Alkali-Activated Mineral Residues in Construction: Case Studies on Bauxite Residue and Steel Slag Pavement Tiles
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Chemical and Mineralogical Characteristics
3.2. Lab-Scale Development and Performance Testing
3.3. Pilot-Scale Production and Performance Testing
3.4. Demo Installation
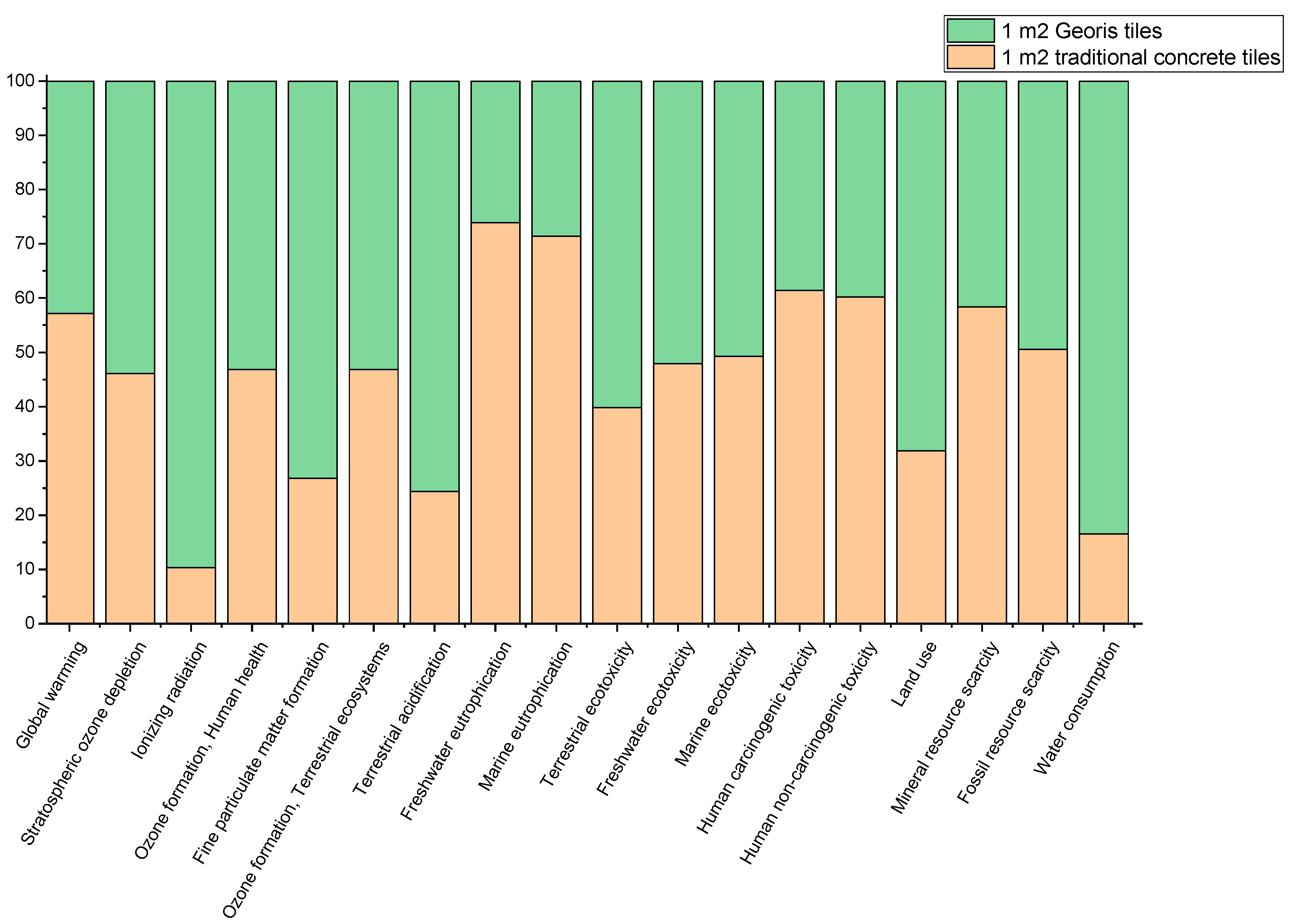
3.5. Life Cycle Impact Assessment of Developed Product
3.6. Economic Analysis of Alkali-Activated Pavers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viola, V.; D’Angelo, A.; Vertuccio, L.; Catauro, M. Metakaolin-Based Geopolymers Filled with Industrial Wastes: Improvement of Physicochemical Properties through Sustainable Waste Recycling. Polymers 2024, 16, 2118. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.; Kaur, P.; Joshi, S.; Shinde, O. Biogenic Treatment Improves the Durability of Steel Slag Amended Mortar Structures. In Proceedings of the Fifth International Conference on Sustainable Construction Materials and Technologies 2019, London, UK, 15–17 July 2019; pp. 241–251.
