Research on the Solidification Structure and Thermoplasticity of CJ5L Recycled Stainless Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results
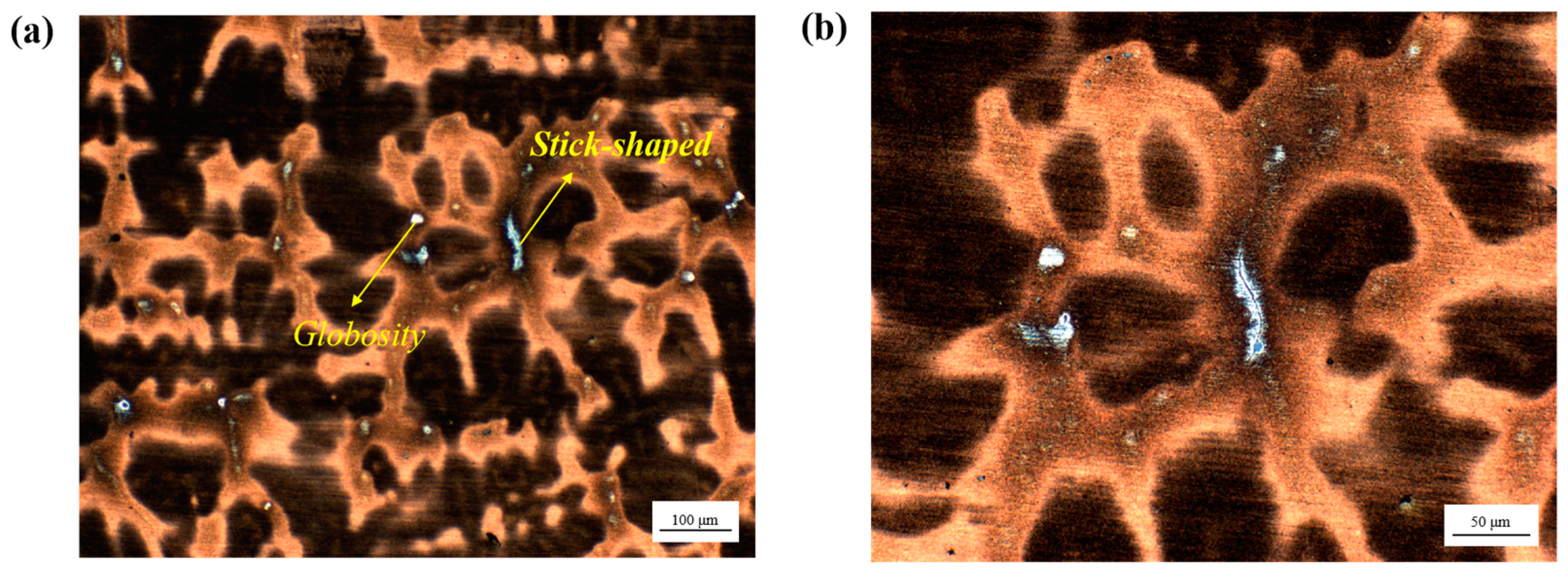
3.1. Solidification Microstructure of CJ5L NRSS Ingot
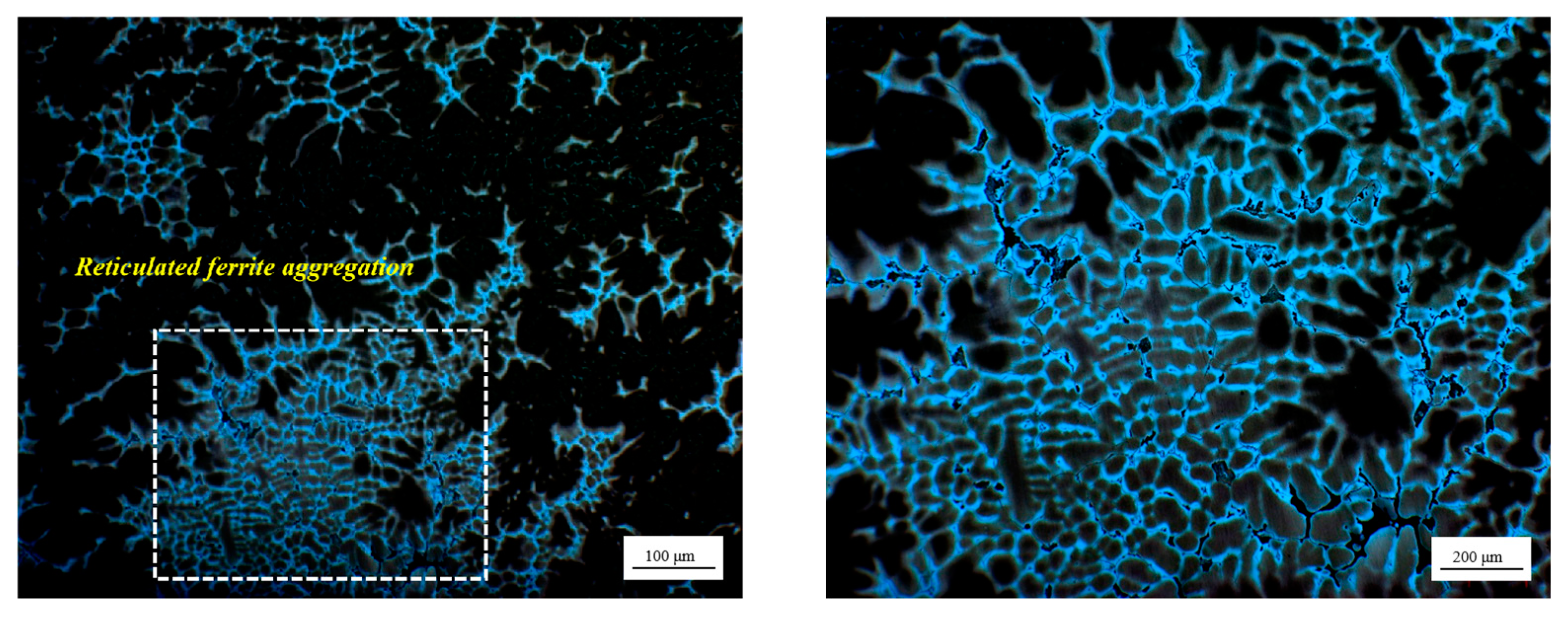
3.2. Solidification Microstructure of RSS Ingot
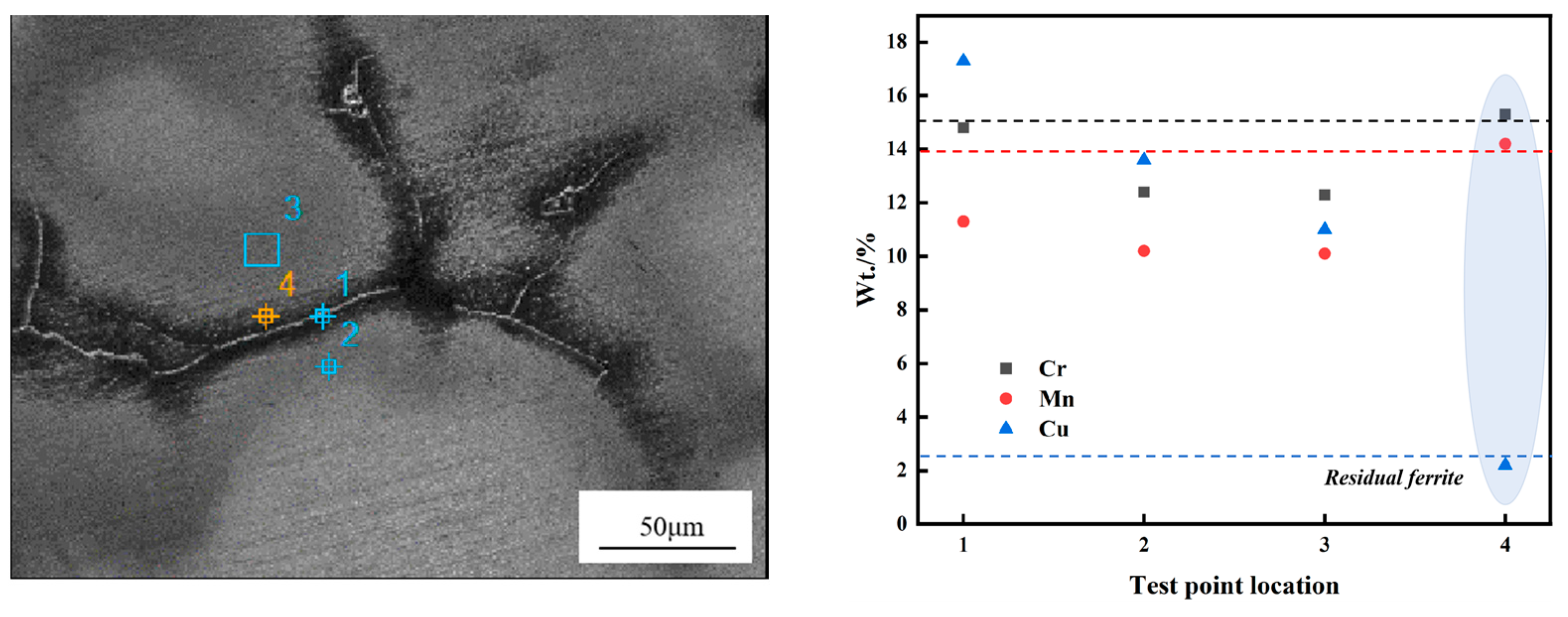
3.3. Residual Ferrite EPMA and EDS Experimental Results
3.4. High-Temperature Tensile Test Results of CJ5L Stainless Steel
4. Discussion
4.1. Prediction and Analysis of Solidification Mode of CJ5L Stainless Steel
AF mode: L → L +γ → L + γ + δ → γ + δ, 1.38 < w(Creq)/w(Nieq) < 1.50;
FA mode: L → δ + γ → L + δ + γ → δ + γ, 1.51 < w(Creq)/w(Nieq) < 2.00;
F mode: L → L + δ → δ → δ + γ, w(Creq)/w(Nieq) > 2.01.
4.2. Analysis of Formation Mechanism of Residual Ferrite in RSS
4.3. Analysis of High Temperature Thermoplasticity of RSS
4.4. Analysis of the Impact of RSS on Achieving the “Dual Carbon” Goals
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Bu, M. Present research and progress on low-nickel and nickel-free austenitic stainless steels. Heat Treat. Met. 2013, 38, 15–20. [Google Scholar]
- Liang, X. The Research on Microstructure and Solidification Mode of Low-Nickel Austenitic Stainless Steel. Master’s Thesis, Mongolia University of Technology, Ulaanbaatar, Mongolia, 2009. [Google Scholar]
- Mukunthan, K.; Hodgson, P.; Strezo, L. Solidification Behaviour and Microstructural Development of Iron- based Alloys under Conditions Pertinent to Strip Casting—200 Series Stainless Steels. ISIJ Int. 2013, 53, 2152–2159. [Google Scholar] [CrossRef]
