Effect of Sulfate Carrier Addition on the Microstructure of Calcined Clay Blended Cements
Abstract
1. Introduction
- The sca in the calcined clay blended cements delayed the early occurrence and reduced the high intensity of the aluminate peak. 1:1-dominated clays required larger amounts of sulfate carrier to adjust the heat flow than 2:1-dominated clays.
- The effect of sca on the mortar compressive strength was more pronounced at 2 days than at 28 days, particularly for 1:1-dominated clays. Therefore, the sca ensuring the highest strength at 2 days was regarded as optimal.
- For blends with 2:1-dominated clays, the Activity Index increased by up to 10% in absolute terms with optimal sca, while oversulfation could already occur with sca exceeding 1 wt%. In contrast, some 1:1-dominated clay blends did not reach oversulfation even with 9 wt% addition, and their Activity Indexes rose by up to 60% in absolute terms. Basically, illitic and smectitic clays had the same strength response to sca.
2. Materials and Methods
2.1. Materials and Binder Compositions
2.2. Thermogravimetric Measurement
2.3. Porosity Measurement
2.4. Scanning Electron Microscopy Images
2.5. Compressive Strength of Calcined Clay Blended Cements with Different Sca
3. Results and Discussion
3.1. Microstructural Investigations of Hardened Calcined Clay Blended Cement Pastes with Different Sca