- Branca, T.A.; Colla, V.; Algermissen, D.; Granbom, H.; Martini, U.; Morillon, A.; Pietruck, R.; Rosendahl, S. Reuse and Recycling of By-Products in the Steel Sector: Recent Achievements Paving the Way to Circular Economy and Industrial Symbiosis in Europe. Metals 2020, 10, 345. [Google Scholar] [CrossRef]
- Bauxite Residue: An Introduction. Available online: https://international-aluminium.org/wp-content/uploads/2022/09/Bauxite-Residue_Final_ENG-1.pdf (accessed on 8 October 2024).
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Singh, N.B.; Middendorf, B. Geopolymers as an Alternative to Portland Cement: An Overview. Constr. Build. Mater. 2020, 237, 117455. [Google Scholar] [CrossRef]
- Assi, L.; Carter, K.; Deaver, E.; Anay, R.; Ziehl, P. Sustainable Concrete: Building a Greener Future. J. Clean. Prod. 2018, 198, 1641–1651. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Sustainable Development; Geopolymer Institute: Saint-Quentin, France, 2005; p. 15. [Google Scholar]
- Albitar, M.; Mohamed Ali, M.S.; Visintin, P.; Drechsler, M. Durability Evaluation of Geopolymer and Conventional Concretes. Constr. Build. Mater. 2017, 136, 374–385. [Google Scholar] [CrossRef]
- Nikoloutsopoulos, N.; Sotiropoulou, A.; Kakali, G.; Tsivilis, S. Physical and Mechanical Properties of Fly Ash Based Geopolymer Concrete Compared to Conventional Concrete. Buildings 2021, 11, 178. [Google Scholar] [CrossRef]
- Bupesh Raja, V.K.; Selvarani, V.; Kannan, S.; Sujan, S.; Sahas, S.; Padmapriya, R.; Kumar, V.G.; Baalamurugan, J. Geopolymer Based Foams from Steel Slag—A Green Technology. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Salman, M.; Cizer, Ö.; Pontikes, Y.; Snellings, R.; Vandewalle, L.; Blanpain, B.; van Balen, K. Investigating the Binding Potential of Continuous Casting Stainless Steel Slag by Alkali Activation. Adv. Cem. Res. 2014, 26, 256–270. [Google Scholar] [CrossRef]
- Salman, M.; Cizer, Ö.; Pontikes, Y.; Snellings, R.; Dijkman, J.; Sels, B.; Vandewalle, L.; Blanpain, B.; Van Balen, K. Alkali Activation of AOD Stainless Steel Slag Under Steam Curing Conditions. J. Am. Ceram. Soc. 2015, 98, 3062–3074. [Google Scholar] [CrossRef]
- Wang, M.; Xu, J.; Zhang, X.; Tan, L.; Mei, Y. Mechanical Performance Optimization and Microstructural Mechanism Study of Alkali-Activated Steel Slag–Slag Cementitious Materials. Buildings 2024, 14, 1204. [Google Scholar] [CrossRef]
- Jing, W.; Jiang, J.; Ding, S.; Duan, P. Hydration and Microstructure of Steel Slag as Cementitious Material and Fine Aggregate in Mortar. Molecules 2020, 25, 4456. [Google Scholar] [CrossRef]
- Hertel, T.; Pontikes, Y. Geopolymers, Inorganic Polymers, Alkali-Activated Materials and Hybrid Binders from Bauxite Residue (Red Mud)—Putting Things in Perspective. J. Clean. Prod. 2020, 258, 120610. [Google Scholar] [CrossRef]
- Guha, R.; Bambier, P.; Ouellet-Plamondon, C.M. Environmental Assessment of Materials in Preparing Alkali-Activated Materials Using Bauxite Residue. IOP Conf. Ser. Earth Environ. Sci. 2024, 1363, 012101. [Google Scholar] [CrossRef]
- Peys, A.; Hertel, T.; Snellings, R. Co-Calcination of Bauxite Residue with Kaolinite in Pursuit of a Robust and High-Quality Supplementary Cementitious Material. Front. Mater. 2022, 9, 913151. [Google Scholar] [CrossRef]
- Hertel, T.; Blanpain, B.; Pontikes, Y. A Proposal for a 100 % Use of Bauxite Residue Towards Inorganic Polymer Mortar. J. Sustain. Metall. 2016, 2, 394–404. [Google Scholar] [CrossRef]
- Santi, M.; ‘Ain, H. Utilization of Bauxite Residue (Red Mud) in the Manufacture of Geopolymer Concrete Bricks. In Proceedings of the First Jakarta International Conference on Multidisciplinary Studies Towards Creative Industries, JICOMS 2022, Jakarta, Indonesia, 16 November 2022. [Google Scholar]
- van Chanh, N.; Shigeishi, M.; Tho, T.Q. Inorganic Composite Material Based on Fly Ash, Red Residue from Bauxite Ore for Road Building Projects in Vietnam. Adv. Mater. Res. 2012, 383–390, 2774–2781. [Google Scholar] [CrossRef]
- EN 196-1:2016; Methods of Testing Cement—Part 1: Determination of Strength. CEN: Brussels, Belgium, 2016.
- EN 12457-4:2002; Characterisation of Waste-Leaching-Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 4: One Stage Batch Test at a Liquid to Solid Ratio of 10 l/kg for Materials with Particle Size below 10 mm (without or with Size Reduction). CEN: Brussels, Belgium, 2002.
