Valorization of Recycled Gypsum from CDW in Green Binder Systems
Abstract
1. Introduction
2. Blended Green Binder Systems Based on Gypsum
3. Materials and Methods
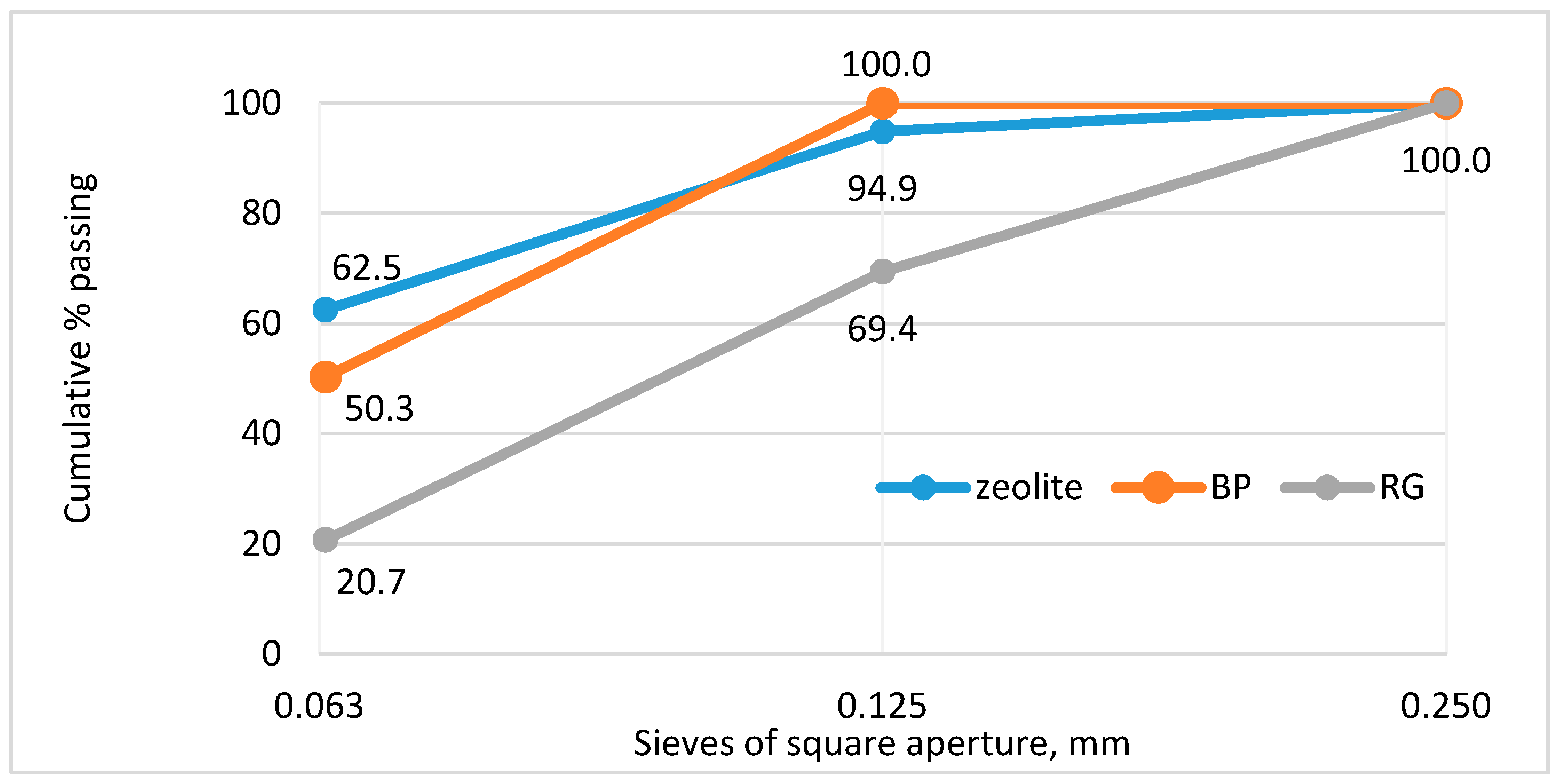
3.1. Materials
3.1.1. Recycled Gypsum (RG)
3.1.2. Portland Cement
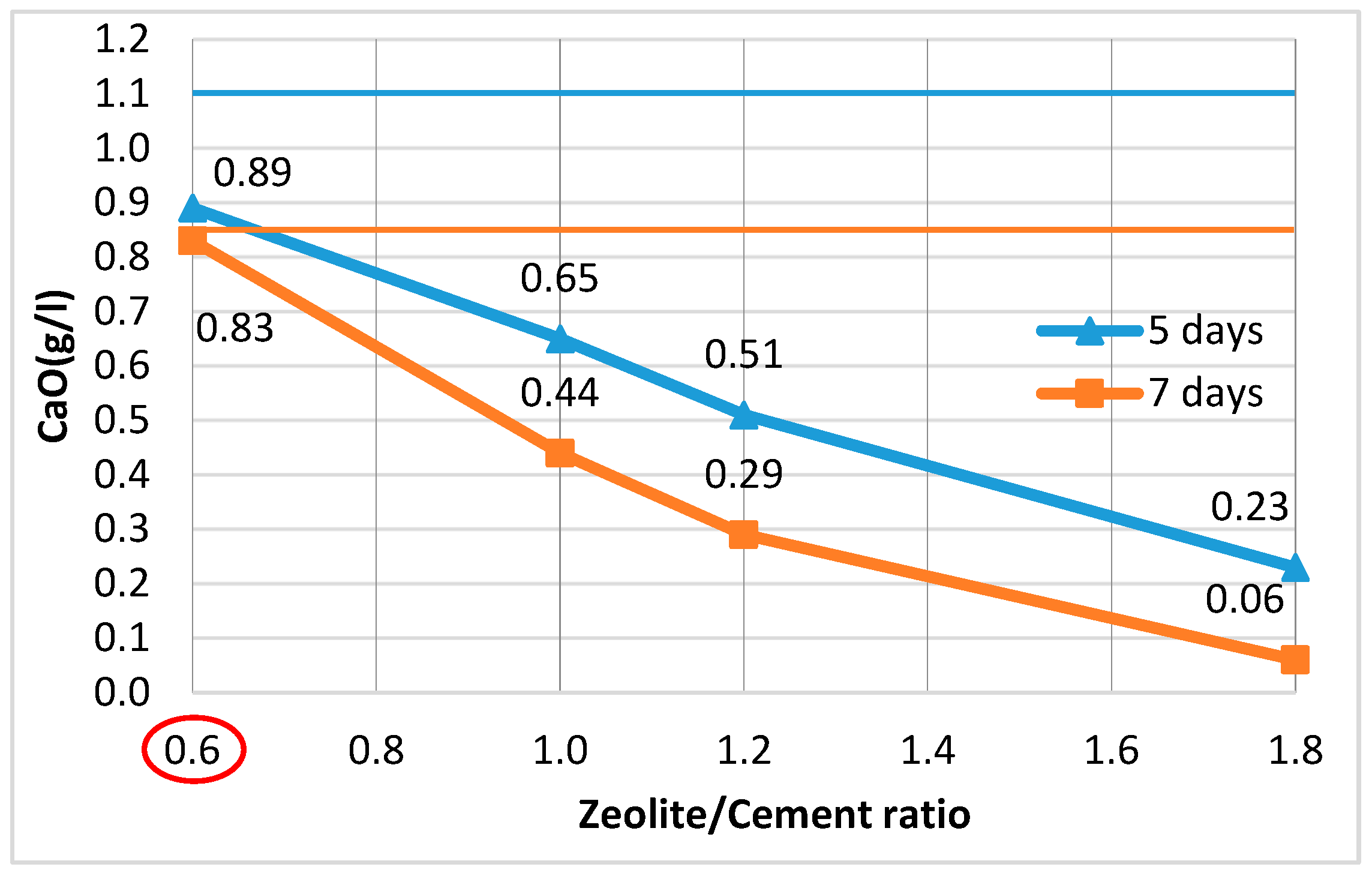
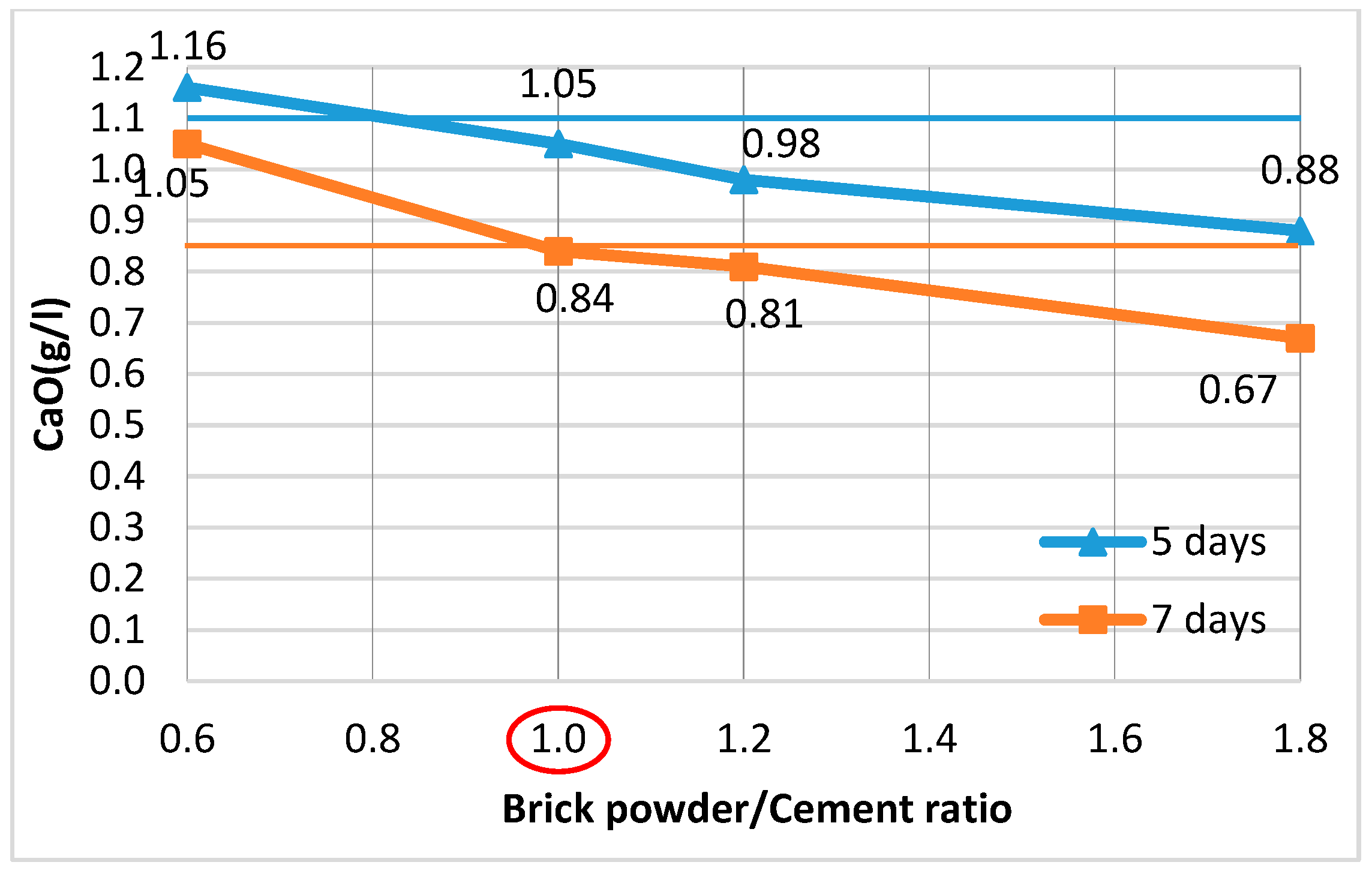
3.1.3. Pozzolanic Additives (PAs)
3.1.4. Chemical Admixture
3.2. Mix Design
3.2.1. Composition Ratio of the Binder Components
- A—the amount of hydrochloric acid used for titration, ml;
- T—the titer of hydrochloric acid (HCl content in g/mL);
- B—the amount of binder subjected to titration, mL.
3.2.2. Water-to-Binder Ratio and Plasticizing Admixture Amount
3.3. Samples Preparation and Treatment
- the first, typically applied to gypsum binders, referred to as “air curing,” at a temperature of 23 ± 2 °C and RH of 50 ± 5%;
- the second, typically applied to Portland cement, referred to as “water curing”, in which the specimens are stored under water at a temperature of 20 ± 1 °C. The samples cured in water are designated with an additional letter W (e.g., GCZ1-W).
3.4. Methods
3.4.1. XRD Analysis
3.4.2. Determination of Properties of Fresh Pastes
3.4.3. Determination of Properties of Hardened Pastes
- —compressive strength of specimens after air curing;
- —compressive strength of specimens after water curing.
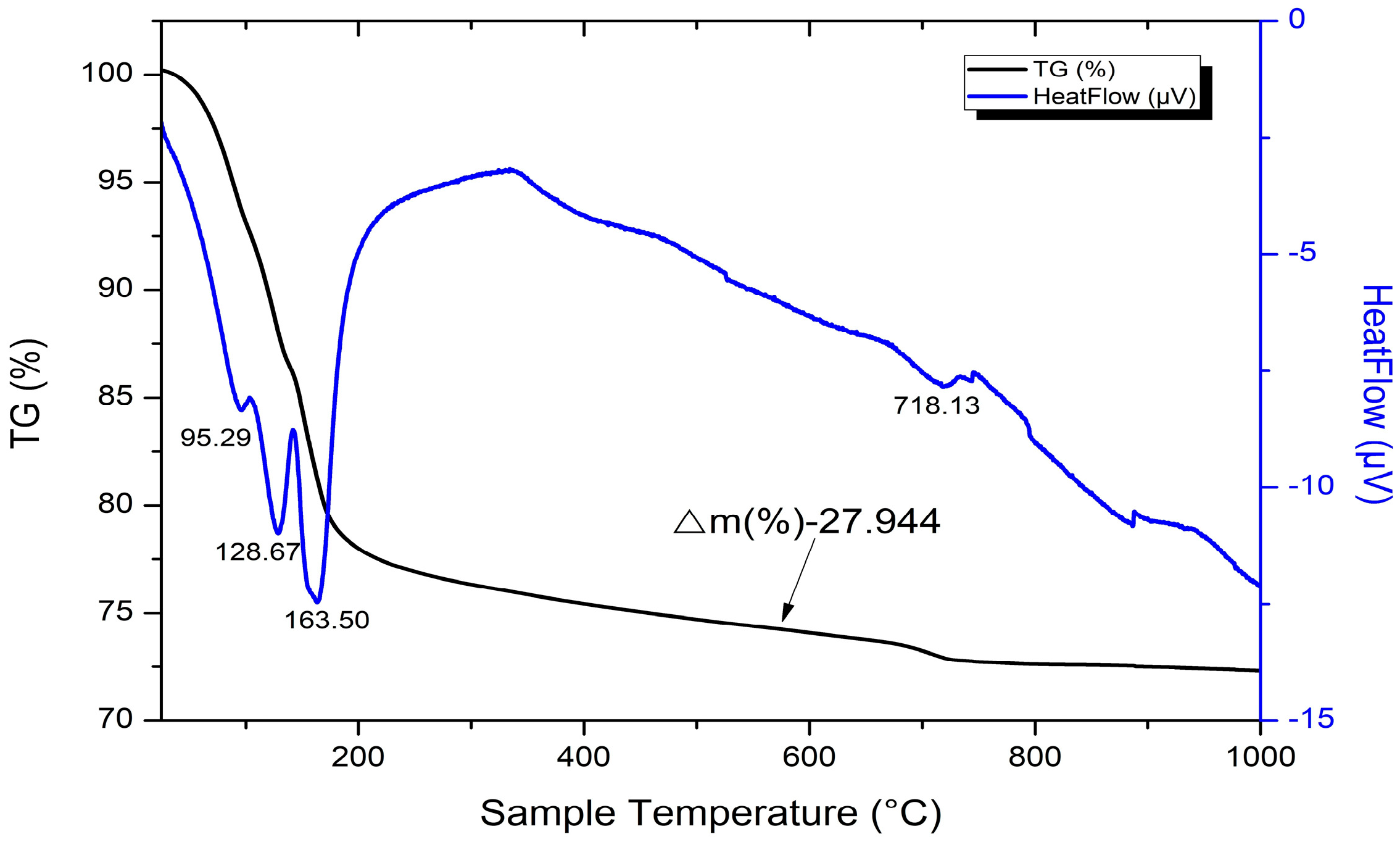
3.4.4. Combined Differential Thermal Analysis and Thermogravimetry (DTA/TG)
4. Results and Discussion
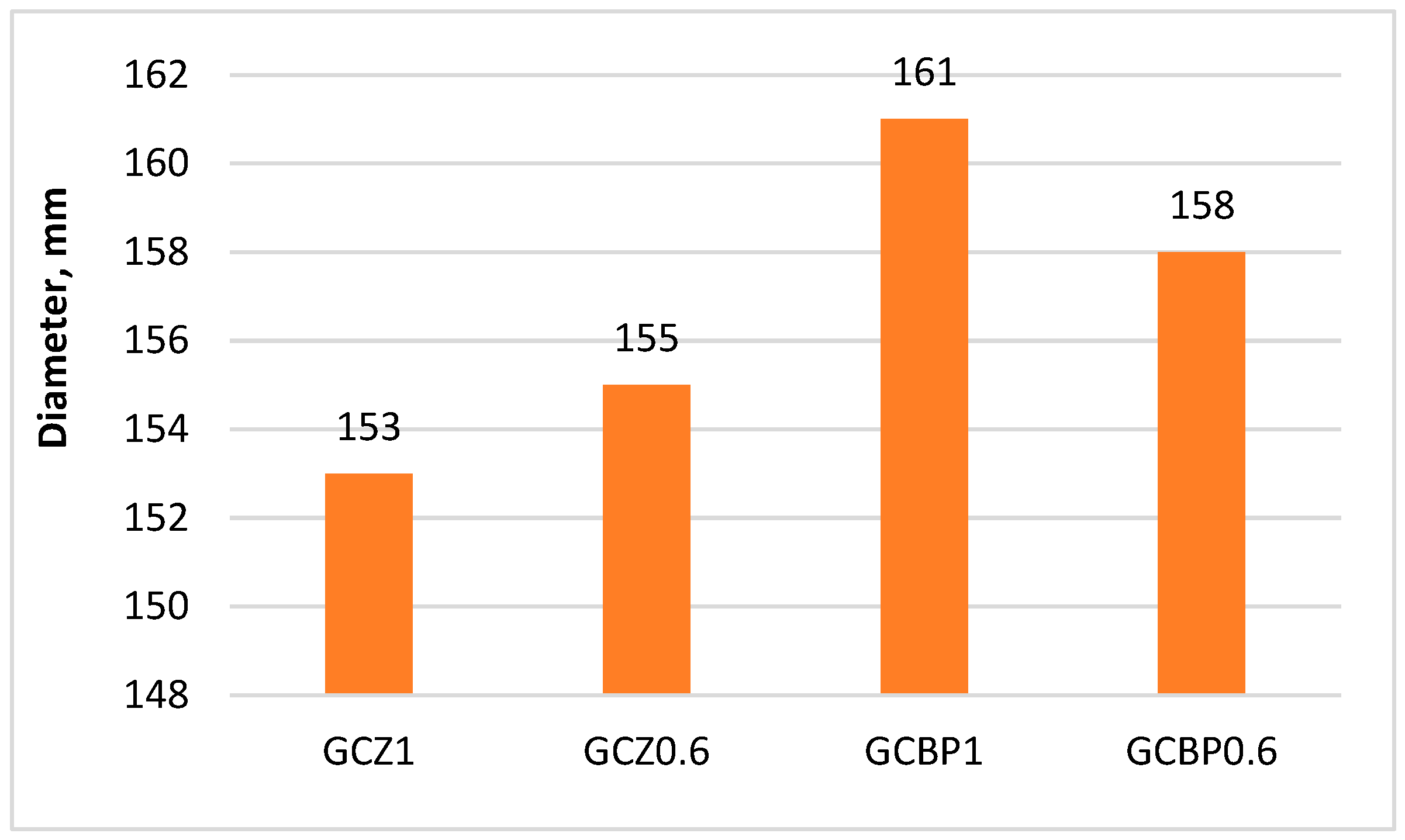