3.1.1. Thermogravimetric Analysis of Hardened Pastes
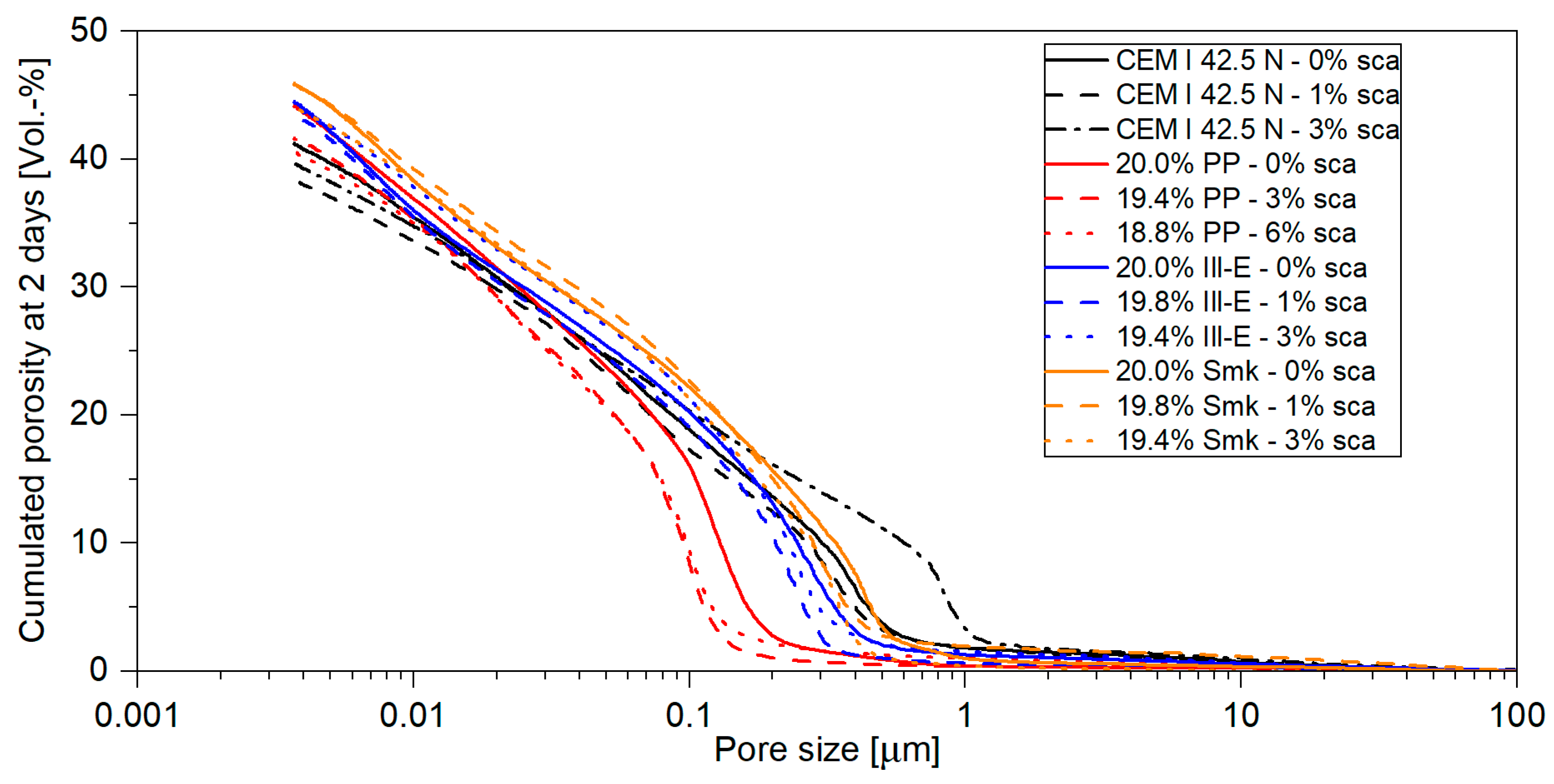
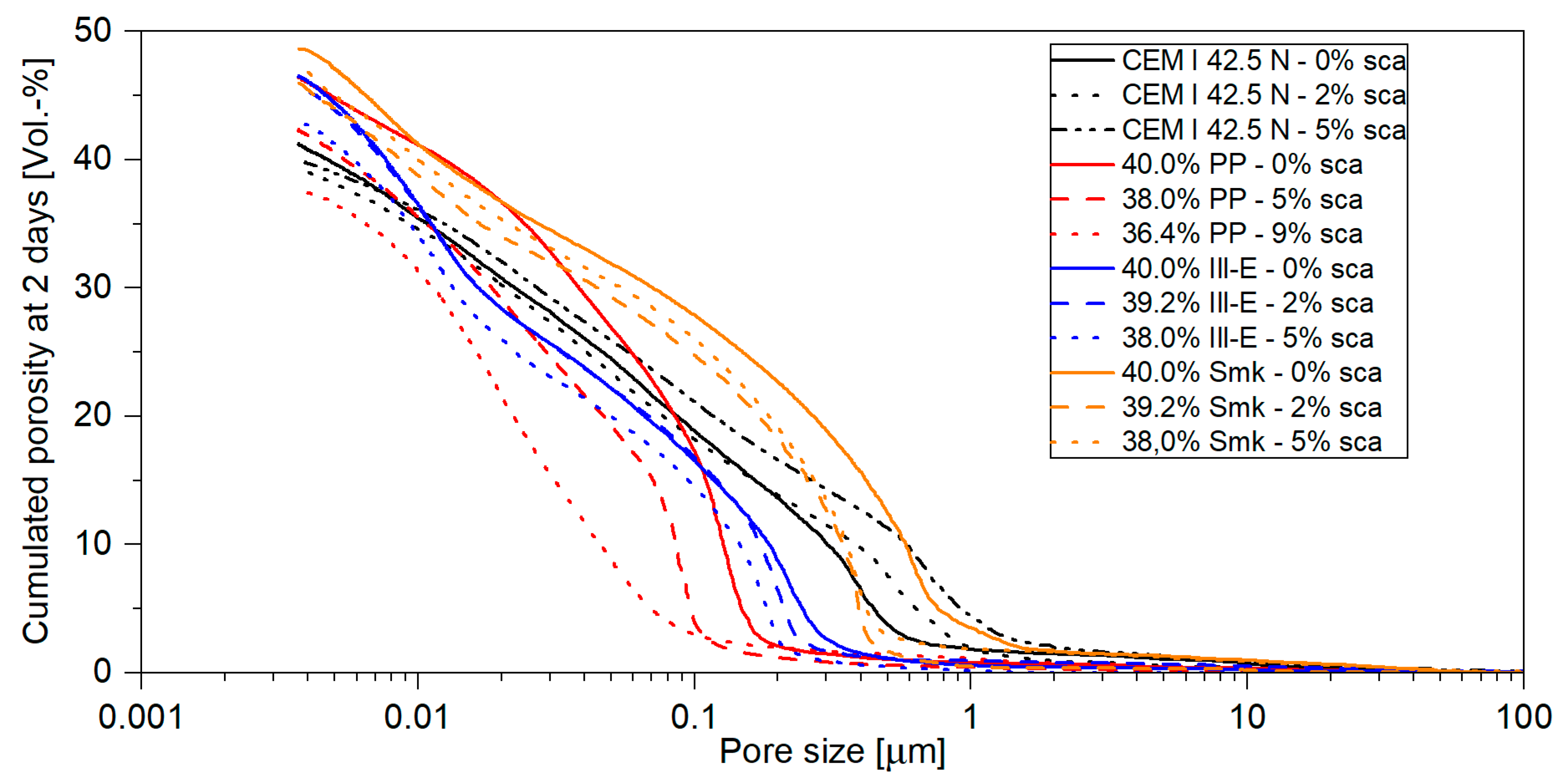
3.1.2. Porosity of Hardened Pastes
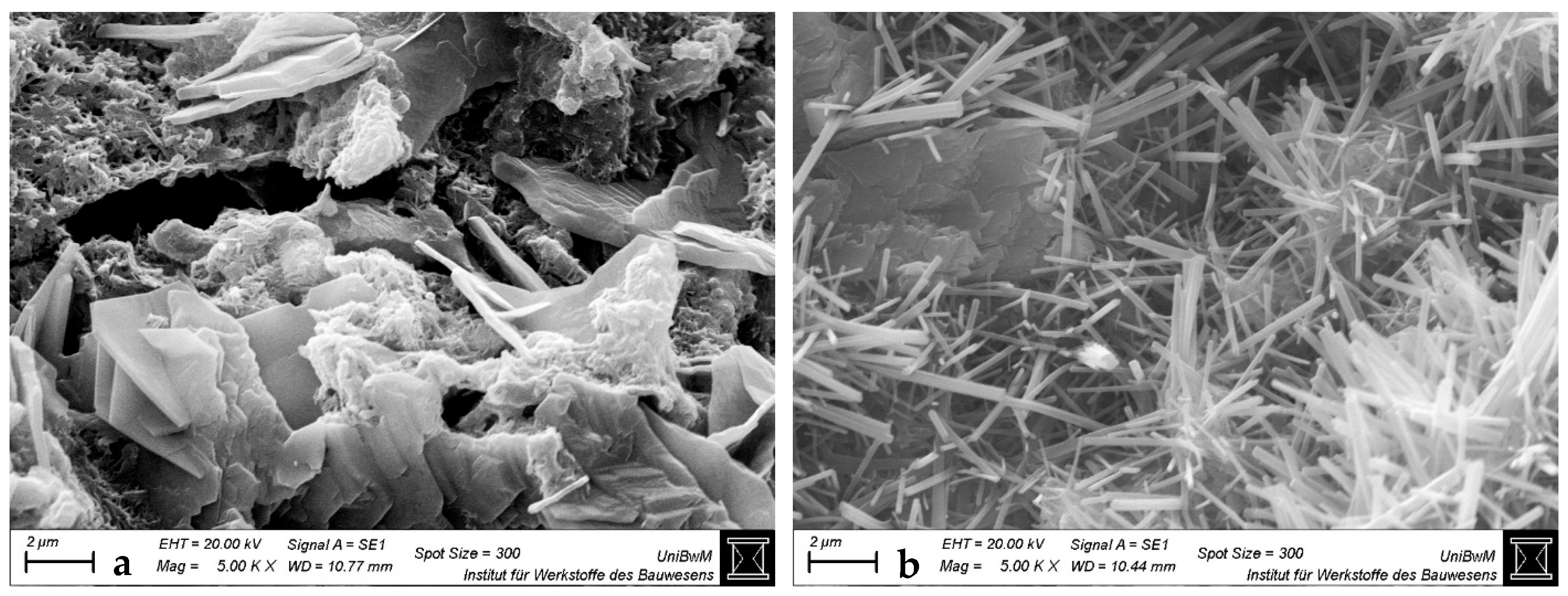
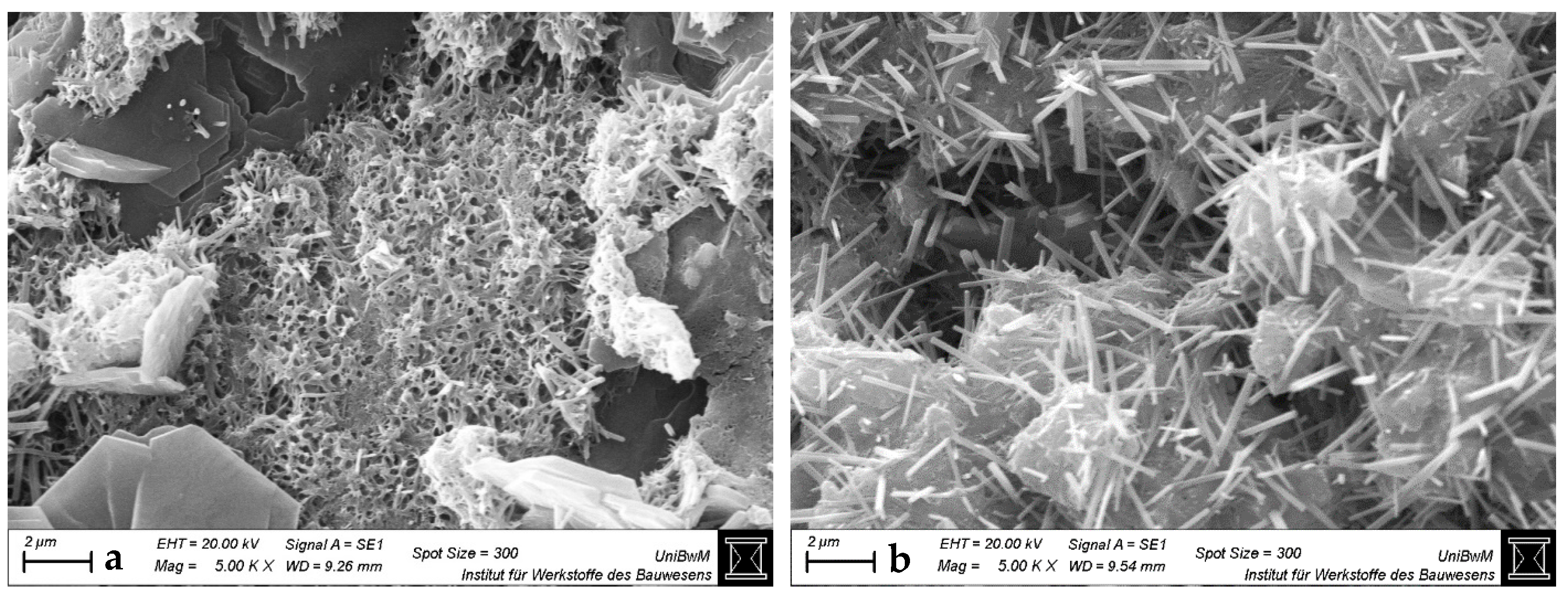
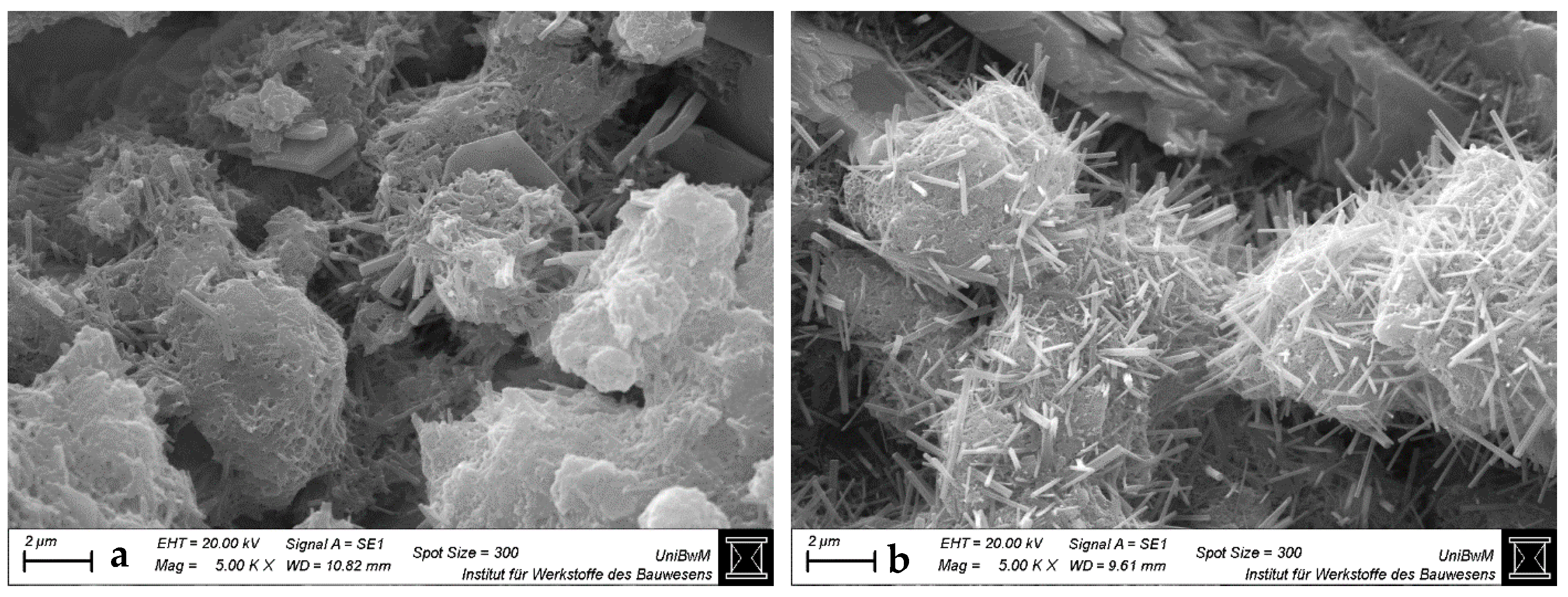
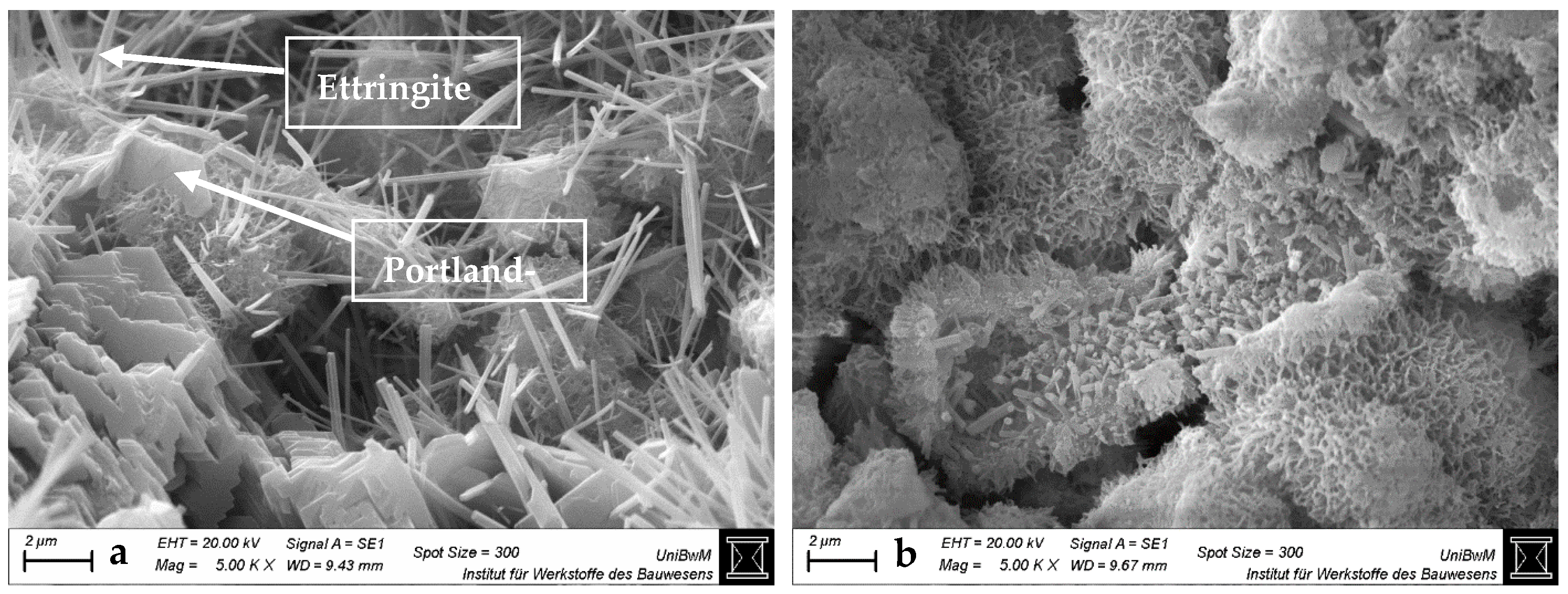
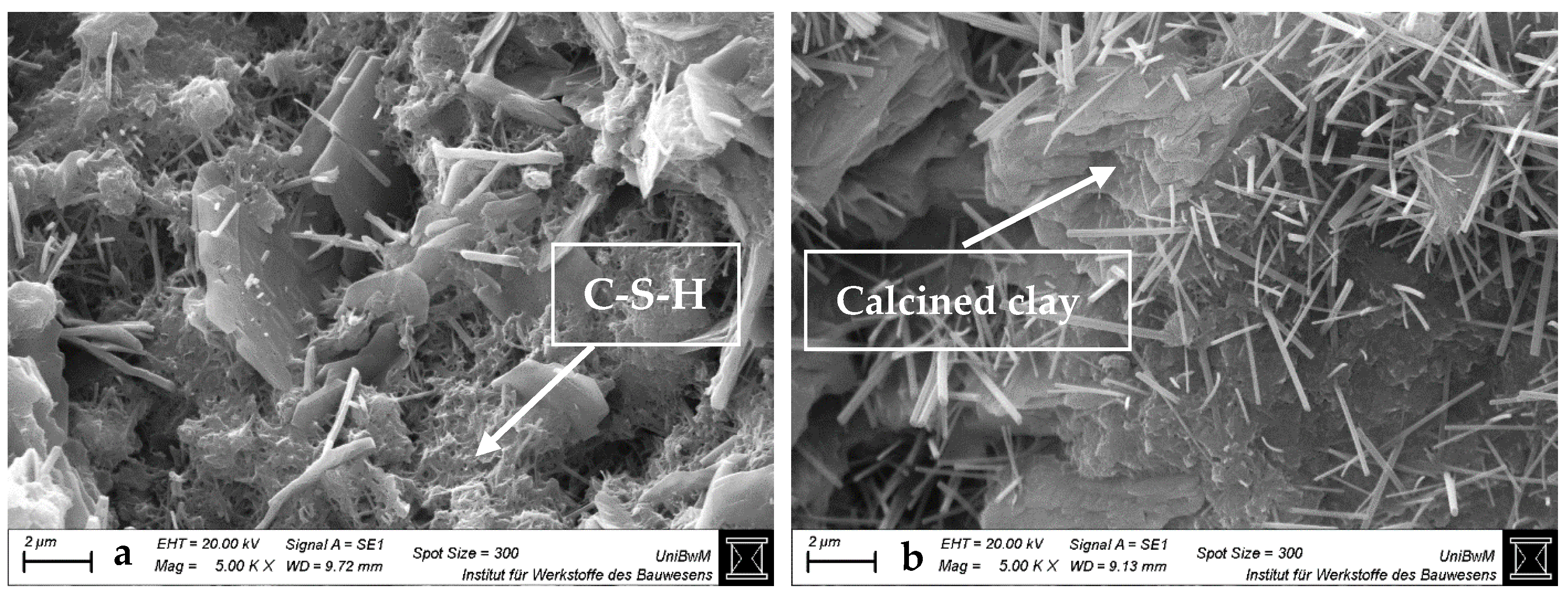
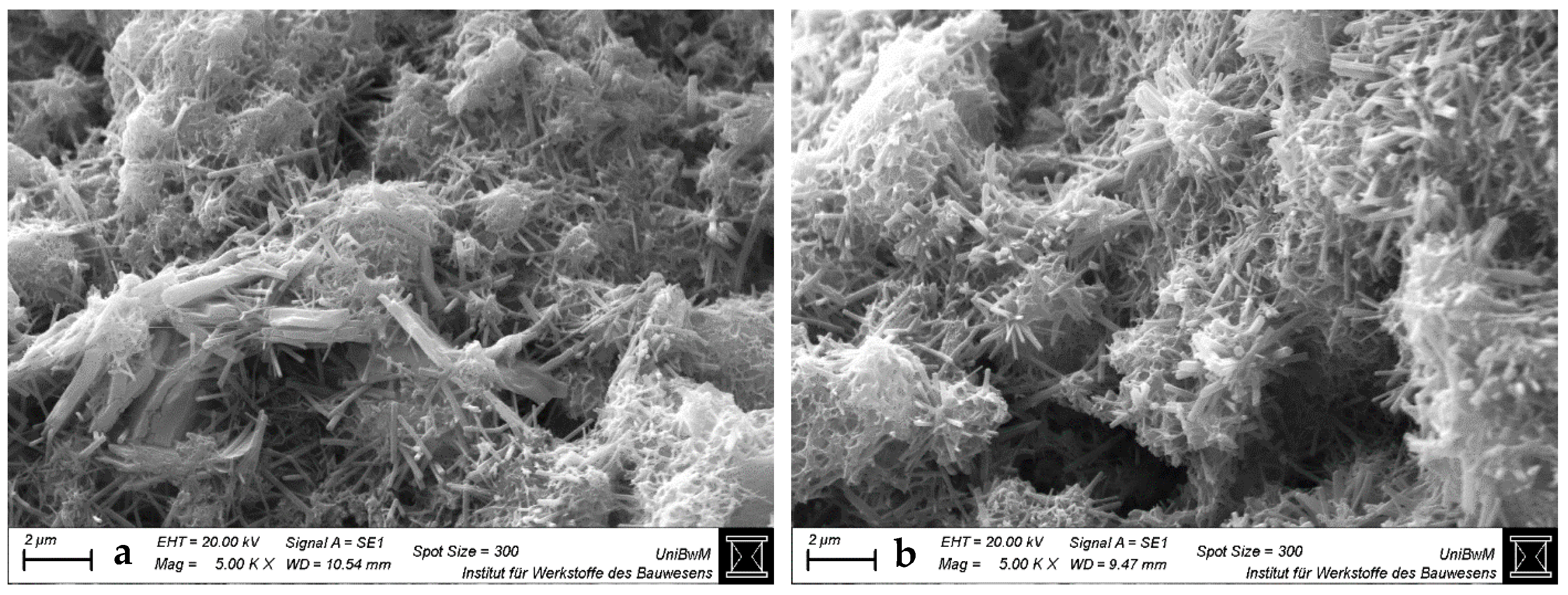
3.1.3. Scanning Electron Microscopy Images of Hardened Pastes
3.2. Correlation Between Microstructural Observations and Compressive Strength of Calcined Clay Blended Cements
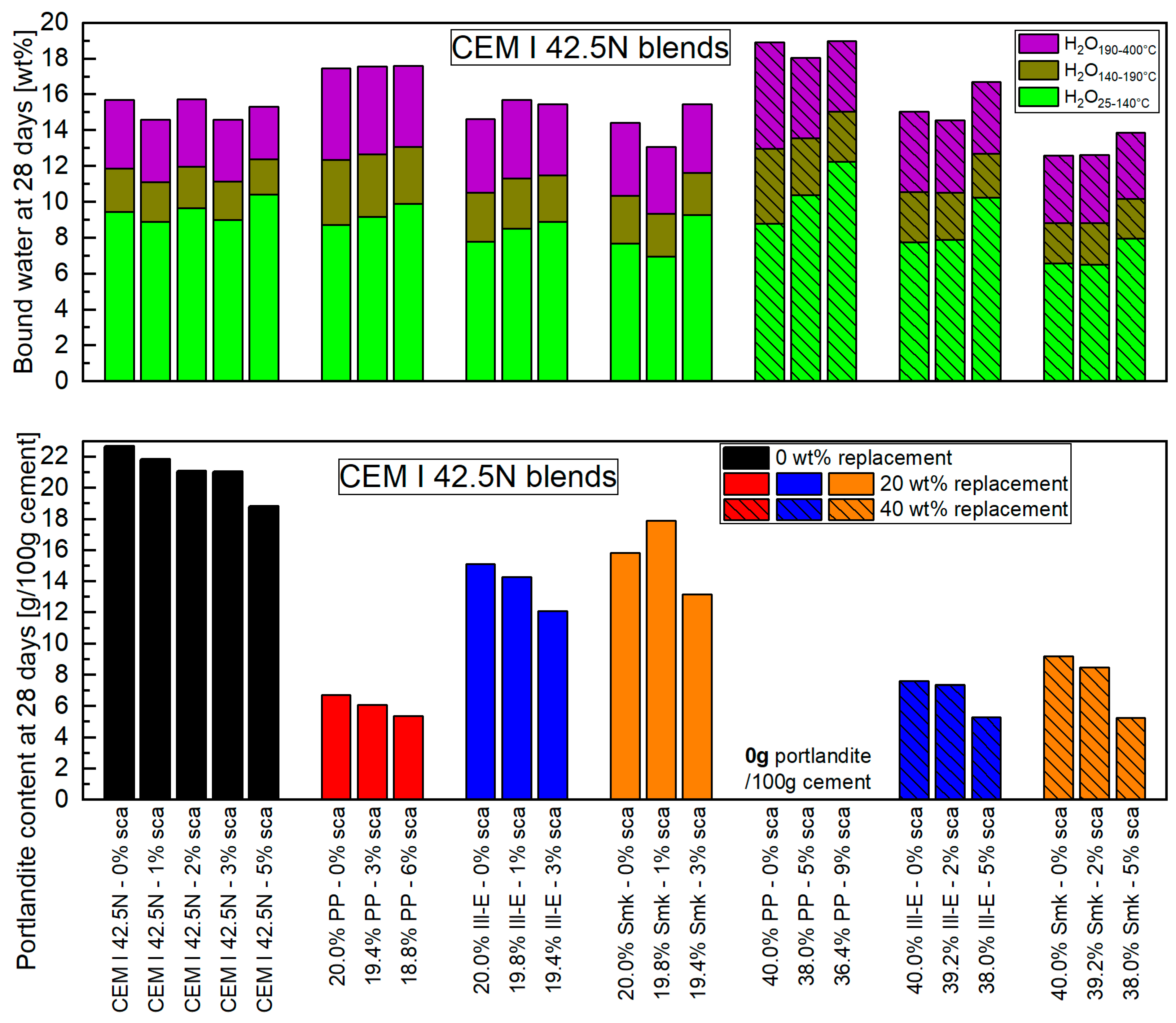