- EN 1338:2003; Concrete Paving Blocks—Requirements and Test Methods. CEN: Brussels, Belgium, 2003.
- SIST 1026:2016; Concrete—Specification, Performance, Production and Conformity—Rules for the Implementation of SIST EN 206SIST: Ljubljana, Slovenia, 2016.
- ASTM C666-97; Standard Test Method for Resistance of Concrete to Rapid Freezing and Thawing. ASTM: West Conshohocken, PA, USA, 2017.
- Kim, M.-J.; Chun, B.; Choi, H.-J.; Shin, W.; Yoo, D.-Y. Effects of Supplementary Cementitious Materials and Curing Condition on Mechanical Properties of Ultra-High-Performance, Strain-Hardening Cementitious Composites. Appl. Sci. 2021, 11, 2394. [Google Scholar] [CrossRef]
- Cai, H.; Liu, X. Freeze-Thaw Durability of Concrete: Ice Formation Process in Pores. Cem. Concr. Res. 1998, 28, 1281–1287. [Google Scholar] [CrossRef]
- Council Directive 1999/31/EC on the Landfill of Waste. FAOLEX. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC038106/ (accessed on 22 October 2024).
- EN 1339:2003/AC:2006; Concrete Paving Flags—Requirements and Test Methods. CEN: Brussels, Belgium, 2006.
- Frankovič, A.; Ducman, V.; Kriskova, L.; Tatsis, E.; Petrica, P.; Pontikes, Y. The Development and Assessment of Alkali Activated Paving Blocks. In Proceedings of the 3rd International Conference on Technologies & Business Models for Circular Economy, 15 December 2020; University of Maribor, University Press, Faculty of Chemistry and Chemical Engineering: Maribor, Slovenia, 2022; pp. 2–9. [Google Scholar]
- SIST-TS CEN/TS 12390-9:2017; Testing Hardened Concrete—Part 9: Freeze-Thaw Resistance with De-Icing Salts. SIST: Ljubljana, Slovenia, 2017.
- SIST-TS CEN/TS 16165:2016; Determination of Slip Resistance of Pedestrian Surfaces—Methods of Evaluation. SIST: Ljubljana, Slovenia, 2016.
- AS 4586-2013; Slip Resistance Classification of New Pedestrian Surface Materials. AS: Sydney, Australia, 2013.
- Seguridad de Utilización y Accesibilidad. Available online: https://www.codigotecnico.org/pdf/Documentos/SUA/DBSUA.pdf (accessed on 13 December 2024).
- Dong, Y.; Wang, Z.; Ren, W.; Jiang, T.; Hou, Y.; Zhang, Y. Influence of Morphological Characteristics of Coarse Aggregates on Skid Resistance of Asphalt Pavement. Materials 2023, 16, 4926. [Google Scholar] [CrossRef]
- Ong, G.; Zhang, L.; Fwa, T. Numerical Study on the Influence of Aggregate Size on Skid Resistance Performance of Porous Pavements. Asian Transp. Stud. 2015, 3, 284–297. [Google Scholar] [CrossRef]
- Uredba o Odpadkih (PISRS). Available online: https://pisrs.si/pregledPredpisa?id=URED8482 (accessed on 11 November 2024).
- SIST EN 1744-3:2002; Tests for Chemical Properties of Aggregates—Part 3: Preparation of Eluates by Leaching of Aggregates. SIST: Ljubljana, Slovenia, 2002.
- SIST EN 16637-2:2024; Construction Products—Assessment of Release of Dangerous Substances—Part 2: Horizontal Dynamic Surface Leaching Test. SIST: Ljubljana, Slovenia, 2024.
- Wang, J.; Zhou, T.; Xu, D.; Zhou, Z.; Du, P.; Xie, N.; Cheng, X.; Liu, Y. Effect of Nano-Silica on the Efflorescence of Waste Based Alkali-Activated Inorganic Binder. Constr. Build. Mater. 2018, 167, 381–390. [Google Scholar] [CrossRef]
- Algaifi, H.A.; Mustafa Mohamed, A.; Alsuhaibani, E.; Shahidan, S.; Alrshoudi, F.; Huseien, G.F.; Bakar, S.A. Optimisation of GBFS, Fly Ash, and Nano-Silica Contents in Alkali-Activated Mortars. Polymers 2021, 13, 2750. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, B.; Zhang, X.; Han, X.; Xing, Y.; Fan, C.; Liu, Z. Effect of Nano Silica on Solidification/Stabilization of Heavy Metal in Alkali-Activated MSWI Fly Ash Solidified Body. Process Saf. Environ. Prot. 2023, 176, 462–474. [Google Scholar] [CrossRef]
- Adsorption of Heavy Metals on Alkali-Activated Zeolite Foams. Available online: https://www.mdpi.com/1996-1944/17/3/685 (accessed on 12 December 2024).