4.1. Setting Time of GCPBs
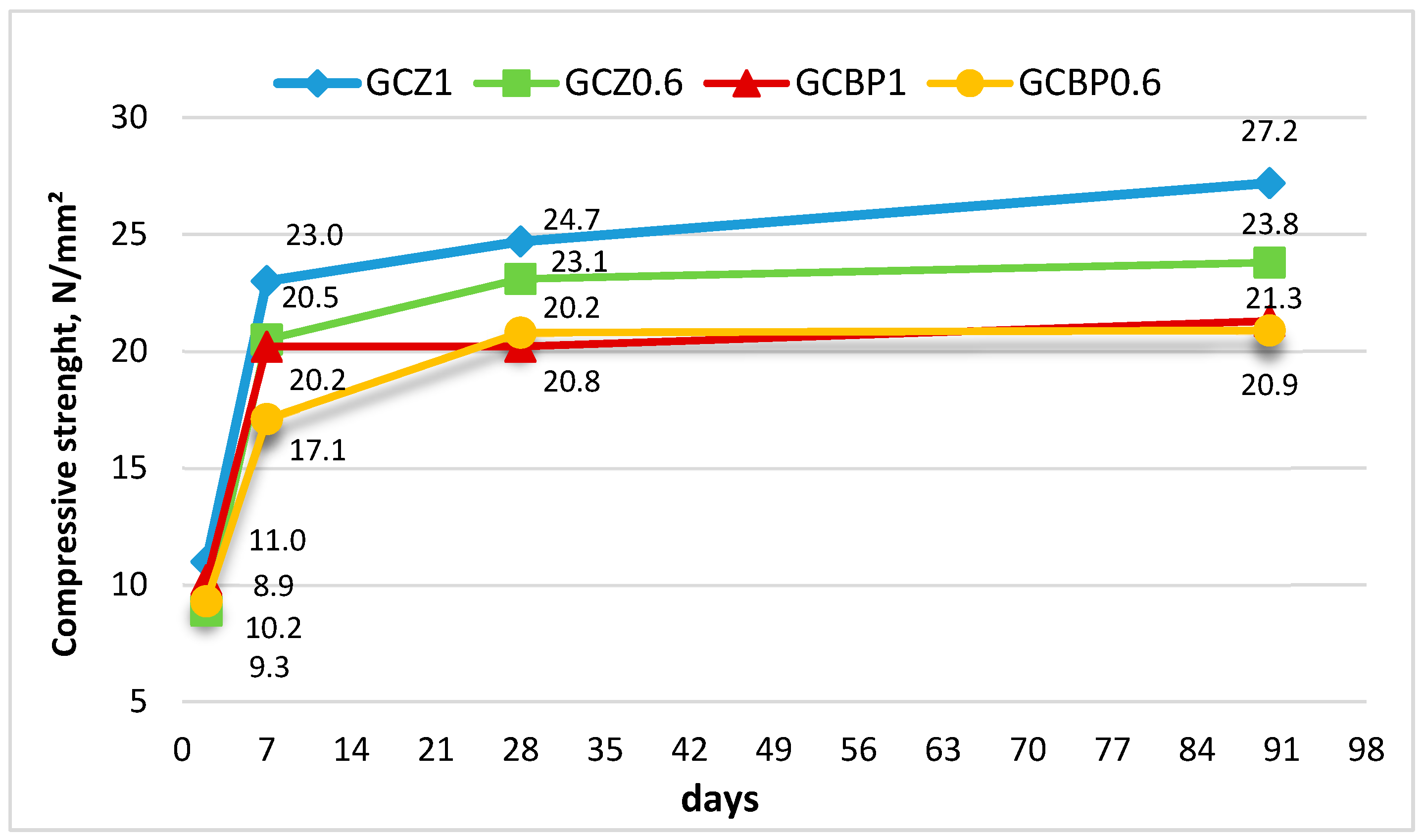
4.2. Compressive Strength Kinetics
4.2.1. Hardening Under Air Curing
4.2.2. Hardening Under Water Curing
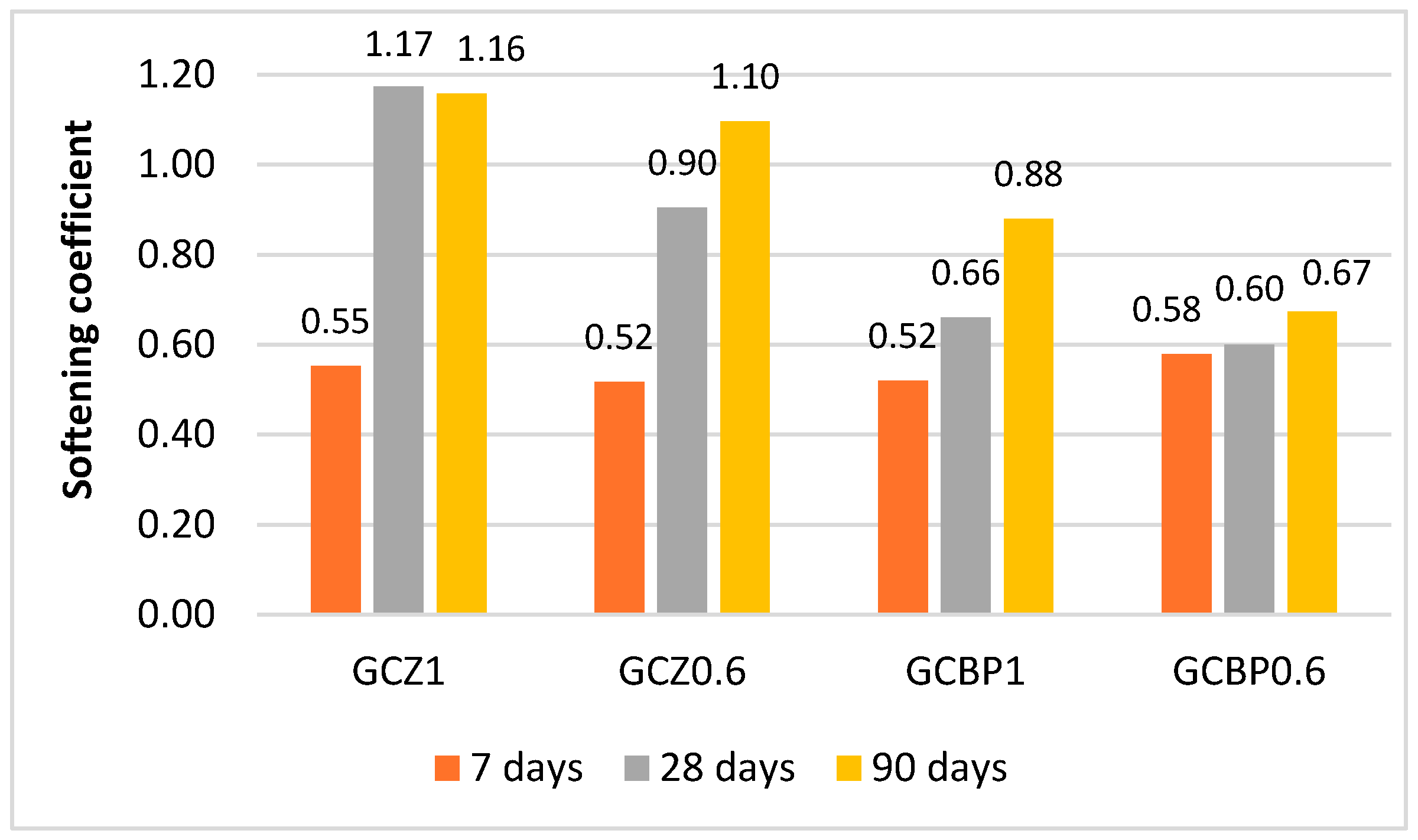
4.3. Water Resistance
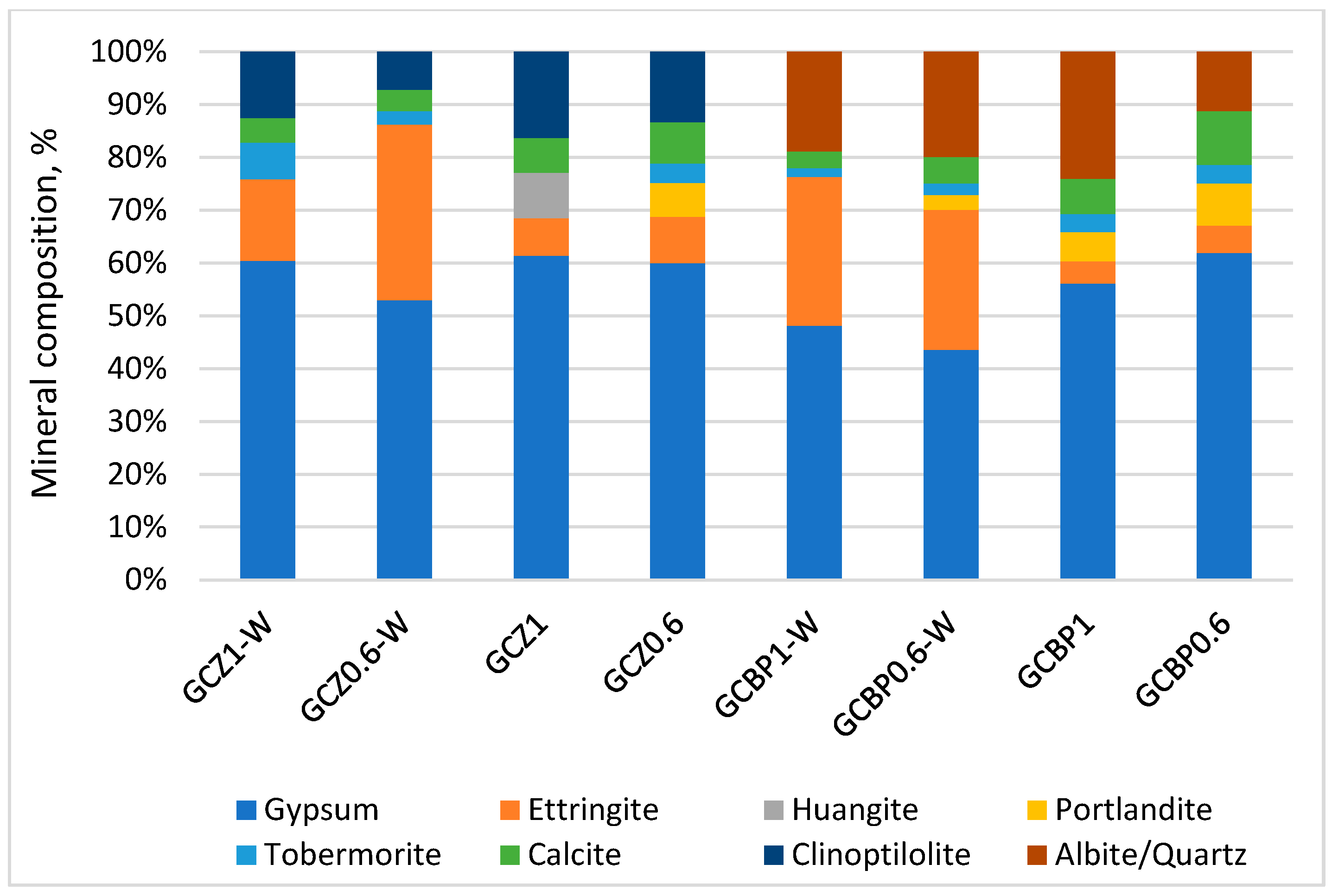
4.4. XRD
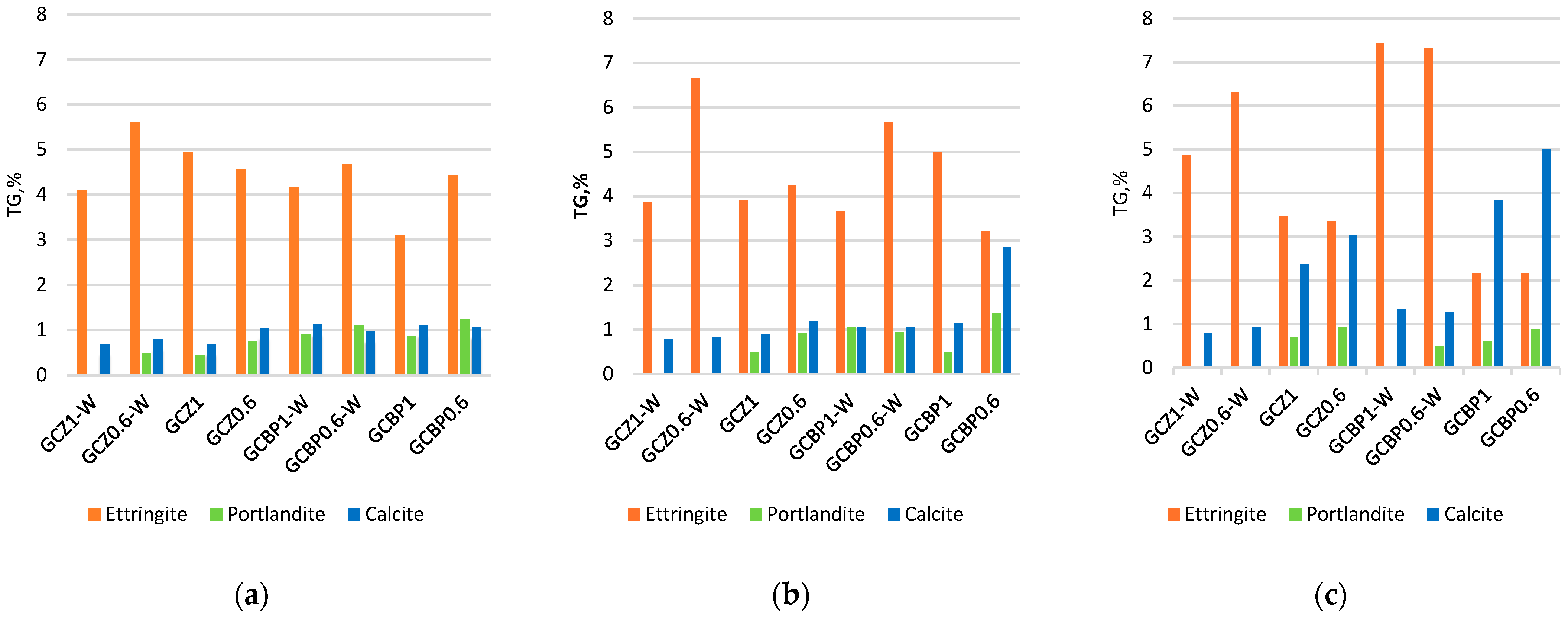
4.5. DTA/TG
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BP | Brick powder |
| CaO | Calcium oxide |
| CCG | Conventional construction gypsum |
| CDW | Construction and demolition waste |
| CH | Calcium hydroxide |
| C-S-H | Calcium Silicate Hydrates |
| HCl | Hydrochloric acid |
| EN | European Norm |
| DTA/TG | Combined Differential Thermal Analysis and Thermogravimetry |
| G | Gypsum |
| GCPB | Gypsum-cement-pozzolan binder(s) |
| OPC | Ordinary Portland cement |
| PA | Pozzolanic additive(s) |
| RG | Recycled gypsum |
| XRD | X-ray diffraction |
| Z | Natural zeolite |
References
- Supriya; Chaudhury, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. Low-CO2 emission strategies to achieve net zero target in cement sector. J. Clean. Prod. 2023, 417, 137466. [Google Scholar] [CrossRef]
- Pitre, V.; La, H.; Bergerson, J.A. Impacts of alternative fuel combustion in cement manufacturing: Life cycle greenhouse gas, biogenic carbon, and criteria air contaminant emissions. J. Clean. Prod. 2024, 475, 143717. [Google Scholar] [CrossRef]
- Salaripoor, H.; Yousefi, H.; Abdoos, M. Life cycle environmental assessment of Refuse-Derived Fuel (RDF) as an alternative to fossil fuels in cement production: A sustainable approach for mitigating carbon emissions. Fuel Commun. 2025, 22, 100135. [Google Scholar] [CrossRef]
