The Effect of Alumina-Rich Spinel Exsolution on the Mechanical Property of Calcium Aluminate Cement-Bonded Corundum Castables
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and the Preparation of Castables and Matrix
2.2. Methods
3. Results and Discussion
3.1. Exsolution of MA-90
3.2. Mechanical Strength
3.3. Thermal Shock Resistance
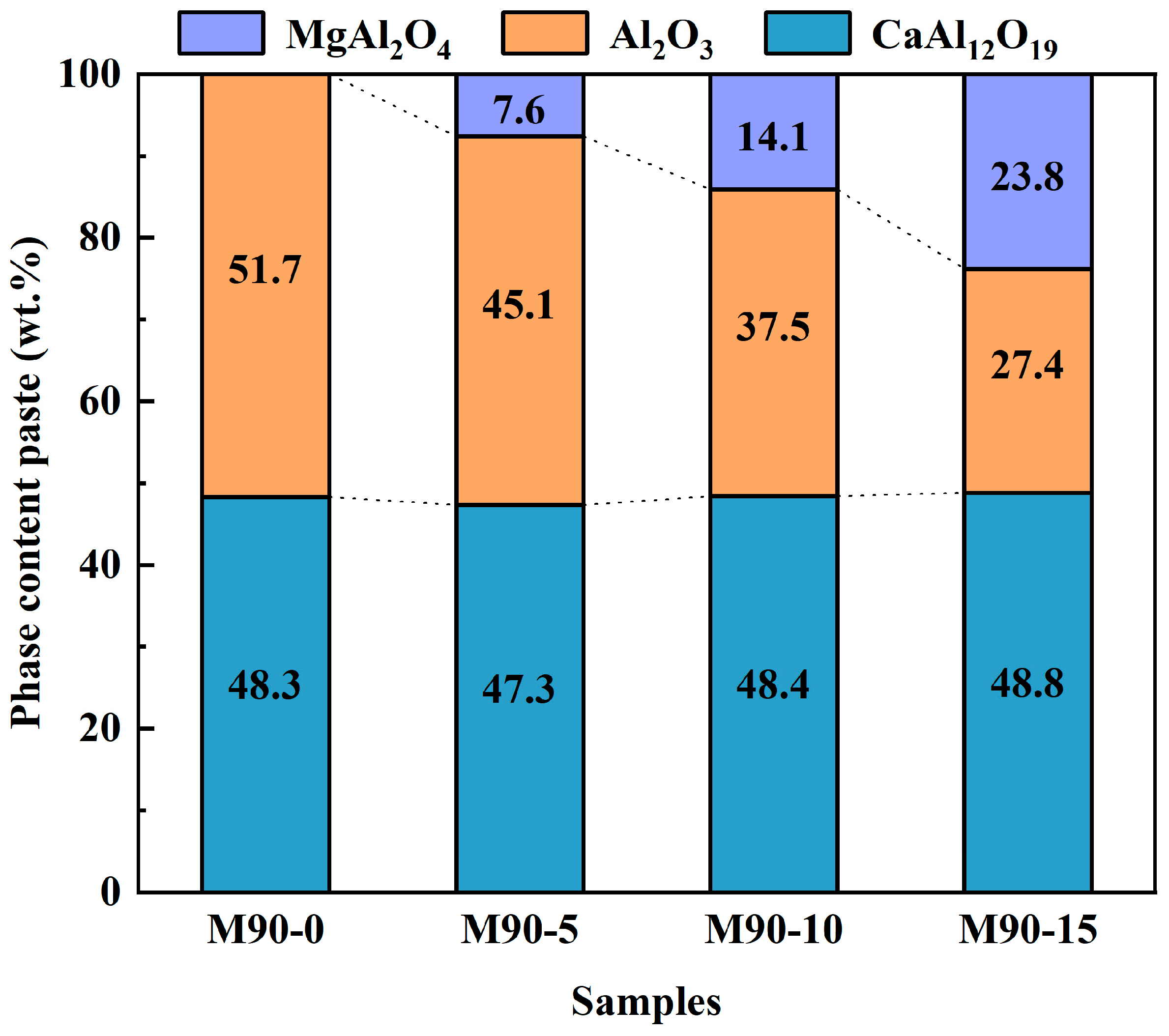
3.4. Phase Composition
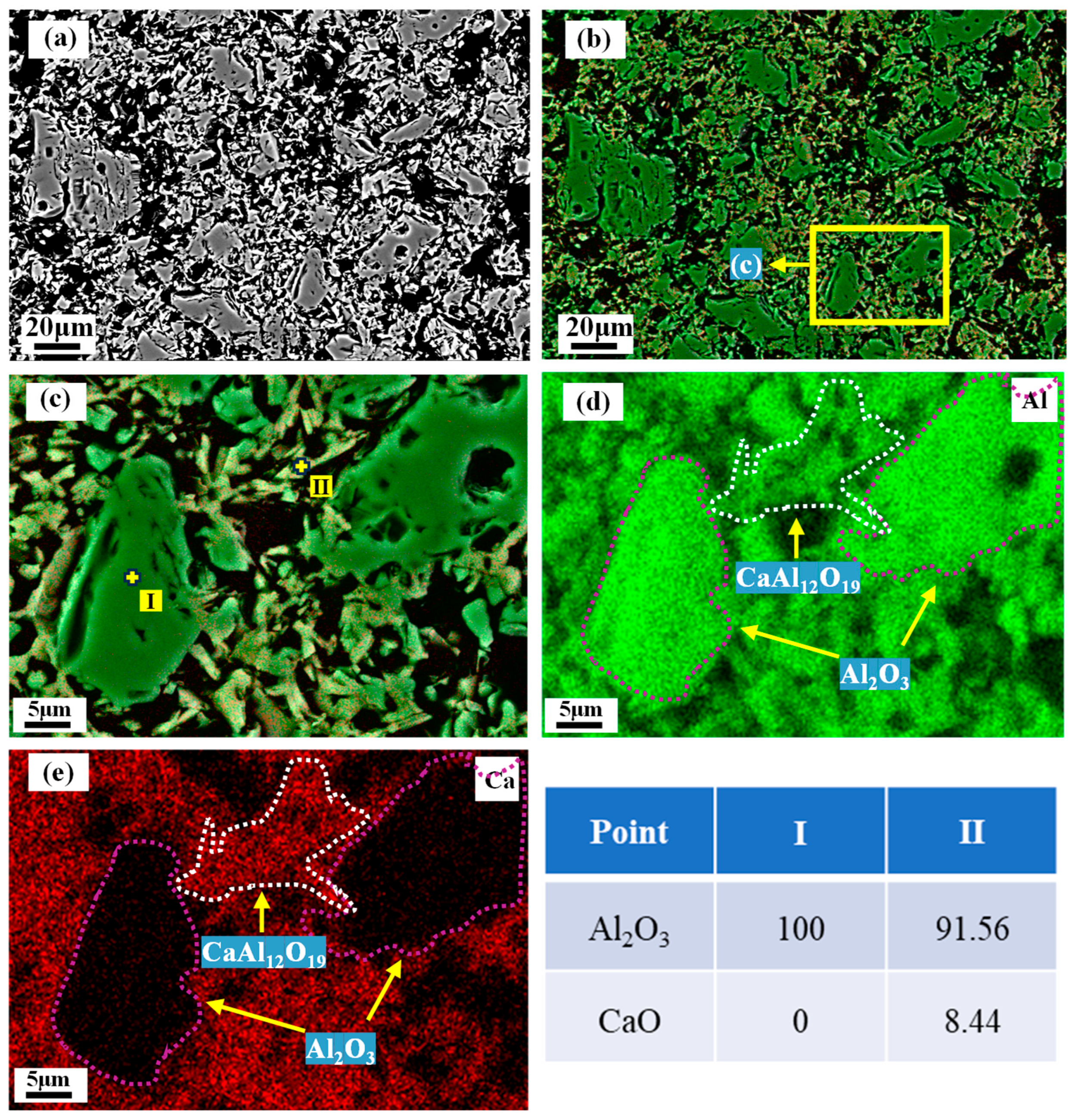
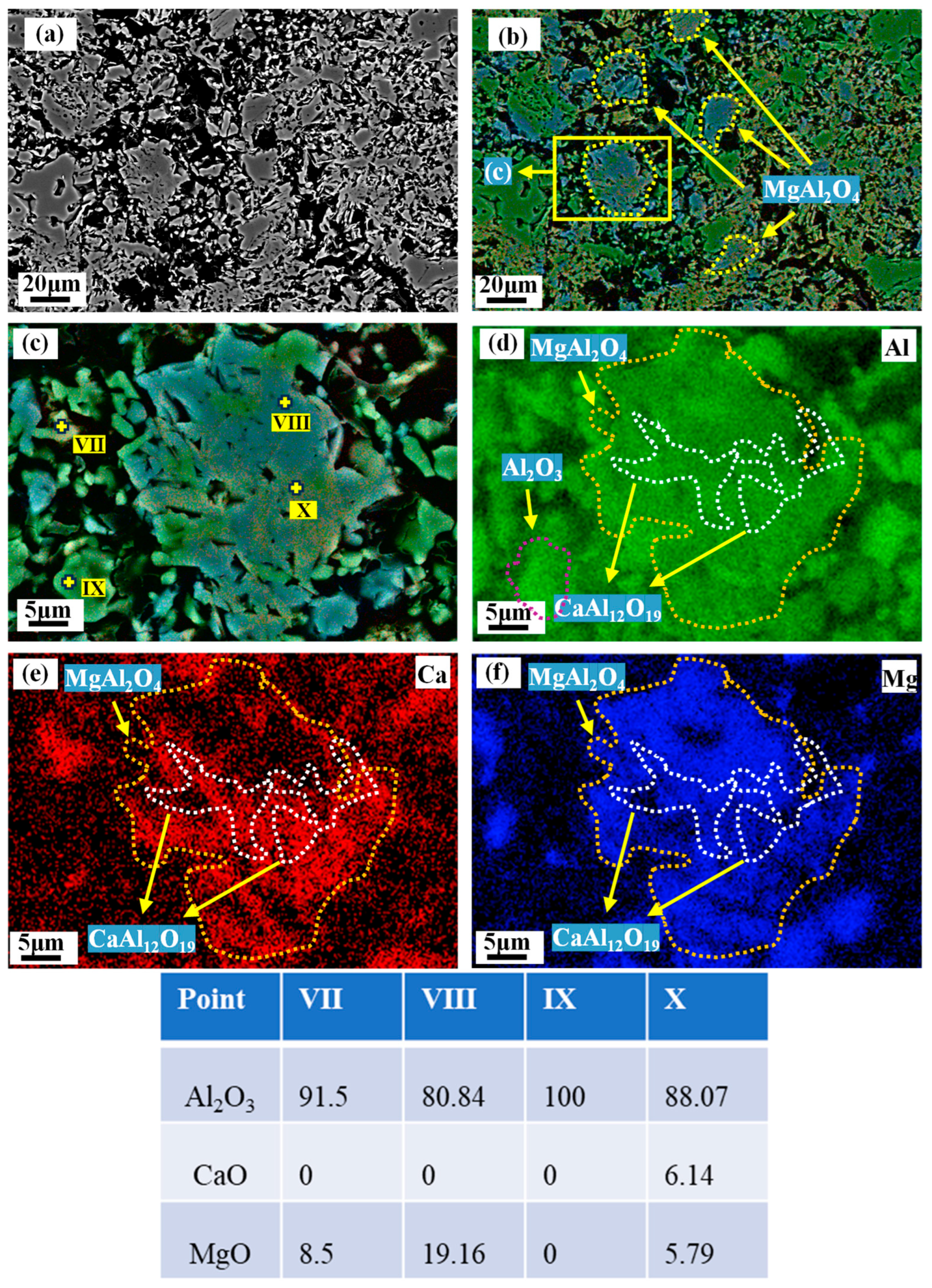
3.5. Microstructure of the Matrix
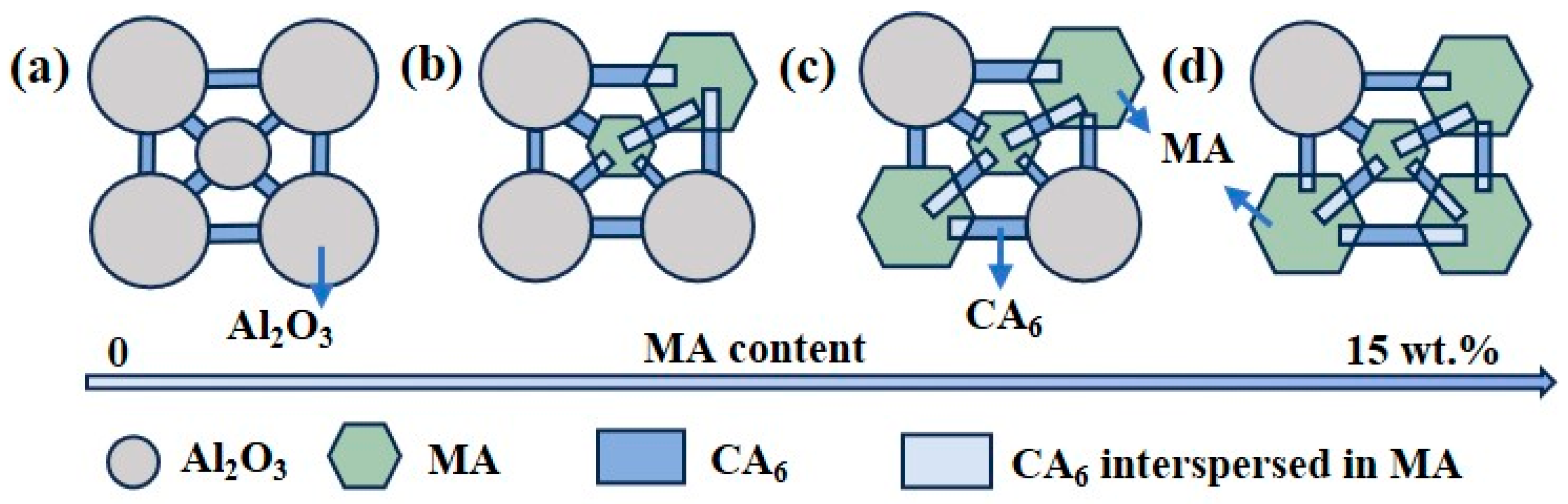
3.6. Mechanism of CA6 Formation and Distribution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Sun, W.; He, H.; Zhang, H.; Xiong, X.; Peng, Z. Effect of composite micro-powder added with α-Al2O3 and zirconium basic carbonate on the structure and properties of corundum castable. Ceram. Int. 2023, 49, 8058–8070. [Google Scholar] [CrossRef]
- Liu, W.; Liao, N.; Nath, M.; Ji, Z.; Dai, Y.; Pan, L.; Li, Y.; Jastrzębska, I.; Szczerba, J. Effects of curing time on the pore structure evolution and fracture behavior of CAC bonded alumina-spinel castables. Ceram. Int. 2022, 48, 25000–25010. [Google Scholar] [CrossRef]
- Liu, W.; Liao, N.; Nath, M.; Li, Y.; Dai, Y.; Pan, L. Effects of characteristic hydrates on the pore structure and fracture behavior of CAC bonded alumina-spinel castables. Constr. Build. Mater. 2023, 389, 131736. [Google Scholar] [CrossRef]