- Chuaiphan, W.; Srijaroenpramong, L. Optimization of TIG welding parameter in dissimilar joints of low nickel stainless steel AISI 205 and AISI 216. J. Manuf. Process. 2020, 58, 163–178. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, K.; Kang, J. Policy and Management of Carbon Peaking and Carbon Neutrality: A Literature Review. Engineering 2022, 14, 52–63. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, X.; Chen, B.; Shang, Y.; Song, M. Challenges toward carbon neutrality in China: Strategies and countermeasures. Resour. Conserv. Recycl. 2022, 176, 105959. [Google Scholar] [CrossRef]
- Dong, H.; Liu, Y.; Zhao, Z.; Tan, X.; Managi, S. Carbon neutrality commitment for China: From vision to action. Sustain. Sci. 2022, 17, 1741–1755. [Google Scholar] [CrossRef]
- Wang, L.; Du, G.; Hu, Z. Crack defect control measures of nickel-saving austenitic stainless steel J5. Metall. Mater. 2023, 43, 151–153. [Google Scholar]
- Li, B.; Huang, R.; Man, Z. Analysis of surface peeling defects on rolled plates of a nickel saving austenitic stainless steel. Sichuan Metall. 2023, 45, 57–62. [Google Scholar]
- Lai, H.H.; Hsieh, H.; Kuo, C.Y.; Wu, W. Solidification cracking nature and sequence of different stainless steels. J. Mater. Res. Technol. 2023, 25, 1030–1040. [Google Scholar] [CrossRef]
- Schino, A.D.; Mecozzi, M.G.; Barteri, M.; Kenny, J.M. Solidification mode and residual ferrite in low-Ni austenitic stainless steels. J. Mater. Sci. 2000, 35, 375–380. [Google Scholar] [CrossRef]
- Ma, R. The Research on Solidification Mode and Elevated Temperature Mechanical Property of Low- Nickel Austenitic Stainless Steel. Master’s Thesis, Lanzhou University of Technology, Lanzhou, China, 2010. [Google Scholar]