- Adediran, A.; Rajczakowska, M.; Steelandt, A.; Novakova, I.; Cwirzen, A.; Perumal, P. Conventional and potential alternative non-conventional raw materials available in Nordic countries for low-carbon concrete: A review. J. Build. Eng 2025, 104, 112384. [Google Scholar] [CrossRef]
- Bumanisa, G.; Vitola, L.; Stipnieceb, L.; Locsb, J.; Korjakinsa, A.; Bajare, D. Evaluation of Industrial by-products as pozzolans: A road map for use in concrete production. Case Stud. Constr. Mater. 2020, 13, e00424. [Google Scholar] [CrossRef]
- Bacatelo, M.; Capucha, F.; Ferrão, P.; Margarido, F. Selection of a CO2 capture technology for the cement industry: An integrated TEA and LCA methodological framework. J. CO2 Util. 2023, 68, 102375. [Google Scholar] [CrossRef]
- Plaza, M.G.; Martínez, S.; Rubiera, F. CO2 Capture, Use, and Storage in the Cement Industry: State of the Art and Expectations. Energies 2020, 13, 5692. [Google Scholar] [CrossRef]
- Elkhaldi, I.; Rozière, E.; Loukili, A. To what extent does decreasing the proportion of clinker in cement production effectively decrease its carbon footprint ? Mater. Today—Proc. 2023. [Google Scholar] [CrossRef]
- Okoye, F.N. Geopolymer binder: A veritable alternative to Portland cement. Mater. Today Proc. 2017, 4, 5599–5604. [Google Scholar] [CrossRef]
- Bezerra, B.P.; Luz, A.P. Geopolymers: A viable binder option for ultra-low-cement and cement-free refractory castables ? J. Eur. Ceram. Soc. 2024, 44, 5241–5251. [Google Scholar] [CrossRef]
