A Comparative Finite Element Analysis of Titanium, Autogenous Bone, and Polyetheretherketone (PEEK)-Based Solutions for Mandibular Reconstruction
Abstract
1. Introduction
2. Materials and Methods
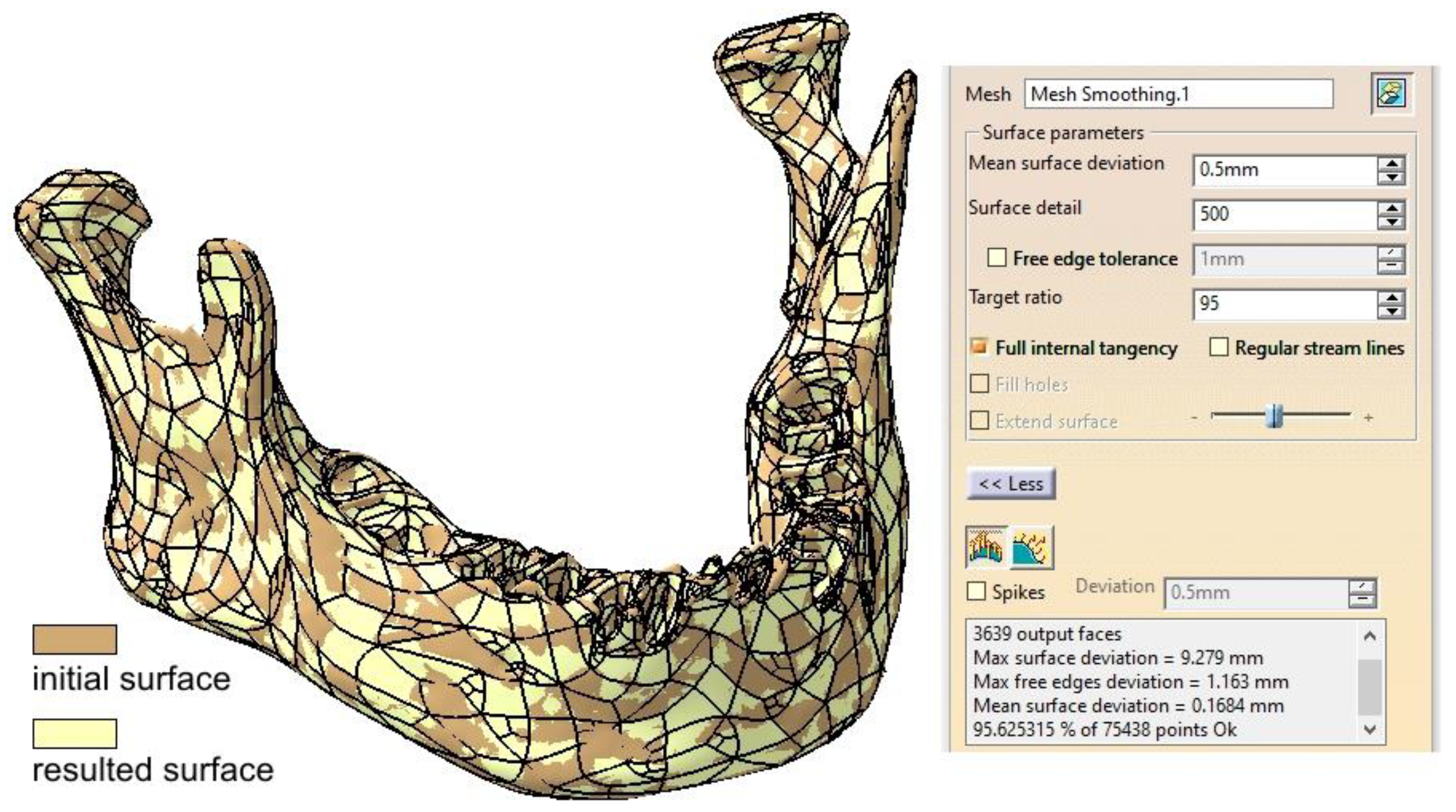
2.1. Optimizing the Mandibular Model for Simulation
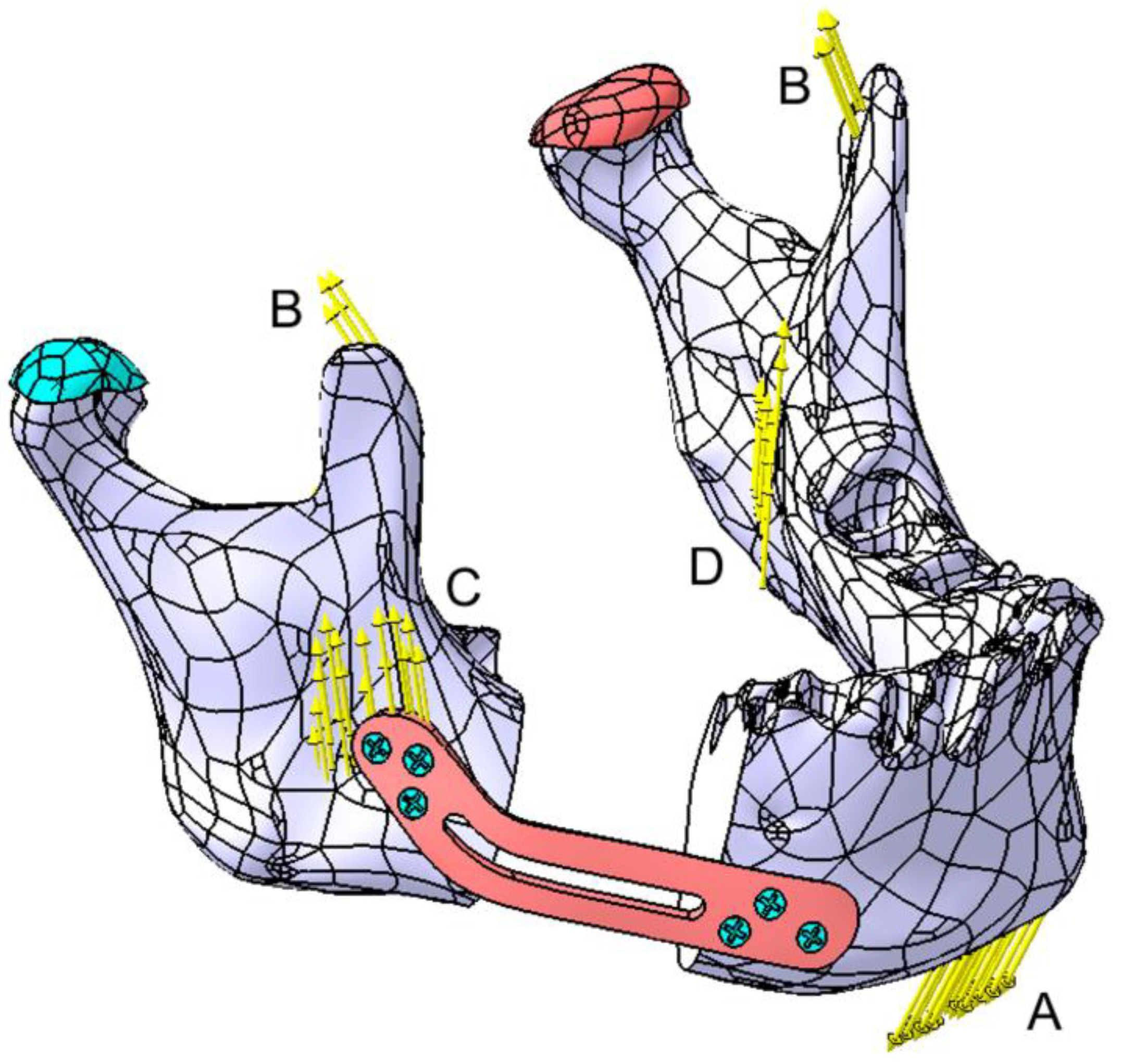
2.2. Post Tumor Ablation Defect Simulation
2.3. Reconstruction Simulation
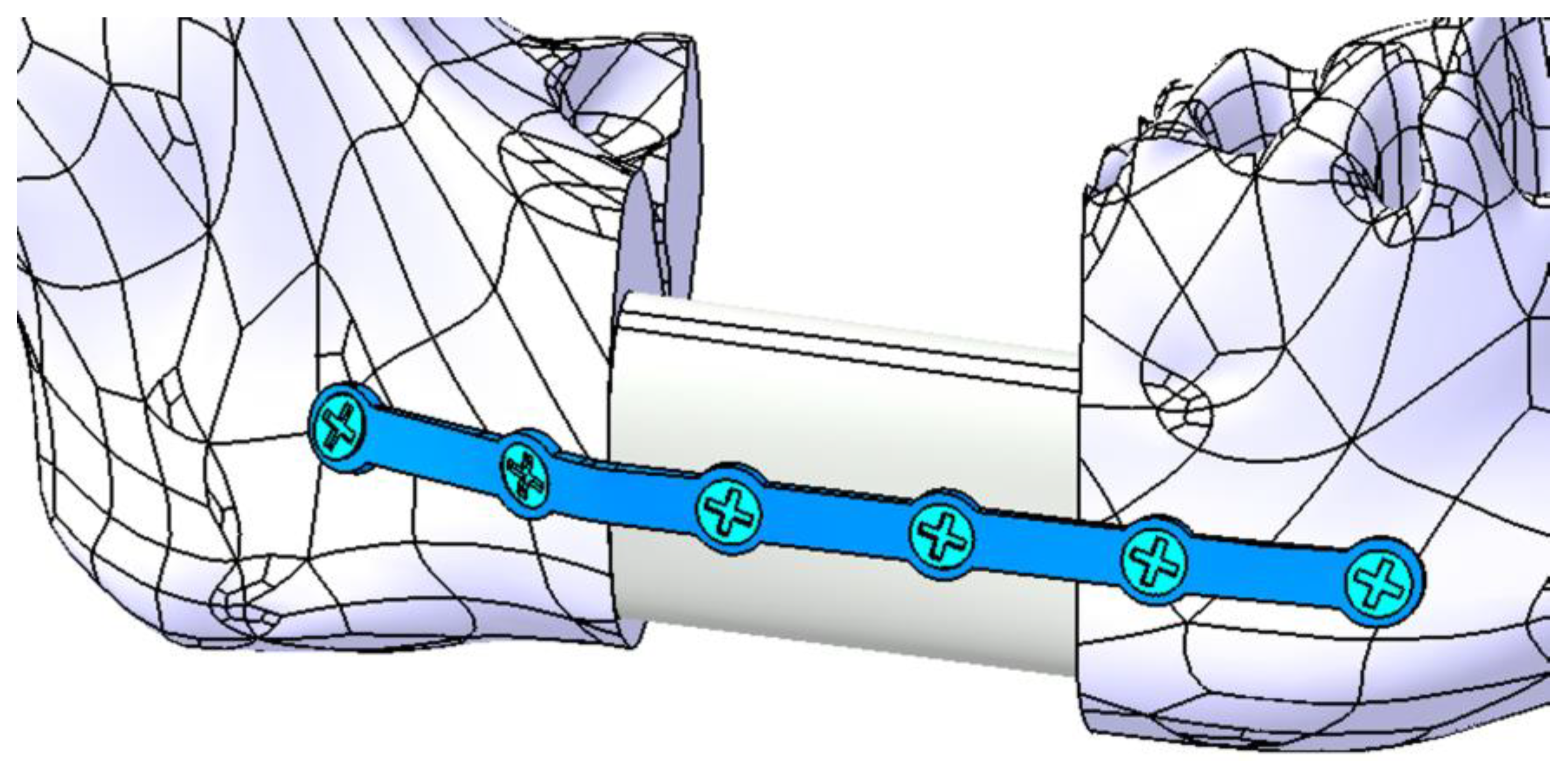
2.3.1. Fixation with a Titanium Osteosynthesis Plate
2.3.2. Reconstruction with a Fibular Autograft
2.3.3. Fixation with a Customized Polyetheretherketone (PEEK) Plate
2.4. Testing Assembly
3. Results
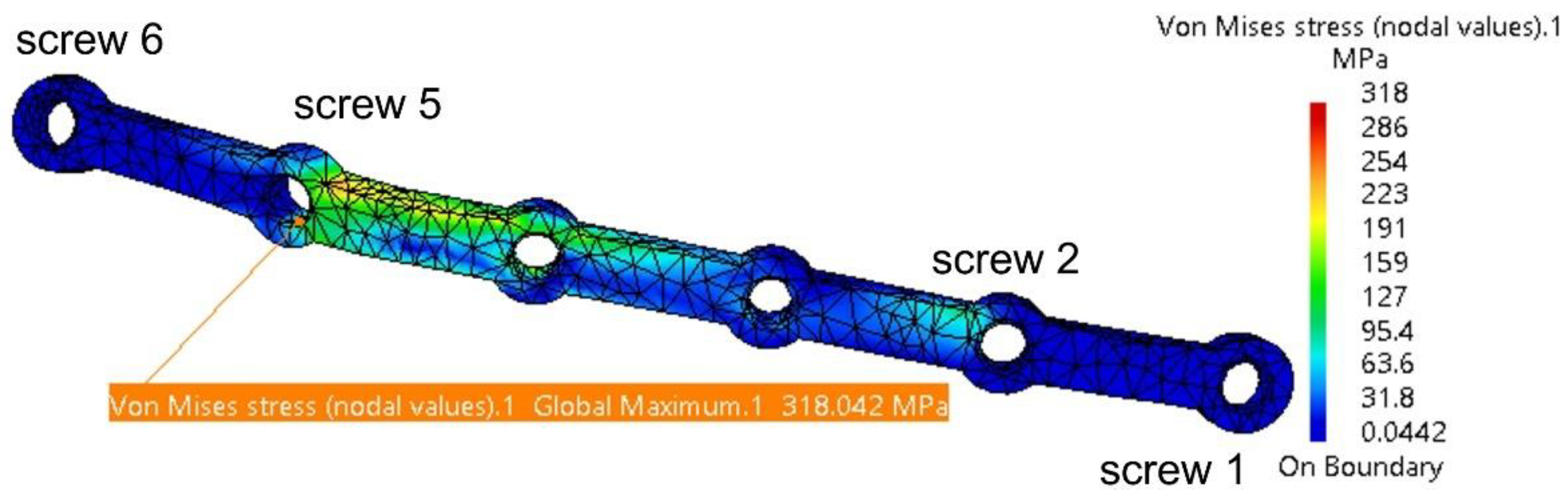
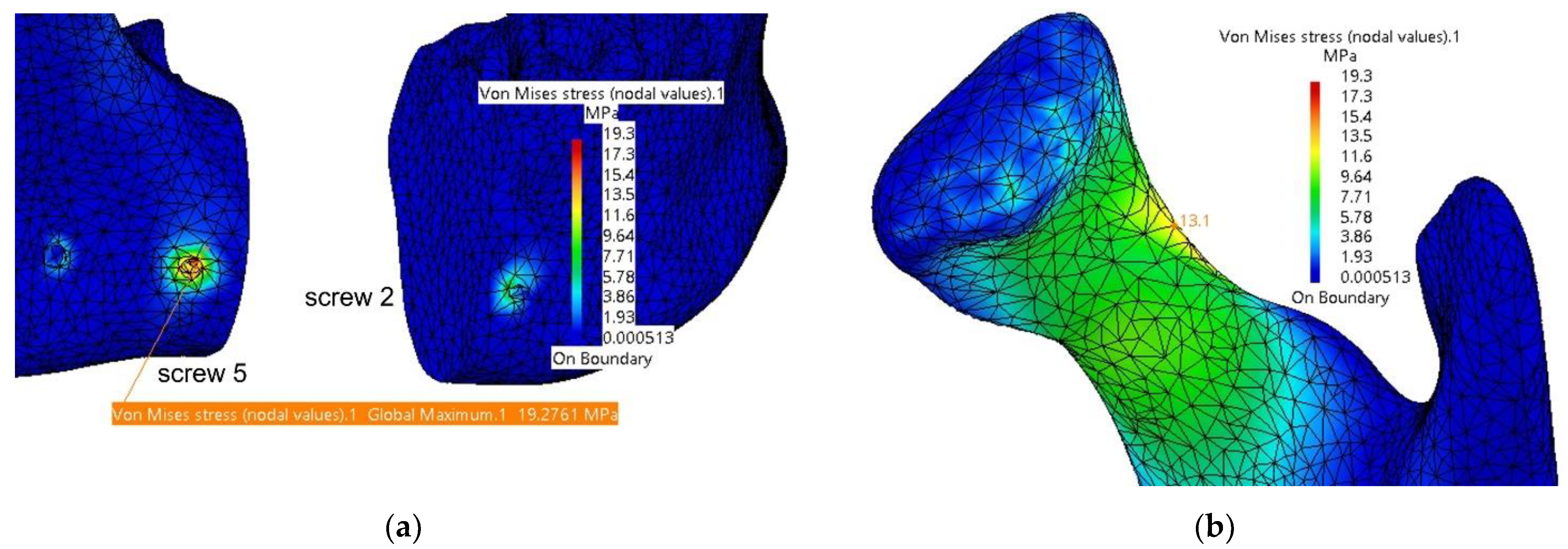
3.1. Fixation with a Titanium Osteosynthesis Plate
3.2. Reconstruction with a Fibular Autograft
3.3. Fixation with a Customized PEEK Plate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Aftabi, H.; Zaraska, K.; Eghbal, A.; McGregor, S.; Prisman, E.; Hodgson, A.; Fels, S. Computational models and their applications in biomechanical analysis of mandibular reconstruction surgery. Comput. Biol. Med. 2024, 169, 107887. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, D.; Cassoni, A.; De Ponti, E.; Moretti, M.; Pucci, R.; Spadoni, D.; Canzi, G.; Novelli, G.; Valentini, V. Effectiveness of Resective Surgery in Complex Ameloblastoma of the Jaws: A Retrospective Multicenter Observational Study. Cancers 2022, 14, 4608. [Google Scholar] [CrossRef] [PubMed]
- Gupta, L.; Mishra, A.; Gurav, S.V.; Dholam, K.; Pal, A.; Kumar, A. Factors associated with mandibular deviation and proposed classification and treatment guidelines for applying mandibular guidance: A retrospective analysis of 185 patients with segmental mandibulectomy. J. Prosthet. Dent. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Pyne, J.M.; Davis, C.M.; Kelm, R.; Bussolaro, C.; Dobrovolsky, W.; Seikaly, H. Advanced mandibular reconstruction with fibular free flap and alloplastic TMJ prosthesis with digital planning. J. Otolaryngol.-Head Neck Surg. 2023, 52, s40463-023. [Google Scholar] [CrossRef]
- Weyh, A.M.; Fernandes, R.P. Narrative review: Fibula free flap, indications, tips, and pitfalls. Front. Oral Maxillofac. Med. 2021, 3, 1–7. [Google Scholar] [CrossRef]