- Van Gerven, T.; Cornelis, G.; Vandoren, E.; Vandecasteele, C. Effects of Carbonation and Leaching on Porosity in Cement-Bound Waste. Waste Manag. 2007, 27, 977–985. [Google Scholar] [CrossRef]
- von Greve-Dierfeld, S.; Lothenbach, B.; Vollpracht, A.; Wu, B.; Huet, B.; Andrade, C.; Medina, C.; Thiel, C.; Gruyaert, E.; Vanoutrive, H.; et al. Understanding the Carbonation of Concrete with Supplementary Cementitious Materials: A Critical Review by RILEM TC 281-CCC. Mater. Struct. 2020, 53, 136. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, Y.; Wang, T.; Wang, J. Characterization of Heavy Metals in Fly Ash Stabilized by Carbonation with Supercritical CO2 Coupling Mechanical Force. J. CO2 Util. 2023, 67, 102308. [Google Scholar] [CrossRef]
- Yuan, Q.; Lu, H.; Guo, X.; Ma, S. Quantitative Effects of Physical Encapsulation during Carbonation Process Based on Scaled up Simulation of Fly Ash Particles. J. Energy Inst. 2024, 115, 101679. [Google Scholar] [CrossRef]
- van Overmeir, A.L.; Figueiredo, S.C.; Šavija, B.; Bos, F.P.; Schlangen, E. Design and Analyses of Printable Strain Hardening Cementitious Composites with Optimized Particle Size Distribution. Constr. Build. Mater. 2022, 324, 126411. [Google Scholar] [CrossRef]
- Geopolymers: The Green Alternative to Traditional Materials for Engineering Applications. Available online: https://www.mdpi.com/2412-3811/8/6/98 (accessed on 21 November 2024).
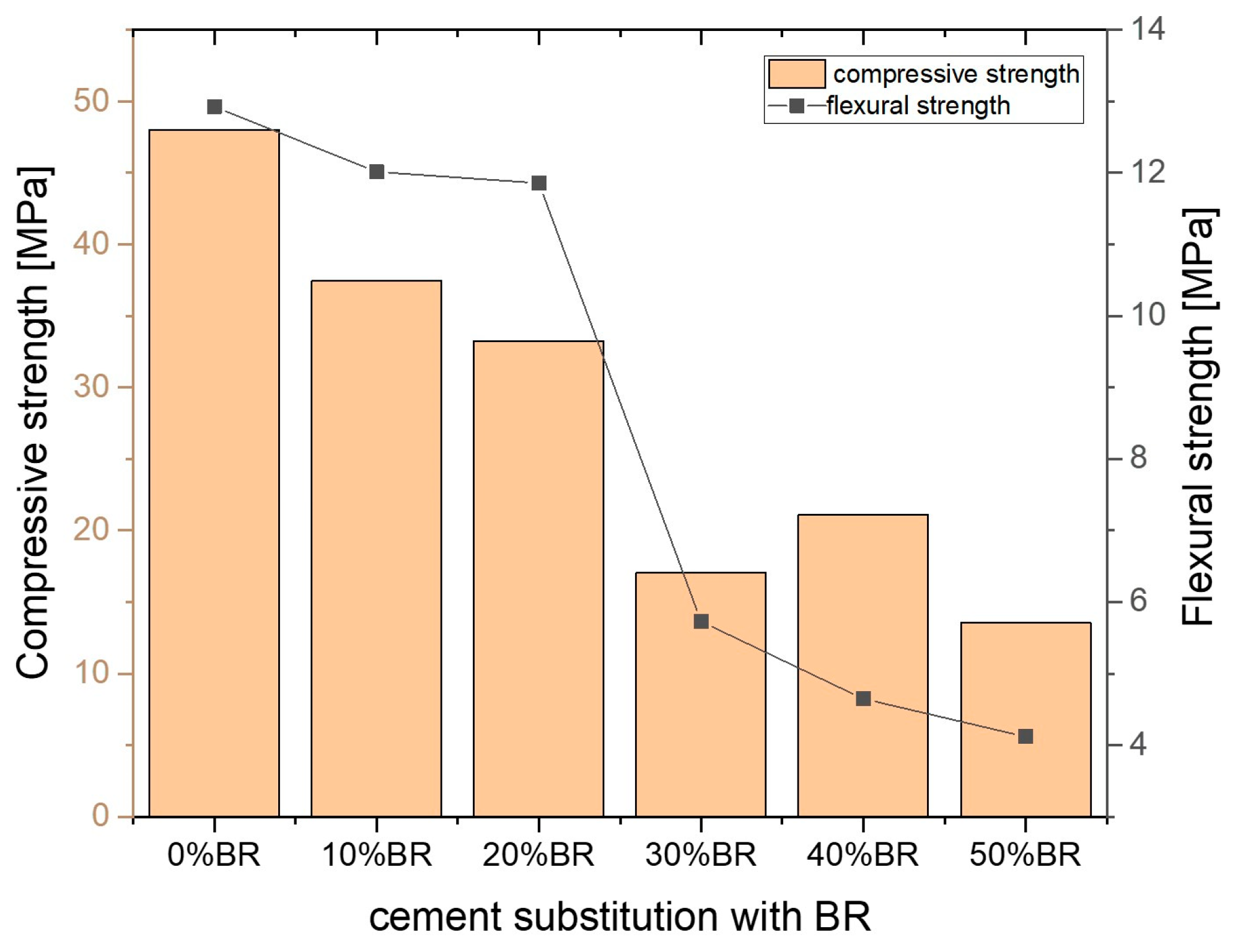
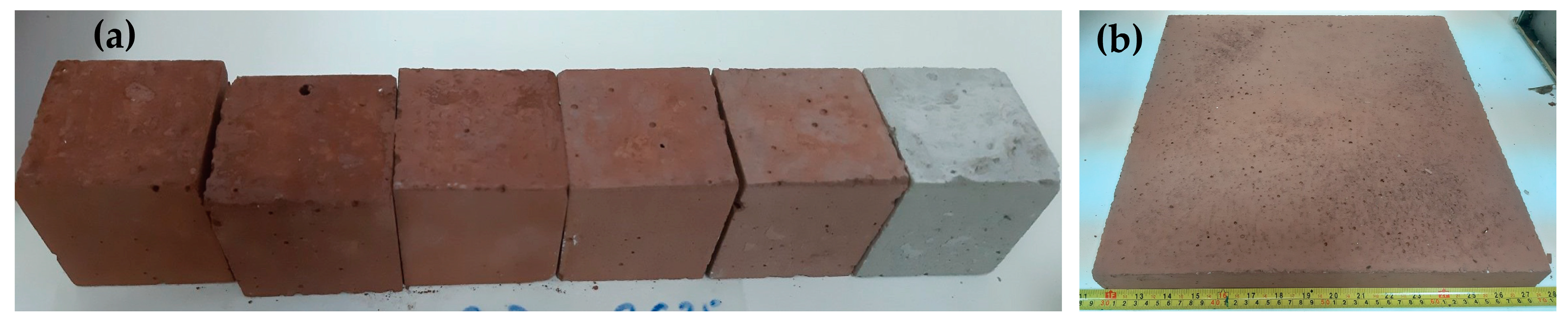
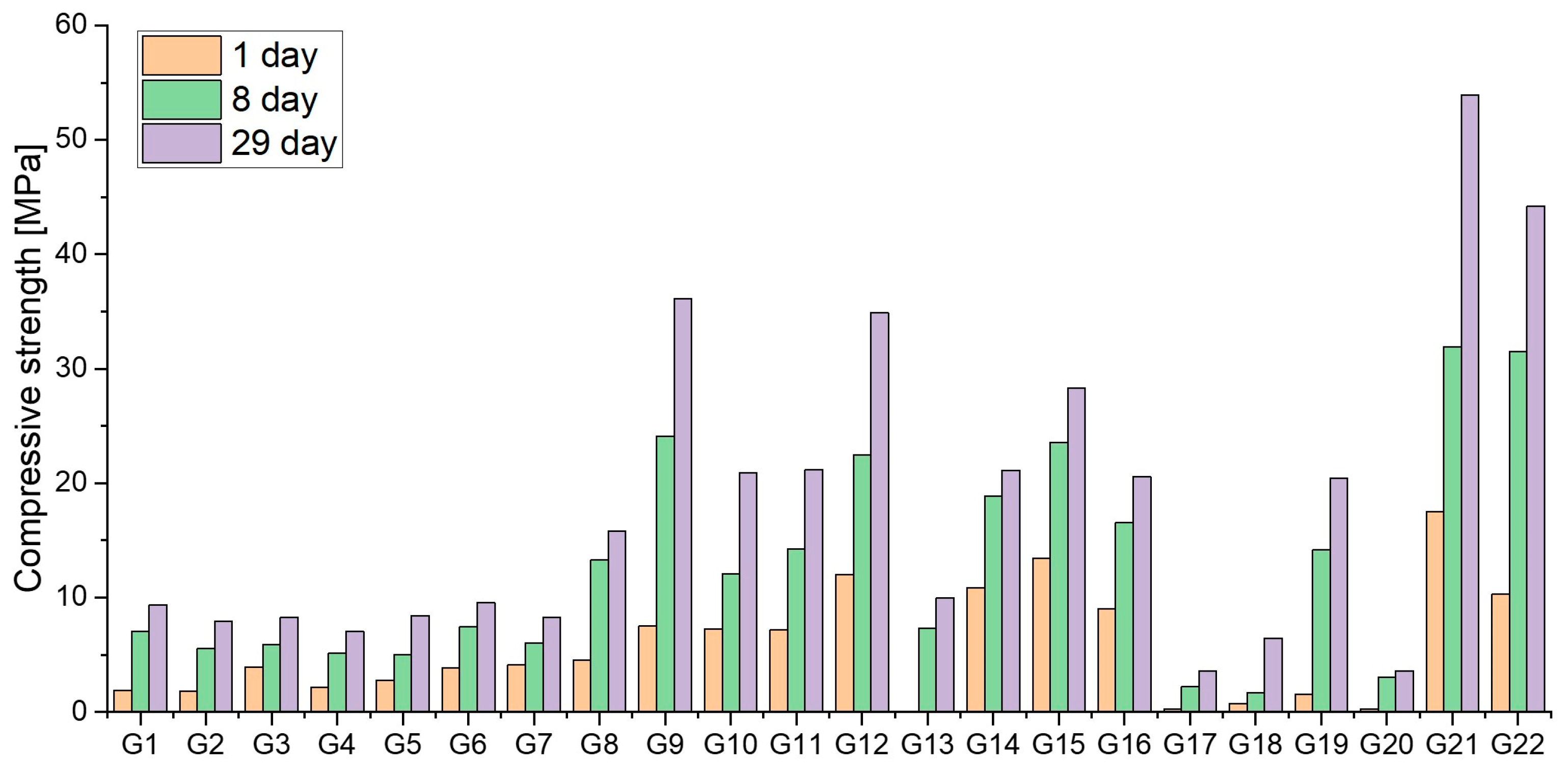


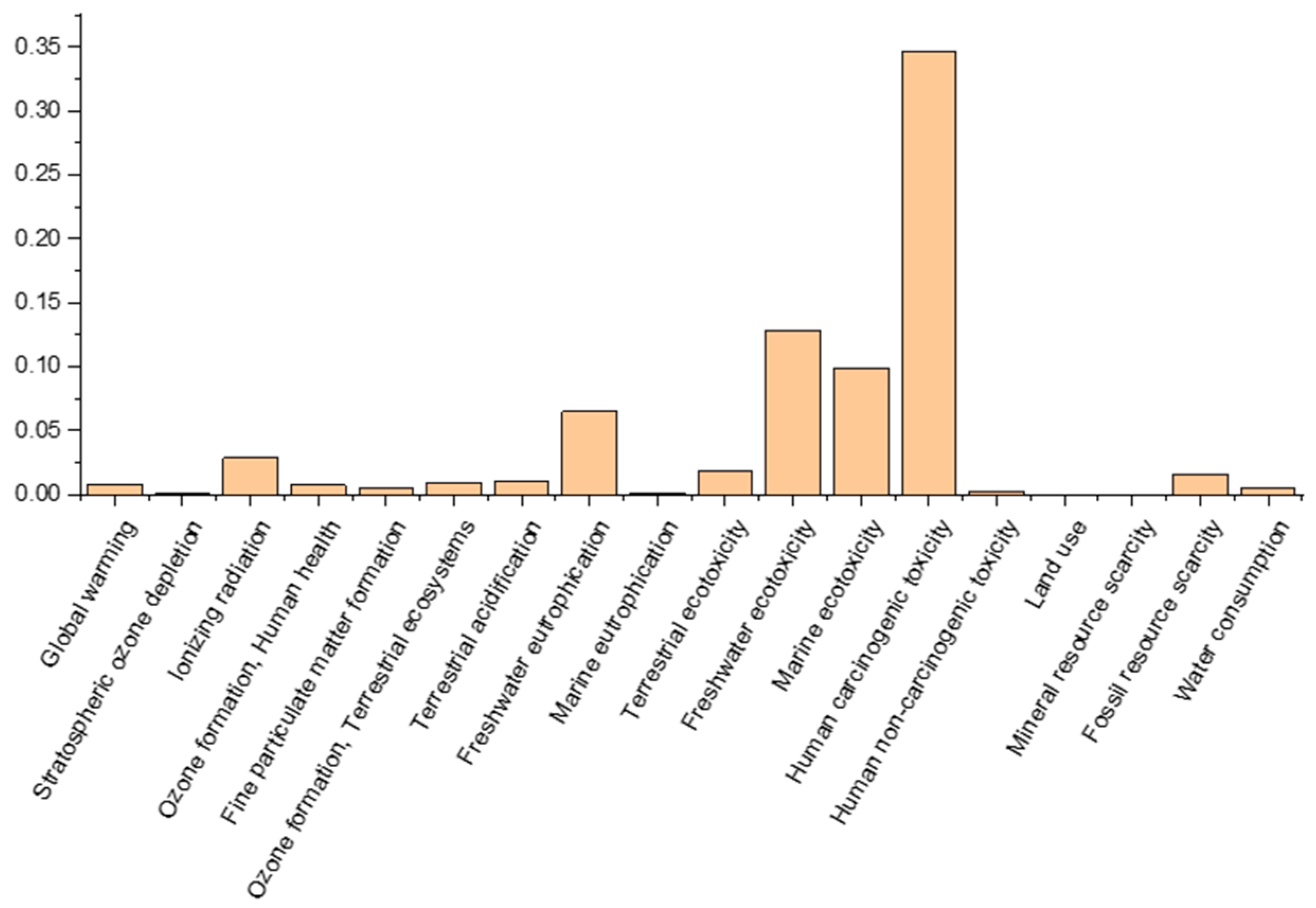
| Bauxite Residue (45 μm) | Portland Cement (42.5 N) | Sand (0–4 mm) | Aggregate (4–16 mm) | 6 M NaOH |