- Snellings, R.; Suraneni, P.; Skibsted, J. Future and emerging supplementary cementitious materials. Cem. Concr. Res. 2023, 171, 107199. [Google Scholar] [CrossRef]
- Velosa, A.L.; Cachim, P.B. Hydraulic-lime based concrete: Strength development using a pozzolanic addition and different curing conditions. Constr. Build. Mater. 2009, 23, 2107–2111. [Google Scholar] [CrossRef]
- Grist, E.R.; Paine, K.A.; Heath, A.; Norman, J.; Pinder, H. Compressive strength development of binary and ternary lime–pozzolan mortars. Mater. Des. 2013, 52, 514–523. [Google Scholar] [CrossRef]
- Scheinherrová, L.; Doleželová, M.; Vimmrová, A.; Vejmelková, E.; Jerman, M.; Pommer, V.; Černý, R. Fired clay brick waste as low cost and eco-friendly pozzolana active filler in gypsum-based binders. J. Clean. Prod. 2022, 368, 133142. [Google Scholar] [CrossRef]
- Wan, Y.; Hui, X.; He, X.; Li, J.; Xue, J.; Feng, D.; Liu, X.; Wang, S. Performance of green binder developed from flue gas desulfurization gypsum incorporating Portland cement and large-volume fly ash. Constr. Build. Mater. 2022, 348, 128679. [Google Scholar] [CrossRef]
- Grist, E.R.; Paine, K.A.; Heath, A.; Norman, J.; Pinder, H. The environmental credentials of hydraulic lime-pozzolan concretes. J. Clean. Prod. 2015, 93, 26–37. [Google Scholar] [CrossRef]
- Zapata, J.F.; Azevedo, A.; Fontes, C.; Monteiro, S.N.; Colorado, H.A. Environmental Impact and Sustainability of Calcium Aluminate Cements. Sustainability 2022, 14, 2751. [Google Scholar] [CrossRef]
