Roles of Water Molecules in the Structures and Magnetic Properties of Coordination Polymers with a Dicarboxylate Ligand
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials and Methods
2.2. Synthesis
- [Zn(nbpdc)(DMF)(H2O)]∞ (1): A suspension of H2nbpdc (0.050 mmol, 0.0145 g) and Zn(NO3)2·6H2O (0.20 mmol, 0.059 g) in DMF/H2O (1:1 (v/v), 2 mL) was stirred thoroughly for 30 min. The mixture was transferred to a 20 mL stainless steel reactor lined with Teflon and placed in an 80 °C oven for 3 days. After slow cooling to room temperature, pale yellow flake crystals of 1 were obtained in a yield of 87% based on Zn(NO3)2·6H2O. Elemental analysis calculated for C17H16ZnN2O8 (%): C 46.36, H 3.66, and N 6.36. Found(%): C 46.39, H 3.83, N 6.14. IR (KBr, cm−1): 3068 (m), 1653 (s), 1611 (s), 1532 (vs), 1488 (m), 1387 (s), 1351 (s), 1250 (w), 1185 (w), 1112 (m), 1005 (w), 919 (w), 862 (m), and 785 (m);
- {[Co(nbpdc)(DMF)(H2O)2]·H2O}∞ (2): The compound was obtained in the form of pink flake crystals by the procedure described above for 1, except that Co(NO3)2·6H2O (0.20 mmol, 0.058 g) was used in the place of Zn(NO3)2·6H2O. Yield: 86% based on Co(NO3)2·6H2O. Elemental analysis calculated for C17H20CoN2O10 (%): C 43.33, H 4.28, and N 5.94. Found (%): C 43.19, H 3.79, N 5.63. IR (KBr, cm−1): 3435 (m), 1667 (s), 1581 (s), 1530 (vs), 1393 (s), 1351 (s), 1249 (w), 1179 (w), 1108 (w), 1003 (w), 787 (m), and 690 (m);
- {[Co(nbpdc)(DMF)(H2O)2]·H2O}∞ (3): The compound was obtained in the form of pale green microcrystals by the procedure described above for 1, except that Ni(NO3)2·6H2O (0.20 mmol, 0.058 g) was used in the place of Zn(NO3)2·6H2O. Yield: 72% based on Ni(NO3)2·6H2O. Elemental analysis calculated for C17H20NiN2O10 (%): C 43.35, H 4.28, and N 5.95. Found(%): C 43.50, H 3.99, N 5.89. IR (KBr, cm−1): 3437 (m), 2973 (w), 1663 (s), 1580 (s), 1530 (vs), 1397 (s), 1351 (s), 1248 (w), 1176 (w), 1110 (w), 1059 (w), 1004 (w), 808 (m), 786 (m), and 692 (m).
2.3. X-Ray Crystallography
3. Results and Discussion
3.1. Synthesis and Structural Characterization
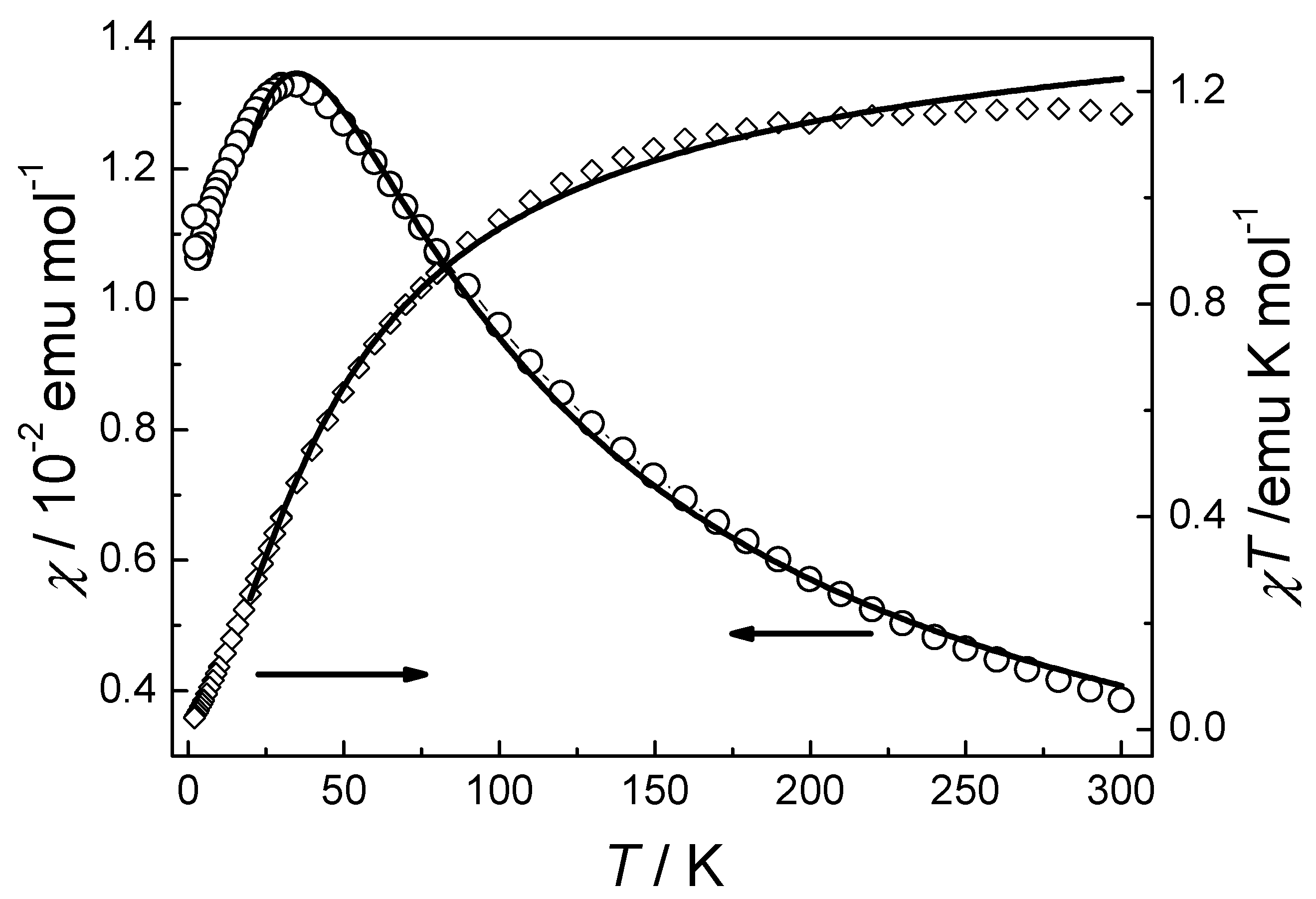
