Effect of Shielding Gas and Post-Welding Heat Treatment on the Mechanical and Corrosion Performances of Duplex and Super Duplex Stainless Steels’ Low Heat-Input Welded Joints
Abstract
1. Introduction
Transformations Occurring During Welding and PWHT
- Allotriomorphic austenite, usually forming at grain boundaries of ferrite δ, requires diffusion of austenite-stabilizing elements towards grain boundaries; it typically occurs in the cooling stage after welding or heat treatment of duplex steels [12];
- Intergranular austenite, which precipitates within the ferrite grains, with nitrides possibly acting as nucleation sites for such austenite type; however, in single pass welding with low heat inputs, it is unlikely to form [13];
- Widmanstätten austenite, forming as needle-like or plate-like structures; it occurs within the austenite phase, and it is favored by slow cooling, and it is commonly observed in the heat-affected zone (HAZ) of welds in duplex stainless steels [14].
2. Materials and Methods
- Open Circuit Potential (OCP) acquisition, i.e., measurement of the voltage in open circuit conditions to determine the equilibrium potential of the sample; this measurement was performed for 300 s, considering the final (stable) potential reached by the sample as the equilibrium potential (OCP);
- Potentiodynamic polarization test, measuring current as a function of the WE potential, varying from −0.4 V (vs. OCP) to 1.6 V (vs. OCP), with a scan rate of 0.0004 V/s, according to ASTM G5 standard.
- “Material” with values D or SD for duplex or super duplex;
- “Shielding gas” with values A for argon or ArN2 for argon + 2% nitrogen;
- “Post welding heat treatment” assuming treated (t) or not treated (nt) values;
- “Region” with values BASE, HAZ, FZ depending on the tested zone.
3. Results and Discussion
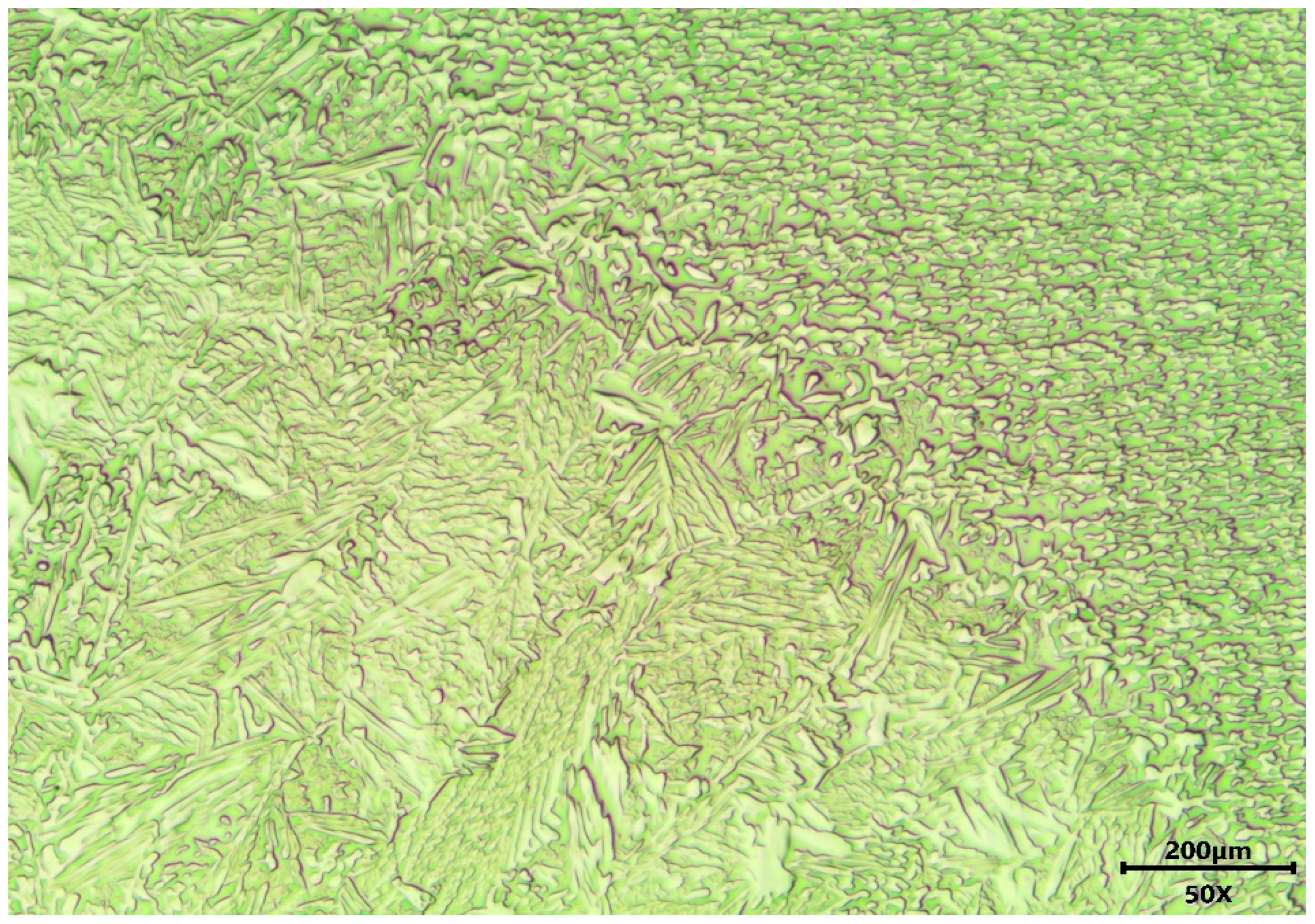
3.1. Microstructure
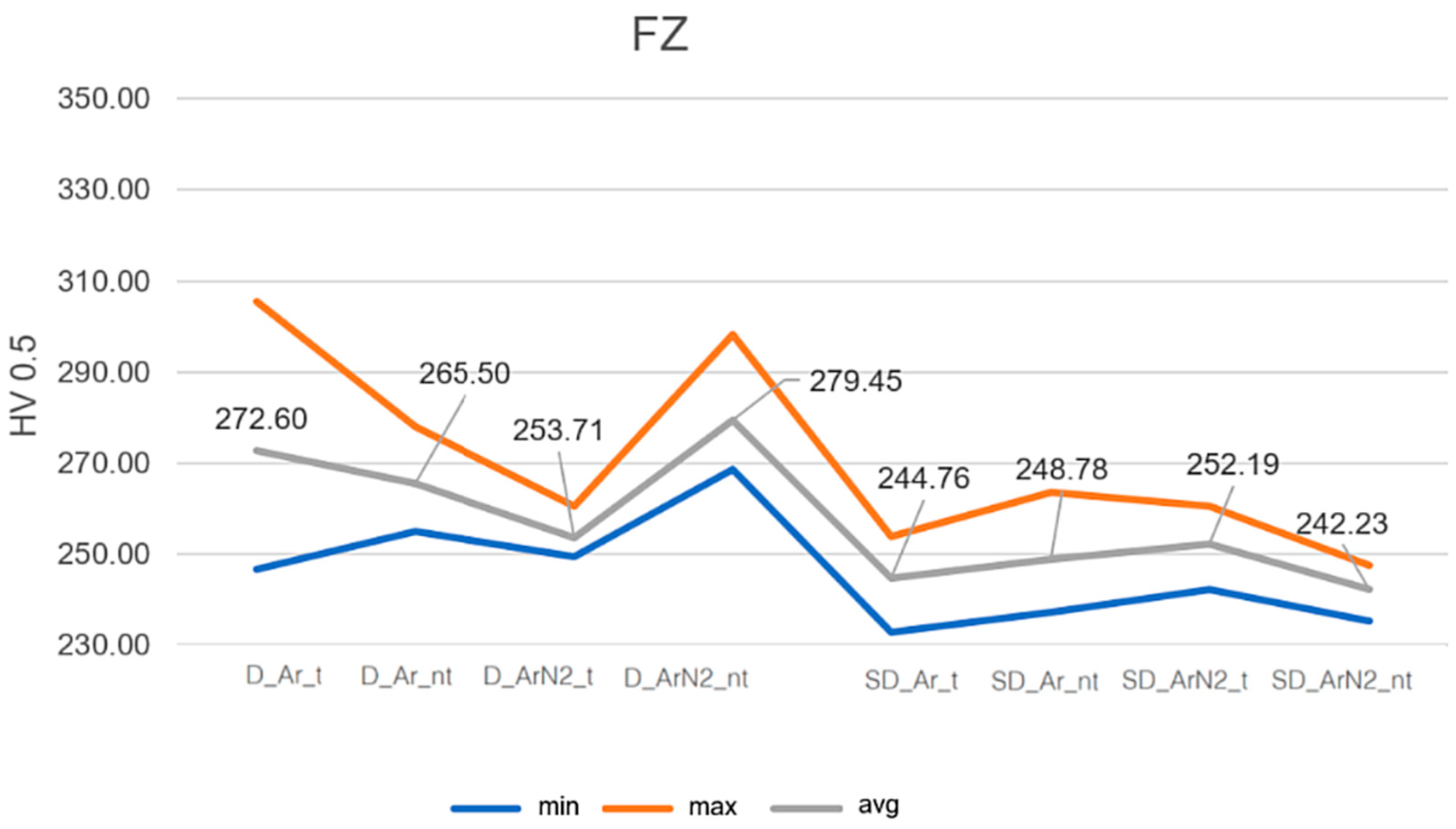
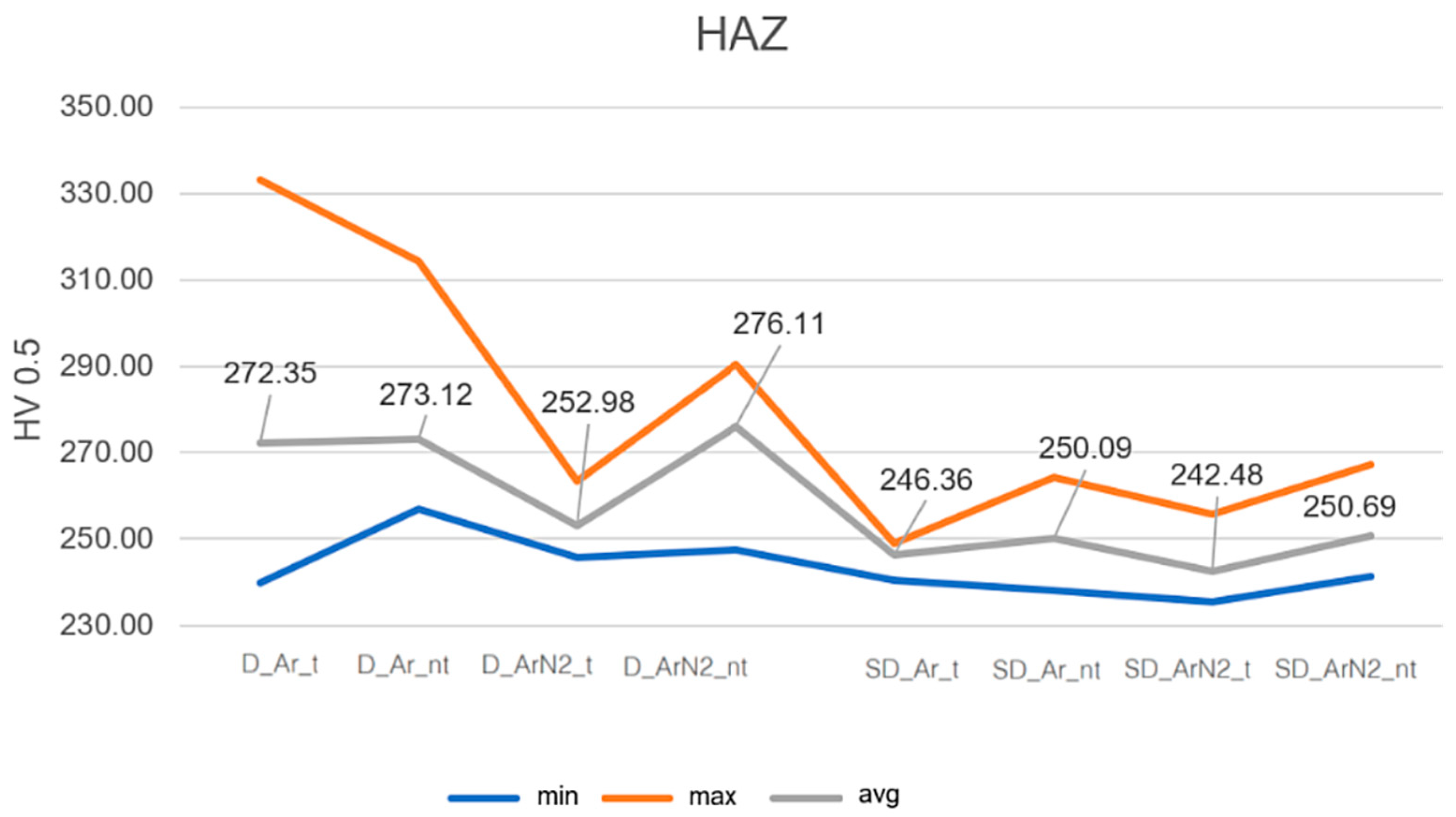