- Akerele, D.D.; Aguayo, F. Evaluating the Impact of CO2 on Calcium SulphoAluminate (CSA) Concrete. Buildings 2024, 14, 2462. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions; The European Green Deal. COM/2019/640 final; European Union: Brussels, Belgium, 2019; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52019DC0640&qid=1754146316522 (accessed on 9 September 2025).
- Walters, J.P.; Véliz, K.; Vargas, M.; Busco, C. A systems-focused assessment of policies for circular economy in construction demolition waste management in the Aysén region of Chile. Sustain. Futures 2024, 7, 100186. [Google Scholar] [CrossRef]
- European Parlament. Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain DIRECTIVES (Text with EEA Relevance); European Union: Brussels, Belgium, 2008; Available online: http://data.europa.eu/eli/dir/2008/98/oj (accessed on 9 September 2025).
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; Roadmap to a Resource Efficient Europe. COM/2011/0571 final; European Union: Brussels, Belgium, 2011; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52011DC0571&qid=1754144311736 (accessed on 9 September 2025).
- European Commission. Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs, EU Construction & Demolition Waste Management Protocol Including Guidelines for Pre-Demolition and Pre-Renovation Audits of Construction Works—Updated Edition 2024; Publications Office of the European Union: Luxembourg, 2024; Available online: https://data.europa.eu/doi/10.2873/77980 (accessed on 9 September 2025).
