Hydrogen Embrittlement Susceptibility of a Newly Developed Grain-Refined Ultra-High Strength Steel
Highlights
- The hydrogen embrittlement susceptibility of 1700 MPa grade ultra-high-strength steel with a grain size of 4 μm was investigated.
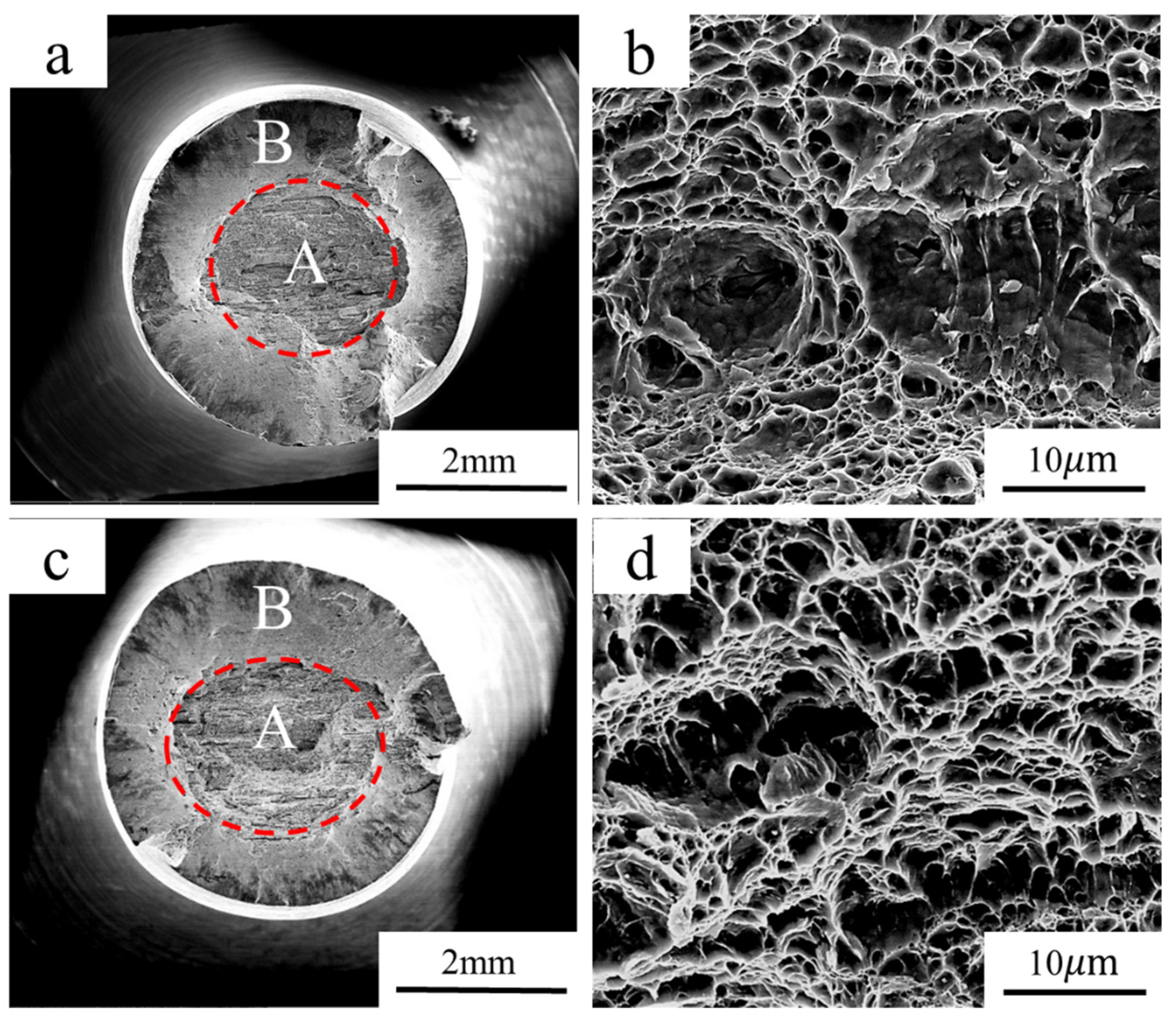
- The fracture mode of the experimental steel remained ductile fracture when the hydrogen content was 0.35 wppm.
- The refined microstructure and good cleanliness show a positive effect on the resistance to HE.