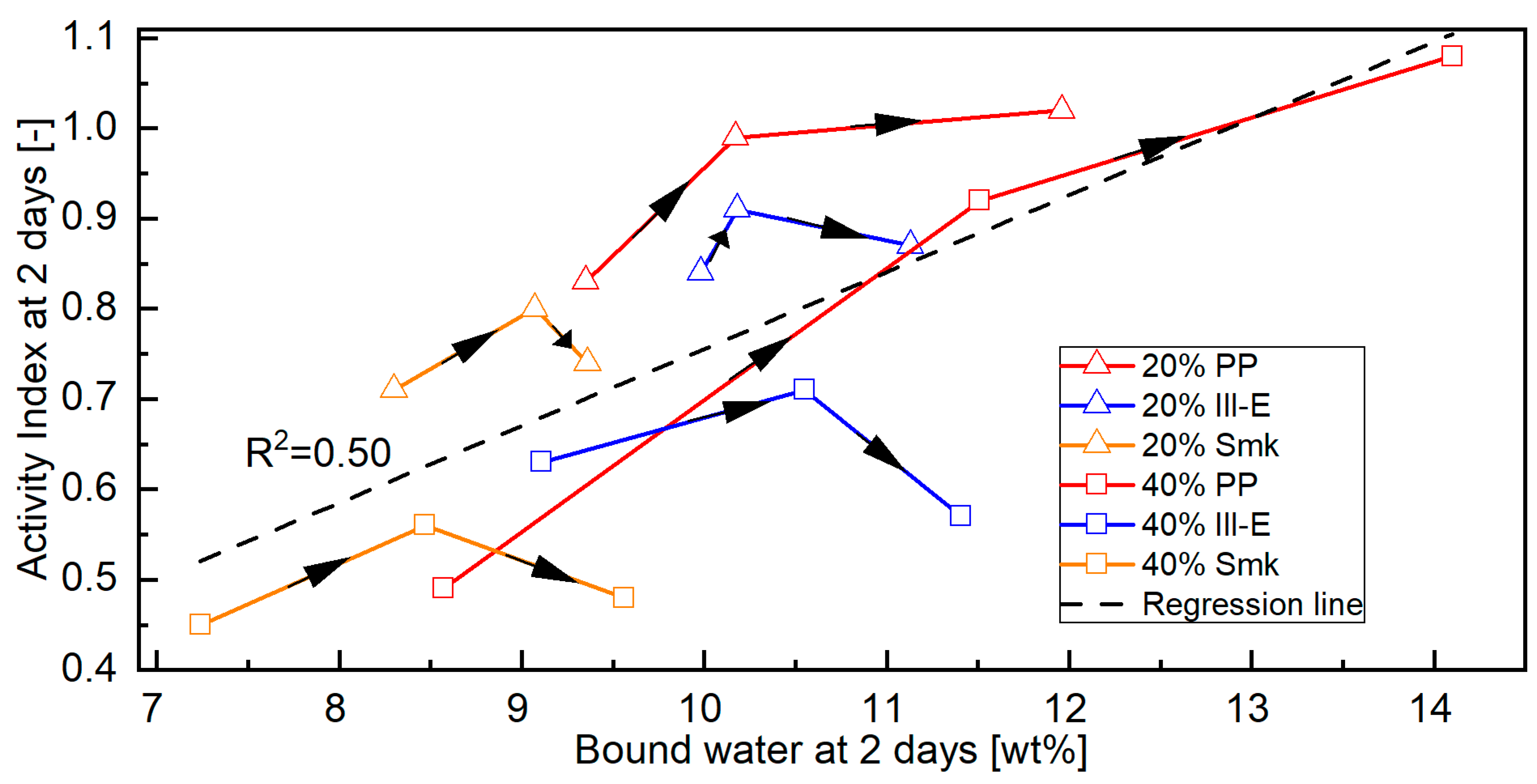
3.2.1. Bound Water of Blended Cements
3.2.2. Porosity of Blended Cements
3.3. Effects of the Optimal Sca on Properties of Calcined Clay Blended Pastes and Mortars at Early Hydration
4. Conclusions
- The impact of the sca on the microstructure is greater in blends with 1:1 than with 2:1-dominated clays. The higher the sca, the lower the porosity and the higher the amount of bound water in the calcined clay blends at 2 days. This is due to the increased ettringite formation, which is also most obvious in SEM images.
- Higher ettringite volume causes early strength increase in 1:1-dominated clay blends up to the highest sca used, but no longer in 2:1-dominated clay blends beyond a certain sca. Despite maximum water binding and minimum porosity at highest sca, their strength has decreased significantly.
- Another reason for the positive effect of sca on the microstructure and strength of 1:1-dominated clay blends is the increase of their alite hydration which is initially reduced by the high amount of aluminum ions from metakaolinite.
- The overall correlation between bound water and Activity Index of the blended cements at 2 days is moderate. The correlation between porosity and Activity Index is better, but still insufficient for a satisfactory prediction.