|---|---|---|---|---|
| - | 280 | 330 | 550 | 132 |
| 28.0 | 252.0 | 330 | 550 | 132 |
| 56.0 | 224.0 | 330 | 550 | 132 |
| 84.0 | 196.0 | 330 | 550 | 132 |
| 112.0 | 168.0 | 330 | 550 | 132 |
| 140.0 | 140.0 | 330 | 550 | 132 |
| Oxide | BR | SS | EAF-C | GGBFS | SCS |
|---|---|---|---|---|---|
| LOI (950 °C) | 4 | 0.1 | |||
| SiO2 | 10 | 17 | 8 | 30 | 25 |
| Al2O3 | 19 | 9 | 5 | 12 | 8 |
| CaO | 11 | 36 | 30 | 43 | 3 |
| MgO | <1 | 13 | 10 | 7 | 1 |
| K2O | <1 | - | <1 | 1 | - |
| Fe2O3 | 45 | 10 | 32 | - | 56 |
| MnO | - | 2 | 5 | - | - |
| TiO2 | 6 | <1 | <1 | - | - |
| Na2O | 5 | - | <1 | - | - |
| SnO3 | - | - | - | 2 | - |
| ZnO | - | <1 | - | - | 6 |
| Cr2O3 | - | 3 | 2 | - | - |
| Phase | Formula | BR | SS |
|---|---|---|---|
| Hematite/magnetite | Fe2O3/Fe3O4 | 33.8/- | -/2.6 |
| Goethite | FeO (OH) | 7.8 | - |
| Diaspore/boehmite | α-AlOOH/γ-AlOOH | 17.1/1.8 | - |
| Bayerite/gibbsite | Al(OH)3 | 1.7/0.9 | - |
| Nordstrandite | Al(OH)3 | 8.9 | |
| Cancrinite | Na6Ca2 (AlSiO4)6(CO3)2 | 12.6 | - |
| Katoite | Ca3Al2(SiO4)1.5(OH)6 | 11.4 | - |
| Calcite | CaCO3 | 7.9 | - |
| Perovskite | CaTiO3 | 3.3 | - |
| Quartz | SiO2 | 0.7 | - |
| Anatase | TiO2 | 0.6 | - |
| Brownmillerite | Ca2(Al,Fe)2O 5 | - | 2.7 |
| Periclase | MgO | 13.2 | |
| β -C2S/γ-C2S | Ca2SiO4 | - | 12.6/21.6 |
| Merwinite | Ca3Mg(SiO4)2 | - | 20.9 |
| Bredigite | Ca7Mg(SiO4) | - | 8.5 |
| Portlandite | Ca(OH)2 | - | 2 |
| Mayenite | 12CaO·7Al2O3 | - | 7.1 |
| Optimization Range | G9 | G12 | G15 | |
|---|---|---|---|---|
| Ekominit S1 | 11–20 wt% | 11.1 wt% | 11.8 wt% | 12.3 wt% |
| Cement | 0–7 wt% | - | - | - |
| GGBFS | 0–11 wt% | 11.1 wt% | 11.8 wt% | 9.9 wt% |