3.2. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Lin, R.-B.; Krishna, R.; Li, H.; Xiang, S.; Wu, H.; Li, J.; Zhou, W.; Chen, B. Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites. Science 2018, 362, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Lippi, M.; Cametti, M. Highly dynamic 1D coordination polymers for adsorption and separation applications. Coord. Chem. Rev. 2021, 430, 213661. [Google Scholar] [CrossRef]
- You, L.-X.; Ren, B.-Y.; He, Y.-K.; Wang, S.-J.; Sun, Y.-G.; Dragutan, V.; Xiong, G.; Ding, F. Structural features of lanthanide coordination polymers with catalytic properties. J. Mol. Struct. 2024, 1304, 137687. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, Y.; Jiang, H.-L.; Xu, Q. Metal–Organic Frameworks as Platforms for Catalytic Applications. Adv. Mater. 2018, 30, 1703663. [Google Scholar] [CrossRef]
- Wang, H.-N.; Meng, X.; Dong, L.-Z.; Chen, Y.; Li, S.-L.; Lan, Y.-Q. Coordination polymer-based conductive materials: Ionic conductivity vs. electronic conductivity. J. Mater. Chem. A 2019, 7, 24059–24091. [Google Scholar] [CrossRef]
- Yang, S.-L.; Li, G.; Guo, M.-Y.; Liu, W.-S.; Bu, R.; Gao, E.-Q. Positive Cooperative Protonation of a Metal–Organic Framework: pH-Responsive Fluorescence and Proton Conduction. J. Am. Chem. Soc. 2021, 143, 8838–8848. [Google Scholar] [CrossRef]
- Yin, H.-Q.; Yin, X.-B. Metal–Organic Frameworks with Multiple Luminescence Emissions: Designs and Applications. Acc. Chem. Res. 2020, 53, 485–495. [Google Scholar] [CrossRef]
- Heine, J.; Müller-Buschbaum, K. Engineering metal-based luminescence in coordination polymers and metal–organic frameworks. Chem. Soc. Rev. 2013, 42, 9232–9242. [Google Scholar] [CrossRef]
- Yue, Q.; Gao, E.-Q. Azide and carboxylate as simultaneous coupler for magnetic coordination polymers. Coord. Chem. Rev. 2019, 382, 1–31. [Google Scholar] [CrossRef]
- Wang, M.; Gou, X.; Shi, W.; Cheng, P. Single-chain magnets assembled in cobalt(II) metal-organic frameworks. Chem. Commun. 2019, 55, 11000–11012. [Google Scholar]
- Foo, M.L.; Matsuda, R.; Kitagawa, S. Functional Hybrid Porous Coordination Polymers. Chem. Mater. 2014, 26, 310–322. [Google Scholar] [CrossRef]
- Sarkar, A.; Dey, S.; Rajaraman, G. Role of Coordination Number and Geometry in Controlling the Magnetic Anisotropy in FeII, CoII, and NiII Single-Ion Magnets. Chem. Eur. J. 2020, 26, 14036–14058. [Google Scholar] [CrossRef]