- Qi, X.; Fu, L.; Zou, Y.; Gu, H.; Huang, A.; Chen, D.; Yang, S. A novel low thermal conductivity refractory aggregate for high-temperature applications: Lightweight microporous alumina-rich spinel (Mg0.4Al2.4O4). Ceram. Int. 2024, 50 Pt B, 3526–3538. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Chen, P.; Zhu, B. Matrix microstructure optimization of alumina-spinel castables and its effect on high temperature properties. Ceram. Int. 2018, 44, 857–868. [Google Scholar] [CrossRef]
- Zhang, D.; Li, C.; Jiang, N.; Gao, J.; Touzo, B.; Yuan, W. Influence of powder characteristics of reactive alumina on properties of alumina-spinel castables. Ceram. Int. 2018, 44, 9984–9990. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, Y.; Li, Y.; Chen, J.; Xiao, J.; Wei, Y.; Liu, G.; Li, G.; Zhang, S.; Li, N. Degradation behaviors of cement-free corundum-spinel castables in Ruhrstahl Heraeus refining ladle: Role of infiltrated steel. Ceram. Int. 2021, 47, 32008–32014. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Zhang, L. Enhancing mechanism of improved slag resistance of Al2O3-spinel castables added with pre-synthesized (Al,Cr)2O3 micro-powder. Ceram. Int. 2021, 47, 33322–33329. [Google Scholar] [CrossRef]
- Xuan, S.; Tian, Y.; Kong, X.; Hao, J.; Wang, X. Enhancement of thermal shock resistance of Al2O3–MgAl2O4 composites by controlling the content and distribution of spinel phase. Ceram. Int. 2023, 49 Pt A, 39908–39916. [Google Scholar] [CrossRef]