Abstract
1. Introduction
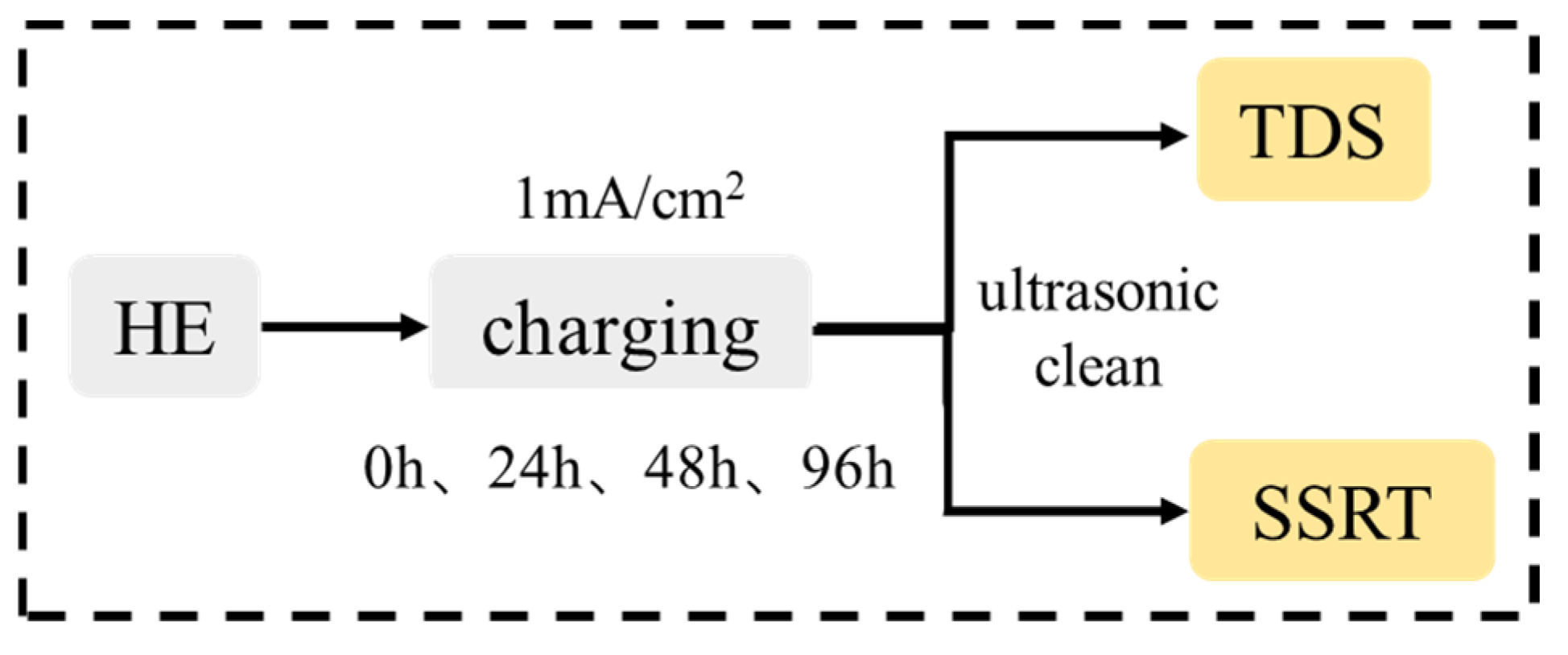
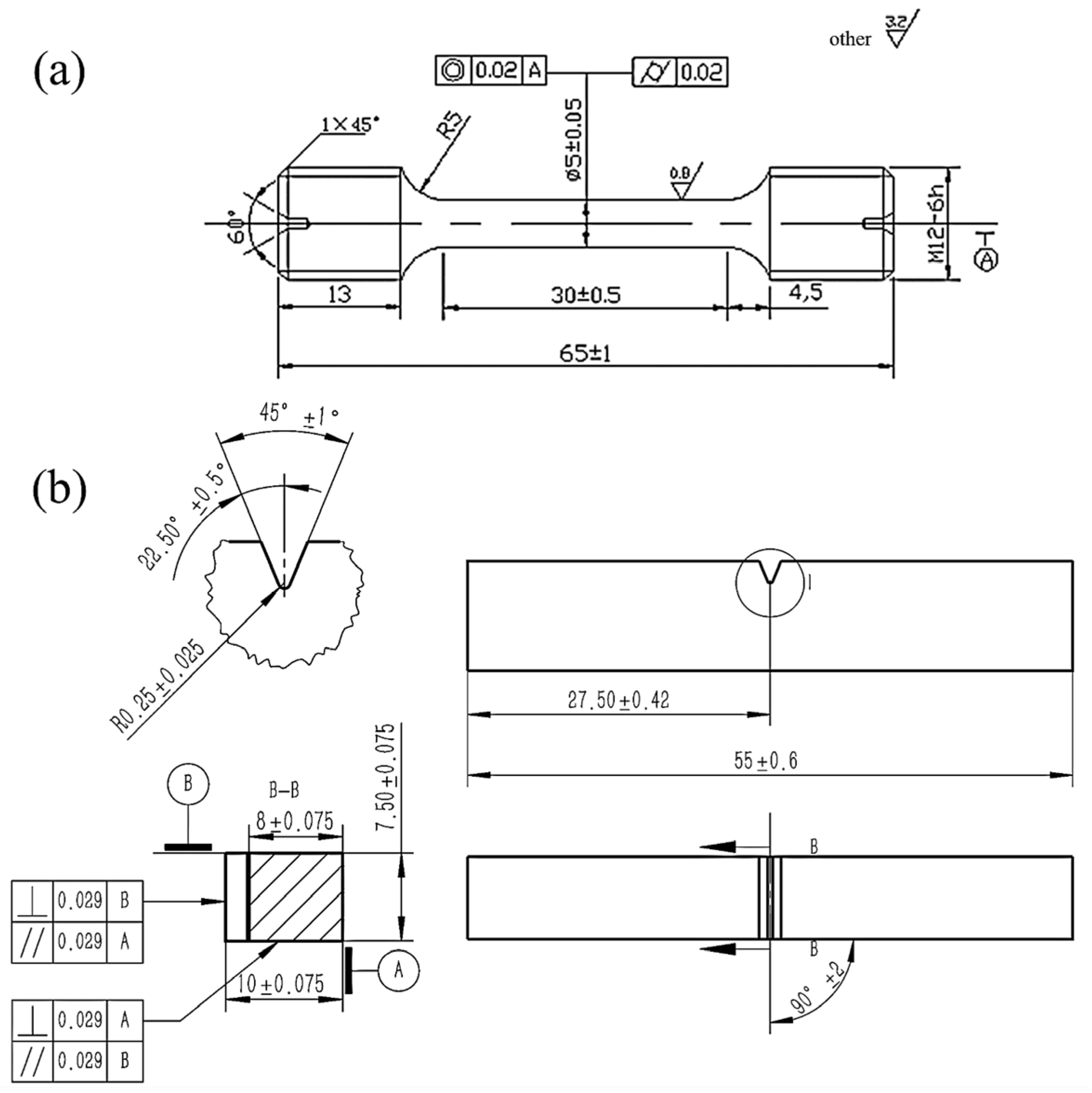
2. Experimental
3. Results
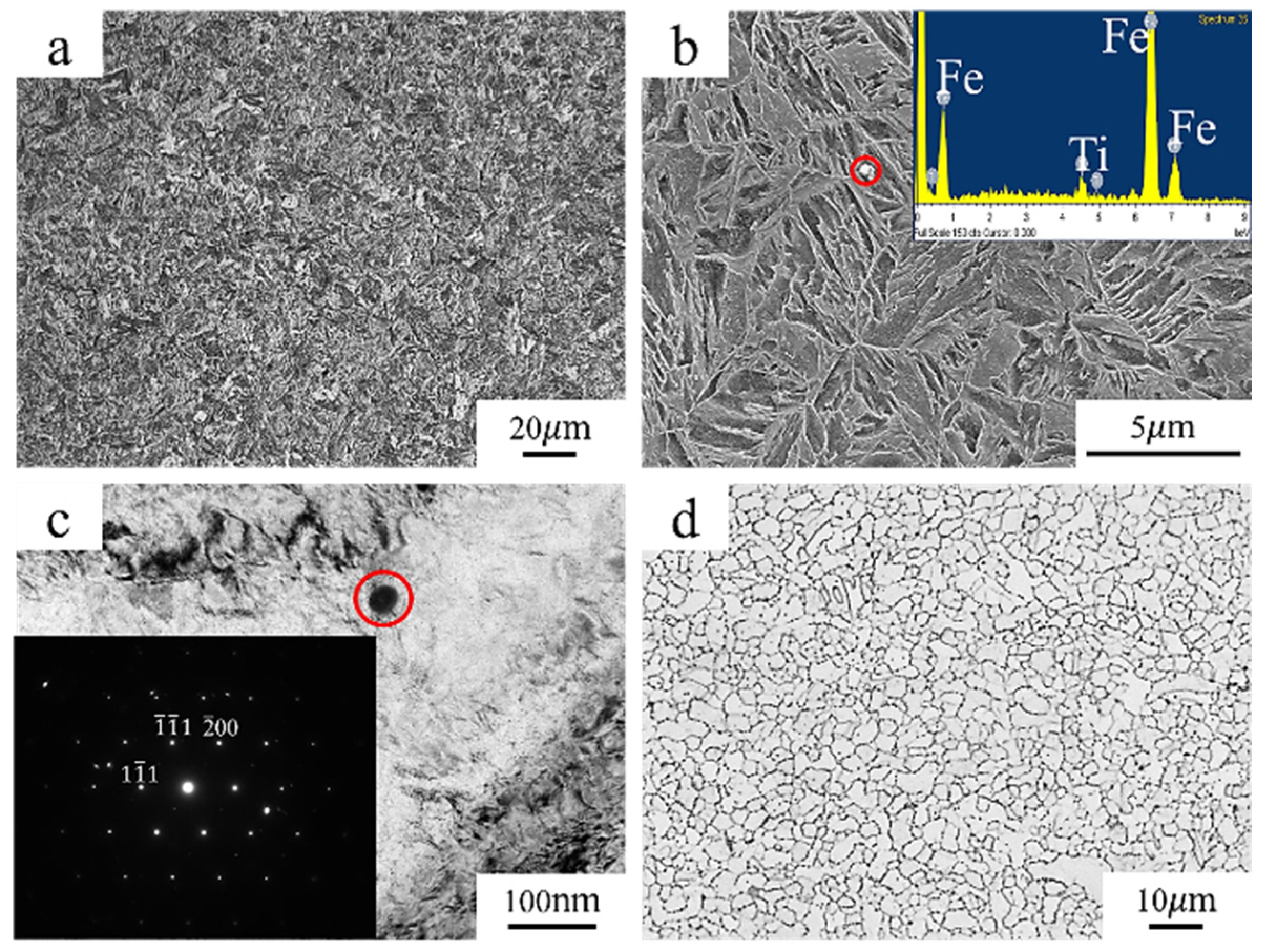
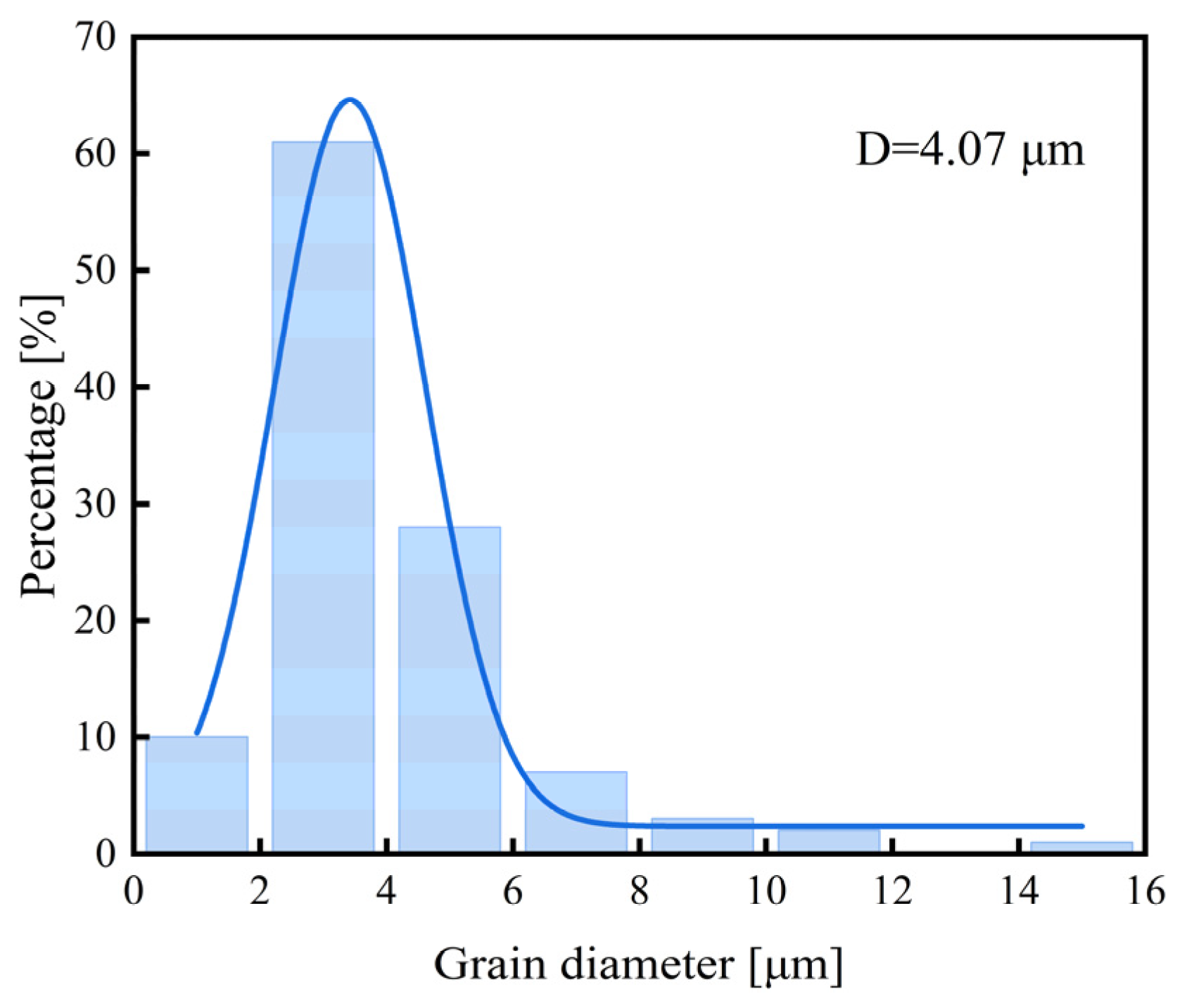
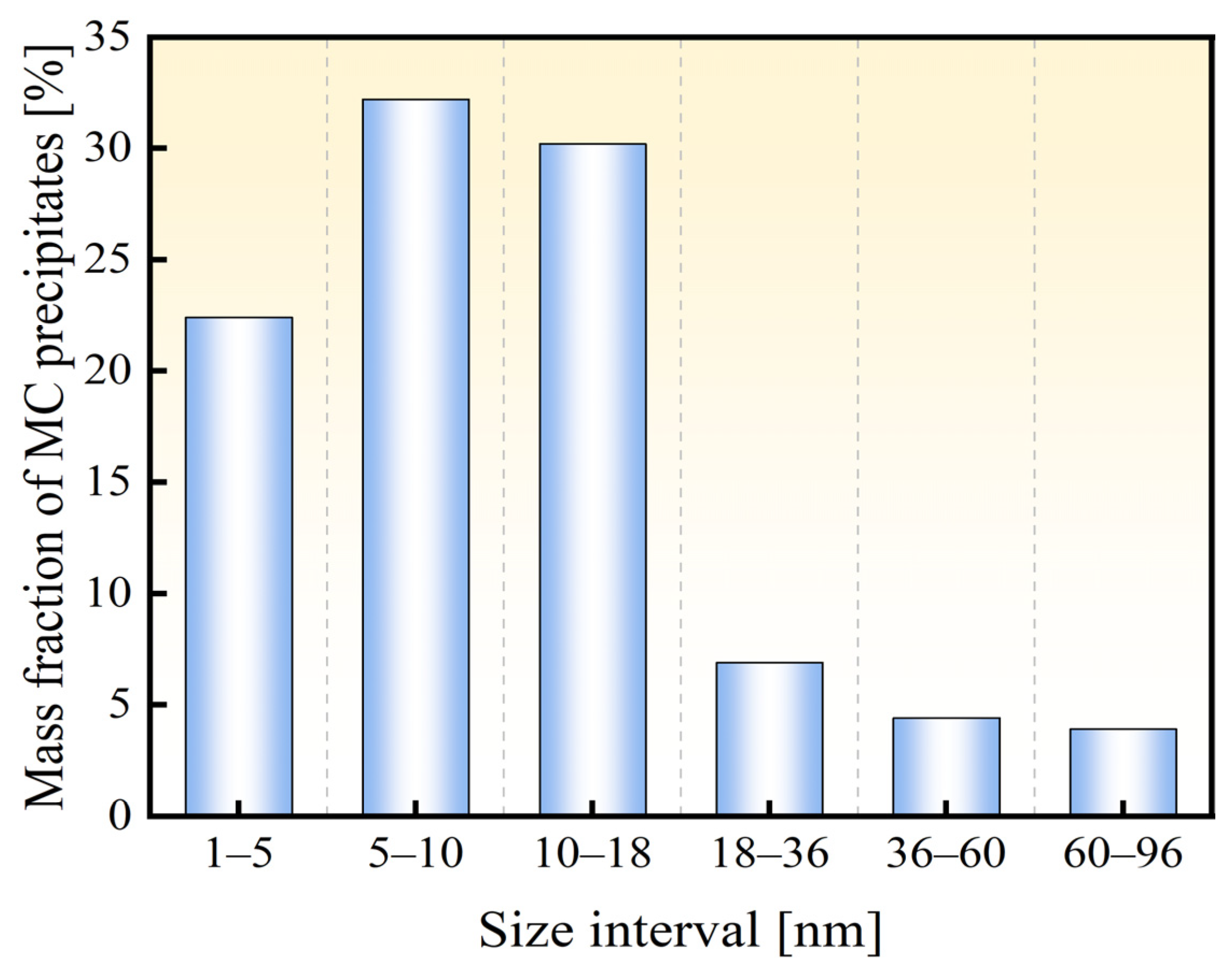
3.1. Microstructure and Mechanical Properties
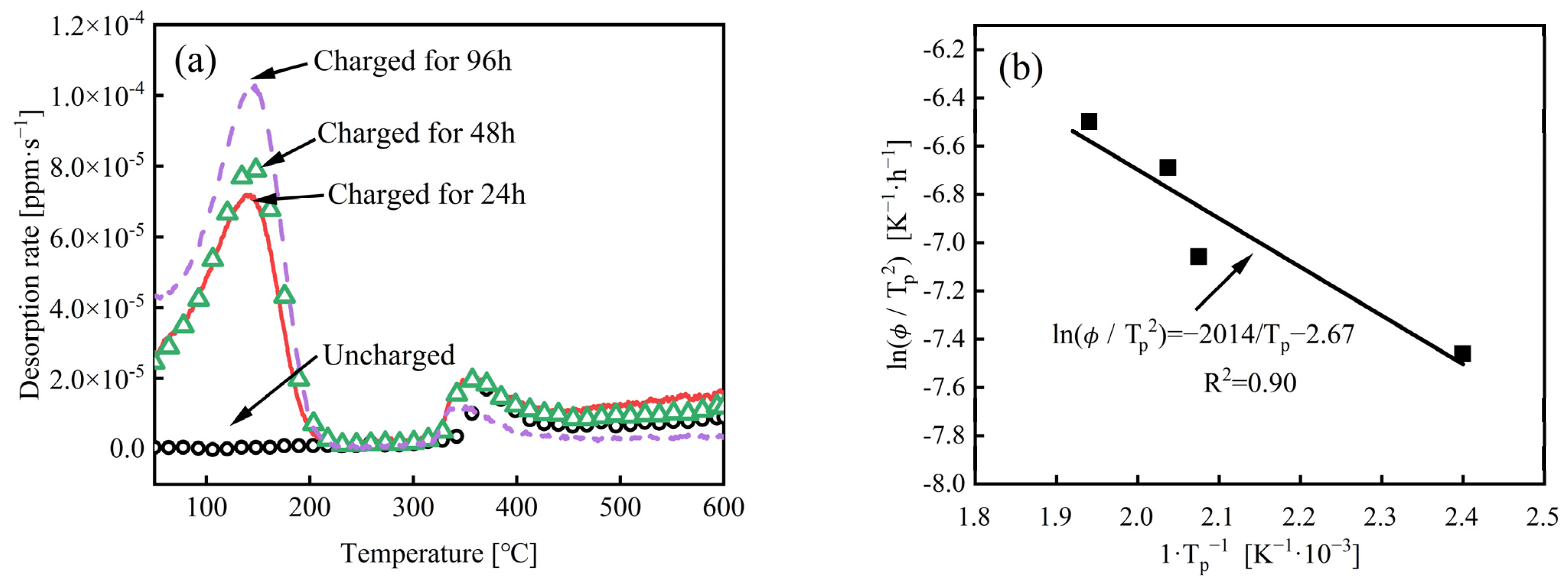