- Kumar, K.S.; Ruben, M. Sublimable Spin-Crossover Complexes: From Spin-State Switching to Molecular Devices. Angew. Chem. Int. Ed. 2021, 60, 7502–7521. [Google Scholar] [CrossRef] [PubMed]
- Khusniyarov, M.M. How to Switch Spin-Crossover Metal Complexes at Constant Room Temperature. Chem.—Eur. J. 2016, 22, 15178–15191. [Google Scholar] [CrossRef] [PubMed]
- Ardizzoia, G.A.; Brenna, S. Hydroxo-bridged copper(II) cubane complexes. Coord. Chem. Rev. 2016, 311, 53–74. [Google Scholar] [CrossRef]
- Escuer, A.; Aromí, G. Azide as a Bridging Ligand and Magnetic Coupler in Transition Metal Clusters. Eur. J. Inorg. Chem. 2006, 4721–4736. [Google Scholar] [CrossRef]
- Frost, J.M.; Harriman, K.L.M.; Murugesu, M. The rise of 3-d single-ion magnets in molecular magnetism: Towards materials from molecules? Chem. Sci. 2016, 7, 2470–2491. [Google Scholar] [CrossRef]
- Andruh, M. Heterotrimetallic complexes in molecular magnetism. Chem. Commun. 2018, 54, 3559–3577. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Clerac, R.; Hearns, N.G.R.; Ray, D. Novel Layering of Aqua and Imidazolidinyl Phenolate Bridged Cationic [CuII2(μ-L)(μ-H2O)·H2O]2 Units Over CuINCS Based One-Dimensional Anionic Parallel Chains as Diamagnetic Coordination Framework Host. Cryst. Growth Des. 2009, 9, 4032–4040. [Google Scholar] [CrossRef]
- Modak, R.; Sikdar, Y.; Mandal, S.; Chatterjee, S.; Bieńko, A.; Mroziński, J.; Goswami, S. Syntheses, crystallographic characterization, catecholase activity and magnetic properties of three novel aqua bridged dinuclear nickel(II) complexes. Inorg. Chim. Acta 2014, 416, 122–134. [Google Scholar] [CrossRef]
- Biswas, R.; Giri, S.; Saha, S.K.; Ghosh, A. One Ferromagnetic and Two Antiferromagnetic Dinuclear Nickel(II) Complexes Derived from a Tridentate N,N,O-Donor Schiff Base Ligand: A Density Functional Study of Magnetic Coupling. Eur. J. Inorg. Chem. 2012, 2916–2927. [Google Scholar] [CrossRef]
- Biswas, R.; Kar, P.; Song, Y.; Ghosh, A. The importance of an additional water bridge in making the exchange coupling of bis(μ-phenoxo) dinickel(ii)complexes ferromagnetic. Dalton Trans. 2011, 40, 5324–5331. [Google Scholar] [CrossRef]
- Oyarzabal, I.; Ruiz, J.; Mota, A.J.; Rodriguez-Dieguez, A.; Seco, J.M.; Colacio, E. An experimental and theoretical magneto-structural study of polynuclear NiII complexes assembled from a versatile bis(salicylaldehyde)diamine polytopic ligand. Dalton Trans. 2015, 44, 6825–6838. [Google Scholar] [CrossRef]