- Aksel, C.; Riley, F.L. Effect of the particle size distribution of spinel on the mechanical properties and thermal shock performance of MgO–spinel composites. J. Eur. Ceram. Soc. 2003, 23, 3079–3087. [Google Scholar] [CrossRef]
- Khalil, N.M. Recent Developments in Magnesia-Spinel Refractory Composites, Part 1. Interceram Int. J. Bricks Struct. Clay Prod. Refract. Pottery Fine Ceram. Abras. Spec. Ceram. 2008, 6, 57. [Google Scholar]
- Mohapatra, D.; Sarkar, D. Effect of in situ spinel seeding on synthesis of MgO-rich MgAl2O4 composite. J. Mater. Sci. 2007, 42, 7286–7293. [Google Scholar] [CrossRef]
- Zubrzycka, P.; Radecka, M.; Graule, T.; Trenczek-Zając, A.; Zientara, D.; Stuer, M. MgAl2O4 spinel with transmittance approaching theoretical value at reduced sintering temperatures. J. Eur. Ceram. Soc. 2024, 44, 6047–6059. [Google Scholar] [CrossRef]
- Zhou, Y.; Ye, D.; Wu, Y.; Zhang, C.; Bai, W.; Tian, Y.; Qin, M. Low-cost preparation and characterization of MgAl2O4 ceramics. Ceram. Int. 2022, 48, 7316–7319. [Google Scholar] [CrossRef]
- Braulio, M.A.L.; Rigaud, M.; Buhr, A.; Parr, C.; Pandolfelli, V.C. Spinel-containing alumina-based refractory castables. Ceram. Int. 2011, 37, 1705–1724. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Hou, Q.; Zeng, J.; Mu, Y.; He, J.; Ye, G.; Tian, J. Effect of solid solution of magnesia-alumina spinel on the microstructure and mechanical properties of CAC-bonded corundum castables. Ceram. Int. 2024. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, L.; Liu, K.; Ding, D.; Zhang, J.; Ye, G. Effect of curing temperature on volume stability of CAC-bonded alumina-based castables. Ceram. Int. 2019, 45, 12066–12071. [Google Scholar] [CrossRef]
- Gungor, A.; Celikcioglu, O.; SAHIN, S. The physical and mechanical properties of alumina-based ultralow cement castable refractories. Ceram. Int. 2012, 38, 4189–4194. [Google Scholar] [CrossRef]
- Parr, C.; Roesky, R.; Whrmeyer, C. Calcium aluminate cements for uushaped refractories. InterCeram Int. Ceram. Rev. 2001, 5, 6–12. [Google Scholar]
- Gao, S.; Zhang, P.; Li, N.; Zhang, J.; Luan, J.; Ye, G.; Liao, G. Effect of CAC content on the strength of castables at temperatures between 300 and 1000 °C. Ceram. Int. 2020, 46 Pt A, 14957–14963. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, Z.; Mu, Y.; Liu, C.; Ye, G. Effect of pre-hydrated CAC suspensions on the hydration behavior of CAC pastes. Cem. Concr. Compos. 2024, 152, 105659. [Google Scholar] [CrossRef]
- Pacewska, B.; Nowacka, M. Studies of conversion progress of calcium aluminate cement hydrates by thermal analysis method. J. Therm. Anal. Calorim. 2014, 117, 653–660. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, G.; Gu, W.; Ding, D.; Chen, L.; Zhu, L. Conversion of calcium aluminate cement hydrates at 60 °C with and without water. J. Am. Ceram. Soc. 2018, 101, 2712–2717. [Google Scholar] [CrossRef]