- Hoffman, G.R.; Islam, S.; Eisenberg, R.L. Microvascular reconstruction of the mouth, jaws, and face: Experience of an australian oral and maxillofacial surgery unit. J. Oral Maxillofac. Surg. 2012, 70, e371–e377. [Google Scholar] [CrossRef]
- Zeller, A.N.; Neuhaus, M.T.; Weissbach, L.V.M.; Rana, M.; Dhawan, A.; Eckstein, F.M.; Gellrich, N.C.; Zimmerer, R.M. Patient-Specific Mandibular Reconstruction Plates Increase Accuracy and Long-Term Stability in Immediate Alloplastic Reconstruction of Segmental Mandibular Defects. J. Maxillofac. Oral Surg. 2020, 19, 609–615. [Google Scholar] [CrossRef]
- Saunders, J.R.; Hirata, R.M.; Jaques, D.A. Definitive mandibular replacement using reconstruction plates. Am. J. Surg. 1990, 160, 387–389. [Google Scholar] [CrossRef]
- Kumar, B.P.; Venkatesh, V.; Kumar, K.A.J.; Yadav, B.Y.; Mohan, S.R. Mandibular Reconstruction: Overview. J. Maxillofac. Oral Surg. 2015, 15, 425–441. [Google Scholar] [CrossRef]
- Kitami, R.; Izumi, M.; Taniguchi, M.; Kozai, Y.; Sakurai, T. Phantom study for CT artifacts of dental titanium implants and zirconia upper structures: The effects of occlusal plane angle setting and SEMAR algorithm. Oral Radiol. 2024, 40, 251–258. [Google Scholar] [CrossRef]
- Huber, F.A.; Sprengel, K.; Müller, L.; Graf, L.C.; Osterhoff, G.; Guggenberger, R. Comparison of different CT metal artifact reduction strategies for standard titanium and carbon-fiber reinforced polymer implants in sheep cadavers. BMC Med. Imaging 2021, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Midthun, P.; Kirkhus, E.; Østerås, B.H.; Høiness, P.R.; England, A.; Johansen, S. Metal artifact reduction on musculoskeletal CT: A phantom and clinical study. Eur. Radiol. Exp. 2023, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Lommen, J.; Schorn, L.; Sproll, C.; Kübler, N.R.; Nicolini, L.F.; Merfort, R.; Dilimulati, A.; Hildebrand, F.; Rana, M.; Greven, J. Mechanical Fatigue Performance of Patient-Specific Polymer Plates in Oncologic Mandible Reconstruction. J. Clin. Med. 2022, 11, 3308. [Google Scholar] [CrossRef] [PubMed]
- Panayotov, I.V.; Orti, V.; Cuisinier, F.; Yachouh, J. Polyetheretherketone (PEEK) for medical applications. J. Mater. Sci. Mater. Med. 2016, 27, 1–11. [Google Scholar] [CrossRef]
