 | 0.06 | 0.13 | 5 |
| Content % wt. dry wool | 0 | 1.9 | 3.5 | 5 | 6.5 | 8 |
| Π7 | 7.1 | 10.7 | 12.6 | 41.2 | 59.1 | 66.9 |
| Before washing | Standard soap | Dry clean | Liquid CO2 | |
| Π7 | 66.9 | 47.8 | 62.9 | 63.7 |
| % wt. oxCNTs@GPEI | Blocking UV-A (%) | Blocking UV-B (%) | UPF | UPF Rate 1 |
|---|---|---|---|---|
| 0 (raw wool) | 70.9 | 90.8 | 7.6 | insufficient |
| 1.9 | 76.3 | 91.5 | 8.4 | insufficient |
| 3.5 | 81.9 | 94.5 | 12.0 | insufficient |
| 5 | 91.0 | 95.4 | 16.9 | good |
| 6.5 | 91.4 | 95.2 | 16.9 | good |
| 8 | 92.9 | 96.1 | 20.3 | good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heliopoulos, N.S.; Lyra, K.-M.; Papavasiliou, A.; Katsaros, F.K.; Stamatakis, K.; Papageorgiou, S.K.; Sideratou, Z. Fabrication of Antibacterial and Ultraviolet Protective Wool Fabric Using Multi-Walled Carbon Nanotubes Functionalized with Guanidinylated Hyperbranched Polyethyleneimine Derivative. Materials 2025, 18, 1993. https://doi.org/10.3390/ma18091993
Heliopoulos NS, Lyra K-M, Papavasiliou A, Katsaros FK, Stamatakis K, Papageorgiou SK, Sideratou Z. Fabrication of Antibacterial and Ultraviolet Protective Wool Fabric Using Multi-Walled Carbon Nanotubes Functionalized with Guanidinylated Hyperbranched Polyethyleneimine Derivative. Materials. 2025; 18(9):1993. https://doi.org/10.3390/ma18091993
Chicago/Turabian StyleHeliopoulos, Nikolaos S., Kyriaki-Marina Lyra, Aggeliki Papavasiliou, Fotios K. Katsaros, Kostas Stamatakis, Sergios K. Papageorgiou, and Zili Sideratou. 2025. "Fabrication of Antibacterial and Ultraviolet Protective Wool Fabric Using Multi-Walled Carbon Nanotubes Functionalized with Guanidinylated Hyperbranched Polyethyleneimine Derivative" Materials 18, no. 9: 1993. https://doi.org/10.3390/ma18091993
APA StyleHeliopoulos, N. S., Lyra, K.-M., Papavasiliou, A., Katsaros, F. K., Stamatakis, K., Papageorgiou, S. K., & Sideratou, Z. (2025). Fabrication of Antibacterial and Ultraviolet Protective Wool Fabric Using Multi-Walled Carbon Nanotubes Functionalized with Guanidinylated Hyperbranched Polyethyleneimine Derivative. Materials, 18(9), 1993. https://doi.org/10.3390/ma18091993








