Research on the Micromorphology of the Native Surface of 2-Inch Aluminum Nitride Single Crystals
Abstract
1. Introduction
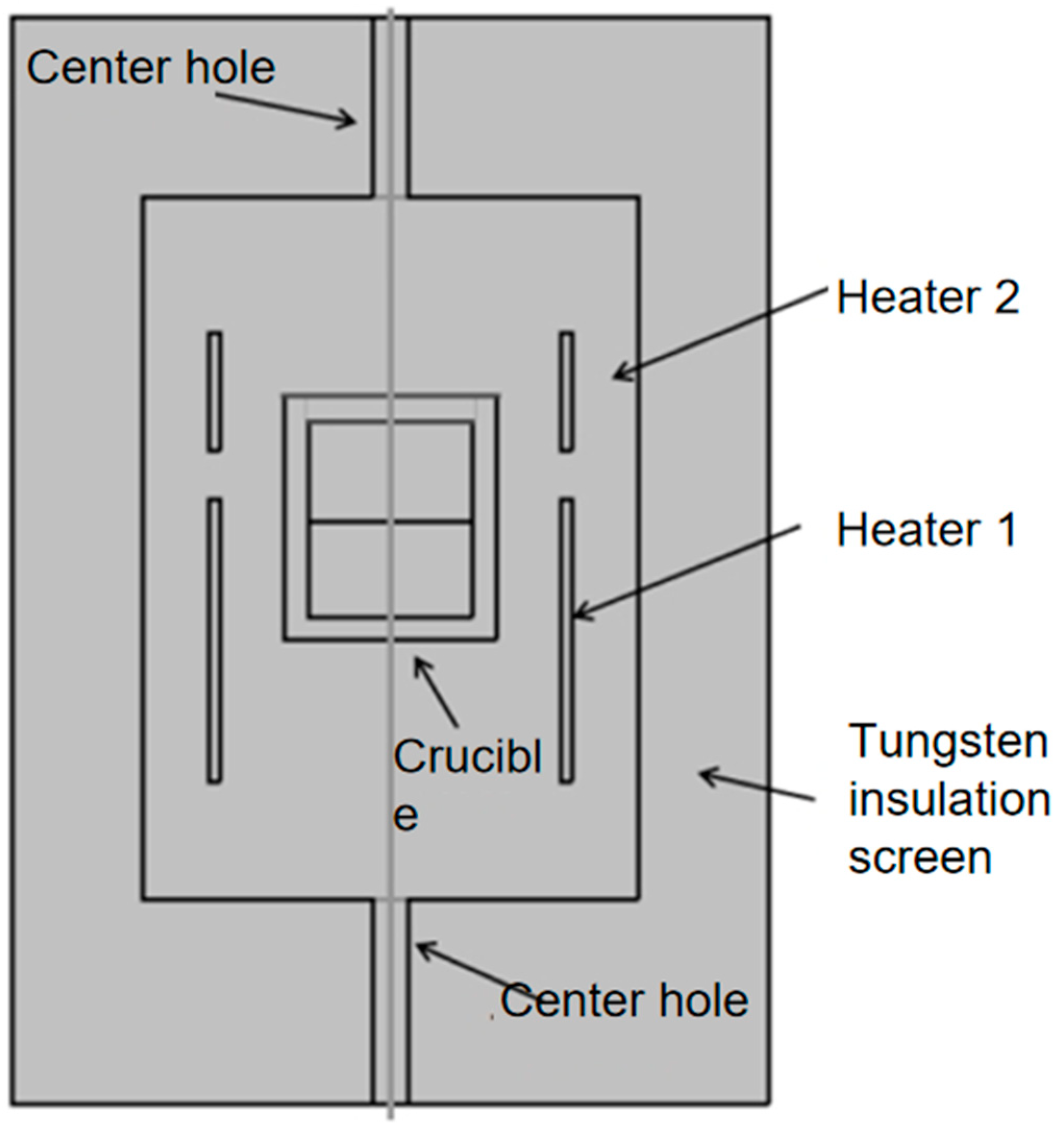
2. Description of AlN Crystal Growth Equipment
3. Discussion
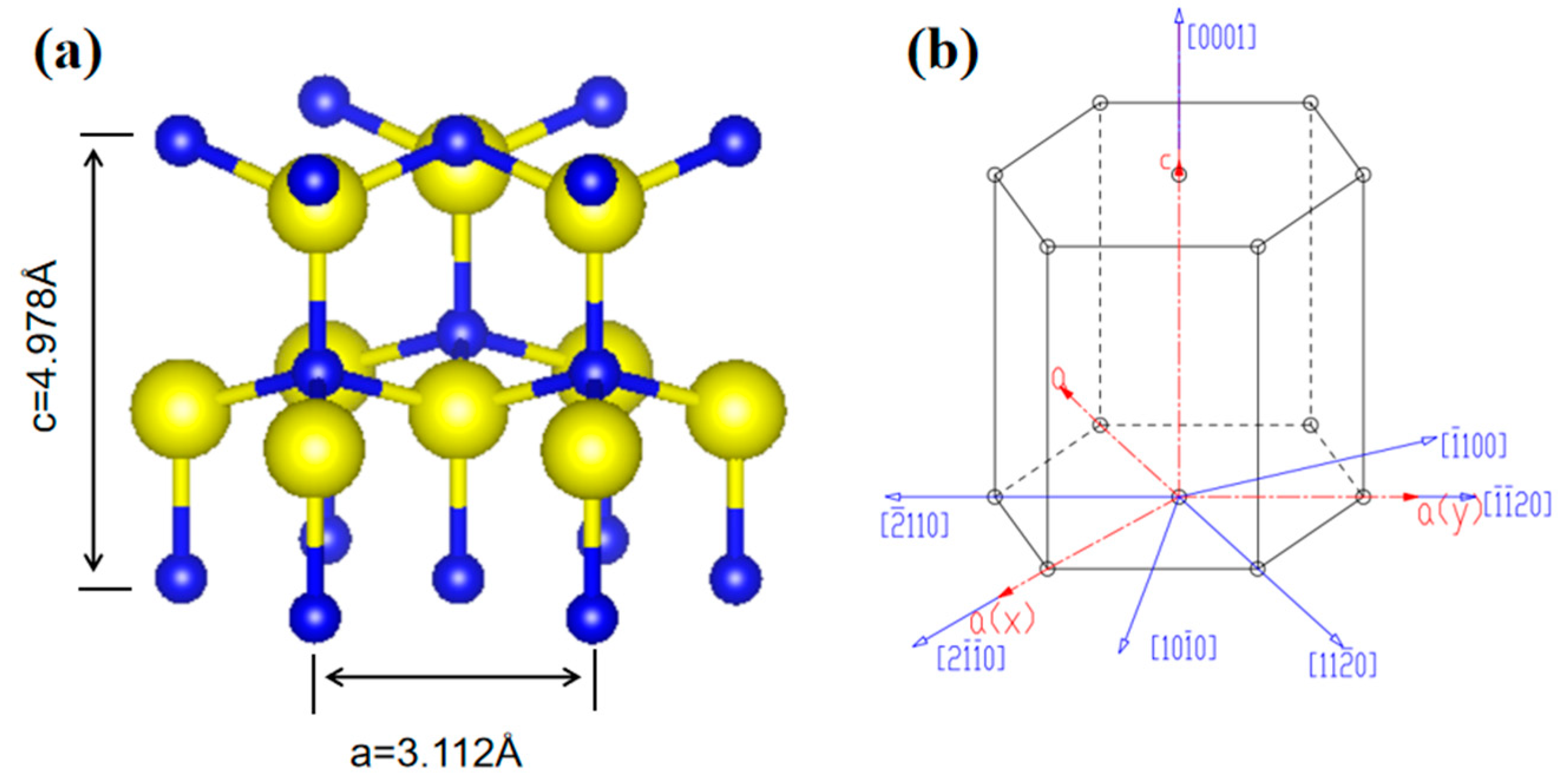
3.1. Surface Dynamics of AlN Crystal Growth Using the PVT Method
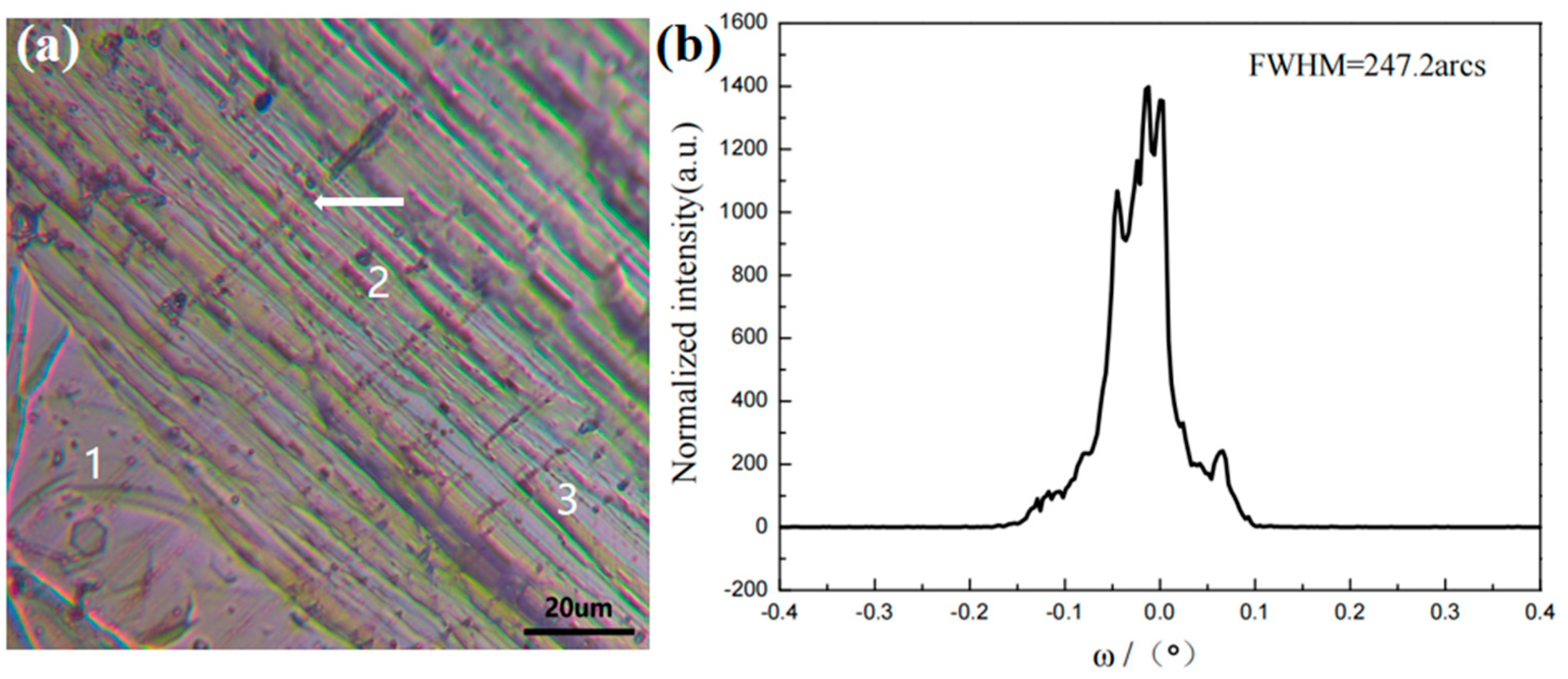
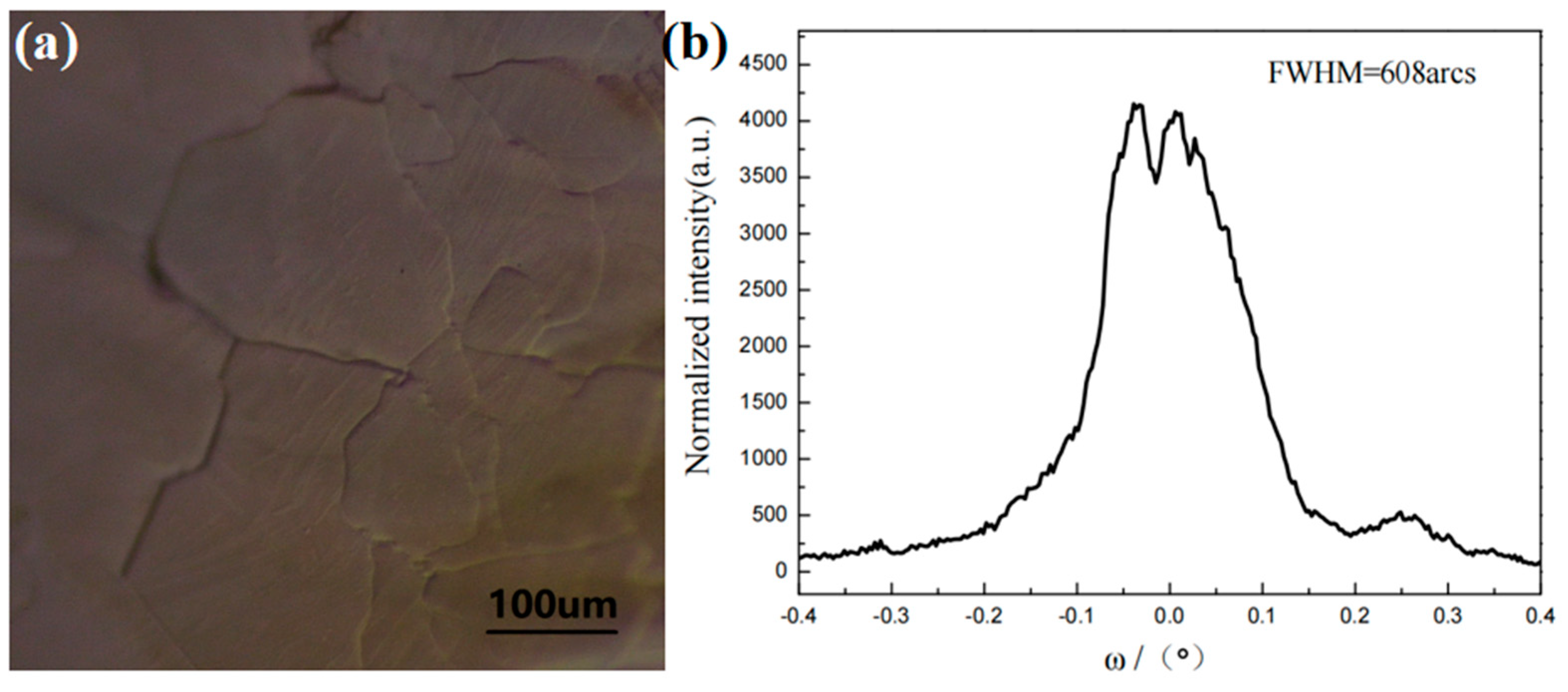
3.2. Analysis of the Surface Morphology of Naturally Grown AlN Crystals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ambacher, O. Growth and applications of group III-nitrides. J. Phys. D Appl. Phys. 1998, 31, 2653–2710. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, F.; Zheng, R.; Wu, H. Low-dimensional structure vacuum-ultraviolet-sensitive (λ < 200 nm) photodetector with fast-response speed based on high-quality AlN micro/nanowire. Adv. Mater. 2015, 27, 3921–3927. [Google Scholar] [PubMed]