- Biswas, R.; Diaz, C.; Ghosh, A. Three nickel(II) complexes derived from a tridentate NNO donor Schiff base ligand: Syntheses, crystal structures and magnetic properties. Polyhedron 2013, 56, 172–179. [Google Scholar] [CrossRef]
- Vrablova, A.; Falvello, L.R.; Campo, J.; Miklovic, J.; Boca, R.; Cernak, J.; Tomas, M. Preparation, First Structure Analysis, and Magnetism of the Long-Known Nickel Benzoate Trihydrate—A Linear Ni···Ni···Ni Polymer and Its Parallels with the Active Site of Urease. Eur. J. Inorg. Chem. 2016, 2016, 928–934. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Jing, X.-H.; Gao, E.-Q. Three-dimensional iron-series coordination networks based on antiferromagnetic and ferromagnetic dinuclear motifs with mixed carboxylate and aqua bridges. Inorg. Chim. Acta 2011, 365, 240–245. [Google Scholar] [CrossRef]
- Jeong, E.; Lee, W.R.; Ryu, D.W.; Kim, Y.; Phang, W.J.; Koh, E.K.; Hong, C.S. Reversible structural transformation and selective gas adsorption in a unique aqua-bridged Mn(II) metal-organic framework. Chem. Commun. 2013, 49, 2329–2331. [Google Scholar] [CrossRef] [PubMed]
- Fontanet, M.; Rodriguez, M.; Romero, I.; Fontrodona, X.; Teixidor, F.; Vinas, C.; Aliaga-Alcalde, N.; Matejicek, P. A water soluble Mn(II) polymer with aqua metal bridges. Dalton Trans. 2013, 42, 7838–7841. [Google Scholar] [CrossRef] [PubMed]
- Caneschi, A.; Ferraro, F.; Gatteschi, D.; Melandri, M.C.; Rey, P.; Sessoli, R. Synthesis, Structure and Magnetic Properties of a Dinuclear Manganese(II) Complex with One μ-Aqua and Two μ-Carboxylato Bridges. Angew. Chem. Int. Ed. 1989, 28, 1365–1367. [Google Scholar] [CrossRef]
- Ye, B.-H.; Mak, T.; Williams, I.D.; Li, X.-y. Novel dimanganese(ii) complexes with (µ-H2O)(µ-OAc)2 bridges. Models for dimanganese enzymes. Chem. Commun. 1997, 1813–1814. [Google Scholar] [CrossRef]
- Abu-Youssef, M.A.M.; Escuer, A.; Langer, V. Synthesis, structure, and magnetic behavior of two new 1D polymeric manganese azido complexes. Eur. J. Inorg. Chem. 2006, 3177–3184. [Google Scholar] [CrossRef]
- Yu, S.B.; Lippard, S.J.; Shweky, I.; Bino, A. Dinuclear manganese(II) complexes with water and carboxylate bridges. Inorg. Chem. 1992, 31, 3502–3504. [Google Scholar] [CrossRef]