- Selvaraj, S.; Tak-Ming, C. Recommendations for Implementing Circular Economy in Construction: Direct Reuse of Steel Structures. J. Constr. Steel Res. 2024, 214, 108439. [Google Scholar] [CrossRef]
- Purchase, C.K.; Al Zulayq, D.M.; O’Brien, B.T.; Kowalewski, M.J.; Berenjian, A.; Tarighaleslami, A.H.; Seifan, M. Circular Economy of Construction and Demolition Waste: A Literature Review on Lessons, Challenges, and Benefits. Construction Circular Economy: Recycling Construction and Demolition Wastes. Materials 2022, 15, 76. [Google Scholar] [CrossRef]
- Likes, L.; Markandeya, A.; Haider, M.M.; Bollinger, D.; McCloy, J.S.; Nassiri, S. Recycled concrete and brick powders as supplements to Portland cement for more sustainable concrete. J. Clean. Prod. 2022, 364, 132651. [Google Scholar] [CrossRef]
- Sakthibala, R.K.; Vasanthi, P.; Hariharasudhan, C.; Partheeban, P. A critical review on recycling and reuse of construction and demolition waste materials. Clean. Waste Sys. 2025, 12, 100375. [Google Scholar] [CrossRef]
- Arshad, M.; Ahmed, M.F. Potential use of reclaimed asphalt pavement and recycled concrete aggregate in base/subbase layers of flexible pavements. Constr. Build. Mater. 2017, 151, 83–97. [Google Scholar] [CrossRef]
- Li, Z.; Xu, K.; Peng, J.; Wang, J.; Zhang, J.; Li, Q. Study on mechanical strength and water resistance of organosilicon waterproofing agent blended recycled gypsum plaster. Case Stud. Constr. Mater. 2021, 14, e00546. [Google Scholar] [CrossRef]
- European Parliament. Directive (EU) 2018/851 of the European Parliament and of the Council of 30 May 2018 Amending Directive 2008/98/EC on Waste (Text with EEA Relevance), PE/11/2018/REV/2, OJ L 150. 14 June 2018, pp. 109–140. Available online: https://data.europa.eu/eli/dir/2018/851/oj (accessed on 14 October 2025).
- Rivero, A.J.; Sathre, R.; Navarro, J.G. Life cycle energy and material flow implications of gypsum plasterboard recycling in the European Union. Resour. Conserv. Recycle. 2016, 108, 171–181. [Google Scholar] [CrossRef]
- Weimann, K.; Adam, C.; Buchert, M.; Sutter, J. Environmental Evaluation of Gypsum, Plasterboard Recycling. Minerals 2021, 11, 101. [Google Scholar] [CrossRef]
- Rumo, D. Forgotten dust: Following plasterboard for non-destructive circular economies. Front. Sustain. 2023, 4, 994452. [Google Scholar] [CrossRef]
- KNAUF. Closing the Loop. News of 30 May 2023. Available online: https://knauf.com/en-GB/news/knauf-gypsum/closing-the-loop (accessed on 11 July 2025).
- Geraldo, R.H.; Pinheiro, S.M.M.; Silva, J.S.; Andrade, H.M.C.; Dweck, J.; Gonçalves, J.P.; Camarini, G. Gypsum plaster waste recycling: A potential environmental and industrial solution. J. Clean. Prod. 2017, 164, 288–300. [Google Scholar] [CrossRef]
- European Commission. Gy.Eco Project. Reference: LIFE10 ENV/IT/000356; European Union: Brussels, Belgium, 2011; Available online: https://webgate.ec.europa.eu/life/publicWebsite/project/LIFE10-ENV-IT-000356/gyproc-eco-friendly (accessed on 9 September 2025).
- European Commission. GtoG Project. Reference: LIFE11 ENV/BE/001039; European Union: Brussels, Belgium, 2013; Available online: https://webgate.ec.europa.eu/life/publicWebsite/project/LIFE11-ENV-BE-001039/gtog-from-production-to-recycling-a-circular-economy-for-the-european-gypsum-industry-with-the-demolition-and-recycling-industry (accessed on 9 September 2025).
- Xu, Q.; Townsend, T.; Bitton, G. Inhibition of hydrogen sulfide generation from disposed gypsum drywall using chemical inhibitors. J. Hazard. Mater. 2011, 191, 204–211. [Google Scholar] [CrossRef]
- Zaharieva, R.; Simonov, B. Influence of Technological Parameters in Recycling of plasterboard Waste on the Properties of Recycled Gypsum. Annu. Univ. Archit. Civ. Eng. Geod. Sofia 2023, 56, 1503–1512. (In Bulgarian) [Google Scholar]
- Scottish Environment Protection Agency (SEPA). Technical Guidance Note. The Disposal in Landfills for Non-Hazardous Waste of Gypsum Wastes. Available online: https://www.sepa.org.uk/media/28998/technical-guidance-note-disposal-of-gypsum-in-landfills.pdf (accessed on 1 August 2025).
- Cordon, H.C.F.; Cagnoni, F.C.; Ferreira, F.F. Comparison of physical and mechanical properties of civil construction plaster and recycled waste gypsum from São Paulo, Brazil. J. Build. Eng. 2019, 22, 504–512. [Google Scholar] [CrossRef]
- Harrell, J.A. Amarna gypsite: A new source of gypsum for ancient Egypt. J. Archaeol. Sci. Rep. 2017, 11, 536–545. [Google Scholar] [CrossRef]
- Bergamonti, L.; Verza, E.; Magnani, R.; Michelini, E.; Ferretti, D.; Lottici, P.P.; Graiff, C. Protection of gypsum artifacts by Mg(OH)2 based super-hydrophobic nanocomposite. Constr. Build. Mater. 2025, 490, 142532. [Google Scholar] [CrossRef]
- Ding, X.; Huang, W.; Li, Y.; Hu, Z.; Shan, Z. Study on retarding feature and retardation mechanism of various retarding materials on gypsum as a construction material: A review. J. Build. Eng. 2023, 72, 106569. [Google Scholar] [CrossRef]
- Arikan, M.; Sobolev, K. The optimization of a gypsum-based composite material. Cem. Concr. Res. 2002, 32, 1725–1728. [Google Scholar] [CrossRef]
- Zaragoza-Benzal, A.; Ferrández, D.; Barrios, A.M.; Morón, C. Water Resistance Analysis of New Lightweight Gypsum-Based Composites Incorporating Municipal Solid Waste. J. Compos. Sci. 2024, 8, 393. [Google Scholar] [CrossRef]
- Potapova, E.; Nyein, A.K.; Tsvetkova, E.; Fischer, H.-B. Modification of the structure of gypsum-cementpozzolanic binder. International Conference on Modern Trends in Manufacturing Technologies and Equipment: Mechanical Engineering and Materials Science (ICMTMTE 2020). MATEC Web Conf. 2020, 329, 04007. [Google Scholar] [CrossRef]
- Marinkovic, S.; Kostic-Pulek, A. Examination of the system fly ash–lime–calcined gypsum–water. J. Phys. Chem. Sol. 2007, 68, 1121–1125. [Google Scholar] [CrossRef]
- Morsy, M.S.; Alsayed, S.H.; Salloumhttps, Y.A. Development of eco-friendly binder using metakaolin-fly ash–lime-anhydrous gypsum. Constr. Build. Mater. 2012, 35, 772–777. [Google Scholar] [CrossRef]
- Yang, L.; Jing, M.; Lu, L.; Zhu, X.; Zhao, P.; Chen, M.; Li, L.; Liu, J. Effects of modified materials prepared from wastes on the performance of flue gas desulfurization gypsum-based composite wall materials. Constr. Build. Mater. 2020, 257, 119519. [Google Scholar] [CrossRef]
- Lesovik, V.; Chernysheva, N.; Fediuk, R.; Amran, M.; Murali, G.; de Azevedo, A.R.G. Optimization of fresh properties and durability of the green gypsum-cement paste. Constr. Build. Mater. 2021, 287, 123035. [Google Scholar] [CrossRef]
- Escalante-Garcia, J.I.; Martínez-Aguilar, O.A.; Gomez-Zamorano, L.Y. Calcium sulphate anhydrite based composite binders; effect of Portland cement and four pozzolans on the hydration and strength. Cem. Concr. Compos. 2017, 82, 227–233. [Google Scholar] [CrossRef]
- Kovler, K. Setting and Hardening of Gypsum-Portland Cement-Silica Fume Blends, Part 2: Early Strength, DTA, XRD, and SEM Observations. Cem. Concr. Res. 1998, 28, 523–531. [Google Scholar] [CrossRef]
- Vimmrová, A.; Keppert, M.; Michalko, O.; Černý, R. Calcined gypsum–lime–metakaolin binders: Design of optimal composition. Cem. Concr. Compos. 2014, 52, 91–96. [Google Scholar] [CrossRef]
- Min, Y.; Jueshi, Q.; Ying, P. Activation of fly ash–lime systems using calcined phosphogypsum, Constr. Build. Mater. 2008, 22, 1004–1008. [Google Scholar] [CrossRef]
- Volzhenskii, A.V.; Burov, Y.S.; Kolokolnikov, V.S. Mineral Binders (Technology and Properties), 3rd ed.; Stroyizdat: Moscow, Russia, 1979; pp. 458–461. (In Russian) [Google Scholar]
- Odintsov, D.S.; Suvorov, A.S.; Estemesov, Z.A.; Barvinov, A.V.; Panigrahi, M. Physico-mechanical and physico-chemical hardening process changing research in gypsum binder and transition in gypsum-cement binder and then to gypsum-cement-pozzolanic using with mechanical tests and x-ray spectroscopy. Her. Kazakh-Br. Tech. Univ.—Chem. Technol. Sci. 2022, 19, 6–16. [Google Scholar] [CrossRef]
- EN 197-1:2011; Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements. European Committee for Standardization: Brussels, Belgium, 2011.