3.2. Microhardness
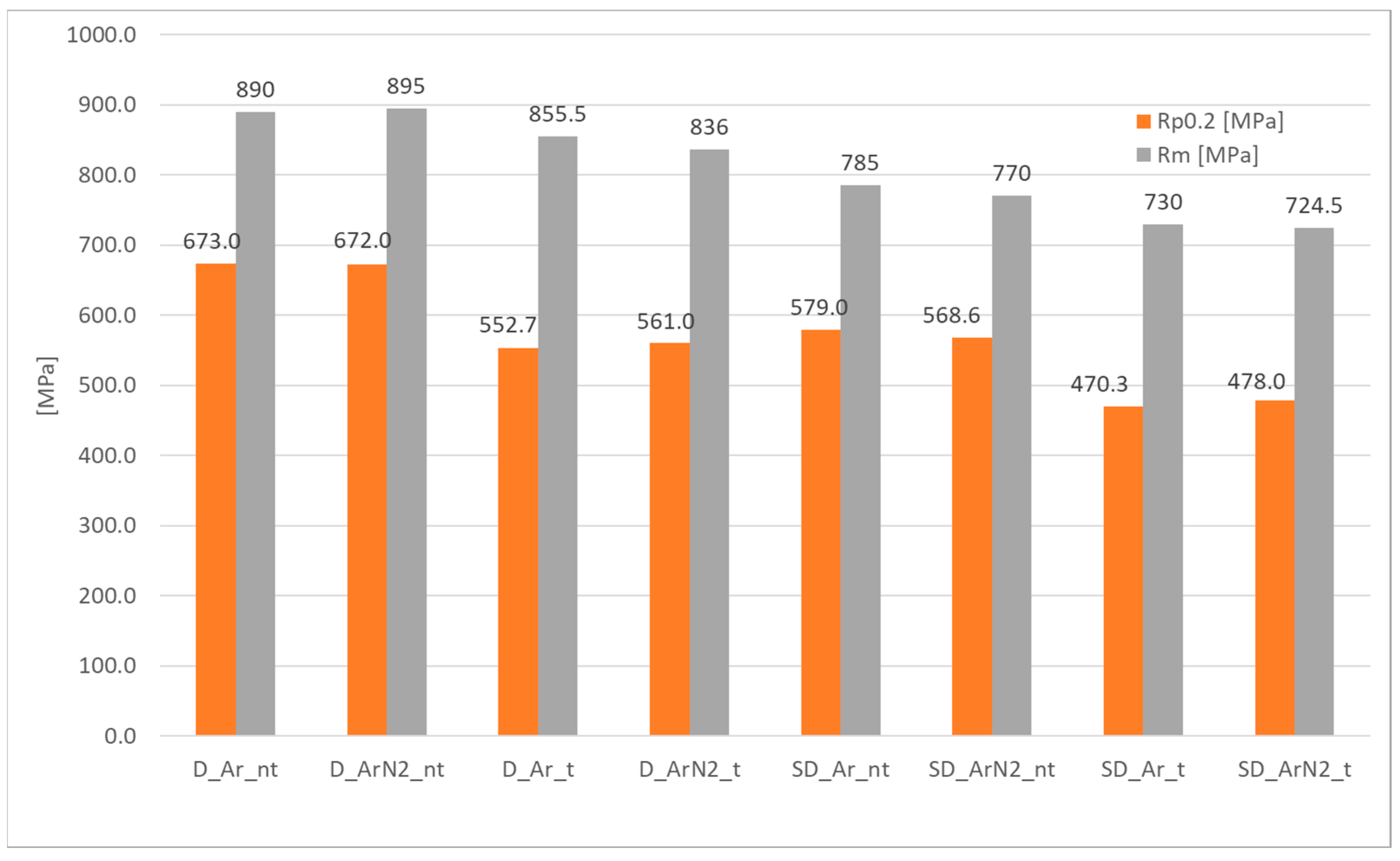
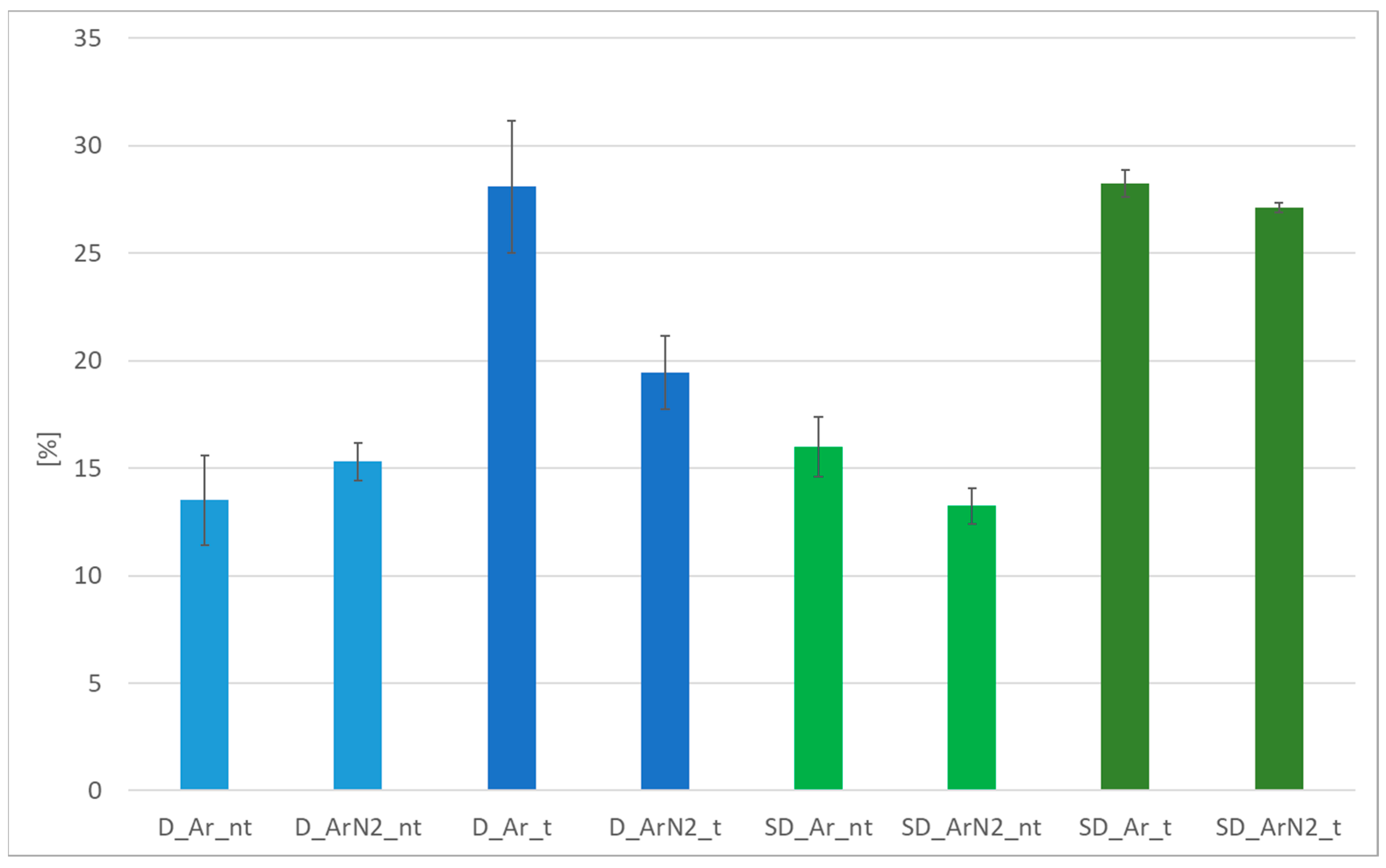
3.3. Tensile Testing
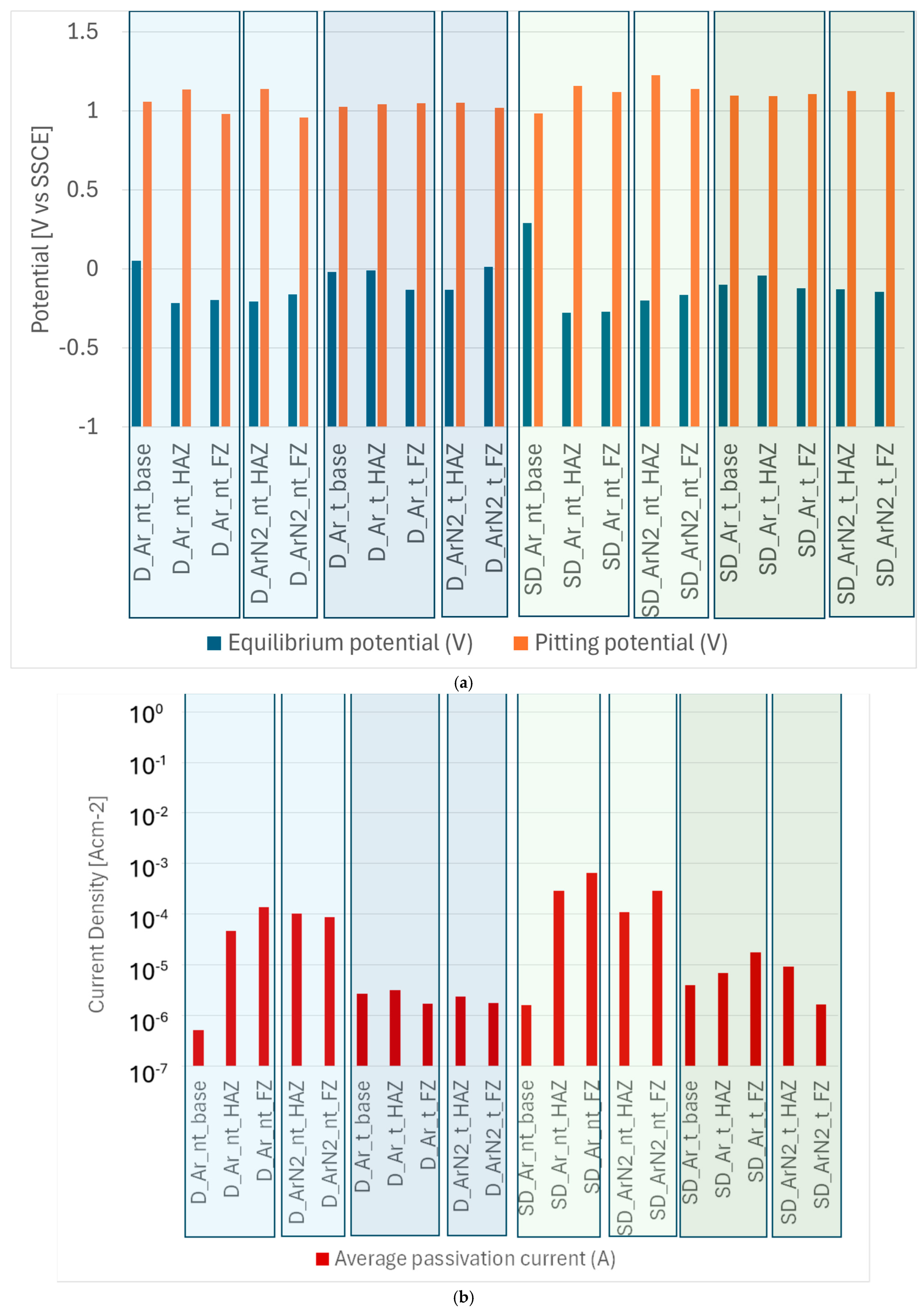
3.4. Corrosion Tests
4. Conclusions
- -
- To produce all the samples, steel plates in DSS and SDSS with Y-shaped edges were chosen so that the welded joint could be made in a single pass. During the execution of the joints, it was found that by using Ar + 2%N2 shielding gas, the welding current can be reduced by 10% compared to when pure Ar is used. However, for all samples produced, the heat input transferred to the material has been set in order to stay within the range of 0.48–0.56 kJ/mm.