- Xu, Z.; Thompson, L.K.; Milway, V.A.; Zhao, L.; Kelly, T.; Miller, D.O. Self-Assembled Dinuclear, Trinuclear, Tetranuclear, Pentanuclear, and Octanuclear Ni(II) Complexes of a Series of Polytopic Diazine Based Ligands: Structural and Magnetic Properties. Inorg. Chem. 2003, 42, 2950–2959. [Google Scholar] [CrossRef] [PubMed]
- Castro, I.; Calatayud, M.L.; Barros, W.P.; Carranza, J.; Julve, M.; Lloret, F.; Marino, N.; De Munno, G. Ligand Effects on the Structure and Magnetic Properties of Alternating Copper(II) Chains with 2,2′-Bipyrimidine- and Polymethyl-Substituted Pyrazolates as Bridging Ligands. Inorg. Chem. 2014, 53, 5759–5771. [Google Scholar] [CrossRef] [PubMed]
- Quartapelle Procopio, E.; Fukushima, T.; Barea, E.; Navarro, J.A.R.; Horike, S.; Kitagawa, S. A Soft Copper(II) Porous Coordination Polymer with Unprecedented Aqua Bridge and Selective Adsorption Properties. Chem.—Eur. J. 2012, 18, 13117–13125. [Google Scholar] [CrossRef] [PubMed]
- Novitchi, G.; Ciornea, V.; Shova, S.; Gulea, A.; Costes, J.-P.; Powell, A.K. Heterometallic M2Cr4 (MII = Sr, Pb) clusters assembled by tris(μ-aqua) bridges. Eur. J. Inorg. Chem. 2008, 1778–1783. [Google Scholar] [CrossRef]
- El Bakkali, H.; Castineiras, A.; Garcia-Santos, I.; Gonzalez-Perez, J.M.; Niclos-Gutierrez, J. Metallo-Supramolecular Structures by Self-Assembly through Weak Interactions in Mixed Ligand Metal Complexes of Adenine and Malonate. Cryst. Growth Des. 2014, 14, 249–260. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Li, X.-B.; Wang, K.; Ma, Y.; Cheng, A.-L.; Gao, E.-Q. Synthesis, structures, and magnetic properties of four copper compounds with 2,2′-dinitrobiphenyl-4,4′-dicarboxylate. Dalton Trans. 2012, 41, 12192–12199. [Google Scholar] [CrossRef]
- Lefebvre, J.; Tyagi, P.; Trudel, S.; Pacradouni, V.; Kaiser, C.; Sonier, J.E.; Leznoff, D.B. Magnetic Frustration and Spin Disorder in Isostructural M(μ-OH2)2[Au(CN)2]2 (M = Mn, Fe, Co) Coordination Polymers Containing Double Aqua-Bridged Chains: SQUID and μSR Studies. Inorg. Chem. 2009, 48, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, J.; Callaghan, F.; Katz, M.J.; Sonier, J.E.; Leznoff, D.B. A New basic motif in cyanometallate coordination polymers: Structure and magnetic behavior of M(μ-OH2)2[Au(CN)2]2 (M = Cu, Ni). Chem. Eur. J. 2006, 12, 6748–6761. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.C.; Price, D.J.; Heath, S.L. Synthesis, structure and magnetism of a novel carboxylate bridged manganese(ii) sheet structure containing the first unsupported aqua bridge. Dalton Trans. 2004, 2833–2835. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.-Y.; Yang, G.-P.; Wang, C.-J.; Wen, G.-L.; Shi, Q.-Z.; Batten, S.R. A series of intriguing metal-organic frameworks with 3,3′,4,4′-benzophenonetetracarboxylic acid: Structural adjustment and pH-dependence. CrystEngComm 2008, 10, 1583–1594. [Google Scholar] [CrossRef]
- Khan, M.R.; Niu, X.; Chen, T.; Liu, Y.; Liu, Z.; Liu, B.; Zhang, Y.; Li, J. Structural diversity and magnetic properties of six ferrocenyl monocarboxylate Mn(II), Ni(II) and Co(II) complexes with 1D aqua, carboxyl or dinuclear hydroxyl bridges. CrystEngComm 2021, 23, 3185–3195. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Z.; Shi, W.; Li, L.; Zou, J.; Cheng, P. An unusual water-bridged homospin CoII single-chain magnet. Chem. Commun. 2014, 50, 6340–6342. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.A.; Sanchiz, J.; Janiak, C. 2∞[Cu2(μ5-btb)(μ-OH)(μ-H2O)]: A two-dimensional coordination polymer built from ferromagnetically coupled Cu2 units (btb = benzene-1,2,3-tricarboxylate). Dalton Trans. 2008, 4877–4884. [Google Scholar] [CrossRef] [PubMed]
- Nesterova, O.V.; Petrusenko, S.R.; Nesterov, D.S.; Kokozay, V.N.; Skelton, B.W.; Jezierska, J.; Linert, W.; Ozarowski, A. A CuIINiII Complex with Ethylenediamine: Crystal Structure and Ferromagnetic Behaviour of an Aqua-Bridged Heterometallic Chain Containing Ambidentate Ni(OAc)42- Blocks. Eur. J. Inorg. Chem. 2010, 3529–3535. [Google Scholar] [CrossRef]
- Jing, X.-H.; Yi, X.-C.; Gao, E.-Q.; Blatov, V.A. Synthesis, structure, topology and magnetic properties of cobalt(ii) coordination polymers with 2-nitrobiphenyl-4,4′-dicarboxylic acid and bis(pyridyl) ligands. Dalton Trans. 2012, 41, 14316–14328. [Google Scholar] [CrossRef]