- Bushnell-Watson, S.M.; Sharp, J.H. The effect of temperature upon the setting behaviour of refractory calcium aluminate cements. Cem. Concr. Res. 1986, 16, 875–884. [Google Scholar] [CrossRef]
- Ye, K.; Huang, G.; Zhao, Y.; Hou, Q.; Chen, J.; Tian, J.; Ye, G.; Mu, Y. Effect of CAC addition and pre-drying on the mechanical property of carbonated C2S(γ) mortar. Constr. Build. Mater. 2024, 456, 139293. [Google Scholar] [CrossRef]
- Tomba Martinez, A.G.; Luz, A.P.; Braulio, M.A.L.; Pandolfelli, V.C. CA6 impact on the corrosion behavior of cement-bonded spinel-containing refractory castables: An analysis based on thermodynamic simulations. Ceram. Int. 2015, 41 Pt B, 4714–4725. [Google Scholar] [CrossRef]
- Ukrainczyk, N.; Matusinović, T. Thermal properties of hydrating calcium aluminate cement pastes. Cem. Concr. Res. 2010, 40, 128–136. [Google Scholar] [CrossRef]
- Auvray, J.-M.; Gault, C.; Huger, M. Evolution of elastic properties and microstructural changes versus temperature in bonding phases of alumina and alumina–magnesia refractory castables. J. Eur. Ceram. Soc. 2007, 27, 3489–3496. [Google Scholar] [CrossRef]
- Díaz, L.A.; Torrecillas, R.; De Aza, A.H.; Pena, P. Effect of spinel content on slag attack resistance of high alumina refractory castables. J. Eur. Ceram. Soc. 2007, 27, 4623–4631. [Google Scholar] [CrossRef]