- Mehle, K.; Eckert, A.W.; Gentzsch, D.; Schwan, S.; Ludtka, C.M.; Knoll, W. Evaluation of a New PEEK Mandibular Reconstruction Plate Design for Continuity Defect Therapy by Finite Element Analysis. Int. J. New Technol. Res. 2016, 2, 263461. [Google Scholar]
- Vayvada, H.; Mola, F.; Menderes, A.; Yilmaz, M. Surgical Management of Ameloblastoma in the Mandible: Segmental Mandibulectomy and Immediate Reconstruction With Free Fibula or Deep Circumflex Iliac Artery Flap (Evaluation of the Long-Term Esthetic and Functional Results). J. Oral Maxillofac. Surg. 2006, 64, 1532–1539. [Google Scholar] [CrossRef]
- Gasparro, R.; Giordano, F.; Campana, M.D.; Aliberti, A.; Landolfo, E.; Dolce, P.; Sammartino, G.; di Lauro, A.E. The Effect of Conservative vs. Radical Treatment of Ameloblastoma on Recurrence Rate and Quality of Life: An Umbrella Review. J. Clin. Med. 2024, 13, 5339. [Google Scholar] [CrossRef]
- Chen, C.; Batstone, M.; Taheri, T.; Johnson, N. Surgical Resection and Reconstruction of Ameloblastoma: A 13-Year Retrospective Review. J. Oral Maxillofac. Surg. 2024, 82, 862–868. [Google Scholar] [CrossRef]
- Xue, R.; Lai, Q.; Xing, H.; Zhong, C.; Zhao, Y.; Zhu, K.; Deng, Y.; Liu, C. Finite element analysis and clinical application of 3D-printed Ti alloy implant for the reconstruction of mandibular defects. BMC Oral Health 2024, 24, 95. [Google Scholar] [CrossRef]
- Shen, Y.W.; Tsai, Y.S.; Hsu, J.T.; Shie, M.Y.; Huang, H.L.; Fuh, L.J. Biomechanical Analyses of Porous Designs of 3D-Printed Titanium Implant for Mandibular Segmental Osteotomy Defects. Materials 2022, 15, 576. [Google Scholar] [CrossRef]
- Kucukguven, M.B.; Akkocaolu, M. Finite element analysis of stress distribution on reconstructed mandibular models for autogenous bone grafts. Technol. Health Care 2020, 28, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Melville, J.C.; Mañón, V.A.; Arribas, A.R.; Wong, M.E. Custom 3D printed titanium reconstruction plate with in-situ tissue engineering for the reconstruction and dental rehabilitation of a severely infected atrophic mandible. A review of technique. Dent. Rev. 2021, 1, 100005. [Google Scholar] [CrossRef]
- Chakraborty, S.; Guha, R.P.; Naskar, S.; Banerjee, R. Custom-Made 3D Titanium Plate for Mandibular Reconstruction in Surgery of Ameloblastoma: A Novel Case Report. Surg. Tech. Dev. 2022, 11, 98–104. [Google Scholar] [CrossRef]