- -
- Although the literature recommends using filler materials with nickel content 2–4% higher than the base materials for welding SDSS and DSS steels, in this study, we investigated the possibility of using rods made of the same alloy of the base material. The purpose of this approach is to produce test specimens with a homogeneous chemical composition which, especially in the case of PWHT, are intended to produce welded parts with high mechanical strength and superior corrosion resistance. This objective was achieved only by the duplex steel samples, as the super duplex steel samples showed lower mechanical properties and corrosion resistance than initially expected.
- -
- The use of shielding gas containing 2% nitrogen compared to traditional argon led to different effects in the weld area between the two materials considered. In the case of DSS, with both shielding gases the ratio between the two phases is approximately 32% ferrite/68% austenite, so no significant benefits can be identified; this aspect is confirmed also from the tensile test data, in which as weld conditions are practically identical. After solution heat treatment, the behavior differs, and the specimen welded with the Ar + 2%N2 mixture shows higher content of ferrite (58% vs. 44%) with respect to the one welded in pure Ar. This difference in phases distribution plays a role mainly on the total elongation, which is significantly higher for the specimen welded in pure Ar; in this case, the elongation reaches 28%, which is above the minimum value required for the raw material UNS S 31803. Instead, in the case of SDSS, it is possible to detect in the specimens in “as weld” condition a significant difference in phase balancing, depending on the shielding gas used. In detail, the use of the Ar + 2%N2 mixture helps in maintaining a better ratio ferrite/austenite (59–41%), while the specimens welded in pure Ar shielding appear to be completely out of balance from a microstructural perspective (22% austenite/78% ferrite). Despite this result, it should be noted that the elongation values specimens in the “as-welded” state are still well below the typical values for this steel and, above all, are almost a half of those specified in the reference standard. After solution heat treatment, the phase balance becomes, in both cases, perfectly balanced, with comparable values and the elongation rises to 27/28%. However, the mechanical characteristics measured with longitudinal tensile tests are approximately 15% lower than those required by the relevant standard.
- -
- The effect of shielding gas can also be considered in relation to corrosion resistance near the welded joint. Corrosion tests evidence that, for DSS steels, no positive contributions are found in the use of Ar + N2 gas; the performance of the welded area improves slightly, while the ZTA is the area with the worst performance. On the other hand, in the case of SDSS, the beneficial effect of Ar + N2 shielding gas is more pronounced, as the average passivation current values are halved. This result can be explained by the decrease in the amount of ferrite observed in SDSS, as highlighted in the previous paragraph.
- -
- In terms of corrosion resistance, the comparison between the two materials under investigation underlined an unexpected behavior: welded DSS using the parameters investigated in the present study has comparable, if not slightly superior, performance compared to SDSS. In SDSS, the corrosion performance of the HAZ and weld area is significantly lower compared to DSS. On the other side, according to the studies by Tavares et al. [28], the percentage of nickel in the filler material should be 2–4% higher than in the base material; this was respected in the present study for both materials, but with a limited excess of Ni (2.3% more for DSS and 3.1% more for SDSS compared to the base materials), and this may have had a negative effect on the corrosion properties of the SDSS-welded samples.
- -
- Considering the effect of the solution heat treatment on the corrosion performance of the samples, both materials improved their performance by an order of magnitude compared to their untreated counterparts. This is a very interesting result as it is not reflected in some of the mentioned literature studies, which report that the post-welding treatment carried out at 1000 °C for less than 10 min, while rebalancing the two phases, did not improve the corrosion resistance properties in the case of TIG welding.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maslak, M.; Stankiewicz, M.; Slazak, B. Duplex Steels Used in Building Structures and Their Resistance to Chloride Corrosion. Materials 2021, 14, 5666. [Google Scholar] [CrossRef]
- Caluscio Dos Santos, D.; Monfrinatti Macarrão, I.; Magnabosco, R. Relation Between Pitting Potential and PREN Values for Ferrite and Austenite in Duplex Stainless Steels. BHM Berg-Und Hüttenmännische Monatshefte 2020, 165, 46–50. [Google Scholar]
- Craig, B. Clarifying the Applicability of PREN Equations: A Short Focused Review. Corrosion 2021, 77, 382–385. [Google Scholar]
- Rodriguez, B.R.; Miranda, A.; Gonzalez, D.; Praga, R.; Hurtado, E. Maintenance of the Austenite/Ferrite Ratio Balance in GTAW DSS Joints Through Process Parameters Optimization. Materials 2020, 13, 780. [Google Scholar] [CrossRef]
- Capello, E.; Chiarello, P.; Previtali, B.; Vedani, M. Laser welding and surface treatment of a 22Cr–5Ni–3Mo duplex stainless steel. Mater. Sci. Eng. A 2003, 351, 334–343. [Google Scholar]
- Shargay, C. Application of Duplex Stainless Steels in Refining–Overview of API 938C. In Proceedings of the Corrosion 2005 (NACE International), Houston, TX, USA, 3–7 April 2005; pp. 1–15. [Google Scholar]
- ISO 15156-3:2020; Petroleum and Natural Gas Industries-Materials for Use in H2S-Containing Environments in Oil and Gas Production-Part 3: Cracking-Resistant CRAs (Corrosion-Resistant Alloys) and Other Alloys. Industries du Pétrole et du Gaz Naturel-Matériaux Pour Utilisation dans des Environnements Contenant de L’hydrogène Sulfuré (H2S) dans la Production de Pétrole et de Gaz-Partie 3: ARC (Alliages Résistants à la Corrosion) et Autres Alliages Résistants à la Fissuration. ISO: Geneva, Switzerland, 2020.