3.2. TDS Curve and Hydrogen Content
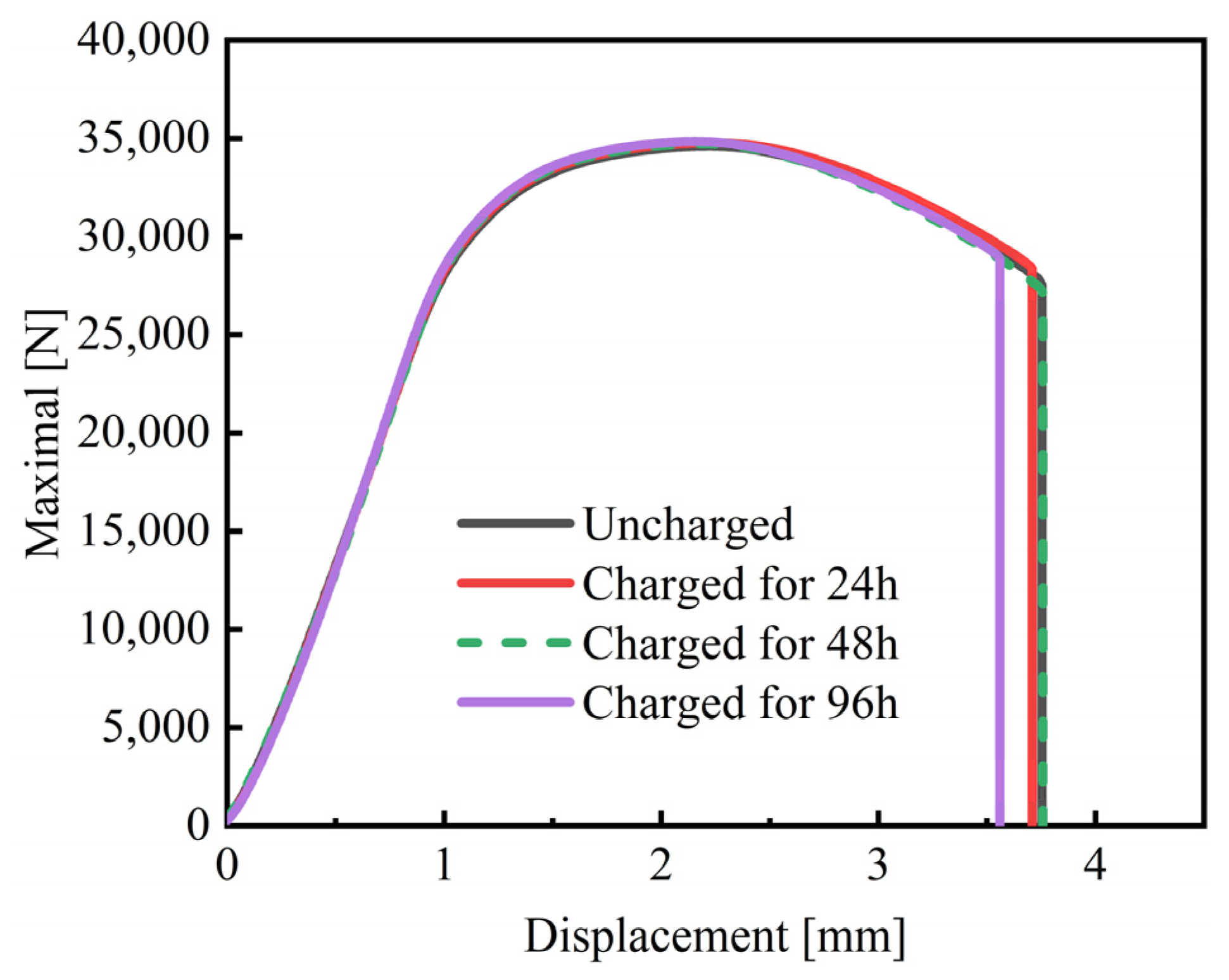
3.3. Slow Strain Rate Tensile Test
4. Discussion
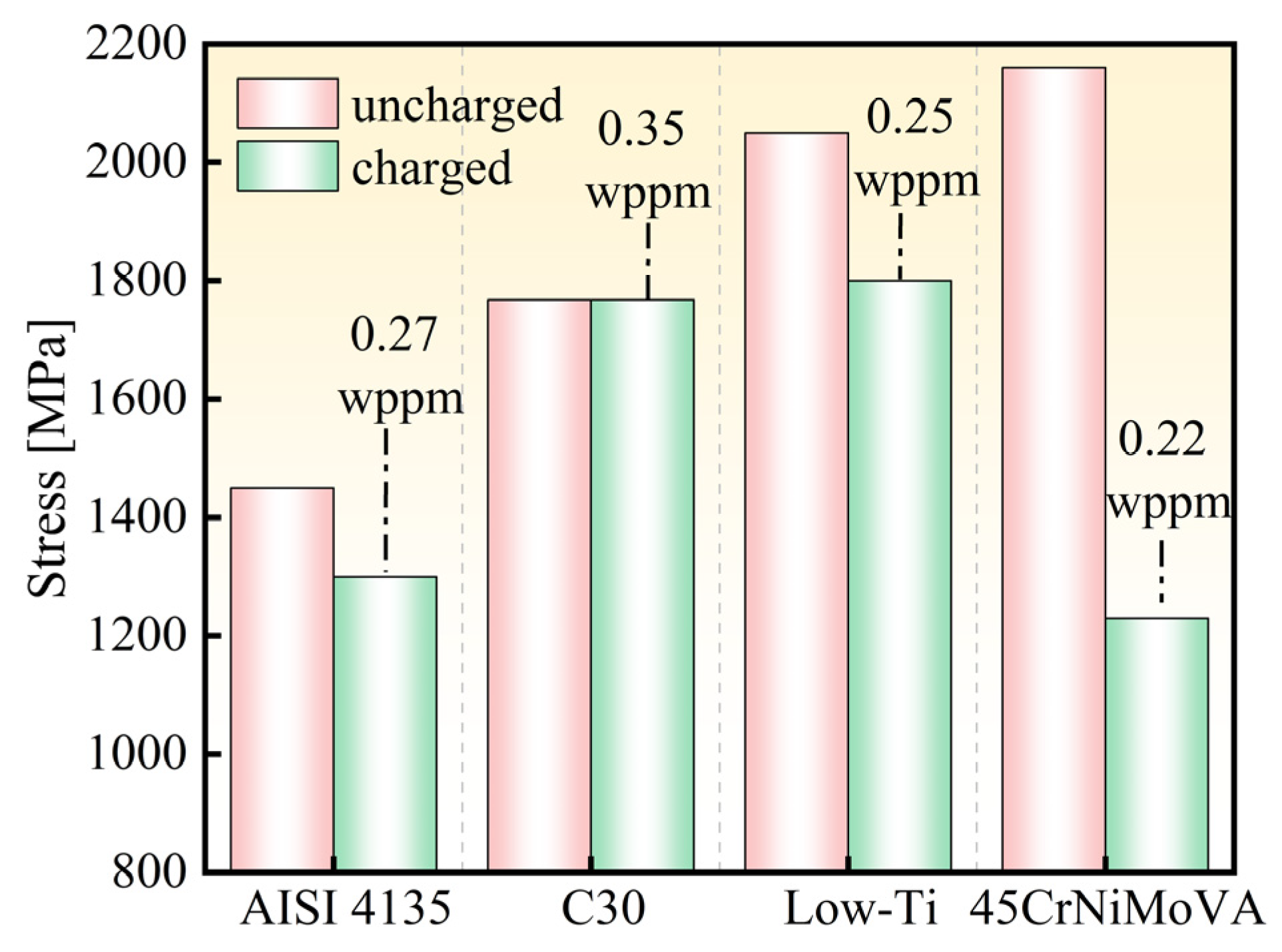
4.1. Hydrogen Embrittlement Susceptibility of Ultra-High-Strength Steels
4.2. Low Hydrogen Embrittlement Susceptibility of 30Mn2MoTi
5. Conclusions
- (1)
- The newly developed 30Mn2MoTi ultra-high-strength steel exhibits good strength and plasticity. Its microstructure primarily consists of fine lath martensite with small-size TiC particles dispersed within the matrix, resulting a refined grain size of about 4 μm.
- (2)
- With the extension of charging time, the hydrogen content in the steel increases. The value was 0.35 wppm when the charging time reached 96 h. On the contrary, the fracture mode of the experimental steel remained ductile fracture, and the SSRT strength, the elongation after fracture, and the section shrinkage showed little decrease.
- (3)
- The fine microstructure of 30Mn2MoTi steel contributes to a large number of grain boundaries, which can act as hydrogen traps, promoting the dispersion of hydrogen atoms and reducing the aggregation of hydrogen inside the grains. Furthermore, due to the low content of impurity elements such as S and P, the hydrogen charged into the steel is difficult to interact with, resulting in low hydrogen embrittlement susceptibility.