- Quan, Z.; Wang, Z.; Liu, H.; Ma, Y.; Wang, X.; Dong, Y.; Deng, C.; Fu, G. Effects of different particle size of spinel and Y2O3 additive of the periclase-spinel refractory on the sintering densification and corrosion resistance to copper smelting slag. Ceram. Int. 2022, 48, 18180–18189. [Google Scholar] [CrossRef]
- Yan, W. Influence of light-burned spinel on the slag resistance of alumina-spinel refractory castables. J. Ceram. Process. Res. 2014, 15, 441–446. (In Chinese) [Google Scholar]
- Chen, H.; Zhao, L.; He, X.; Fang, W.; Lei, Z.X.; Chen, H. The fabrication of porous corundum spheres with core-shell structure for corundum-spinel castables. Mater. Des. 2015, 85, 574–581. [Google Scholar] [CrossRef]
- Pilli, V.; Sarkar, R. Effect of spinel content on the properties of Al2O3–SiC–C based trough castable. Ceram. Int. 2016, 42 Pt B, 2969–2982. [Google Scholar] [CrossRef]
- Fedorov, M.V.; Vasilieva, M.I. Study of phase transitions of ultradispersed spinel powder during solid-phase sintering at 1450 °C. Procedia Struct. Integr. 2022, 40, 145–152. [Google Scholar] [CrossRef]
- GB/T 3001-2017; Refractory Products-Determination of Modulus of Rupture at Ambient Temperture. National Standard of the People’s Republic of China, Standardization Administration of China (SAC): Beijing, China, 2017.