- Li, L.Y.; Sun, C.H.; Ruan, Y.; Wei, B. Duplex corrosion mechanisms correlated to α/γ phase selection in containerlessly processed Fe-Cr-Ni-Mo alloy. Corros. Sci. 2023, 221, 111311. [Google Scholar] [CrossRef]
- Messer, B.; Wright, A.; Oprea, V. Duplex stainless steel welding: Best practices. Stainl. Steel World 2007, 53, 53–63. [Google Scholar]
- Zhang, W.; DebRoy, T.; Palmer, T.A.; Elmer, J. Modeling of ferrite formation in a duplex stainless steel weld considering non-uniform starting microstructure. Acta Mater. 2005, 53, 4441–4453. [Google Scholar] [CrossRef]
- Ciuffini, A.; Barella, S.; Di Cecca, C.; Gruttadauria, A.; Mapelli, C.; Mombelli, D. Isothermal Austenite–Ferrite Phase Transformations and Microstructural Evolution during Annealing in Super Duplex Stainless Steels. Metals 2017, 7, 368. [Google Scholar] [CrossRef]
- Magalhães, C.H.X.M.; Faria GLde Lagoeiro, L.E.; Silva, J.D. Characterization of the Austenite Reformation Mechanisms as a Function of the Initial Ferritic State in a UNS S32304 Duplex Stainless Steel. Mater. Res. 2017, 20, 1470–1479. [Google Scholar] [CrossRef]
- Karlsson, L. Welding duplex stainless steels: A review of current recommendations. Zavarivanje i Zavarene Konstrukcije 2018, 63, 29–35. [Google Scholar] [CrossRef]
- Wu, X.; Song, Z.; He, J.; Feng, H.; Wang, B.; Wu, M. Effect of newly formed Widmanstätten austenite during high temperature cooling on mechanical properties in UNS S32750 duplex stainless steel. Mater. Sci. Eng. A 2022, 851, 143654. [Google Scholar] [CrossRef]
- Muthupandi, V.; Bala Srinivasan, P.; Seshadri, S.K.; Sundaresan, S. Effect of weld metal chemistry and heat input on the structure and properties of duplex stainless steel welds. Mater. Sci. Eng. A 2003, 358, 9–16. [Google Scholar] [CrossRef]
- Pettersson, N.; Pettersson, R.F.A.; Wessman, S. Precipitation of Chromium Nitrides in the Super Duplex Stainless Steel 2507. Metall. Mater. Trans. A 2015, 46, 1062–1072. [Google Scholar] [CrossRef]
- Jacob, A.; Povoden-Karadeniz, E. Predictive computations of intermetallic σ phase evolution in duplex steel. II) Thermo-kinetic simulation in duplex and hyper duplex stainless steels. Calphad 2020, 71, 101810. [Google Scholar] [CrossRef]
- Du Toit, M.; Pistorius, P.C. Nitrogen Control During the Autogenous ARC Welding of Stainless Steel. Weld. World 2003, 47, 30–43. [Google Scholar] [CrossRef]
- Karlsson, L.; Börjesson, J. Orientation relationships of intragranular austenite in duplex stainless steel weld metals. Sci. Technol. Weld. Join. 2014, 19, 318–323. [Google Scholar] [CrossRef]
- Pimenta, A.R.; Diniz, M.G.; Perez, G.; Solórzano-Naranjo, I.G. Nitrogen Addition to the Shielding Gas for Welding Hyper-duplex Stainless Steel. Soldag. Inspeção 2020, 25, e2512. [Google Scholar] [CrossRef]
- Pamuk, S.; Sojiphan, K. Effects of argon-nitrogen backing gas ratios on microstructure and corrosion resistance of duplex stainless steel pipe ASTM A790 welds by gas tungsten arc welding process. Mater. Today Proc. 2018, 5, 9512–9518. [Google Scholar] [CrossRef]
- Devendranath Ramkumar, K.; Mishra, D.; Ganesh Raj, B.; Vignesh, M.K.; Thiruvengatam, G.; Sudharshan, S.P.; Arivazhagan, N.; Sivashanmugam, N.; Rabel, A.M. Effect of optimal weld parameters in the microstructure and mechanical properties of autogeneous gas tungsten arc weldments of super-duplex stainless steel UNS S32750. Mater. Des. (1980–2015) 2015, 66, 356–365. [Google Scholar] [CrossRef]
- Palmer, T.A.; Elmer, J.W.; Babu, S.S. Observations of ferrite/austenite transformations in the heat affected zone of 2205 duplex stainless steel spot welds using time resolved X-ray diffraction. Mater. Sci. Eng. A 2004, 374, 307–321. [Google Scholar] [CrossRef]
- Nishimoto, K.; Saida, K.; Katsuyama, O. Prediction of Sigma Phase Precipitation in Super Duplex Stainless Steel Weldments. Weld. World 2006, 50, 13–28. [Google Scholar] [CrossRef]
- Liu, J.; Das, Y.; King, S.M.; Jonsson, J.Y.; Wessman, S.; Hedström, P. Effect of Cooling Rate after Solution Treatment on Subsequent Phase Separation Evolution in Super Duplex Stainless Steel 25Cr-7Ni (wt.%). Metals 2022, 12, 890. [Google Scholar] [CrossRef]
- Kısasöz, A.; Karaaslan, A. Variation of Microstructure and Mechanical Properties of 2205 Duplex Stainless Steel for Solution Treatment at 1150 °C, Intl Conf on Engineering Technology and Innovation (ICETI 2017), Sarajevo, Bosnia-Erxegovina. 2017. Available online: https://www.researchgate.net/publication/318100816_Variation_of_Microstructure_and_Mechanical_Properties_of_2205_Duplex_Stainless_Steel_for_Solution_Treatment_at_1150_C#full-text (accessed on 8 September 2025).