- (4)
- This manuscript studies the HE susceptibility of the newly developed 30Mn2MoTi ultra-high-strength steel. the results show a positive effect of fine structure, micro-alloy carbides, and cleanliness on resistance to HE, which could provide a new reference for research on HE susceptibility in ultra-high-strength metallic materials. A further investigation will be carried out to clarify the hydrogen diffusion coefficients and to find the critical hydrogen content of the test steel by charging more hydrogen into the material to reveal the mechanism of its better hydrogen embrittlement resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, B.; Hui, W.; Song, H.; Zhang, Y.; Zhao, X.; Xu, L. Hydrogen embrittlement of a V+ Nb- microalloyed medium-carbon bolt steel subjected to different tempering temperatures. J. Int. J. Hydrog. Energy 2024, 81, 458–470. [Google Scholar] [CrossRef]
- Wang, M.-Q.; Akiyama, E.; Tsuzaki, K. Effect of hydrogen on the fracture behavior of high strength steel during slow strain rate test. J. Corros. Sci. 2007, 49, 4081–4097. [Google Scholar] [CrossRef]
- Aiello, F.; Beghini, M.; Belardini, C.M.; Bertini, L.; Macoretta, G.; Monelli, B.D.; Valentini, R. Proposal of a hydrogen embrittlement index for a martensitic advanced high-strength steel. J. Corros. Sci. 2023, 222, 111357. [Google Scholar] [CrossRef]
- Oh, D.K.; Kim, S.G.; Shin, S.H.; Hwang, B. Hydrogen Embrittlement Behavior of API X70 Linepipe Steel under Ex Situ and In Situ Hydrogen Charging. Materials 2024, 17, 4887. [Google Scholar] [CrossRef]
- Xu, P.; Li, C.; Li, W.; Zhu, M.; Zhang, K. Effect of microstructure on hydrogen embrittlement susceptibility in quenching-partitioning-tempering steel. J. Mater. Sci. Eng. A 2022, 831, 142046. [Google Scholar] [CrossRef]
- Lynch, S. Hydrogen embrittlement phenomena and mechanisms. J. Corros. Rev. 2012, 30, 105–123. [Google Scholar] [CrossRef]
- Cui, Q.; Wu, J.; Xie, D.; Wu, X.; Huang, Y.; Li, X. Effect of Nanosized NbC Precipitates on Hydrogen Diffusion in X80 Pipeline Steel. J. Mater. 2017, 10, 721. [Google Scholar] [CrossRef]
- Zafra, A.; Belzunce, J.; Rodríguez, C.; Fernández-Pariente, I. Hydrogen embrittlement of the coarse grain heat affected zone of a quenched and tempered 42CrMo4 steel. J. Int. J. Hydrog. Energy 2020, 45, 16890–16908. [Google Scholar] [CrossRef]
- Takasawa, K.; Ikeda, R.; Ishikawa, N.; Ishigaki, R. Effects of grain size and dislocation density on the susceptibility to high-pressure hydrogen environment embrittlement of high-strength low-alloy steels. J. Int. J. Hydrog. Energy 2011, 37, 2669–2675. [Google Scholar] [CrossRef]
- Depover, T.; Verbeken, K. The effect of TiC on the hydrogen induced ductility loss and trapping behavior of Fe-C-Ti alloys. J. Corros. Sci. 2016, 112, 308–326. [Google Scholar] [CrossRef]
- Mitsuhiro, O.; Yuki, T. Hydrogen embrittlement characteristics of the hot- and cold-stamped 22MnB5 steel. J. Int. J. Fract. 2023, 240, 243–255. [Google Scholar]
- Liu, Y.; Wang, M.-Q.; Liu, G. Effect of hydrogen on ductility of high strength 3Ni–Cr–Mo–V steels. J. Mater. Sci. Eng. A 2014, 594, 40–47. [Google Scholar] [CrossRef]
- Rudomilova, D.; Prosek, T.; Luckeneder, G. Techniques for investigation of hydrogen embrittlement of advanced high strength steels. J. Corros. Rev. 2018, 36, 413–434. [Google Scholar] [CrossRef]
- Herlach, D.; Kottler, C.; Wider, T.; Maier, K. Hydrogen embrittlement of metals. J. Physica B Phys. Condens. Matter 2000, 289, 443–446. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Q.; Gu, S.; He, Z.; Wang, Q.; Zhang, F. Sulfide Stress Cracking Behavior of a Martensitic Steel Controlled by Tempering Temperature. J. Mater. 2018, 11, 412. [Google Scholar] [CrossRef]
- Wu, J.-H.; Gan, J.-S.; Liu, M.; Sun, L.-Y.; Li, Y.-L.; Xu, G. Microstructure and property of HB500 low alloy high strength wear-resistant steel. J. Iron Steel 2025, 1–11. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Yu, W.; Shi, J.; Wang, M.; Wu, R. Microstructure characteristics and strengthening mechanism of martensitic steel at two quenching rates. J. Mater. Eng. Perform. 2022, 31, 8711–8720. [Google Scholar] [CrossRef]
- ISO 6892-1: 2019; Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature. The International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 148-1: 2016; Metallic Materials—Charpy Pendulum Impact Test—Part 1: Test Method. The International Organization for Standardization: Geneva, Switzerland, 2016.
- Wang, M.-Q.; Akiyama, E.; Tsuzaki, K. Crosshead speed dependence of the notch tensile strength of a high strength steel in the presence of hydrogen. J. Scr. Mater. 2005, 53, 713–718. [Google Scholar] [CrossRef]
- Choo, W.Y.; Lee, J.Y. Thermal analysis of trapped hydrogen in pure iron. J. Metall. Mater. Trans. A. 1982, 13, 135–140. [Google Scholar] [CrossRef]
- Zhang, Y.; Hui, W.; Zhao, X.; Wang, C.; Dong, H. Effects of Hot Stamping and Tempering on Hydrogen Embrittlement of a Low-Carbon Boron-Alloyed Steel. J. Mater. 2018, 11, 2507. [Google Scholar] [CrossRef]
- Yoo, J.; Jo, M.C.; Jo, M.C.; Kim, S.; Oh, J.; Bian, J.; Sohn, S.S.; Lee, S. Effects of Ti alloying on resistance to hydrogen embrittlement in (Nb + Mo) alloyed ultra-high-strength hot-stamping steels. J. Mater. Sci. Eng. A 2020, 791, 139763. [Google Scholar] [CrossRef]