| Gypsum | 0–1 wt% | - | - | - |
| Molarity of NaOH solution | 0–6 | 3 M/8.9 wt% | 3 M/3.1 wt%+ 6 M/5.8 wt% | 3 M/8.9 wt% |
| Na-silicate in the solution | 0 and 11 wt% | 2.2 wt% | 2.2 wt% | 2.2 wt% |
| Standard sand | 65–67 wt% g | 67 wt% | 67 wt% | 67 wt% |
| Powder-to-solution mass ratio | 0.47–0.5 | 0.5 | 0.47 | 0.5 |
| BR-Based Mix Design | SS-Based Mix Design | ||
|---|---|---|---|
| Total water absorption | 5.8% (<6%) | Porosity | 21% |
| Tensile splitting strength | 4 N/mm2 | Flexural strength—after 300 cycles | 2.4 MPa |
| Unpolished slip resistance | USRVwet 62.4 SRVdry 64.5 | Freeze–thaw resistance | No visual damage after 300 cycles |
| Flexural strength | 3.7 MPa | ||
| Abrasion | 6553.2 mm3 | Abrasion | 19.3 mm |
| Freeze/thaw resistance with de-icing salt | 0.4 kg/m2 | Freeze/thaw resistance with de-icing salt | 3.9 mg/mm2 |
| BR-Based Mix Design | SS-Based Mix Design | ||
|---|---|---|---|
| GGBFS | 23.0 | Ekominit S1 | 11.5 |
| BR | 23.0 | SCS | 11.5 |
| 8M NaOH | 8.0 | GGBFS | 4.1 |
| Sodium silicate (SiO2/Na2O MR~3.4) | 8.0 | M800-fine quartz sand | 8.0 |
| Limestone sand (0–2 mm) | 16.0 | EAF slag | 51.6 |
| Limestone gravel (2–6 mm) | 16.0 | 1.65 NS 65 | 13.0 |
| Shrinkage-reducing agent | 0.4 | ||
| Solid/liquid | 2.72 | Binder/liquid | 2.08 |
| Parameter | LoQ (mg/kg) | BR-Based Tile (mg/kg) |
|---|---|---|
| Ba | 0.01 | <LoQ |
| AS | 0.01 | <LoQ |
| Cd | 0.005 | <LoQ |
| Crtot | 0.05 | <LoQ |
| Cr+6 | 0.05 | <LoQ |
| Cu | 0.1 | <LoQ |
| Hg | 0.0002 | <LoQ |
| Mo | 0.01 | <LoQ |
| Ni | 0.01 | <LoQ |
| Pb | 0.02 | <LoQ |
| Sb | 0.01 | <LoQ |
| Se | 0.001 | <LoQ |
| Zn | 0.5 | <LoQ |
| Cl− | 50 | 390 |