- Sim, B.-M.; Hong, T.-S.; Hanim, M.A.-A.; Tchan, E.-J.N.; Talari, M.-K. The Influence of Post Weld Heat Treatment Precipitation on Duplex Stainless Steels Weld Overlay towards Pitting Corrosion. Materials 2019, 12, 3285. [Google Scholar] [CrossRef] [PubMed]
- Tavares, S.S.M.; Pardal, J.M.; Lima, L.D.; Bastos, I.N.; Nascimento, A.M.; de Souza, J.A. Characterization of microstructure, chemical composition, corrosion resistance and toughness of a multipass weld joint of superduplex stainless steel UNS S32750. Mater. Charact. 2007, 58, 610–616. [Google Scholar] [CrossRef]
- Akbarzadeh, E.; Yurtışık, K.; Hakan Gür, C.; Saeid, T.; Tavangar, R. Influence of Shielding Gas on the Microstructure and Mechanical Properties of Duplex Stainless Steel in Wire Arc Additive Manufacturing. Met. Mater. Int. 2024, 30, 1977–1996. [Google Scholar] [CrossRef]
- Wiktorowicz, R.; Crouch, J. Shielding gas developments for TIG welding of duplex and super duplex stainless steels. Weld. Res. Abroad 1996, 42, 33–36. [Google Scholar]
- Hosseini, V.A. Super Duplex Stainless Steels—Microstructure and Properties of Physically Simulated Base and Weld Metal. Ph.D. Thesis, University West, Trollhättan, Sweden, 2018. [Google Scholar]
- Tromellini, C.M.; Ciuffini, A.F.; Gruttadauria, A.; Barella, S.; Di Cecca, C.; Mapelli, C. Mechanical properties evolution on heat treated severe cold rolled UNS S32760 Super Duplex Stainless Steel. Metall. Ital. 2018, 1, 20–27. [Google Scholar]
- Melotti, G.; Bertelli, R.; Zanesco, M.; Calliari, I.; Ramous, E. Il trattamento di solubilizzazione degli acciai inossidabili duplex. La Metall. Ital. 2005, 4, 39–42. [Google Scholar]
- ISO 6892-1:2019; Metallic Materials—Tensile Testing Part 1: Method of Test at Room Temperature, Edition 3. ISO: Geneva, Switzerland, 2019.
- ASTM G5-13; Standard Reference Test Method for Making Potentiodynamic Anodic Polarization Measurements. ASTM: West Conshohocken, PA, USA, 2013. Available online: https://www.astm.org/g0005-14r21.html (accessed on 8 September 2025).
- Mohammed, G.; Ishak, M.; Aqida, S.; Abdulhadi, H. Effects of Heat Input on Microstructure, Corrosion and Mechanical Characteristics of Welded Austenitic and Duplex Stainless Steels: A Review. Metals 2017, 7, 39. [Google Scholar] [CrossRef]
- Devendranath Ramkumar, K.; Thiruvengatam, G.; Sudharsan, S.P.; Mishra, D.; Arivazhagan, N.; Sridhar, R. Characterization of weld strength and impact toughness in the multi-pass welding of super-duplex stainless steel UNS 32750. Mater. Des. 2014, 60, 125–135. [Google Scholar] [CrossRef]
- Asif M, M.; Shrikrishna, K.A.; Sathiya, P.; Goel, S. The impact of heat input on the strength, toughness, microhardness, microstructure and corrosion aspects of friction welded duplex stainless steel joints. J. Manuf. Process 2015, 18, 92–106. [Google Scholar]
- Da Fonseca, G.S.; Barbosa, L.O.R.; Ferreira, E.A.; Xavier, C.R.; De Castro, J.A. Microstructural, Mechanical, and Electrochemical Analysis of Duplex and Superduplex Stainless Steels Welded with the Autogenous TIG Process Using Different Heat Input. Metals 2017, 7, 538. [Google Scholar] [CrossRef]
- Brytan, Z.; Niagaj, J. Corrosion Resistance and Mechanical Properties of TIG and A-TIG Welded Joints of Lean Duplex Stainless Steel S82441/1.4662. Arch. Metall. Mater. 2016, 61, 771–784. [Google Scholar] [CrossRef]
- Chacón-Fernández, S.; Martín, A.P.; García, A.P. Influence of post-weld heat treatments on the formation of the sigma phase and the corrosion resistance of S32001 steel. Mater. Today Commun. 2023, 37, 107197. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y.; Xu, Z.; Sun, Y.; Wang, P.; Cui, L.; Wang, T. Characterization of σ-phase precipitation and effect on performance in duplex stainless steel S31803. Eng. Fail. Anal. 2024, 157, 107836. [Google Scholar] [CrossRef]