- Tanaka, E. Biomechanical and tribological properties of the temporomandibular joint. Front. Oral Maxillofac. Med. 2021, 3, 15. [Google Scholar] [CrossRef]
- De Stefano, M.; Ruggiero, A. A Critical Review of Human Jaw Biomechanical Modeling. Appl. Sci. 2024, 14, 3813. [Google Scholar] [CrossRef]
- Ghionea, I.G.; Vatamanu, O.E.B.; Cristescu, A.M.; David, M.; Stancu, I.C.; Butnarasu, C.; Cristache, C.M. A Finite Element Analysis of a Tooth-Supported 3D-Printed Surgical Guide without Metallic Sleeves for Dental Implant Insertion. Appl. Sci. 2023, 13, 9975. [Google Scholar] [CrossRef]
- Pinheiro, M.; Willaert, R.; Khan, A.; Krairi, A.; Van Paepegem, W. Biomechanical evaluation of the human mandible after temporomandibular joint replacement under different biting conditions. Sci. Rep. 2021, 11, 14034. [Google Scholar] [CrossRef]
- Ghionea, I.G. CATIA V5 Practical Studies Using Finite Element Analysis; CRC Press: Boca Raton, FL, USA, 2024; ISBN 9781032711645. [Google Scholar] [CrossRef]
- Ashcroft, I.A.; Mubashar, A. Numerical Approach: Finite Element Analysis. In Handbook of Adhesion Technology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 629–660. [Google Scholar] [CrossRef]
- Tang, Y.; Qing, H. Finite element formulation for higher-order shear deformation beams using two-phase local/nonlocal integral model. Arch. Appl. Mech. 2024, 94, 1243–1262. [Google Scholar] [CrossRef]
- Hilber, N.; Reichmann, O.; Schwab, C.; Winter, C. Finite Element Methods for Parabolic Problems. In Computational Methods for Quantitative Finance: Finite Element Methods for Derivative Pricing; Springer: Berlin/Heidelberg, Germany, 2013; pp. 27–45. [Google Scholar] [CrossRef]
- Kimura, A.; Nagasao, T.; Kaneko, T.; Tamaki, T.; Miyamoto, J.; Nakajima, T. Adaquate fixation of plates for stability during mandibular reconstruction. J. Cranio-Maxillofac. Surg. 2006, 34, 193–200. [Google Scholar] [CrossRef]
- Seebach, M.; Theurer, F.; Foehr, P.; von Deimling, C.; Burgkart, R.; Zaeh, M.F. Design of Bone Plates for Mandibular Reconstruction Using Topology and Shape Optimization. In Advances in Structural and Multidisciplinary Optimization; Springer: Cham, Switzerland, 2018; pp. 2086–2096. [Google Scholar] [CrossRef]