- Neville, A.M. Properties of Concrete, 5th ed.; Pearson Education Ltd.: Harlow, UK, 2011; pp. 83–84. ISBN 978-0-273-75580-7. [Google Scholar]
- Janina, S.; Alona, G.; Juhnevica, I. Effect of Pozzolanic Additives on Structure and Chemical Durability of Concrete. Procedia Eng. 2013, 57, 1005–1012. [Google Scholar] [CrossRef]
- Doleželová, M.; Krejsová, J.; Scheinherrová, L.; Keppert, M.; Vimmrová, A. Investigation of environmentally friendly gypsum based composites with improved water resistance. J. Clean. Prod. 2022, 370, 133278. [Google Scholar] [CrossRef]
- Wansom, S.; Chintasongkro, P.; Srijampan, W. Water resistant blended cements containing flue-gas desulfurization gypsum, Portland cement and fly ash for structural applications. Cem. Concr. Compos. 2019, 103, 134–148. [Google Scholar] [CrossRef]
- Yao, G.; Cui, T.; Zhang, J.; Wang, J.; Lyu, X. Effects of mechanical grinding on pozzolanic activity and hydration properties of quartz. Adv. Powder Technol. 2020, 31, 4500–4509. [Google Scholar] [CrossRef]
- He, Z.; Hu, L.; Li, Y.; Hu, J.; Shao, Y. Use of sandstone powder as a mineral additive for concrete. Constr. Build. Mater. 2018, 186, 276–286. [Google Scholar] [CrossRef]
- Gomez-Zamorano, L.Y.; Escalante-Garcia, J.I.; Mendoza-suarez, G. Geothermal waste: An alternative replacement material of Portland cement. J. Mater. Sci. 2004, 39, 4021–4025. [Google Scholar] [CrossRef]
- Nežerka, V.; Němeček, J.; Slížková, Z.; Tesárek, P. Investigation of crushed brick-matrix interface in lime-based ancient mortar by microscopy and nanoindentation. Cem. Concr. Compos. 2015, 55, 122–128. [Google Scholar] [CrossRef]
- Torres, I.; Matias, G.; Faria, P. Natural hydraulic lime mortars—The effect of ceramic residues on physical and mechanical behaviour. J. Build. Eng. 2020, 32, 101747. [Google Scholar] [CrossRef]
- Liu, Q.; Tong, T.; Liu, S.; Yang, D.; Yu, Q. Investigation of using hybrid recycled powder from demolished concrete solids and clay bricks as a pozzolanic supplement for cement. Constr. Build. Mater. 2014, 73, 754–763. [Google Scholar] [CrossRef]
- Maaze, M.R.; Shrivastava, S. Development of framework in the selection and reuse of concrete waste and brick waste powder as pozzolanic material in cement concrete application using analytical hierarchy process technique. Constr. Build. Mater. 2023, 393, 132056. [Google Scholar] [CrossRef]
- Yue, W.; Wang, B. Ceramic-added lime and cement mortars: A review of applications in building products. Sci. Prog. 2024, 107, 1–28. [Google Scholar] [CrossRef]
- Erbs, A.; Nagalli, A.; de Carvalho, K.Q.; Mazer, W.; Erbs, M.d.M.; Paz, D.H.F.; Lafayette, K.P.V. Development of plasterboard sheets exclusively from waste. J. Build. Eng. 2021, 44, 102524. [Google Scholar] [CrossRef]
- EN 520:2004+A1:2009; Gypsum Plasterboards—Definitions, Requirements and Test Methods. European Committee for Standardization: Brussels, Belgium, 2009.
- Boccarusso, L.; Durante, M.; Iucolano, F.; Langella, A.; Minutolo, F.M.C.; Mocerino, D. Recyclability Process of Standard and Foamed Gypsum. Procedia Manuf. 2020, 47, 743–748. [Google Scholar] [CrossRef]
- Bumanis, G.; Zorica, J.; Korjakins, A.; Bajare, D. Processing of Gypsum Construction and Demolition Waste and Properties of Secondary Gypsum Binder. Recycling 2022, 7, 30. [Google Scholar] [CrossRef]
- EN 933-1:2012; Tests for Geometrical Properties of Aggregates—Part 1: Determination of Particle Size Distribution—Sieving Method. European Committee for Standardization: Brussels, Belgium, 2012.
- EN 13279-1:2008; Gypsum Binders and Gypsum Plasters—Part 1: Definitions and Requirements. European Committee for Standardization: Brussels, Belgium, 2008.
- EN 459-2:2021; Building lime—Part 2: Test methods. European Committee for Standardization: Brussels, Belgium, 2021.
- EN 13279-2:2014; Gypsum Binders and Gypsum Plasters—Part 2: Test Methods. European Committee for Standardization: Brussels, Belgium, 2014.
- prEN 13279-2:1998; Gypsum Binders and Gypsum Plasters—Part 2: Test Methods. European Committee for Standardization: Brussels, Belgium, 1998.
- EN 12390-7:2019; Testing Hardened Concrete—Part 7: Density of Hardened Concrete. European Committee for Standardization: Brussels, Belgium, 2019.
- Erbs, A.; Nagalli, A.; de Carvalho, K.Q.; Mymrin, V.; Passig, F.H.; Mazer, W. Properties of recycled gypsum from gypsum plasterboards and commercial gypsum throughout recycling cycles. J. Clean. Prod. 2018, 183, 1314–1322. [Google Scholar] [CrossRef]
- EN 196-6:2019; Methods of Testing Cement—Part 6: Determination of Fineness. European Committee for Standardization: Brussels, Belgium, 2019.
- EN 196-1:2016; Methods of Testing Cement—Part 1: Determination of Strength. European Committee for Standardization: Brussels, Belgium, 2016.
- BDS 166:1972; Active Mineral Additives to Binding Substances. Bulgarian Institute for Standardization: Sofia, Bulgaria, 1972. (In Bulgarian)
- BDS 16720:1987; Active Mineral Additives of Natural Origin for Cement. Technical Requirements. Methods of Test. Bulgarian Institute for Standardization: Sofia, Bulgaria, 1987. (In Bulgarian)
- Kumar, B.N.; Rushikesh, M.; Kumar, A.A. An experimental study on high strength self-compacting concrete inclusion of zeolite and silica fume as a potential alternative sustainable cementitious materials. Mater. Today—Proc. 2024. [Google Scholar] [CrossRef]
- Chmielewská, E. Natural Zeolites as Sustainable and Environmental Inorganic Resourcesover the History to Present. Gen. Chem. 2019, 5, 190001. [Google Scholar] [CrossRef]
- Uzal, B.; Turanl, L.; Yücel, H.; Göncüoğlu, M.C.; Çulfaz, A. Pozzolanic activity of clinoptilolite: A comparative study with silica fume, fly ash and a non-zeolitic natural pozzolan. Cem. Concr. Res. 2010, 40, 398–404. [Google Scholar] [CrossRef]
- Yao, G.; Cui, T.; Jia, Z.; Sun, S.; Anning, C.; Qiu, J.; Lyu, X. Effect of anhydrite on hydration properties of mechanically activated muscovite in the presence of calcium oxide. Appl. Clay Sci. 2020, 196, 105742. [Google Scholar] [CrossRef]
- Yao, G.; Wang, Z.; Yao, J.; Cong, X.; Anning, C.; Lyu, X. Pozzolanic activity and hydration properties of feldspar after mechanical activation. Powder Technol. 2021, 383, 167–174. [Google Scholar] [CrossRef]
- TC 21-31-62-89; Gypsum-Cement-Pozzolanic Binder. Technical Conditions. Moscow Civil Engineering Institute “V.V. Kuibishev”: Moscow, Russia, 1989. (In Russian)
- Naidenov, V. Rapid hardening cement-gypsum composites for shotcreting on the base of Bulgarian raw materials: Part I. Introduction, materials, design of the compositions, strength and deformability. Cement Concr. Res. 1991, 21, 896–904. [Google Scholar] [CrossRef]
- EN 1015-3:2001/A1:2006; Methods of Testing Mortar for Masonry. Part 3: Determination of the Consistency of a Fresh Solution (by Shaking Table). European Committee for Standardization: Brussels, Belgium, 2006.