- Wu, R.F.; Zhang, T.L.; Qiao, X.J. Synthesis and characterization of an intermediary of new heat-resistant energetic materials. Chin. Chem. Lett. 2010, 21, 1007–1010. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, Program for Empirical Absorption Correction; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELX-2014; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Brandenburg, K.; Putz, H. Diamond 3.2k-Crystal and Molecular Structure Visualization; Crystal Impact: Bonn, Germany, 2014. [Google Scholar]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Lloret, F.; Julve, M.; Cano, J.; Ruiz-Garcia, R.; Pardo, E. Magnetic properties of six-coordinated high-spin cobalt(II) complexes: Theoretical background and its application. Inorg. Chim. Acta 2008, 361, 3432–3445. [Google Scholar] [CrossRef]
- Estes, W.E.; Gavel, D.P.; Hatfield, W.E.; Hodgson, D.J. Magnetic and structural characterization of dibromo- and dichlorobis(thiazole)copper(II). Inorg. Chem. 1978, 17, 1415–1421. [Google Scholar] [CrossRef]
- Weng, D.F.; Wang, Z.M.; Gao, S. Framework-structured weak ferromagnets. Chem. Soc. Rev. 2011, 40, 3157–3181. [Google Scholar] [CrossRef] [PubMed]
- Kahn, O. Molecular Magnetism; VCH: New York, NY, USA, 1993. [Google Scholar]
- Sun, Q.; Cheng, A.L.; Wang, Y.Q.; Ma, Y.; Gao, E.Q. Magnetic ordering in three-dimensional metal-organic frameworks based on carboxylate bridged square-grid layers. Inorg. Chem. 2011, 50, 8144–8152. [Google Scholar] [CrossRef] [PubMed]
- Beghidja, A.; Welter, R.; Rabu, P.; Rogez, G. Synthesis, structure and magnetic properties of a new family of chiral metal(II) citramalates. Inorg. Chim. Acta 2007, 360, 1111–1120. [Google Scholar] [CrossRef]
- Rettig, S.J.; Thompson, R.C.; Trotter, J.; Xia, S. Crystal structure and magnetic properties of polybis(formamide)bis(µ-formato)cobalt(II): An extended two-dimensional square lattice material which exhibits spontaneous magnetization below 9 K. Inorg. Chem. 1999, 38, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- Fabelo, O.; Pasán, J.; Cañadillas-Delgado, L.; Delgado, F.S.; Lloret, F.; Julve, M.; Ruiz-Pérez, C. Crystal structure and magnetic properties of two isomeric three-dimensional pyromellitate-containing cobalt(II) complexes. Inorg. Chem. 2008, 47, 8353–8361. [Google Scholar] [CrossRef]
- Rueff, J.-M.; Paulsen, C.; Souletie, J.; Drillon, M.; Rabu, P. Very-low temperature magnetic ordering and dimensionality of the square planar system cobalt(II) phenoxyacetate. Solid State Sci. 2005, 7, 431–436. [Google Scholar] [CrossRef]