- Wang, Y.; Chen, L.; Mu, W. Solidification mode and residual ferrite characteristics of high nickel 316L stainless steel continuous casting billet. Iron Steel 2024, 59, 80–91. [Google Scholar]
- Wu, C.; Li, S.; Zhang, C.; Wang, X. Microstructural evolution in 316LN austenitic stainless steel during solidification process under different cooling rates. J. Mater. Sci. 2016, 51, 2529–2539. [Google Scholar] [CrossRef]
- Cicutti, C.; Boeri, R. On the relationship between primary and secondary dendrite arm spacing in continuous casting products. Scr. Mater. 2001, 45, 1455–1460. [Google Scholar] [CrossRef]
- Tian, L.; Jiang, H. The Effect of Solidification Structures on Central Segregation of Slabs. Contin. Cast. 2010, 26–29. [Google Scholar] [CrossRef]
- Wang, Y.; Mu, W. Effect of Cooling Rate on Crystallization Behavior during Solidification of Hyper Duplex Stainless Steel S33207: An In Situ Confocal Microscopy Study. Crystals 2023, 13, 1114. [Google Scholar] [CrossRef]
- Li, Y. High Temperature Mechanical Properties of 2205 Duple Stainless Steel Billet. Master’s Thesis, Xi’an University of Architecture and Technology, Xi’an, China, 2012. [Google Scholar]
- Chen, S.; Li, J.; Cheng, G.; Li, L.; Xiang, Y.; Hu, B. Thermoplasticity and influence mechanism of continuous casting billet of Cr18Mn6Ni4N austenitic stainless steel. Contin. Cast. 2019, 44, 1–6. [Google Scholar]
- Suutala, N.; Takalo, T.; Moisio, T. Ferritic-austenitic solidification mode in austenitic stainless steel welds. Metall. Trans. A 1980, 11, 717–725. [Google Scholar] [CrossRef]
- Brooks, J.A.; Williams, J.C.; Thompson, A.W. Microstructural Origin of the Skeletal Ferrite Morphology of Austenitic Stainless Steel Welds. Metall. Trans. A 1983, 14, 1271–1281. [Google Scholar] [CrossRef]