- Kang, S.; Bin, X.; Le, X. Hydrogen embrittlement susceptibility of 2000 MPa grade martensitic steels. J. Trans. Mater. Heat Treat. 2017, 38, 76–82. [Google Scholar]
- Wei, G.-F.; Tsuzaki, K. Quantitative Analysis on Hydrogen Trapping of TiC Particles in Steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2006, 37, 331–353. [Google Scholar] [CrossRef]
- Tong, Z.; Wang, H.; Zheng, W.; Zhou, H. Change in Hydrogen Trapping Characteristics and Influence on Hydrogen Embrittlement Sensitivity in a Medium-Carbon, High-Strength Steel: The Effects of Heat Treatments. J. Mater. 2024, 17, 1854. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.-F.; Hara, T.; Tsuzaki, K. Precise Determination of the Activation Energy for Desorption of Hydrogen in Two Ti-Added Steels by a Single Thermal-Desorption Spectrum. Metall. Mater. Trans. 2004, 35, 587–597. [Google Scholar] [CrossRef]
- Wang, M.-Q.; Eiji, A.; Kaneaki, T. Hydrogen degradation of a boron-bearing steel with 1050 and 1300 MPa strength levels. Scr. Mater. 2004, 52, 403–408. [Google Scholar] [CrossRef]
- Xiaokun, J.; Le, X.; Wenchao, Y.; Kefu, Y.; Jie, S.; Maoqiu, W. Effect of Hydrogen on the Very High Cycle Fatigue Properties of Quenched and Tempered Steels Containing (Ti, Mo) C Precipitates. J. Rare Met. Mater. Eng. 2021, 50, 458–468. [Google Scholar]
- Xiaokun, J.; Le, X.; Wenchao, Y.; Kefu, Y.; Jie, S.; Maoqiu, W. Hydrogen Trapping and Desorption in a Martensitic Steel with Mixed (Ti, Mo) C Precipitates. J. Rare Met. Mater. Eng. 2020, 49, 1901–1906. [Google Scholar]
- Haixia, Z.; Xiaoying, C.; Heng, L.; Heping, S. Effect of tempering temperature on microstructure and hydrogen embrittlement sensibility of a new type mooring chain steel. J. Heat Treat. Met. 2015, 40, 114–119. [Google Scholar]
- Wu, C.; Yan, C.; Zhang, S.; Zhou, L.; Shen, M.; Tian, Z. Research on Hydrogen-Induced Induced Cracking Sensitivity of X80 Pipeline Steel under Different Heat Treatments. J. Mater. 2024, 17, 1953. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Yu, W.; Wang, M.; Wu, R. Effect of cooling rate on martensitic transformation initiation temperature and hardness of super high strength martensitic steel. Heat Treat. Met. 2022, 47, 183–189. [Google Scholar]
- Cang, K.-D.; Gu, J.-L.; Fang, H.-S. Effect of heat treatment process on hydrogen embrittlement susceptibility of new bainite/martensite dual-phase high strength steel. J. World Iron Steel 2002, 2, 23–27. [Google Scholar]
- Shao, Y.-B.; Liu, X.; Song, Y.-W.; Zhang, Z.; Wang, P.; Xing, X.; Peng, W.; Xing, S.; Bian, J.; Cao, X.; et al. Experimental study on slow tensile, fatigue, and impact on X42 steel and #20 carburizing steel. Int. J. Press. Vessel. Pip. 2024, 208, 105139. [Google Scholar]
| Steel | C | Si | Mn | S | P | Ti | Mo | B | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 30Mn2MoTi | 0.30 | 0.4 | 2.0 | 0.002 | 0.009 | 0.06 | 0.35 | 0.002 | Bal. |
| Steel | Rm [MPa] | Rp0.2 [MPa] | A [%] | Z [%] | KV2 [−40 °C, J] | Yield Ratio |
|---|---|---|---|---|---|---|
| 30Mn2MoTi | 1769 ± 3 | 1520 ± 2 | 12.5 ± 0.5 | 44.5 ± 1.0 | 26 ± 1 | 0.86 |
| Charging Time [h] | Peak 1 [°C] | Peak 2 [°C] | Peak 1 [wppm] | Peak 2 [wppm] |
|---|---|---|---|---|
| 0 | — | 370.1 | — | 0.03 |
| 24 | 142.1 | 354.0 | 0.25 | 0.05 |
| 48 | 144.7 | 352.9 | 0.28 | 0.05 |
| 96 | 145.8 | 342.0 | 0.35 | 0.03 |
| Charging Time [h] | Maximal Loading [N] | Displacement at Failure [mm] | Rm [MPa] | A [%] | Z [%] |
|---|---|---|---|---|---|
| 0 | 34,600 | 3.75 | 1769 ± 3 | 12.5 ± 0.5 | 44.5 ± 0.5 |
| 24 | 34,636 | 3.70 | 1764 ± 2 | 12.0 ± 1.0 | 43 ± 1.0 |
| 48 | 34,714 | 3.75 | 1768 ± 4 | 12.5 ± 0.5 | 44 ± 0.5 |
| 96 | 34,773 | 3.55 | 1771 ± 3 | 11.5 ± 0.5 | 42 ± 1.0 |
| Steel | Rm [MPa] (Uncharged) | Hydrogen Content [wppm] | Rm [MPa] (Charged) | A [%] | Fracture Mode | Ref. |
|---|---|---|---|---|---|---|
| AISI 4135 | 1450 | 0.27 | 1310 | — | brittle fracture | [2] |
| Low-Ti | 2050 | 0.25 | 1803 | 1.9 | brittle fracture | [23] |
| 45CrNiMoVA | 2160 | 0.22 | 1230 | 0 | brittle fracture | [24] |
| 30Mn2 MoTi | 1769 ± 3 | 0.35 | 1768 | 11.5 | ductile fracture | This article |
| Steel | M3C | MC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe | Mn | Mo | C | Σ | Mo | Ti | C | Σ | |
| 30Mn2MoTi | 0.426 | 0.006 | 0.036 | 0.032 | 0.490 | 0.044 | 0.060 | 0.021 | 0.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, W.; Yu, W.; Wu, Z.; Yan, Y.; Shi, J.; Wang, M. Hydrogen Embrittlement Susceptibility of a Newly Developed Grain-Refined Ultra-High Strength Steel. Materials 2025, 18, 987. https://doi.org/10.3390/ma18050987
Lv W, Yu W, Wu Z, Yan Y, Shi J, Wang M. Hydrogen Embrittlement Susceptibility of a Newly Developed Grain-Refined Ultra-High Strength Steel. Materials. 2025; 18(5):987. https://doi.org/10.3390/ma18050987
Chicago/Turabian StyleLv, Wanqing, Wenchao Yu, Zhifang Wu, Yongming Yan, Jie Shi, and Maoqiu Wang. 2025. "Hydrogen Embrittlement Susceptibility of a Newly Developed Grain-Refined Ultra-High Strength Steel" Materials 18, no. 5: 987. https://doi.org/10.3390/ma18050987
APA StyleLv, W., Yu, W., Wu, Z., Yan, Y., Shi, J., & Wang, M. (2025). Hydrogen Embrittlement Susceptibility of a Newly Developed Grain-Refined Ultra-High Strength Steel. Materials, 18(5), 987. https://doi.org/10.3390/ma18050987