- Yang, F.X.; Jin, L.; Sun, L.J.; Ren, X.C.; Duan, X.L.; Cheng, H.J.; Xu, Y.K.; Zhang, X.T.; Lai, Z.P.; Chen, W.; et al. Free-standing 2D hexagonal aluminum nitride dielectric crystals for high-performance organic field-effect transistors. Adv. Mater. 2018, 30, 1801891. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.J.; Lienhard, B.; Jeong, K.Y.; Moon, H.; Iranmanesh, A.; Grosso, G.; Englund, D. Bright high-purity quantum emitters in aluminum nitride integrated photonics. ACS Photonics 2020, 7, 2650–2657. [Google Scholar] [CrossRef]
- Chen, Z.L.; Liu, Z.Q.; Wei, T.B.; Yang, S.Y.; Dou, Z.P.; Wang, Y.Y.; Ci, H.; Chang, H.L.; Qi, Y.; Yan, J.H.; et al. Improved epitaxy of AlN film for deep-ultraviolet light-emitting diodes enabled by graphene. Adv. Mater. 2019, 31, 1807345. [Google Scholar] [CrossRef] [PubMed]
- Bikhert, Y.V.; Kozlovskiy, A.L.; Popov, A.; Zdorovets, M.V. Study of the Relationship between Changes in the Structural, Optical, and Strength Properties of AlN Ceramics Subjected to Irradiation with Heavy Xe23+ Ions. Materials 2023, 16, 6362. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kerrigan, S.; Bourham, M.; Jiang, X. AlN Single Crystal Accelerometer for Nuclear Power Plants. IEEE Trans. Ind. Electron. 2020, 68, 5346–5354. [Google Scholar] [CrossRef]
- Grandusky, J.R.; Smart, J.A.; Mendrick, M.C.; Schowalter, L.J.; Chen, K.X.; Schubert, E.F. Pseudomorphic growth of thick n-type AlxGa1−xN layers on low-defect-density bulk AlN substrates for UV LED applications. Cryst. Growth 2009, 311, 2864–2866. [Google Scholar] [CrossRef]
- Tokuda, Y.; Kojima, J.; Hara, K. 4H-SiC Bulk Growth Using High-Temperature Gas Source Method. Mater. Sci. Forum. 2014, 778–780, 51–54. [Google Scholar] [CrossRef]
- Nagamatsu, K.; Okada, N.; Kato, N.; Sumii, T.; Bandoh, A.; Iwaya, M.; Kamiyama, A.; Amano, H.; Akasaki1et, I. Effect of c-plane sapphire misorientation on the growth of AlN by high-temperature MOVPE. Phys. Status Solidis C 2008, 5, 3048–3050. [Google Scholar] [CrossRef]
- Bryan, I.; Bryan, Z.; Bobea, M.; Hussey, L.; Kirste, R.; Collazo, R.; Sitar, Z. Homoepitaxial AlN thin films deposited on m-plane (1-100) AlN substrates by metalorganic chemical vapor deposition. J. Appl. Phys. 2014, 116, 133517. [Google Scholar] [CrossRef]
- Bryan, I.; Bryan, Z.; Mita, S.; Rice, A.; Tweedie, J.; Collazo, R.; Sitaret, Z. Surface kinetics in AlN growth: A universal model for the control of surface morphology in III-nitrides. J. Cryst. Growth 2016, 438, 81–89. [Google Scholar] [CrossRef]
- Qin, Z.; Jin, L.; Li, W.; Tan, J.; He, G.; Wu, H. Regulation of AlN Crystal Growth Mode by PVT Method. J. Synth. Cryst. 2024, 53, 1542. [Google Scholar]
- Xu, Y.; Ching, W. Electronic, optical, and structural properties of some wurtzite crystals. Phys. Rev. B 1993, 7, 4335–4351. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, F.; Fiorentini, V.; Vanderbilt, D. Spontaneous polarization and piezoelectric constants of III-V nitrides. Phys. Rev. B 1997, 56, 10024. [Google Scholar] [CrossRef]