- The use of the optimal sca is particularly beneficial for early hydration. Blends with the 1:1-dominated clay benefit the most from improved properties (bound water, porosity, strength), but also have a significantly reduced portlandite content. With 2:1-dominated clays, the optimal sca improves the properties of their blends not as much, but the portlandite content is higher.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| sca | Sulfate carrier addition. |
| SEM | Scanning electron microscopy. |
| AI | Activity Index. |
Appendix A
| Mineralogical Composition [wt%] | CEM I 42.5 N | Chemical Composition [wt%] | CEM I 42.5 N | Physical Parameter | CEM I 42.5 N |
|---|---|---|---|---|---|
| C3S | 60.4 | SiO2 | 20.1 | Particle density [g/cm3] | 3.15 |
| C2S | 18.2 | Al2O3 | 5.2 | BET SSA [m2/g] | 0.8 |
| C3A | 6.5 | Fe2O3 | 2.8 | d’ [µm] | 31.9 |
| C4AF | 8.3 | CaO | 62.4 | Water demand [%] | 27 |
| Calcite | 0.8 | MgO | 1.5 | Blaine SSA [cm2/g] | 2742 |
| Anhydrite | 1.5 | Na2O | 1.5 | f2d [N/mm2] | 19.2 |
| Hemihydrate | 0.7 | K2O | 1.9 | f28d [N/mm2] | 54.9 |
| Dihydrate | 0.2 | TiO2 | 0.1 | ||
| SO3 | 2.8 | ||||
| LOI | 1.8 | ||||
| Raw Clay | Clay Group | Mineralogy [wt%] | SO3 [wt%] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Kaolinite | Smectite | Smectite-Illite | Illite | Muscovite | Inert | ||||
| Quartz | Others | ||||||||
| PP | 1:1-dominated clay | 93 | 5 | 2 | 0.1 | ||||
| Ill-E | 2:1-dominated clay | 10 | 67 | 7 | 12 | 4 | 0.2 | ||
| Smk | 2:1-dominated clay | 7 | 54 | 4 | 17 | 18 | 0.1 | ||
| Material | Grain Size [µm] | BET SSA [m2/g] | Water Demand [%] | Particle Density [g/cm3] | ||
|---|---|---|---|---|---|---|
| d10 | d50 | d90 | ||||
| PP | 1.0 | 4.8 | 39.2 | 18 | 57 | 2.61 |
| Ill-E | 1.9 | 15.8 | 54.6 | 71 | 53 | 2.71 |
| Smk | 1.4 | 14.4 | 60.6 | 38 | 45 | 2.71 |
| Sulfate carrier | 0.6 | 6.9 | 52.1 | 5 | 12 | 2.91 |
| 0 wt% Replacement | 20 wt% Replacement | 40 wt% Replacement | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sca [wt%] | AI2d [-] | AI28d [-] | Sca [wt%] | AI2d [-] | AI28d [-] | Sca [wt%] | AI2d [-] | AI28d [-] | ||||
| CEM I 42.5 N | 0 | 1.00 | 1.00 | |||||||||
| 1 | 0.97 | 0.98 | ||||||||||
| 2 | 0.91 | 0.97 | ||||||||||
| 3 | 0.87 | 0.94 | ||||||||||
| 4 | 0.85 | 0.93 | ||||||||||
| 5 | 0.82 | 0.89 | ||||||||||
| PP | 0 | 0.83 | 1.25 | PP | 0 | 0.49 | 1.00 | |||||
| 1 | 0.92 | 1.27 | 1 | 0.61 | 1.10 | |||||||
| 2 | 0.97 | 1.31 | 2 | 0.67 | 1.14 | |||||||
| 3 | 0.99 | 1.32 | 3 | 0.78 | 1.17 | |||||||
| 4 | 1.02 | 1.35 | 4 | 0.84 | 1.18 | |||||||
| 5 | 1.01 | 1.32 | 5 | 0.92 | 1.20 | |||||||
| 6 | 1.02 | 1.31 | 6 | 1.00 | 1.16 | |||||||
| 7 | 1.08 | 1.14 | ||||||||||
| 8 | 1.09 | 1.11 | ||||||||||
| 9 | 1.08 | 1.05 | ||||||||||
| Ill-E | 0 | 0.84 | 0.94 | Ill-E | 0 | 0.63 | 0.75 | |||||
| 1 | 0.91 | 1.01 | 1 | 0.67 | 0.78 | |||||||
| 2 | 0.90 | 1.00 | 2 | 0.71 | 0.81 | |||||||
| 3 | 0.87 | 0.98 | 3 | 0.66 | 0.81 | |||||||
| 4 | 0.64 | 0.78 | ||||||||||
| 5 | 0.57 | 0.75 | ||||||||||
| Smk | 0 | 0.71 | 1.01 | Smk | 0 | 0.45 | 0.81 | |||||
| 1 | 0.80 | 1.08 | 1 | 0.54 | 0.85 | |||||||
| 2 | 0.76 | 1.03 | 2 | 0.56 | 0.87 | |||||||
| 3 | 0.74 | 0.99 | 3 | 0.57 | 0.85 | |||||||
| 4 | 0.50 | 0.80 | ||||||||||
| 5 | 0.48 | 0.78 | ||||||||||
References
- International Energy Agency (IEA). Technology Roadmap—Low-Carbon Transition in the Cement Industry; International Energy Agency (IEA): Paris, France, 2018; p. 66. [Google Scholar]
- Panzer, M.; Scherb, S.; Beuntner, N.; Thienel, K.-C. Effect of sulfate carrier addition on the strength of calcined clay blended cements. Constr. Build. Mater. 2025, 477, 140915. [Google Scholar] [CrossRef]
- Maier, M.; Sposito, R.; Beuntner, N.; Thienel, K.-C. Particle characteristics of calcined clays and limestone and their impact on the early hydration and sulfate demand of blended cement. Cem. Concr. Res. 2022, 154, 15. [Google Scholar] [CrossRef]
- Maier, M.; Scherb, S.; Neißer-Deiters, A.; Beuntner, N.; Thienel, K.-C. Hydration of cubic tricalcium aluminate in the presence of calcined clays. J. Am. Ceram. Soc. 2021, 104, 3619–3631. [Google Scholar] [CrossRef]
- Scherb, S.; Maier, M.; Köberl, M.; Beuntner, N.; Thienel, K.-C. Reaction kinetics during early hydration of calcined phyllosilicates in model cement systems. Cem. Concr. Res. 2024, 175, 107356. [Google Scholar] [CrossRef]
- Scherb, S.; Maier, M.; Mathias, K.; Beuntner, N.; Thienel, K.-C. Reaction kinetics of calcined smectite in a clinker-free model and a synthetic cement system in comparison with selected calcined phyllosilicates. Cem. Concr. Res. 2025, 189, 107766. [Google Scholar] [CrossRef]
- Sowoidnich, T.; Cölfen, H.; Rößler, C.; Damidot, D.; Ludwig, H.-M. The impact of metakaolin on the hydration of tricalcium silicate: Effect of C-A-S-H precipitation. Front. Mater. 2023, 10, 1159772. [Google Scholar] [CrossRef]
- Maier, M.; Beuntner, N.; Thienel, K.-C. Mineralogical characterization and reactivity test of common clays suitable as supplementary cementitious material. Appl. Clay Sci. 2021, 202, 105990. [Google Scholar] [CrossRef]
- Panzer, M.; Scherb, S.; Beuntner, N.; Thienel, K.-C. An approach to estimate the strength contribution of calcined clays in blended cements. Constr. Build. Mater. 2024, 447, 137800. [Google Scholar] [CrossRef]
- Avet, F.; Snellings, R.; Alujas Diaz, A.; Ben Haha, M.; Scrivener, K. Development of a new rapid, relevant and reliable (R3) test method to evaluate the pozzolanic reactivity of calcined kaolinitic clays. Cem. Concr. Res. 2016, 85, 1–11. [Google Scholar] [CrossRef]
- DIN EN 17979; Entwurf. Reaktivität von Zementbestandteilen—Verfahren zur Bestimmung der Hydratationswärme und des Chemisch Gebundenen Wassers (Reactivity of Cement Constituents—Heat of Hydration and Bound Water Methods). Beuth-Verlag GmbH: Berlin, Germany, 2023; p. 31. [CrossRef]
- Werling, N.; Kaltenbach, J.; Weidler, P.G.; Schuhmann, R.; Dehn, F.; Emmerich, K. Solubility of Calcined Kaolinite, Montmorillonite, and Illite in High Molar NaOH and Suitability as Precursors for Geopolymers. Clays Clay Miner. 2022, 70, 270–289. [Google Scholar] [CrossRef]
- Trümer, A. Calcinierte Tone als Puzzolane der Zukunft—Von den Rohstoffen bis zur Wirkung im Beton. Ph.D. Thesis, Fakultät Bauingenieurwesen, Bauhaus-Universität Weimar, Weimar, Germany, 2020; p. XXI, 200. [Google Scholar]
- Alujas Diaz, A.; Almenares Reyes, R.S.; Hanein, T.; Irassar, E.F.; Juenger, M.; Kanavaris, F.; Maier, M.; Marsh, A.T.M.; Sui, T.; Thienel, K.-C.; et al. Properties and occurrence of clay resources for use as supplementary cementitious materials: A paper of RILEM TC 282-CCL. Mater. Struct. 2022, 55, 139. [Google Scholar] [CrossRef]
- Msinjili, N.S.; Gluth, G.J.G.; Sturm, P.; Vogler, N.; Kühne, H.-C. Comparison of calcined illitic clays (brick clays) and low-grade kaolinitic clays as supplementary cementitious materials. Mater. Struct. 2019, 52, 94. [Google Scholar] [CrossRef]
- Danner, T.; Norden, G.; Justnes, H. Calcareous smectite clay as a pozzolanic alternative to kaolin. Eur. J. Environ. Civ. Eng. 2019, 25, 1647–1664. [Google Scholar] [CrossRef]
- He, C.; Osbæck, B.; Makovicky, E. Pozzolanic reactions of six principal clay minerals: Activation, reactivity assessments and technological effects. Cem. Concr. Res. 1995, 25, 1691–1702. [Google Scholar] [CrossRef]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Danner, T. Reactivity of Calcined Clays. Ph.D. Thesis, Faculty of Natural Science and Technology, Department of Natural Sciences and Engineering, NTNU, Trondheim, Norway, 2013; p. 229. [Google Scholar]