- GB/T 5072-2023; Refractory Products—Determination of Cold Compressive Strength. National Standard of the People’s Republic of China, Standardization Administration of China (SAC): Beijing, China, 2023.
- YB 4018-1991; Test Method for Thermal Shock Resistance of Refractory Products. National Standard of the People’s Republic of China, Standardization Administration of China (SAC): Beijing, China, 1991.
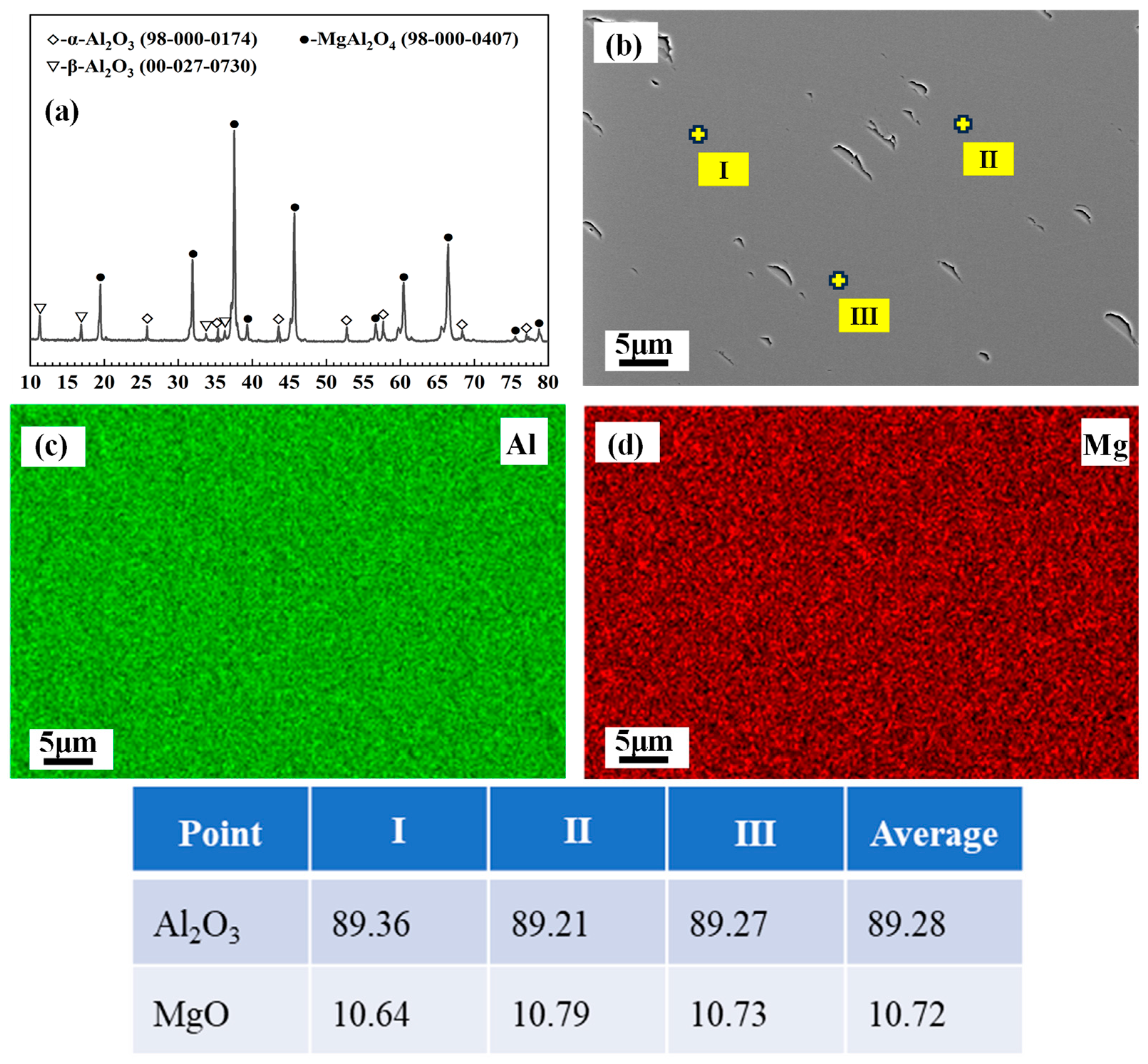
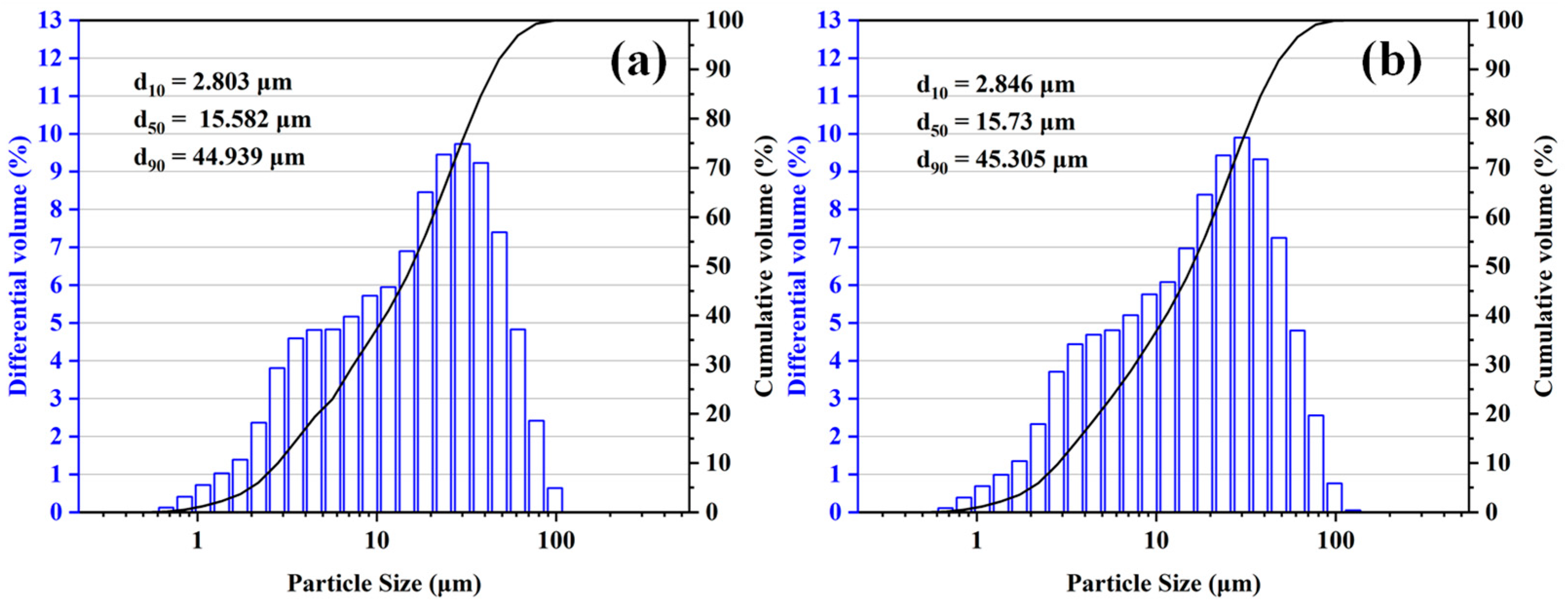
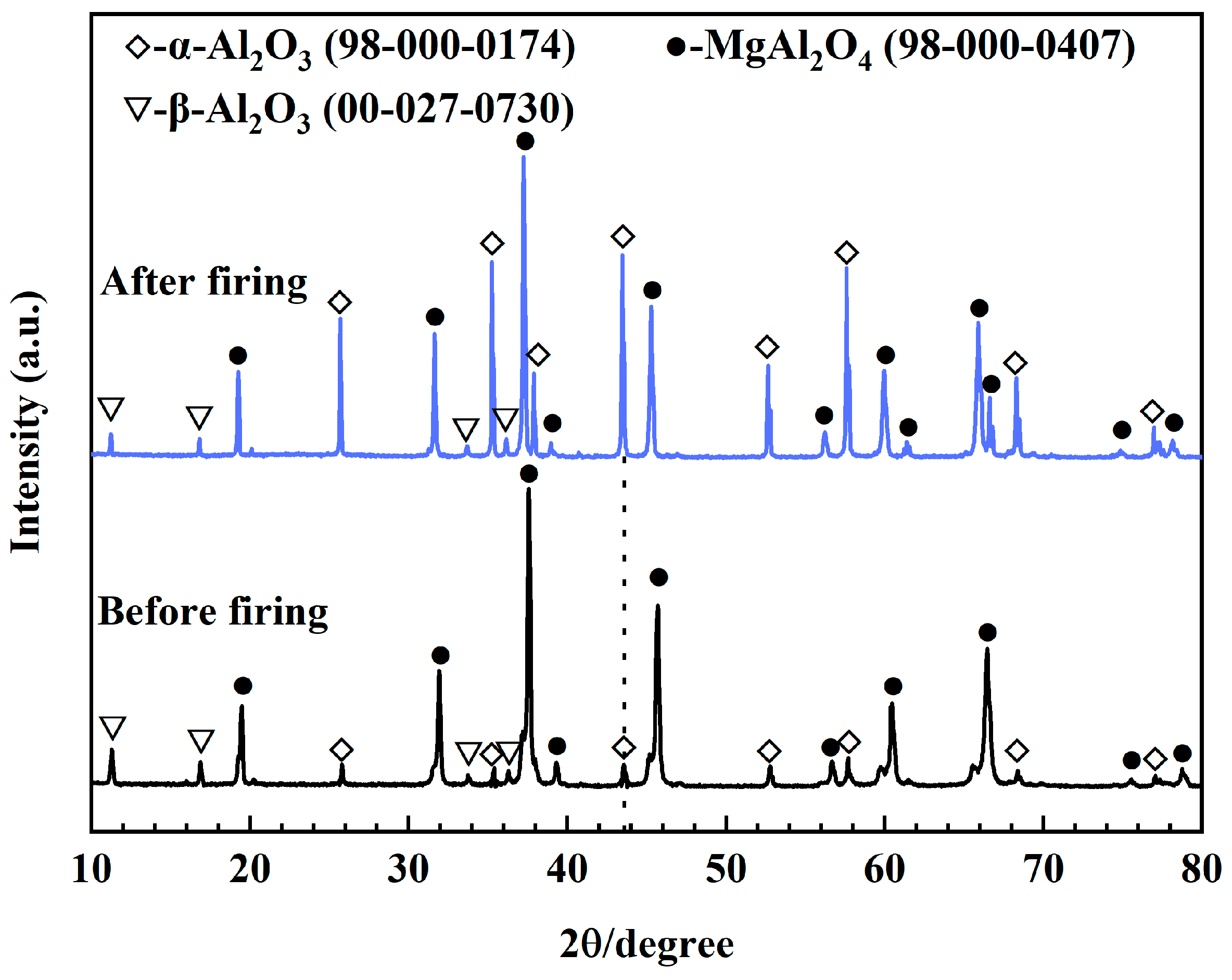

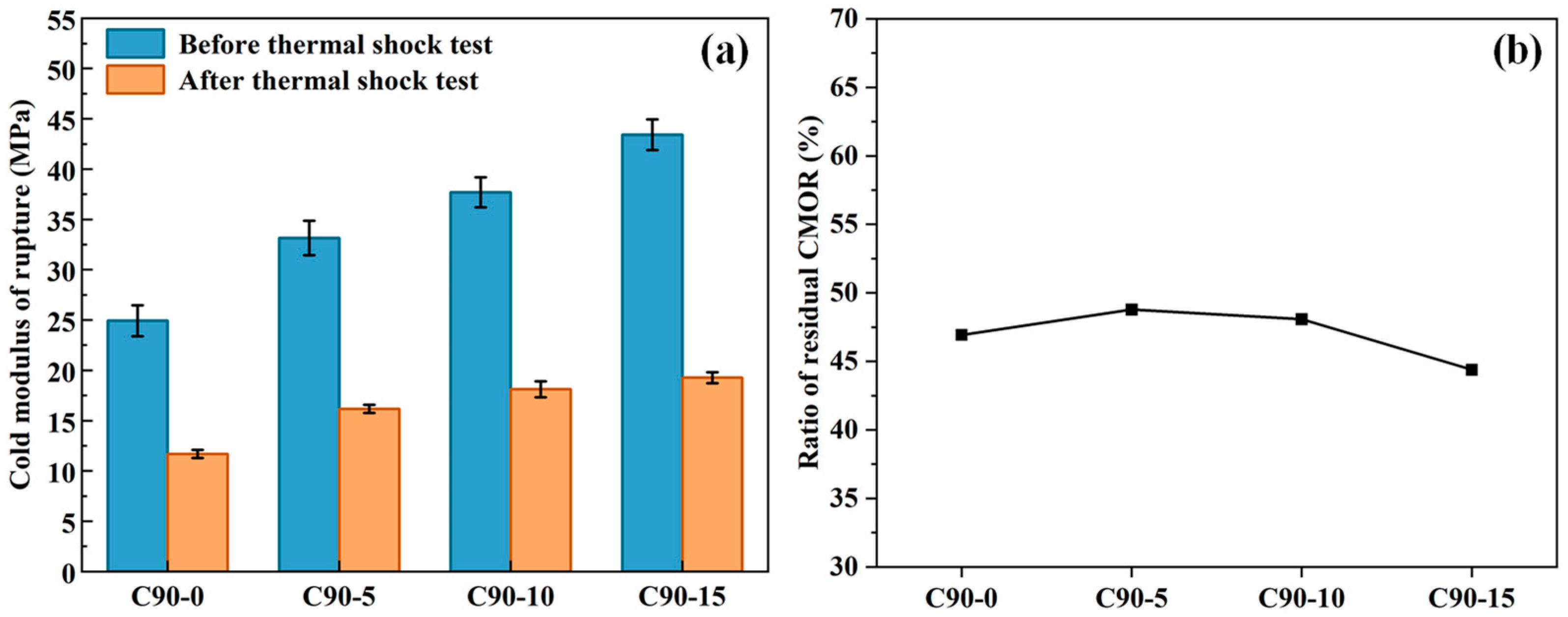



| Al2O3 | SiO2 | Fe2O3 | Na2O |
|---|---|---|---|
| 99.56 | 0.09 | 0.05 | 0.30 |
| Raw Materials | Castable Samples | ||||
|---|---|---|---|---|---|
| C90-0 | C90-5 | C90-10 | C90-15 | ||
| Tabular alumina | 6-3 mm | 28 | 28 | 28 | 28 |
| 3-1 mm | 22 | 22 | 22 | 22 | |
| 1-0 mm | 20 | 20 | 20 | 20 | |
| d50 = 15.582 μm | 20 | 15 | 10 | 5 | |
| Reactive alumina | HA115 | 6 | 6 | 6 | 6 |
| Cement | Secar 71 | 4 | 4 | 4 | 4 |
| MA-90 | d50 = 15.73 μm | 0 | 5 | 10 | 15 |
| Dispersant | ADW1 | +0.4 | +0.4 | +0.4 | +0.4 |
| ADS1 | +0.1 | +0.1 | +0.1 | +0.1 | |
| Raw Materials | Matrix Samples | ||||
|---|---|---|---|---|---|
| M90-0 | M90-5 | M90-10 | M90-15 | ||
| Tabular alumina | d50 = 15.582 μm | 67 | 50 | 33.5 | 17 |
| Reactive alumina | HA115 | 20 | 20 | 20 | 20 |
| Cement | Secar 71 | 13 | 13 | 13 | 13 |
| MA-90 | d50 = 15.73 μm | 0 | 17 | 33.5 | 50 |
| Dispersant | ADW1 | +0.4 | +0.4 | +0.4 | +0.4 |