- Li, Z.X.; Zhao, J.P.; Sanudo, E.C.; Ma, H.; Pan, Z.D.; Zeng, Y.F.; Bu, X.H. New 3D coordination polymers constructed from pillared metal-formate Kagome layers exhibiting spin canting only in the nickel(II) complex. Inorg. Chem. 2009, 48, 11601–11607. [Google Scholar] [CrossRef]
- Beghidja, A.; Hallynck, S.; Welter, R.; Rabu, P. Syntheses, Structures and Magnetic Properties of Layered Metal(II) Mandelates. Eur. J. Inorg. Chem. 2005, 662–669. [Google Scholar] [CrossRef]
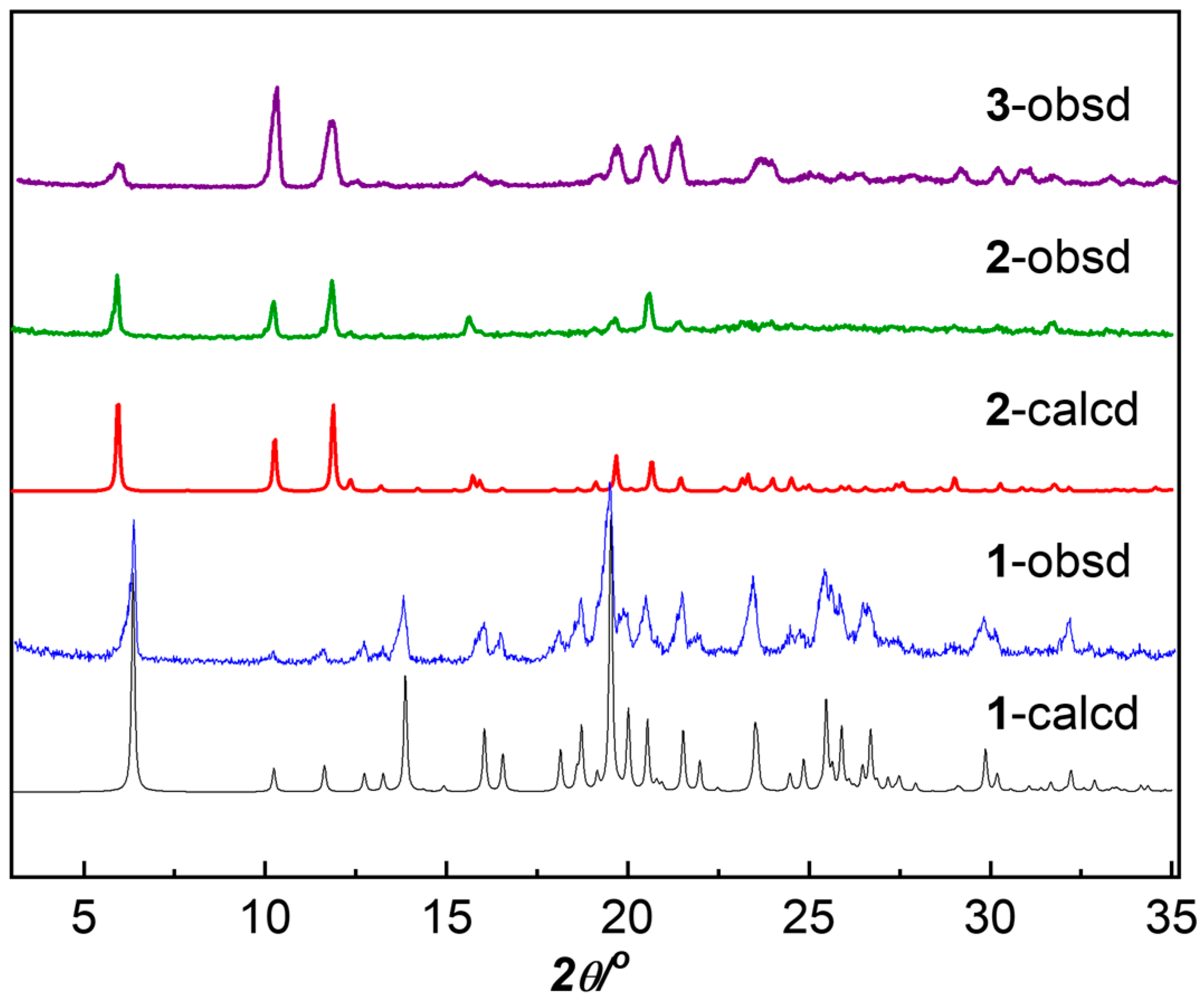
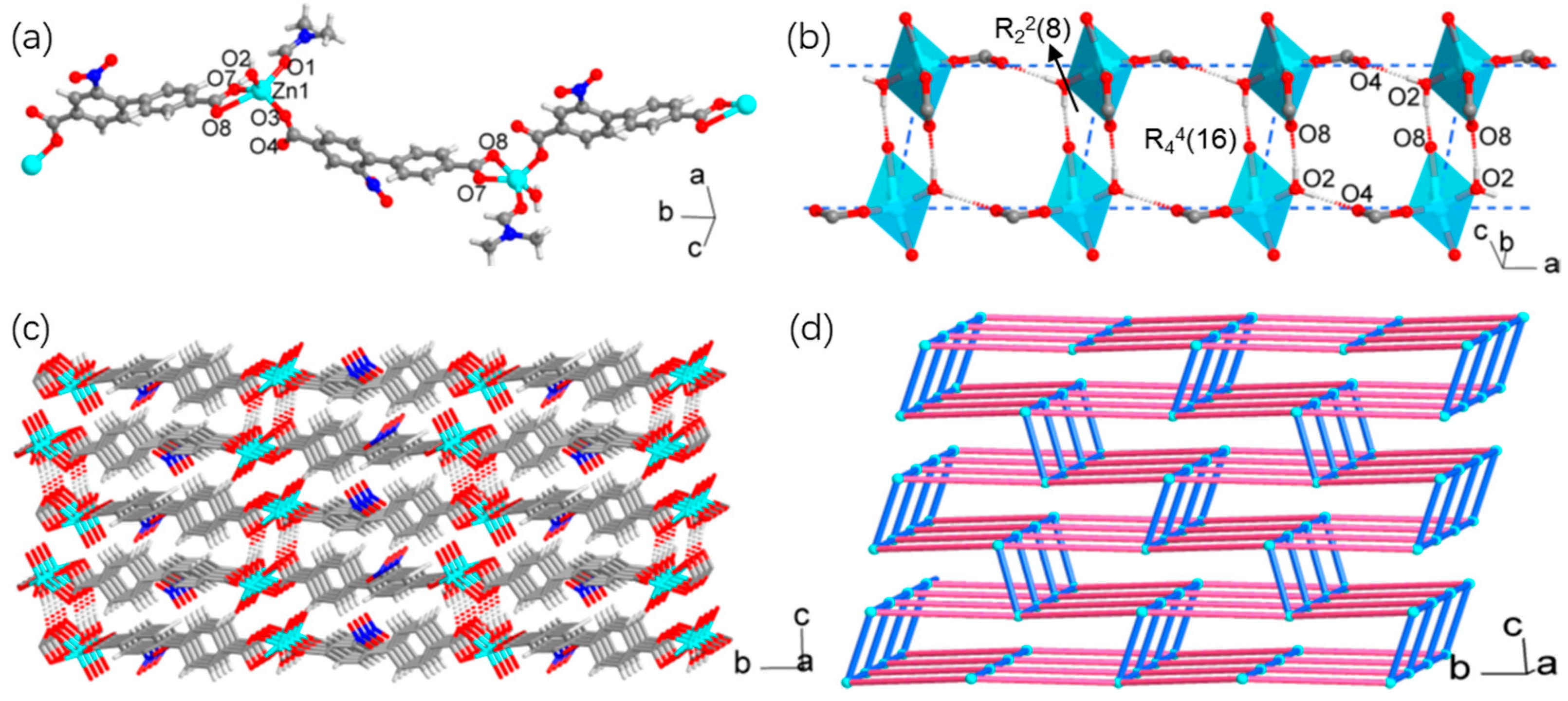
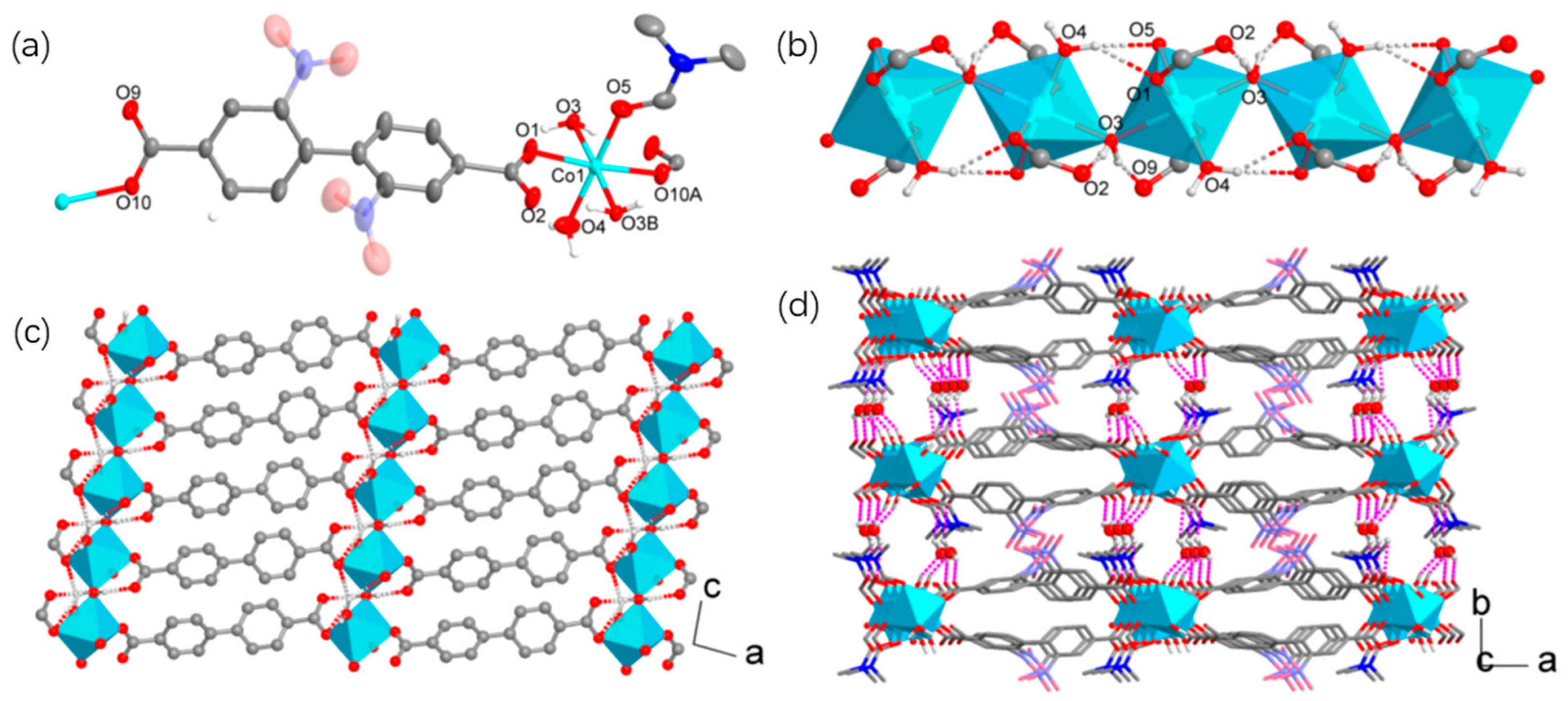
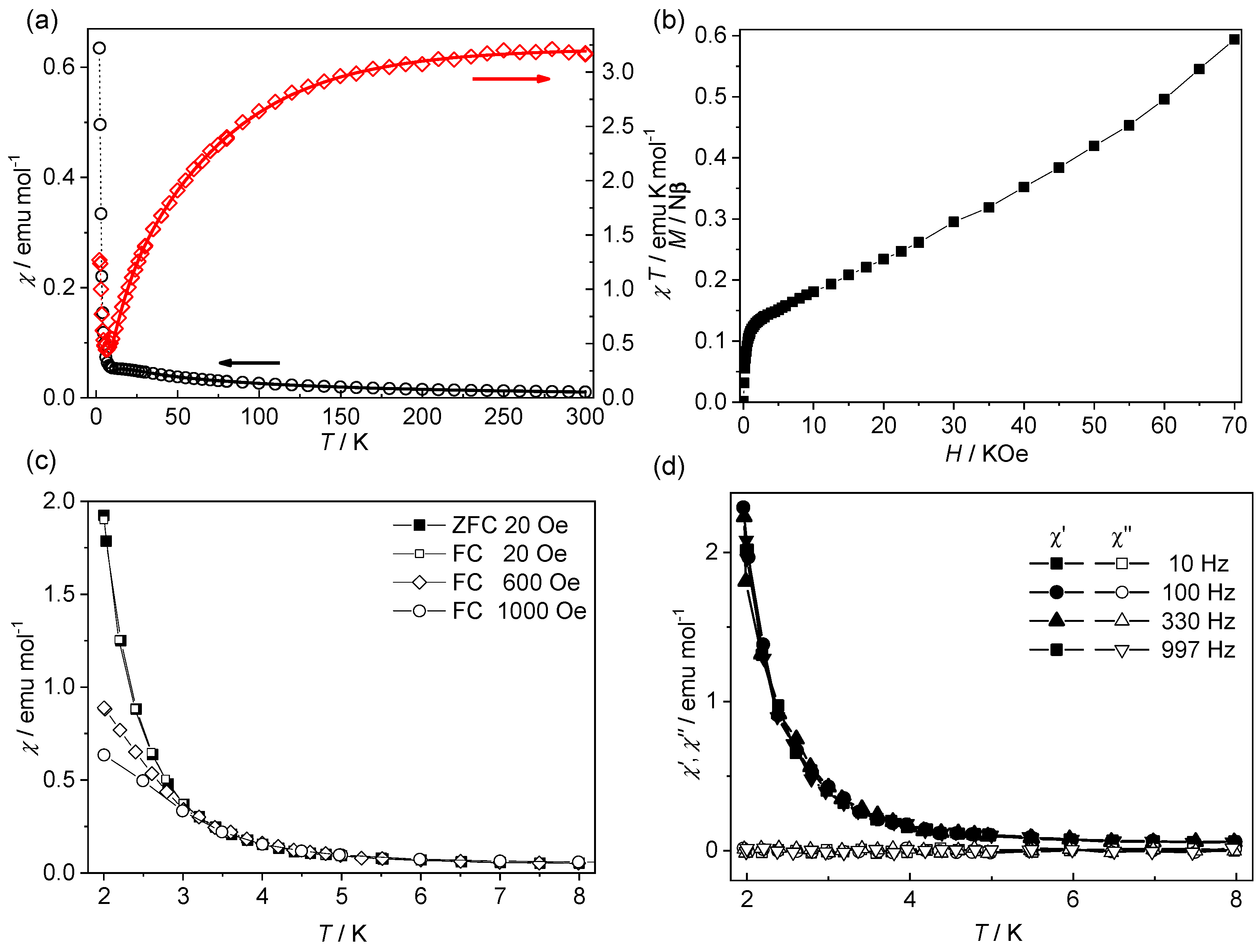
| Compound | 1 | 2 |
|---|---|---|
| Formula | C17H16ZnN2O8 | C17H20CoN2O10 |
| CCDC | 2406943 | 2406942 |
| Formula Weight | 441.69 | 471.28 |
| Temperature, K | 293 | 293 |
| Crystal system | Monoclinic | Monoclinic |
| space group | P21/c | P21/c |
| a, Å | 7.238(4) | 14.947(2) |
| b, Å | 27.774(13) | 17.226(2) |
| c, Å | 9.562(5) | 7.9198(11) |
| α, deg | 90 | 90 |
| β, deg | 108.233(6) | 95.989(2) |
| γ, deg | 90 | 90 |
| V, Å3 | 1825.9(15) | 2028.1(5) |
| Z | 4 | 4 |
| Dc, g cm−3 | 1.607 | 1.543 |
| μ, mm−1 | 1.394 | 0.904 |
| Reflns collected | 8144 | 9013 |
| Unique reflns/ Rint | 3591/0.0331 | 3949/0.0484 |
| R1 [I > 2σ(I)] | 0.0383 | 0.0725 |
| wR2 (all data) | 0.0941 | 0.1870 |
| GOF | 1.082 | 1.118 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, D.; Gao, E.-Q.; Zhang, D. Roles of Water Molecules in the Structures and Magnetic Properties of Coordination Polymers with a Dicarboxylate Ligand. Materials 2025, 18, 1089. https://doi.org/10.3390/ma18051089
Zong D, Gao E-Q, Zhang D. Roles of Water Molecules in the Structures and Magnetic Properties of Coordination Polymers with a Dicarboxylate Ligand. Materials. 2025; 18(5):1089. https://doi.org/10.3390/ma18051089
Chicago/Turabian StyleZong, Dehui, En-Qing Gao, and Dawei Zhang. 2025. "Roles of Water Molecules in the Structures and Magnetic Properties of Coordination Polymers with a Dicarboxylate Ligand" Materials 18, no. 5: 1089. https://doi.org/10.3390/ma18051089
APA StyleZong, D., Gao, E.-Q., & Zhang, D. (2025). Roles of Water Molecules in the Structures and Magnetic Properties of Coordination Polymers with a Dicarboxylate Ligand. Materials, 18(5), 1089. https://doi.org/10.3390/ma18051089