- Sowoidnich, T.; Hoffmann, C.; Rößler, C.; Ludwig, H.-M. Reaktivitätsuntersuchungen von calcinierten Tonen mit geringem Metakaolingehalt. Ce/Pap. 2023, 6, 400–404. [Google Scholar] [CrossRef]
- Antoni, M.; Rossen, J.; Martirena, F.; Scrivener, K. Cement substitution by a combination of metakaolin and limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Rodriguez, C.; Tobon, J.I. Influence of calcined clay/limestone, sulfate and clinker proportions on cement performance. Constr. Build. Mater. 2020, 251, 119050. [Google Scholar] [CrossRef]
- Hay, R.; Li, L.; Celik, K. Shrinkage, hydration, and strength development of limestone calcined clay cement (LC3) with different sulfation levels. Cem. Concr. Compos. 2022, 127, 104403. [Google Scholar] [CrossRef]
- Canbek, O.; Szeto, C.; Washburn, N.R.; Kurtis, K.E. A quantitative approach to determining sulfate balance for LC3. Cement 2023, 12, 100063. [Google Scholar] [CrossRef]
- Dhoopadahalli, G.R.; Krishnan, S.; Bishnoi, S. Influence of Calcium Sulphate on Hydration of Cements Containing Calcined Clay. In Calcined Clays for Sustainable Concrete; Bishnoi, S., Ed.; Springer: Singapore, 2020; Volume 25, pp. 315–322. [Google Scholar]
- Zunino, F.; Scrivener, K. The reaction between metakaolin and limestone and its effect in porosity refinement and mechanical properties. Cem. Concr. Res. 2021, 140, 106307. [Google Scholar] [CrossRef]
- Silva, M.R.; Andrade Neto, J.; Walkley, B.; Kirchheim, A. Exploring sulfate optimization techniques in Limestone Calcined Clay Cements (LC3): Limitations and insights. Cem. Concr. Res. 2023, 175, 107375. [Google Scholar] [CrossRef]
- Silva, M.; Neto, J.S.A.; Walkley, B.; Kirchheim, A.P. Effects of kaolinite and montmorillonite calcined clays on the sulfate balance, early hydration, and artiifcial pore solution of limestone calcined clay cements (LC3). Mater. Struct. 2024, 57, 187. [Google Scholar] [CrossRef]
- Scrivener, K.; Avet, F.; Maraghechi, H.; Zunino, F.; Ston, J.; Hanpongpun, W.; Favier, A. Impacting factors and properties of limestone calcined clay cements (LC3). Green Mater. 2019, 7, 3–14. [Google Scholar] [CrossRef]
- Sandberg, P.; Bishnoi, S. Sulphate Optimization of Binders with Calcined Clay Using Isothermal Calorimetry. In Calcined Clays for Sustainable Concrete—Proceedings of the 2nd International Conference on Calcined Clays for Sustainable Concrete; Martirena, F., Favier, A., Scrivener, K., Eds.; Springer Nature: La Havanna, Cuba, 2018; pp. 422–426. [Google Scholar] [CrossRef]
- Atasever, M.; Erdoğan, S.T. Effects of clay type and component fineness on the hydration and properties of limestone calcined clay cement. Mater. Struct. 2024, 57, 183. [Google Scholar] [CrossRef]
- Goldenberg Py, L.; Andrade Neto, J.; Longhi, M.; Kirchheim, A. Effects of different calcined kaolinite clays on the sulfate demand of LC3 cements. In Proceedings of the 16th International Congress on the Chemistry of Cement 2023 (ICCC 2023), Bangkok, Thailand, 18–22 September 2023. [Google Scholar]
- Py, L.G.; Neto, J.S.A.; Longhi, M.A.; Kirchheim, A.P. Evaluation of ultrafine calcined clays on LC3 cements on the sulfate requirement, water demand and strength development. Mater. Struct. 2024, 57, 13. [Google Scholar] [CrossRef]
- Hesse, C. Der Reaktionsverlauf der Frühen Hydratation von Portlandzement in Relation zur Temperatur. Ph.D. Thesis, Naturwissenschaftliche Fakultät, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 2009. [Google Scholar]
- Lin, R.-S.; Park, K.-B.; Wang, X.-Y.; Zhang, G.-Y. Increasing the early strength of high-volume Hwangtoh–cement systems using bassanite. J. Build. Eng. 2020, 30, 101317. [Google Scholar] [CrossRef]
- Maier, M.; Scherb, S.; Thienel, K.-C. Sulfate consumption during the hydration of Alite and its influence by SCMs. Ce/Pap. 2023, 6, 2–7. [Google Scholar] [CrossRef]
- Shi, Z.; Ferreiro, S.; Lothenbach, B.; Geiker, M.R.; Kunther, W.; Kaufmann, J.; Herfort, D.; Skibsted, J. Sulfate resistance of calcined clay—Limestone—Portland cements. Cem. Concr. Res. 2019, 116, 238–251. [Google Scholar] [CrossRef]
- Avet, F.; Scrivener, K. Investigation of the calcined kaolinite content on the hydration of Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 124–135. [Google Scholar] [CrossRef]
- Beuntner, N. Zur Eignung und Wirkungsweise Calcinierter Tone als Reaktive Bindemittelkomponente in Zement (On the Suitability and Mode of Action of Calcined Clays as Reactive Binder Components in Cement). Ph.D. Thesis, Fakultät für Bauingenieurwesen und Umweltwissenschaften, Universität der Bundeswehr München, Neubiberg, Germany, 2017; p. 207. [Google Scholar]
- Tang, J.; Wei, S.; Li, W.; Ma, S.; Ji, P.; Shen, X. Synergistic effect of metakaolin and limestone on the hydration properties of Portland cement. Constr. Build. Mater. 2019, 223, 177–184. [Google Scholar] [CrossRef]
- Sabir, B.B.; Wild, S.; Bai, J. Metakaolin and calcined clays as pozzolans for concrete: A review. Cem. Concr. Compos. 2001, 23, 441–454. [Google Scholar] [CrossRef]
- Hollanders, S.; Adriaens, R.; Skibsted, J.; Cizer, Ö.; Elsen, J. Pozzolanic reactivity of pure calcined clays. Appl. Clay Sci. 2016, 132–133, 552–560. [Google Scholar] [CrossRef]
- Mishra, G.; Emmanuel, A.C.; Bishnoi, S. Influence of temperature on hydration and microstructure properties of limestone-calcined clay blended cement. Mater. Struct. 2019, 52, 13. [Google Scholar] [CrossRef]
- Zunino, F.; Martirena Hernández, F.; Scrivener, K. Limestone Calcined Clay Cements (LC3). ACI Mater. J. 2021, 118, 49–60. [Google Scholar] [CrossRef]
- Sharma, M.; Bishnoi, S.; Martirena, F.; Scrivener, K. Limestone calcined clay cement and concrete: A state-of-the-art review. Cem. Concr. Res. 2021, 149, 106564. [Google Scholar] [CrossRef]
- Zunino, F.; Scrivener, K. Recent advances in understanding the hydration of limestone calcined clay cements (LC3). In Proceedings of the 16th International Congress on the Chemistry of Cement 2023 (ICCC 2023), Bangkok, Thailand, 18–22 September 2023. [Google Scholar]
- Lin, R.S.; Lee, H.S.; Han, Y.; Wang, X.Y. Experimental studies on hydration–strength–durability of limestone-cement-calcined Hwangtoh clay ternary composite. Constr. Build. Mater. 2021, 269, 121290. [Google Scholar] [CrossRef]
- Zunino, F.A. Limestone Calcined Clay Cements (LC3): Raw Material Processing, Sulfate Balance and Hydration Kinetics. Ph.D. Thesis, Faculté des Sciences et Techniques de L’ingénieur, EPFL, Lausanne, Switzerland, 2020; p. 211. [Google Scholar]
- Ito, A.; Wagai, R. Global distribution of clay-size minerals on land surface for biogeochemical and climatological studies. Sci. Data 2017, 4, 170103. [Google Scholar] [CrossRef]
- Buchwald, A.; Kriegel, R.; Kaps, C.; Zellmann, H.-D. Untersuchung zur Reaktivität von Metakaolinen für die Verwendung in Bindemittelsystemen. In Proceedings of the 5. Tagung Bauchemie, München, Germany, 9–10 October 2003; Gesellschaft Deutscher Chemiker e.V.: Frankfurt, Germany, 2003; Volume 27, pp. 91–97. [Google Scholar]
- Marsh, B.K.; Day, R.L. Pozzolanic and cementitious reactions of fly ash in blended cement pastes. Cem. Concr. Res. 1988, 18, 301–310. [Google Scholar] [CrossRef]
- Bazzoni, A.; Berodier, E.M.J.; Bizzozero, J.; Bowen, P.; de Weerdt, K.; Durdziński, P.; Geiker, M.; Herfort, D.; Kazemi-Kamyab, H.; Lothenbach, B.; et al. A Practical Guide to Microstructural Analysis of Cementitious Materials, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016; p. 558. [Google Scholar]
- DIN 66133; Bestimmung der Porenvolumenverteilung und der Spezifischen Oberfläche von Feststoffen Durch Quecksilberintrusion (Determination of Pore Volume Distribution and Specific Surface Area of Solids by Mercury Intrusion). Beuth Verlag: Berlin, Germany, 1993; p. 3.