- Inoue, H.; Koseki, T. Solidification mechanism of austenitic stainless steels solidified with primary ferrite. Acta Mater. 2017, 124, 430–436. [Google Scholar] [CrossRef]
- Hammar, O.; Svensson, U. Solidification and Casting of Metals; The Metals Society: London, UK, 1979; pp. 401–410. [Google Scholar]











| Sample | C | Ni | Cr | Mn | Cu | Si | P | S | N | Mo |
|---|---|---|---|---|---|---|---|---|---|---|
| NRSS | 0.153 | 1.12 | 13.38 | 9.17 | 0.202 | 0.449 | 0.052 | 0.003 | 0.138 | 0.001 |
| RSS | 0.153 | 1.06 | 13.29 | 9.11 | 0.224 | 0.587 | 0.044 | 0.002 | 0.127 | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Li, X.; Huang, L.; Ling, R.; Chen, C.; Tang, Z.; Yu, H. Research on the Solidification Structure and Thermoplasticity of CJ5L Recycled Stainless Steel. Materials 2025, 18, 1156. https://doi.org/10.3390/ma18051156
Dong X, Li X, Huang L, Ling R, Chen C, Tang Z, Yu H. Research on the Solidification Structure and Thermoplasticity of CJ5L Recycled Stainless Steel. Materials. 2025; 18(5):1156. https://doi.org/10.3390/ma18051156
Chicago/Turabian StyleDong, Xianbang, Xiang Li, Lei Huang, Rui Ling, Chengkang Chen, Zhenguang Tang, and Hao Yu. 2025. "Research on the Solidification Structure and Thermoplasticity of CJ5L Recycled Stainless Steel" Materials 18, no. 5: 1156. https://doi.org/10.3390/ma18051156
APA StyleDong, X., Li, X., Huang, L., Ling, R., Chen, C., Tang, Z., & Yu, H. (2025). Research on the Solidification Structure and Thermoplasticity of CJ5L Recycled Stainless Steel. Materials, 18(5), 1156. https://doi.org/10.3390/ma18051156