| F− | 1 | 5.5 |
| SO4−2 | 100 | 660 |
| Ba | 0.01 | <LoQ |
| Tested Property | Obtained Value |
|---|---|
| Density | 2.6 kg/m3 |
| 7-day compressive strength—EN 196-1 [22] | 58 N/mm2 |
| Total water absorption | 12.4 |
| Tensile splitting strength | 4.9 N/mm2 |
| Unpolished slip resistance value | USRVwet 37 SRVdry 81 |
| Abrasion | 2052 mm3 |
| Freeze/thaw resistance with de-icing salt | 1.1 kg/m2 |
| Testing Parameter | Standard | Result | Reference Concrete Product [31] |
|---|---|---|---|
| Freeze–thaw resistance | ASTM 666 [26] | No visual change after 150 cycles | Non-resistant |
| Freeze–thaw resistance in the presence of de-icing salts | SIST-TS CEN/TS 12390:9 [32] | 0.01–0.03 mg/mm2 | Non-resistant |
| Skid resistance | SIST-TS CEN/TS 16165:2016, Annex C [33] | 64 PTV | 69 PTV |
| Abrasion resistance | EN 1338 [24] | 15.5 mm | 22.1 mm |
| Original Recipe | Optimistic Scenario | ||||||
|---|---|---|---|---|---|---|---|
| Material | Required Quantities of Raw Materials (kg/m2) | Cost of Raw Materials (EUR/t) | Potential Supplier | Cost of Raw Materials (EUR/m2) | Cost of Raw Materials (%) | Potential Supplier | Cost of Raw Materials (EUR/m2) |
| SS | 13.6 | 0 | Acroni | 0 | 0 | Acroni | 0 |
| SCS | 13.6 | 77 | Aurubis | 1 | 77 | Aurubis | 1 |
| GGBFS | 4.8 | 236 | ECOCEM | 1.1 | 236 | ECOCEM | 1.1 |
| Sand (M800) | 9.5 | 1850 | Agitrade | 17.5 | 500 | Termit | 4.7 |
| EAF C slag | 60.9 | 0 | Acroni | 0 | 0 | Acroni | 0 |
| Activator | 15.4 | 1376 | Kefo, ECP | 21.1 | 486 | Silkem, ECP | 7.5 |
| 2 methyl | 0.7 | 3520 | Chamatek | 2.5 | 3520 | Chamatek | 2.5 |
| TOTAL PRICE of raw materials (EUR/m2) | 43.3 | 16.9 |