- Setzer, M.J. Zum Mikrogefüge des Zementsteins und dessen Einfluss auf das mechanische Verhalten des Betons. Zem. Und Beton 1975, 85/86, 29–35. [Google Scholar]
- Zhang, Y.; Wu, K.; Yang, Z.; Ye, G. A reappraisal of the ink-bottle effect and pore structure of cementitious materials using intrusion-extrusion cyclic mercury porosimetry. Cem. Concr. Res. 2022, 161, 106942. [Google Scholar] [CrossRef]
- Tibbetts, C.M.; Tao, C.; Paris, J.M.; Ferraro, C.C. Mercury intrusion porosimetry parameters for use in concrete penetrability qualification using the Katz-Thompson relationship. Constr. Build. Mater. 2020, 263, 119834. [Google Scholar] [CrossRef]
- Canbek, O.; Washburn, N.R.; Kurtis, K.E. Relating LC3 microstructure, surface resistivity and compressive strength development. Cem. Concr. Res. 2022, 160, 106920. [Google Scholar] [CrossRef]
- Shao, Z.; Cao, M.; Zheng, X. Limestone particle sizes and sulfate impact on the early hydration of Limestone Calcined Clay Cement. J. Build. Eng. 2024, 97, 110848. [Google Scholar] [CrossRef]
- Tironi, A.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Incorporation of Calcined Clays in Mortars: Porous Structure and Compressive Strength. Procedia Mater. Sci. 2012, 1, 366–373. [Google Scholar] [CrossRef]
- Ioannidou, K. Mesoscale Structure and Mechanics of C-S-H. In Handbook of Materials Modeling; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Kundt, G.; Krentz, H.; Glass, Ä. Epidemiologie und Medizinische Biometrie (Epidemiology and Medical Biometry); Berichte aus der Statistik; Shaker Verlag: Aachen, Germany, 2011; p. 246. [Google Scholar]
- Tironi, A.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Kaolinitic calcined clays: Factors affecting its performance as pozzolans. Constr. Build. Mater. 2012, 28, 276–281. [Google Scholar] [CrossRef]
- Antoni, M. Investigation of Cement Substitution by Blends of Calcined Clays and Limestone. Ph.D. Thesis, Faculté des Sciences et Techniques de L’ingénieur, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 2013; p. 254. [Google Scholar]





| Material | Cumulative Heat Release [J/g Calcined Clay] | Ion Solubility in Alkaline Solution at 20 h [mmol/L] | ||
|---|---|---|---|---|
| 24 h | 168 h | Al | Si | |
| PP | 776 | 917 | 14.6 | 15.6 |
| Ill-E | 150 | 242 | 2.7 | 5.9 |
| Smk | 123 | 380 | 2.3 | 5.5 |
| Replacement | CEM I 42.5 N | PP | Ill-E | Smk | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sca [wt%] | 0 | 1 | 2 | 3 | 4 | 5 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 0 | 1 | 2 | 3 | 4 | 5 | 0 | 1 | 2 | 3 | 4 | 5 | |
| Microstructural investigations | 0 wt% | X | X | X | X | X | |||||||||||||||||||||||
| 20 wt% | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||
| 40 wt% | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||
| 0 wt% Replacement | 20 wt% Replacement | 40 wt% Replacement | |||
|---|---|---|---|---|---|
| Sca [wt%] | Cement [wt%] | Cement [wt%] | Calcined Clay [wt%] | Cement [wt%] | Calcined Clay [wt%] |
| 0 | 100.0 | 80.0 | 20.0 | 60.0 | 40.0 |
| 1 | 99.0 | 79.2 | 19.8 | 59.4 | 39.6 |
| 2 | 98.0 | 78.4 | 19.6 | 58.8 | 39.2 |
| 3 | 97.0 | 77.6 | 19.4 | 58.2 | 38.8 |
| 5 | 95.0 | 76.0 | 19.0 | 57.0 | 38.0 |
| 6 | 75.2 | 18.8 | 56.4 | 37.6 | |
| 9 | 54.6 | 36.4 | |||
| Variations of Sca for Microstr. Invest. | No Sca | Medium Sca | Highest Sca | Optimal Sca | ||||
|---|---|---|---|---|---|---|---|---|
| Replacement Level of Calcined Clay | 20 wt% | 40 wt% | 20 wt% | 40 wt% | 20 wt% | 40 wt% | 20 wt% | 40 wt% |
| PP blends | 0 | 3 | 5 | 6 | 9 | 6 | 9 | |
| Ill-E blends/Smk blends | 0 | 1 | 2 | 3 | 5 | 1 | 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panzer, M.; Scherb, S.; Beuntner, N.; Thienel, K.-C. Effect of Sulfate Carrier Addition on the Microstructure of Calcined Clay Blended Cements. Materials 2025, 18, 4972. https://doi.org/10.3390/ma18214972
Panzer M, Scherb S, Beuntner N, Thienel K-C. Effect of Sulfate Carrier Addition on the Microstructure of Calcined Clay Blended Cements. Materials. 2025; 18(21):4972. https://doi.org/10.3390/ma18214972
Chicago/Turabian StylePanzer, Maximilian, Sebastian Scherb, Nancy Beuntner, and Karl-Christian Thienel. 2025. "Effect of Sulfate Carrier Addition on the Microstructure of Calcined Clay Blended Cements" Materials 18, no. 21: 4972. https://doi.org/10.3390/ma18214972
APA StylePanzer, M., Scherb, S., Beuntner, N., & Thienel, K.-C. (2025). Effect of Sulfate Carrier Addition on the Microstructure of Calcined Clay Blended Cements. Materials, 18(21), 4972. https://doi.org/10.3390/ma18214972