- Lang, J.J.; Bastian, M.; Foehr, P.; Seebach, M.; Weitz, J.; Von Deimling, C.; Schwaiger, B.J.; Micheler, C.M.; Wilhelm, N.J.; Grosse, C.U.; et al. Improving mandibular reconstruction by using topology optimization, patient specific design and additive manufacturing?—A biomechanical comparison against miniplates on human specimen. PLoS ONE 2021, 16, e0253002. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zhang, J.; Zheng, J.; Wang, L.; Li, D.; Liu, S. 3D-printed PEEK implant for mandibular defects repair—A new method. J. Mech. Behav. Biomed. Mater. 2021, 116, 104335. [Google Scholar] [CrossRef] [PubMed]
- Batstone, M.D. Reconstruction of major defects of the jaws. Aust. Dent. J. 2018, 63 (Suppl. S1), S108–S113. [Google Scholar] [CrossRef] [PubMed]
- Likhterov, I.; Roche, A.M.; Urken, M.L. Contemporary Osseous Reconstruction of the Mandible and the Maxilla. Oral Maxillofac. Surg. Clin. North Am. 2019, 31, 101–116. [Google Scholar] [CrossRef]
- Chen, L.; Gao, L.; Cui, H.; Guo, X.; Han, J.; Liu, J.; Yao, Y. Finite element comparison of titanium and polyetheretherketone materials for mandibular defect reconstruction. Am. J. Transl. Res. 2024, 16, 6097–6105. [Google Scholar] [CrossRef]
- Cassari, L.; Balducci, C.; Messina, G.M.L.; Iucci, G.; Battocchio, C.; Bertelà, F.; Lucchetta, G.; Coward, T.; Silvio, L.D.; Marletta, G.; et al. Polyetheretherketone Double Functionalization with Bioactive Peptides Improves Human Osteoblast Response. Biomimetics 2024, 9, 767. [Google Scholar] [CrossRef]
- Jones, E.A.; Sigurjónsson, Ó.E.; Weng, X.; Zhu, W.; Chai, H.; Wang, W.; Yuan, X.; Zhu, C. Bio-Activated PEEK: Promising Platforms for Improving Osteogenesis through Modulating Macrophage Polarization. Bioengineering 2022, 9, 747. [Google Scholar] [CrossRef]
- Dondani, J.R.; Iyer, J.; Tran, S.D. Surface Treatments of PEEK for Osseointegration to Bone. Biomolecules 2023, 13, 464. [Google Scholar] [CrossRef]
- Lommen, J.; Schorn, L.; Sproll, C.; Haussmann, J.; Kübler, N.R.; Budach, W.; Rana, M.; Tamaskovics, B. Reduction of CT Artifacts Using Polyetheretherketone (PEEK), Polyetherketoneketone (PEKK), Polyphenylsulfone (PPSU), and Polyethylene (PE) Reconstruction Plates in Oral Oncology. J. Oral Maxillofac. Surg. 2022, 80, 1272–1283. [Google Scholar] [CrossRef]