- Zaharieva, R.; Evlogiev, D.; Kerenchev, N.; Stanimirova, T. Modification of Quaternary Clays Using Recycled Fines from Construction and Demolition Waste. Processes 2022, 10, 1062. [Google Scholar] [CrossRef]
- Szudek, W.; Szydłowski, J.; Buchała, I.; Kapeluszna, E. Synthesis and Characterization of Calcium Sulfoaluminate Hydrates—Ettringite (AFt) and Monosulfate (AFm). Materials 2024, 17, 5216. [Google Scholar] [CrossRef]
- EN 196-3:2016; Methods of Testing Cement. Part 3: Determination of Contact Times and Volume Constancy. European Committee for Standardization: Brussels, Belgium, 2016.
- Naidenov, V. Rapid hardening cement-gypsum composites for shotcreting on the base of bulgarian raw materials Part II. Structural Investigations, Tests in Production Conditions, conclusions. Cement Concr. Res. 1991, 21, 1028–1034. [Google Scholar] [CrossRef]
- Suherman, P.M.; van Riessen, A.; Fairhurst, H.; O’Connor, B.; Li, D.; Bolton, D. Determination of amorphous phase levels in Portland cement clinker. Powder Diffr. 2002, 17, 178–185. [Google Scholar] [CrossRef]
- Evlogiev, D. Opportunities for recycling gypsum in Bulgaria. In Proceedings of the I Youth Scientific Conference with International Participation “Design and Construction of Buildings and Facilities”, Sofia, Bulgaria, 4–5 November 2021. ISSN 2738-7887 (online); ISSN 2738-7879 (CD-ROM). (In Bulgarian, abstract in English). [Google Scholar]


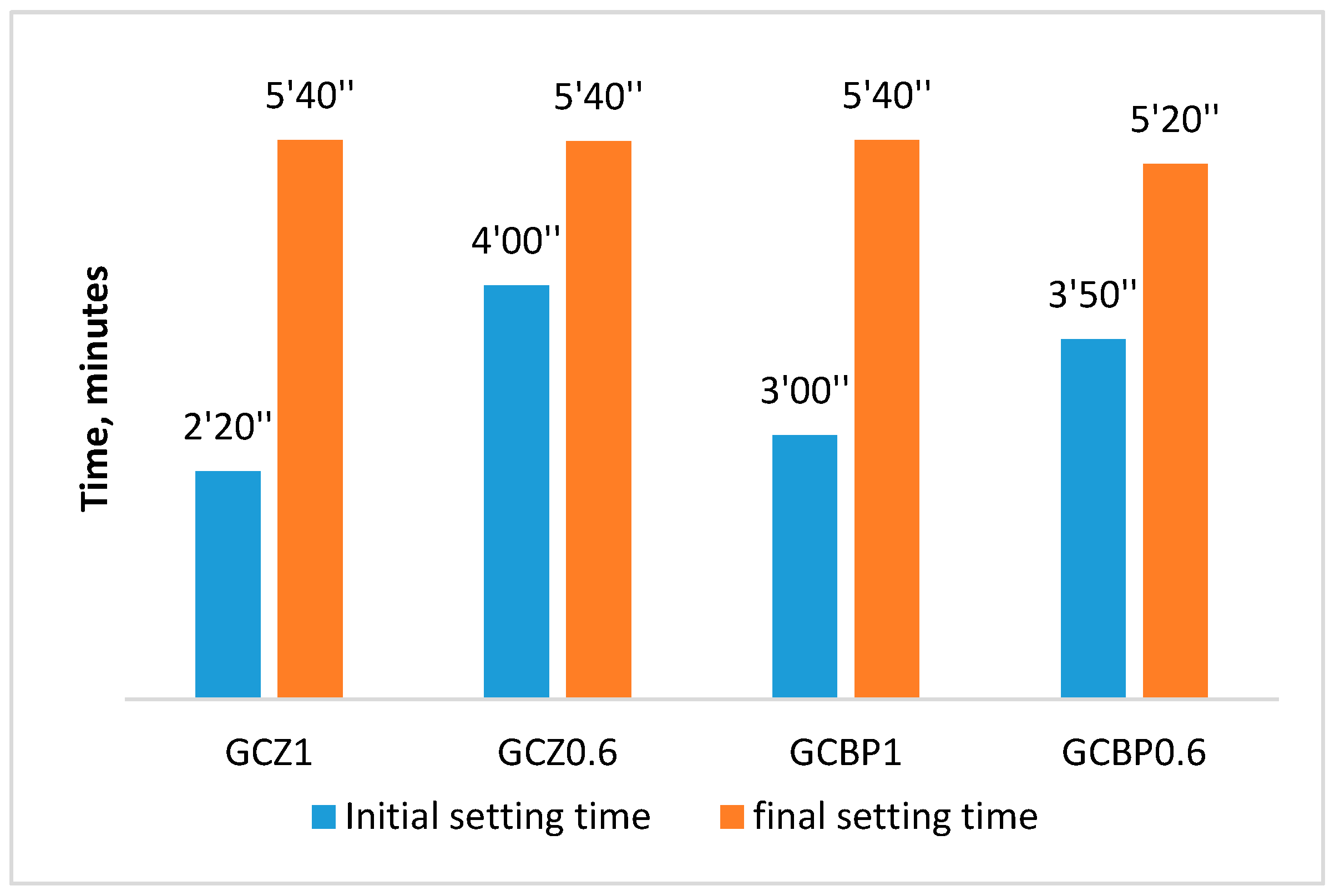






| Properties | Units | Methods | Recycled Gypsum (RG) | Binder Type “A” (CCG) |
|---|---|---|---|---|
| Bulk density | kg/m3 | EN 459-2 [77] | 720 | 875 |
| Water/plaster ratio | - | EN 13279-2 (4.3.1) [78] | 0.99 | 0.66 |
| Initial setting time | min | EN 13279-2 (4.4.1) [78] | 3′25″ | 6′38″ |
| Final setting time | min | prEN 13279-2 [79] | 7′36″ | 13′51″ |
| Flexural strength | N/mm2 | EN 13279-2 [78] | 2.5 | 3.3 |
| Compressive strength | 11.8 | 14.2 | ||
| Density of hardened paste | kg/m3 | EN 12390-7 [80] | 863 | 1105 |
| Properties | Units | Methods | Values | |
|---|---|---|---|---|
| Fineness | cm2/g | EN 196-6 [82] | 3930 | |
| Flexural strength | 2 days | N/mm2 | EN 196-1 [83] | 6.5 |
| 28 days | 9.8 | |||
| Compressive strength | 2 days | 31.5 | ||
| 28 days | 53.9 | |||
| Amount of CaO Bound by 1 g Additive After 30 Days [mg] | Additive Activity |
|---|---|
| below 50 | low activity |
| 50–80 | medium activity |
| 80–150 | high activity |
| Amorphous Phase | Clinoptilolite-Na | Heulandite-Ca | Cristobalite-Low |
|---|---|---|---|
| 23.0 | 43.5 | 55.3 | 1.3 |
| Amorphous Phase | Quartz | Мuskovite | Calcite | Albite | Microcline | Hematite |
|---|---|---|---|---|---|---|
| 27.5 | 50.8 | 9.1 | 2.0 | 15.5 | 21.6 | 1.0 |
| Mix Designation | RG, % Per Total Binder | OPC, % Per Total Binder | PA, % Per Total Binder | Water/Binder | Admixture, % Per Mass of OPC and RG |
|---|---|---|---|---|---|
| GCZ1 | 45.0 | 27.50 | 27.50 | 0.7 | 1.2 |
| GCZ0.6 | 45.0 | 34.37 | 20.63 | 0.7 | 0.8 |
| GCBP1 | 45.0 | 27.50 | 27.50 | 0.7 | 0.8 |
| GCBP0.6 | 45.0 | 34.37 | 20.63 | 0.7 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaharieva, R.; Simonov, B. Valorization of Recycled Gypsum from CDW in Green Binder Systems. Materials 2025, 18, 4849. https://doi.org/10.3390/ma18214849
Zaharieva R, Simonov B. Valorization of Recycled Gypsum from CDW in Green Binder Systems. Materials. 2025; 18(21):4849. https://doi.org/10.3390/ma18214849
Chicago/Turabian StyleZaharieva, Roumiana, and Borislav Simonov. 2025. "Valorization of Recycled Gypsum from CDW in Green Binder Systems" Materials 18, no. 21: 4849. https://doi.org/10.3390/ma18214849
APA StyleZaharieva, R., & Simonov, B. (2025). Valorization of Recycled Gypsum from CDW in Green Binder Systems. Materials, 18(21), 4849. https://doi.org/10.3390/ma18214849