- Heuell, P.; Kulakov, M.; Bullemer, B. Stepped morphology on 4H and 15R silicon carbide: Modelling by a random walk. Surf. Sci. 1995, 331, 965–970. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, R.; Zhao, G.; Jiang, K.; Tang, W.; Zhang, L.; Xia, W. Research on the Micromorphology of the Native Surface of 2-Inch Aluminum Nitride Single Crystals. Materials 2025, 18, 1147. https://doi.org/10.3390/ma18051147
Yu R, Zhao G, Jiang K, Tang W, Zhang L, Xia W. Research on the Micromorphology of the Native Surface of 2-Inch Aluminum Nitride Single Crystals. Materials. 2025; 18(5):1147. https://doi.org/10.3390/ma18051147
Chicago/Turabian StyleYu, Ruixian, Gang Zhao, Kai Jiang, Wenjing Tang, Lei Zhang, and Wei Xia. 2025. "Research on the Micromorphology of the Native Surface of 2-Inch Aluminum Nitride Single Crystals" Materials 18, no. 5: 1147. https://doi.org/10.3390/ma18051147
APA StyleYu, R., Zhao, G., Jiang, K., Tang, W., Zhang, L., & Xia, W. (2025). Research on the Micromorphology of the Native Surface of 2-Inch Aluminum Nitride Single Crystals. Materials, 18(5), 1147. https://doi.org/10.3390/ma18051147