| Required Quantities for 1 Panel (50 cm × 50 cm × 4 cm) (kg) | Required Quantities for 1 m2 (kg) | Component Price (EUR/kg) | Component Price (EUR/m2) | |
|---|---|---|---|---|
| Bauxite residues | 2.44 | 9.76 | 0.05 | 0.49 |
| GGBFS | 1.83 | 7.32 | 0.08 | 0.59 |
| Metakaolin | 0.31 | 1.24 | 0.16 | 0.2 |
| River sand | 3.05 | 12.2 | 0.01 | 0.06 |
| Fibers | 0.1 | 0.4 | 4.2 | 1.68 |
| Activator | 3.67 | 14.68 | 1.5 | 22.02 |
| Surfactant | 0.04 | 0.16 | 9 | 1.44 |
| TOTAL: | 26.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kriskova, L.; Ducman, V.; Loncnar, M.; Tesovnik, A.; Žibret, G.; Skentzou, D.; Georgopoulos, C. Alkali-Activated Mineral Residues in Construction: Case Studies on Bauxite Residue and Steel Slag Pavement Tiles. Materials 2025, 18, 257. https://doi.org/10.3390/ma18020257
Kriskova L, Ducman V, Loncnar M, Tesovnik A, Žibret G, Skentzou D, Georgopoulos C. Alkali-Activated Mineral Residues in Construction: Case Studies on Bauxite Residue and Steel Slag Pavement Tiles. Materials. 2025; 18(2):257. https://doi.org/10.3390/ma18020257
Chicago/Turabian StyleKriskova, Lubica, Vilma Ducman, Mojca Loncnar, Anže Tesovnik, Gorazd Žibret, Dimitra Skentzou, and Christos Georgopoulos. 2025. "Alkali-Activated Mineral Residues in Construction: Case Studies on Bauxite Residue and Steel Slag Pavement Tiles" Materials 18, no. 2: 257. https://doi.org/10.3390/ma18020257
APA StyleKriskova, L., Ducman, V., Loncnar, M., Tesovnik, A., Žibret, G., Skentzou, D., & Georgopoulos, C. (2025). Alkali-Activated Mineral Residues in Construction: Case Studies on Bauxite Residue and Steel Slag Pavement Tiles. Materials, 18(2), 257. https://doi.org/10.3390/ma18020257