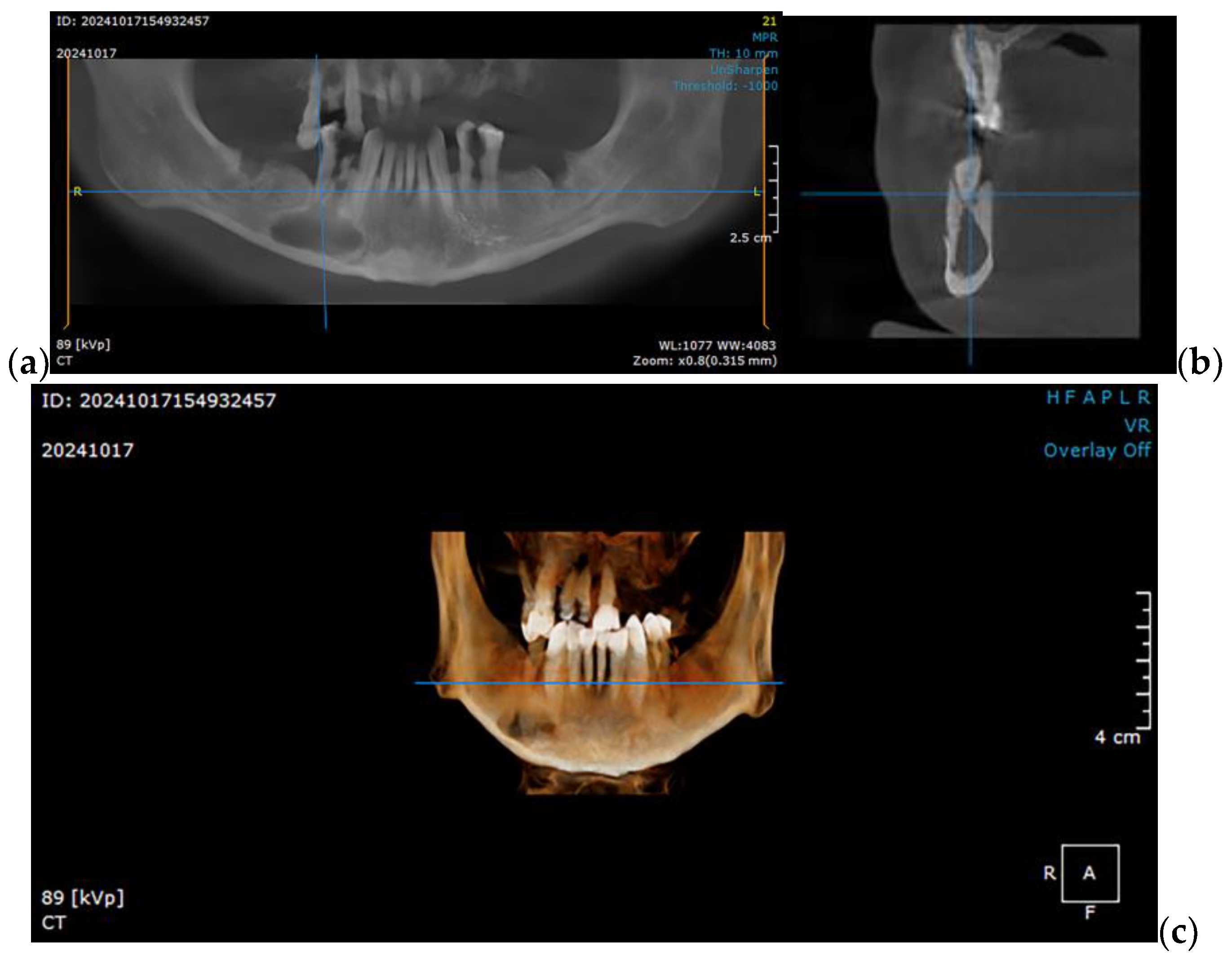
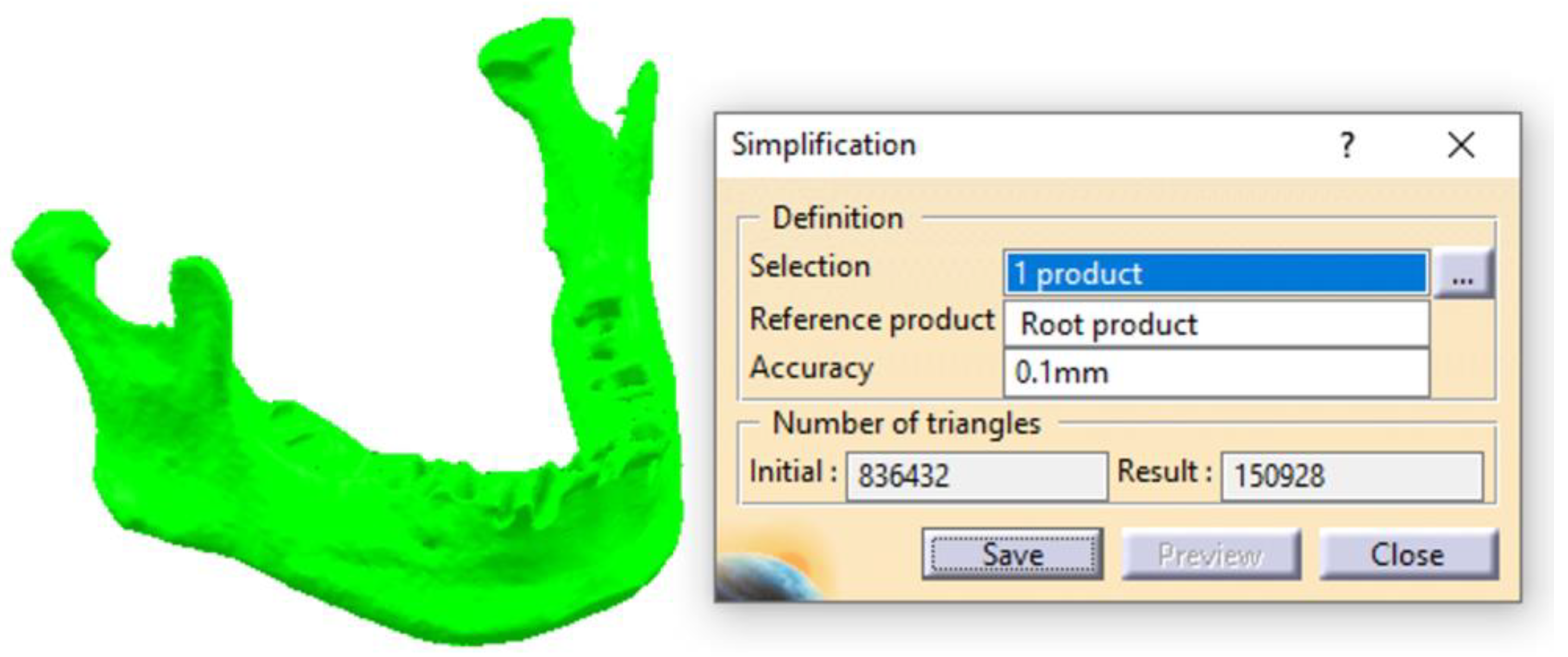
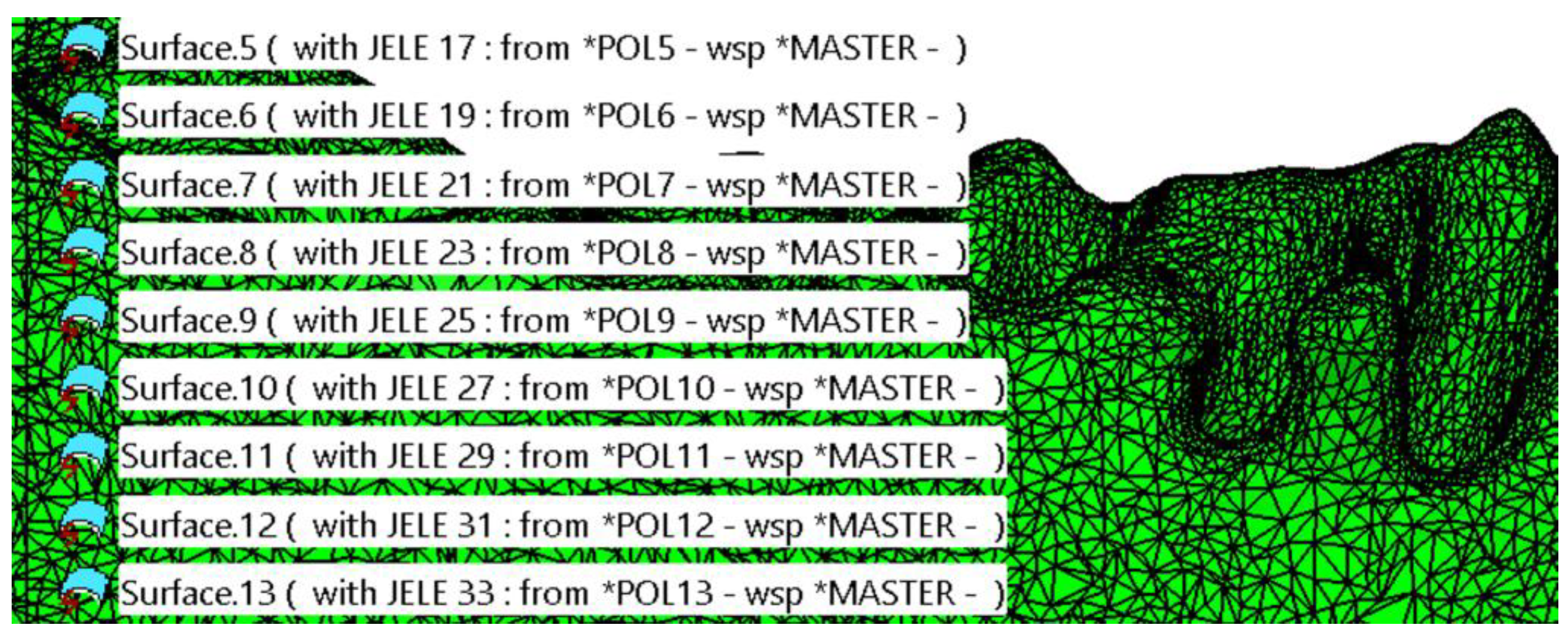

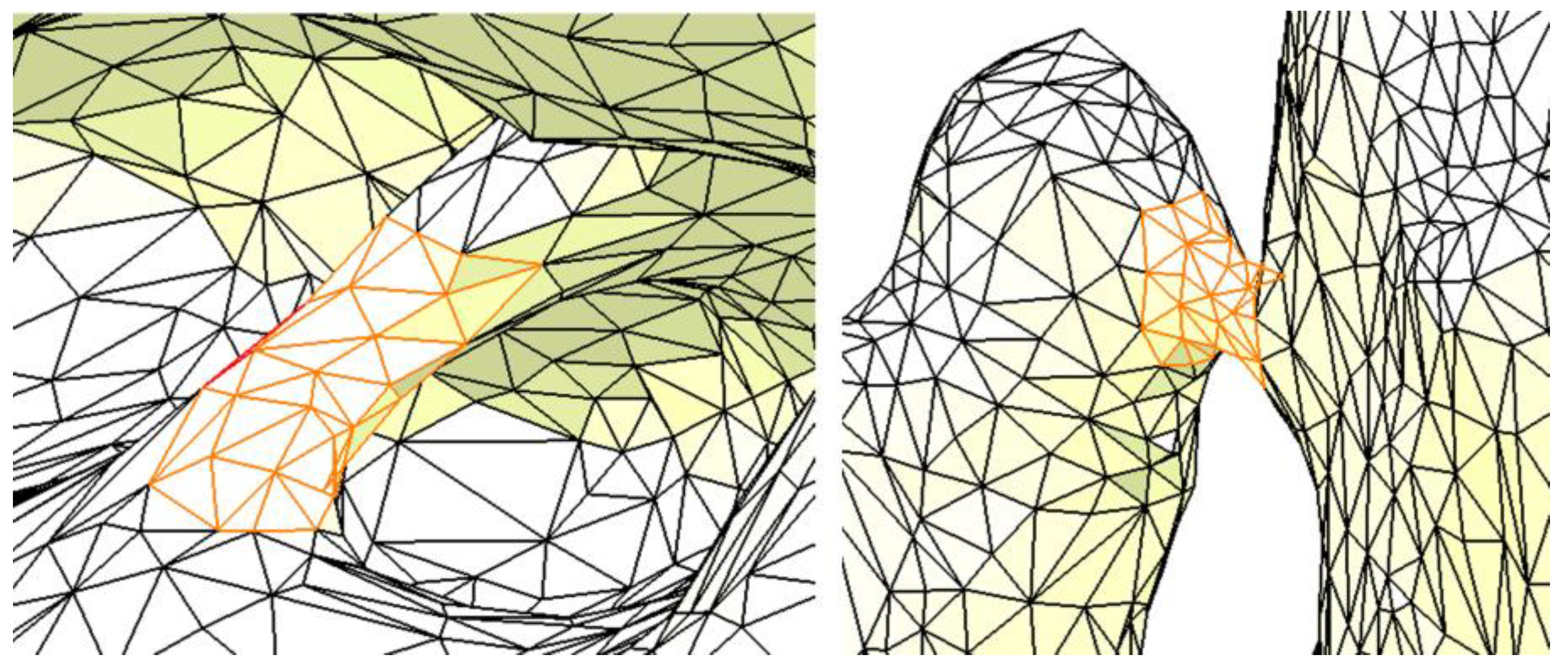





| Component | Finite Element Size, mm | Absolute Sag, mm | Finite Element Type | Material |
|---|---|---|---|---|
| Mandible | 1.5 | 0.8 | Parabolic | Bone |
| Grafted autogenous bone | 1.2 | 0.5 | Parabolic | Bone |
| TMJ disc | 0.8 | 0.1 | Parabolic | Cartilage |
| Fixing plate titanium | 1.5 | 0.2 | Parabolic | Ti-Grade-4 (TI75A) |
| Fixing plate PEEK | 1 | 0.5 | Parabolic | PEEK |
| Screws | 0.5 | 0.2 | Linear | Ti-Grade-4 (TI75A) |
| Component | Von Mises Stress, MPa | Translational Displacement Vector, mm | Component | Von Mises Stress, MPa | Translational Displacement Vector, mm |
|---|---|---|---|---|---|
| Plate thickness = 2 mm | |||||
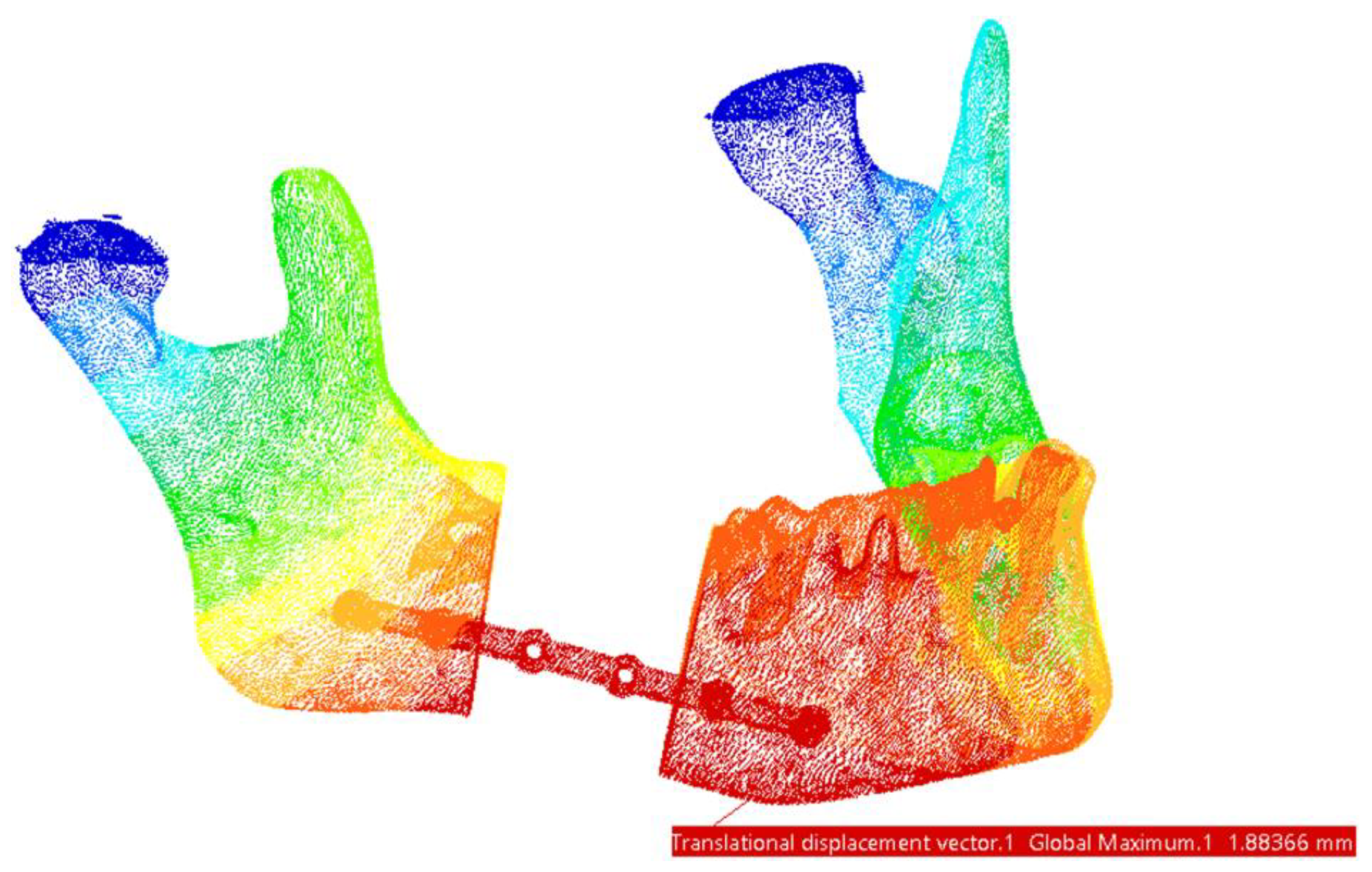
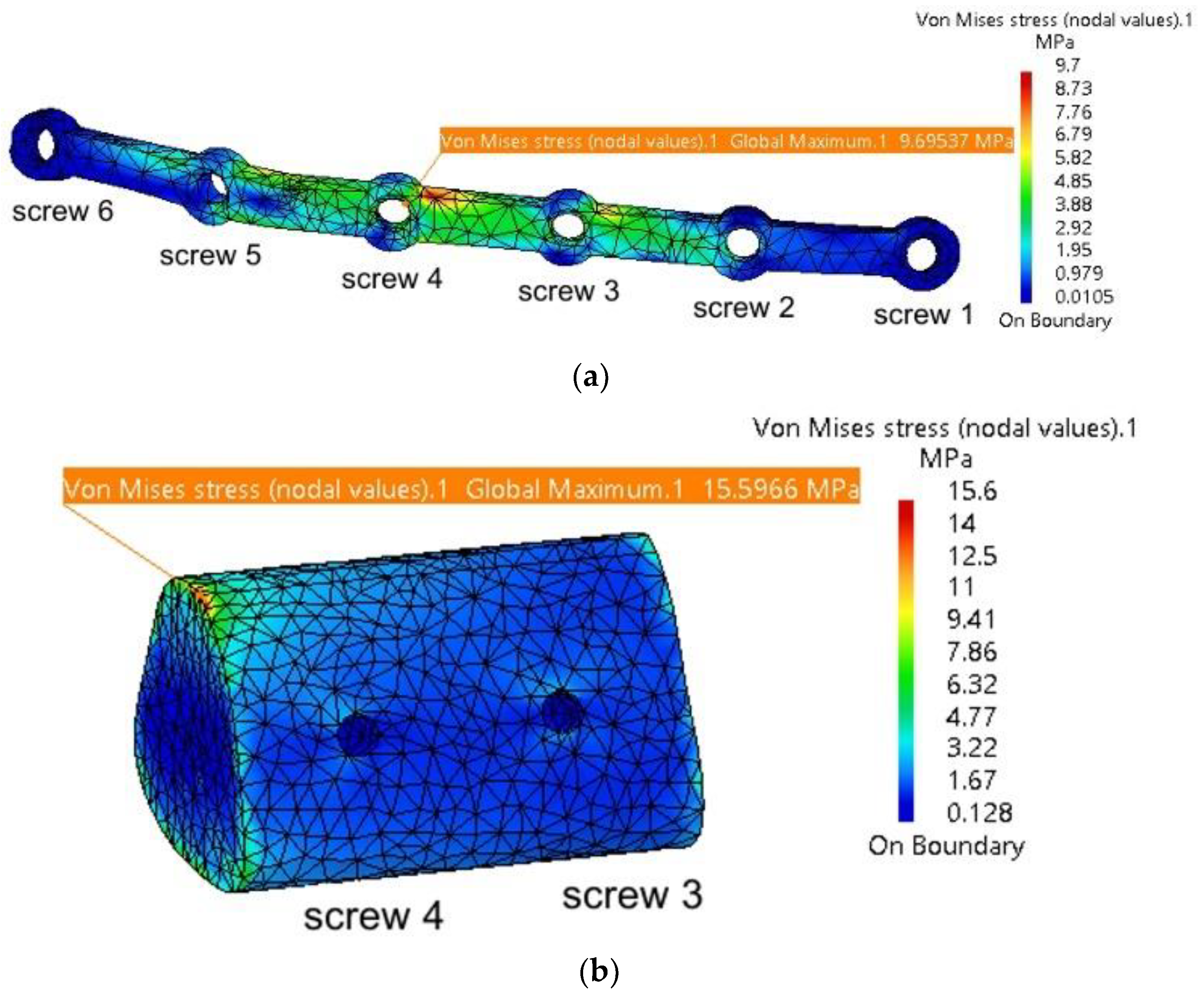
| Mandible | 19.3 | 1.9 | Fixing plate | 257 | 1.89 |
| TMJ 1 | 2.58 | 0.197 | Screw 2 | 10.7 | - |
| TMJ 2 | 1.3 | 0.175 | Screw 5 | 20.4 | - |
| Plate thickness = 1.8 mm | |||||
| Mandible | 19.4 | 1.89 | Fixing plate | 307 | 1.89 |
| TMJ 1 | 2.63 | 0.199 | Screw 2 | 11.8 | - |
| TMJ 2 | 1.5 | 0.173 | Screw 5 | 27.1 | - |
| Plate thickness = 1.6 mm | |||||
| Mandible | 19.3 | 1.88 | Fixing plate | 318 | 1.88 |
| TMJ 1 | 2.67 | 0.201 | Screw 2 | 15.5 | - |
| TMJ 2 | 1.61 | 0.171 | Screw 5 | 27.3 | - |
| Plate thickness = 1.4 mm | |||||
| Mandible | 19.2 | 1.87 | Fixing plate | 349 | 1.86 |
| TMJ 1 | 2.69 | 0.204 | Screw 2 | 18 | - |
| TMJ 2 | 2.09 | 0.168 | Screw 5 | 26.5 | - |
| Plate thickness = 1.2 mm | |||||
| Mandible | 19 | 1.87 | Fixing plate | 431 | 1.86 |
| TMJ 1 | 2.69 | 0.204 | Screw 2 | 24.2 | - |
| TMJ 2 | 2.98 | 0.168 | Screw 5 | 33.1 | - |
| Component | Von Mises Stress, MPa | Translational Displacement Vector, mm | Component | Von Mises Stress, MPa | Translational Displacement Vector, mm |
|---|---|---|---|---|---|
| Plate thickness = 1.2 mm | |||||
| Mandible | 40.7 | 1.61 | Fixing plate | 12 | 1.53 |
| Cartilage 1 | 2.62 | 0.118 | Screw 2 | 2.11 | 1.41 |
| TMJ 2 | 1.17 | 0.191 | Screw 3 | 2.69 | 1.29 |
| Bone insert | 15.7 | 1.37 | Screw 4 | 2.29 | 1.17 |
| Plate thickness = 1.4 mm | |||||
| Mandible | 40.3 | 1.61 | Fixing plate | 10.8 | 1.53 |