- Paulraj, P.; Garg, R. Effect of intermetallic phases on corrosion behavior and mechanical properties of duplex stainless steel and super-duplex stainless steel. Adv. Sci. Technol. Res. J. 2015, 9, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Li, X.G.; Dong, C.F.; Xiao, K. Effect of solution treatment on pitting behavior of 2205 duplex stainless steel. Arab. J. Chem. 2017, 10, S90–S94. [Google Scholar] [CrossRef]
- Fargas, G.; Anglada, M.; Mateo, A. Effect of the annealing temperature on the mechanical properties, formability and corrosion resistance of hot-rolled duplex stainless steel. J. Mater. Process Technol. 2009, 209, 1770–1782. [Google Scholar] [CrossRef]
- Liang, T.; Hu, X.Q.; Kang, X.H.; Li, D.Z. Effect of The Sigma Phase on the Mechanical Properties of a Cast Duplex Stainless Steel during the Ageing Treatment at 850 °C. Adv. Mater. Res. 2013, 684, 325–329. [Google Scholar] [CrossRef]
- Cho, D.M.; Park, J.S.; Hong, S.G.; Kim, S.J. Corrosion behaviors according to the welding process of superduplex stainless steel welded tubes: Gas tungsten arc welding vs. laser beam welding. Corros. Sci. 2023, 216, 111108. [Google Scholar] [CrossRef]
- Wang, R. Precipitation of sigma phase in duplex stainless steel and recent development on its detection by electrochemical potentiokinetic reactivation: A review. Corros. Commun. 2021, 2, 41–54. [Google Scholar] [CrossRef]
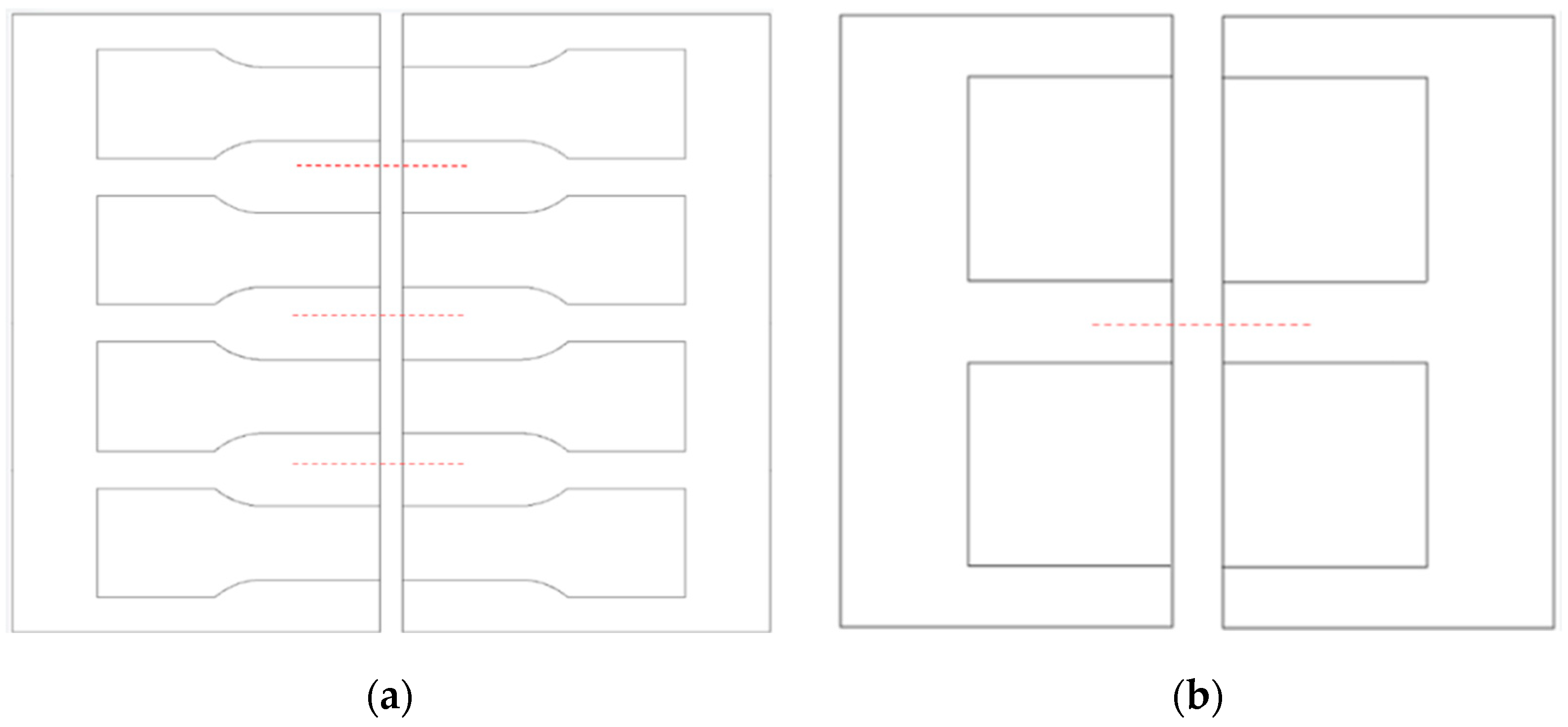


| C % | Mn % | P % | S % | Si % | Cr % | Ni % | Mo % | N % | Cu % | W % | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Duplex UNS S31803 | 0.019 | 1.33 | 0.027 | 0.001 | 0.40 | 22.5 | 5.6 | 3.2 | 0.18 | - | - |
| Super duplex UNS S32760 | 0.015 | 0.67 | 0.028 | 0.001 | 0.32 | 25.19 | 6.99 | 3.50 | 0.272 | 0.55 | 0.53 |
| C % | Si % | Mn % | P % | S % | Cr % | Ni % | Mo % | N % | |
|---|---|---|---|---|---|---|---|---|---|
| S31803–ER 2209 | 0.009 | 0.549 | 1.5 | 0.022 | 0.001 | 22.96 | 8.79 | 3.1 | 0.16 |
| S32760-ER 2594 | 0.014 | 0.41 | 0.6 | 0.013 | 0.001 | 25.17 | 9.33 | 4.02 | 0.26 |
| Materials and Gases | Current [A] | Voltage [V] | Welding Speed [mm/s] | Arc Linear Energy Density [kJ/mm] |
|---|---|---|---|---|
| Duplex Ar | 100 | 9.5 | 2 | 0.48 |
| Duplex Ar + N2 | 90 | 9 | 1.5 | 0.54 |
| Super Duplex Ar | 110 | 10.5 | 2 | 0.58 |
| Super Duplex Ar + N2 | 90 | 9 | 1.5 | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, E.; Colombini, E.; Giovanardi, R.; Zaniboni, F.; Gaiani, S.; Veronesi, P. Effect of Shielding Gas and Post-Welding Heat Treatment on the Mechanical and Corrosion Performances of Duplex and Super Duplex Stainless Steels’ Low Heat-Input Welded Joints. Materials 2025, 18, 4818. https://doi.org/10.3390/ma18214818
Ferrari E, Colombini E, Giovanardi R, Zaniboni F, Gaiani S, Veronesi P. Effect of Shielding Gas and Post-Welding Heat Treatment on the Mechanical and Corrosion Performances of Duplex and Super Duplex Stainless Steels’ Low Heat-Input Welded Joints. Materials. 2025; 18(21):4818. https://doi.org/10.3390/ma18214818
Chicago/Turabian StyleFerrari, Elisa, Elena Colombini, Roberto Giovanardi, Francesco Zaniboni, Silvia Gaiani, and Paolo Veronesi. 2025. "Effect of Shielding Gas and Post-Welding Heat Treatment on the Mechanical and Corrosion Performances of Duplex and Super Duplex Stainless Steels’ Low Heat-Input Welded Joints" Materials 18, no. 21: 4818. https://doi.org/10.3390/ma18214818
APA StyleFerrari, E., Colombini, E., Giovanardi, R., Zaniboni, F., Gaiani, S., & Veronesi, P. (2025). Effect of Shielding Gas and Post-Welding Heat Treatment on the Mechanical and Corrosion Performances of Duplex and Super Duplex Stainless Steels’ Low Heat-Input Welded Joints. Materials, 18(21), 4818. https://doi.org/10.3390/ma18214818