| ADS1 | +0.1 | +0.1 | +0.1 | +0.1 | |
| Sample | Phase Quantification/wt.% | Agreement Factors | ||
|---|---|---|---|---|
| MA | α-Al2O3 | β-Al2O3 | RWP/% | |
| Before firing | 88.3 | 6.3 | 5.4 | 11.47 |
| After firing | 51.3 | 42.9 | 5.8 | 12.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Q.; Zhang, Z.; Zhao, Y.; Ye, K.; Tian, J.; Mu, Y.; He, J.; Ye, G. The Effect of Alumina-Rich Spinel Exsolution on the Mechanical Property of Calcium Aluminate Cement-Bonded Corundum Castables. Materials 2025, 18, 405. https://doi.org/10.3390/ma18020405
Hou Q, Zhang Z, Zhao Y, Ye K, Tian J, Mu Y, He J, Ye G. The Effect of Alumina-Rich Spinel Exsolution on the Mechanical Property of Calcium Aluminate Cement-Bonded Corundum Castables. Materials. 2025; 18(2):405. https://doi.org/10.3390/ma18020405
Chicago/Turabian StyleHou, Qiqi, Zhongzhuang Zhang, Yaning Zhao, Kaiwei Ye, Jiajia Tian, Yuandong Mu, Jian He, and Guotian Ye. 2025. "The Effect of Alumina-Rich Spinel Exsolution on the Mechanical Property of Calcium Aluminate Cement-Bonded Corundum Castables" Materials 18, no. 2: 405. https://doi.org/10.3390/ma18020405
APA StyleHou, Q., Zhang, Z., Zhao, Y., Ye, K., Tian, J., Mu, Y., He, J., & Ye, G. (2025). The Effect of Alumina-Rich Spinel Exsolution on the Mechanical Property of Calcium Aluminate Cement-Bonded Corundum Castables. Materials, 18(2), 405. https://doi.org/10.3390/ma18020405