| TMJ 1 | 2.68 | 0.118 | Screw 2 | 2.09 | 1.41 |
| TMJ 2 | 1.22 | 0.191 | Screw 3 | 2.49 | 1.29 |
| Bone insert | 15.6 | 1.37 | Screw 4 | 2.23 | 1.17 |
| Plate thickness = 1.6 mm | |||||
| Mandible | 40.2 | 1.61 | Fixing plate | 10.2 | 1.53 |
| TMJ 1 | 2.64 | 0.118 | Screw 2 | 1.77 | 1.41 |
| TMJ 2 | 1.34 | 0.191 | Screw 3 | 2.18 | 1.29 |
| Bone insert | 15.6 | 1.37 | Screw 4 | 2.19 | 1.17 |
| Plate thickness = 1.8 mm | |||||
| Mandible | 40.1 | 1.61 | Fixing plate | 9.7 | 1.53 |
| TMJ 1 | 2.78 | 0.118 | Screw 2 | 1.79 | 1.41 |
| TMJ 2 | 1.88 | 0.191 | Screw 3 | 2.16 | 1.29 |
| Bone insert | 15.6 | 1.37 | Screw 4 | 2.16 | 1.17 |
| Component | Von Mises Stress, MPa | Translational Displacement Vector, mm | Component | Von Mises Stress, MPa | Translational Displacement Vector, mm |
|---|---|---|---|---|---|
| Plate thickness = 2 mm | |||||
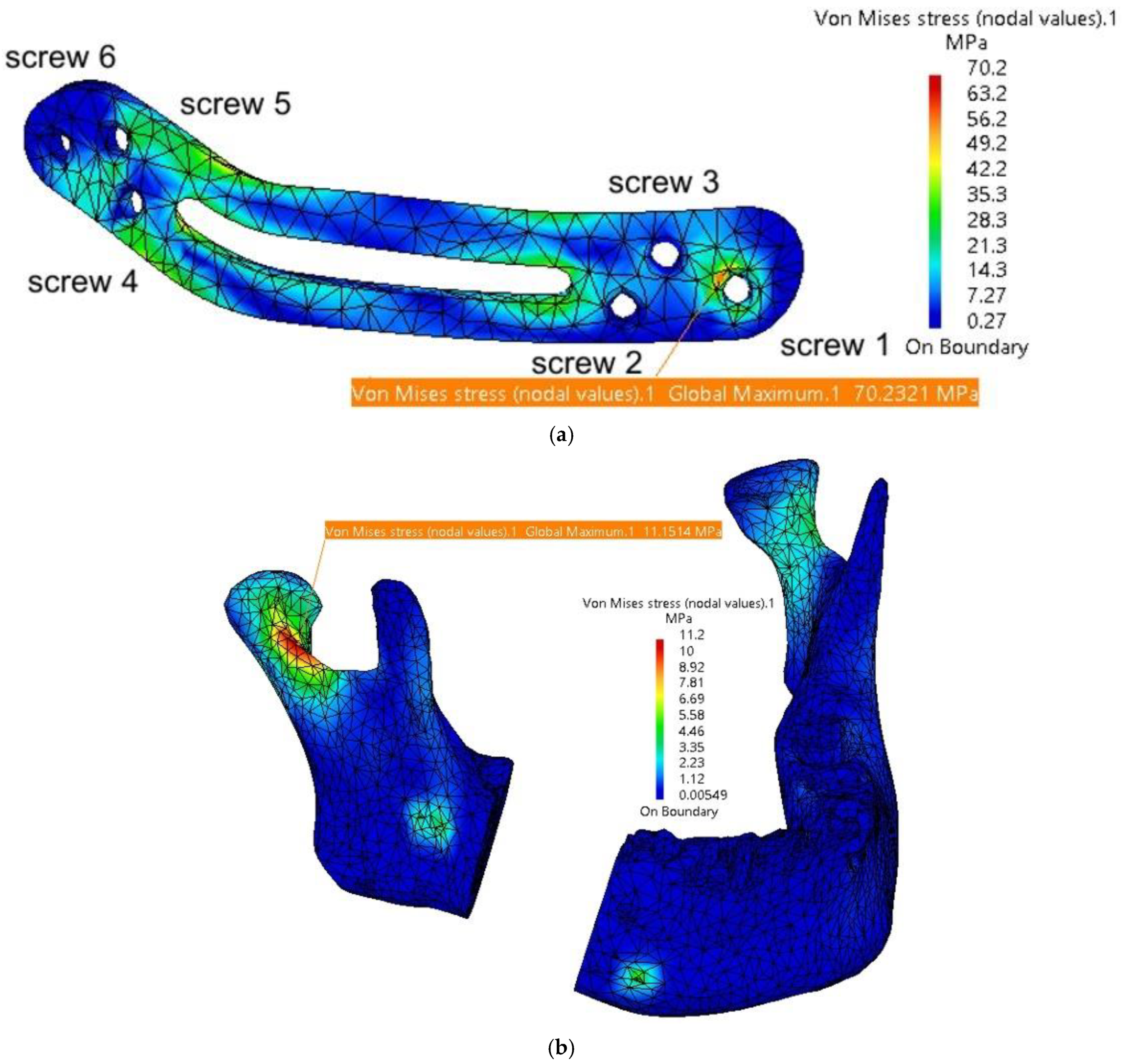
| Mandible | 11.2 | 1.14 | Screw 1 and 2 | 35 and 12.8 | max 0.11 |
| TMJ 1 | 2.98 | 0.2 | Screw 3 and 4 | 24.2 and 26.3 | max 0.09 |
| TMJ 2 | 3.31 | 0.2 | Screw 5 | 28.4 | 0.1 |
| Fixing plate | 70.2 | 1.12 | Screw 6 | 18.2 | 0.09 |
| Plate thickness = 1.8 mm | |||||
| Mandible | 16.8 | 1.32 | Screw 1 and 2 | 38 and 16.2 | max 0.13 |
| TMJ 1 | 3.1 | 0.2 | Screw 3 and 4 | 28.4 and 32.1 | max 0.11 |
| TMJ 2 | 3.49 | 0.2 | Screw 5 | 34.2 | 0.18 |
| Fixing plate | 81.29 | 1.29 | Screw 6 | 12 | 0.1 |
| Plate thickness = 1.6 mm | |||||
| Mandible | 20.6 | 1.46 | Screw 1 and 2 | 46 and 22.6 | max 0.16 |
| TMJ 1 | 3.48 | 0.22 | Screw 3 and 4 | 34 and 42.4 | max 0.14 |
| TMJ 2 | 3.88 | 0.22 | Screw 5 | 46 | 0.22 |
| Fixing plate | 92.7 | 1.38 | Screw 6 | 16 | 0.12 |
| Plate thickness = 1.4 mm | |||||
| Mandible | 32.6 | 1.85 | Screw 1 and 2 | 58 and 28.8 | max 0.23 |
| TMJ 1 | 3.79 | 0.24 | Screw 3 and 4 | 41.9 and 49.5 | max 0.2 |
| TMJ 2 | 4.22 | 0.22 | Screw 5 | 51.8 | 0.24 |
| Fixing plate | 130.4 | 1.7 | Screw 6 | 28.4 | 0.17 |
| Characteristic | Titanium Plates | PEEK Plates |
|---|---|---|
| Mechanical strength | High mechanical stability, with superior load-bearing capacity. | Sufficient strength for physiological loads, but thinner plates (<1.8 mm) risk plastic deformation. |
| Elastic modulus | Very high (104 GPa), leading to stress shielding and reduced bone remodeling. | Closer to cortical bone (3.9 GPa), minimizing stress shielding and promoting better load distribution. |
| Imaging artifacts [43] | Causes significant streaking and blooming artifacts in CT and MRI imaging. | Radiolucent with minimal imaging artifacts, allowing for a clear postoperative assessment. |
| Weight | Denser and heavier, which may reduce patient comfort. | Lightweight, improving patient comfort and reducing load on surrounding tissues. |
| Plate thickness | Minimum thickness recommended: 1.2–1.6 mm for mechanical stability. | Minimum thickness recommended: 1.8–2.0 mm to avoid plastic deformation under physiological loads. |
| Biocompatibility | Highly biocompatible but can enhance radiation dose in adjacent tissues during radiotherapy. | Biocompatible and inert; does not interfere with radiotherapy or adjacent tissue assessment. |
| Manufacturing | 3D printing or sintering; higher production cost. | Can be manufactured using additive techniques, offering reduced production costs and patient-specific design. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghionea, I.G.; Tarba, C.I.; Cristache, C.M.; Filipov, I.; Beuran, I.A. A Comparative Finite Element Analysis of Titanium, Autogenous Bone, and Polyetheretherketone (PEEK)-Based Solutions for Mandibular Reconstruction. Materials 2025, 18, 314. https://doi.org/10.3390/ma18020314
Ghionea IG, Tarba CI, Cristache CM, Filipov I, Beuran IA. A Comparative Finite Element Analysis of Titanium, Autogenous Bone, and Polyetheretherketone (PEEK)-Based Solutions for Mandibular Reconstruction. Materials. 2025; 18(2):314. https://doi.org/10.3390/ma18020314
Chicago/Turabian StyleGhionea, Ionut Gabriel, Cristian Ioan Tarba, Corina Marilena Cristache, Iulian Filipov, and Irina Adriana Beuran. 2025. "A Comparative Finite Element Analysis of Titanium, Autogenous Bone, and Polyetheretherketone (PEEK)-Based Solutions for Mandibular Reconstruction" Materials 18, no. 2: 314. https://doi.org/10.3390/ma18020314
APA StyleGhionea, I. G., Tarba, C. I., Cristache, C. M., Filipov, I., & Beuran, I. A. (2025). A Comparative Finite Element Analysis of Titanium, Autogenous Bone, and Polyetheretherketone (PEEK)-Based Solutions for Mandibular Reconstruction. Materials, 18(2), 314. https://doi.org/10.3390/ma18020314









