Integration of Multi-Scale Predictive Tools of Bone Fragility: A Structural and Material Property Perspective
Abstract
1. Introduction
- Summarizing the current state of predictive tools developed across multiple biological scales.
- Identifying gaps in their integration.
- Outlining future directions for unified multiscale frameworks capable of more accurately evaluating bone fragility.
2. Recent Advances in Multi-Scale Predictors of Bone Fragility
2.1. Molecular Level
2.2. Cellular Level
2.3. Tissue Level
2.4. Organ Level
2.5. Whole-Body Level
| Author | Tools/ Techniques | Samples Size | Population (Sex, Age) | Follow-Up Periods (Years) | Key Outcomes |
|---|---|---|---|---|---|
| Ulivieri et al. [48] | BSI | 143 | 121 F and 22 M, ~60–67.9 y | - | The BSI hazard ratio of refracture with a 95% confidence interval was 1.201, and the p-value was 0.982−1.468. |
| Zhang et al. [70] | Transcription and Epigenetics | 38 GWAS | -, - | - | The result indicated that from the 38 BMD GWAS, 14 osteoporosis-related control SNPs are linked to 5 genes. |
| Liaw et al. [73] | SNPs (GWAS) | 29,084 used for GWAS and 18,918 for replications | -, - | - | One of the three SNPs found is rs78827626, which showed a lower certainty in its estimation with an information value of only 0.359. |
| Altınsoy K E, Unat B. [87] | CTX | 520 | -, ≥50 | 3 | They found that CTX, NTX, DPD, and TRAP are important biomarkers that are essential for assessing bone health, tracking the efficacy of treatment, and identifying pathological fractures in the setting of osteoporosis. |
| Gama et al. [90] | HR-pQCT | 129 | M and F, 20–82.3 y | - | TBS and the trabecular and cortical bone characteristics determined by HR-pQCT showed a substantial correlation. |
| Xie et al. [97] | μ-CT | 67 | 41 F and 26 M, >50 | - | The result showed that Age and BMI weren’t significantly different between postmenopausal women and men over 50. |
| Villamor et al. [99] | DXA-based FEA | 137 | F, 81.4 ± 6.95 y | - | This novel approach brought around a 14 pp increase in precision compared to the gold-standard BMD. |
| Hsieh et al. [100] | DXA | 5164 and 18,175 patients with pelvis/lumbar spine radiographs and Hologic DXA | 77.4% of F in the hip evaluation and 79.6% in the spine evaluation set. | - | Comparing the developed tool to 3008 DXA performance during the identical investigation duration, 5206 (84.8%) subjects are classified as having a 95% either positive or negative outcome for osteoporosis. |
| Sornay-Rendu et al. [101] | BSI | 846 | F, 60 ± 15 y | - | There is 1.23 ± 0.28 Neck BSI, 1.13 ± 0.22 Tot Hip BSI, and 1.94 ± 0.60 Spine BSI observed using the developed predicted tool. |
| Landi et al. [104] | Physical Activity | 2005 | 1559 F and 446 M, ≥65 y | 1 | Within the following twelve-month period, 15% or 370 participants experienced a disability. |
| Cheung et al. [112] | FRAX | 2266 | All F, 62.1 ± 8.5 | 4.5 ± 2.8 years | They observed the ideal cut-off value of 9.95% for FRAX with BMD. |
| Pulskiewicz et al. [116] | FRAX | 457 | F, 64.21 ± 5.94 y | 10 | The result shows that 6.3% for large FRAX fractures, 20.0% for any fractures in Garvan, and 18.0% for any fractures in POL-RISK. |
| Garnero et al. [119] | P1NP | 473 | M and F, 30–65 y | - | The observed bone formation marker has good efficiency and ease of use, which make it a potential tool for assessing osteoporosis victims. |
| Chalhoub et al. [120] | DXA | 3301 | All M, ≥65 y | - | The majority of fractures were linked to a higher risk when trabecular volumetric BMD and low aBMD were present. |
| McLean et al. [121] | DXA | 1978 | F, 50–93 y | 8 | The results indicated that women who have lesser lean mass as determined by DXA do not have a higher risk of hip fractures. |
| Messina et al. [122] | BSI | 234 | M and F, ~60–70 y | - | BSI hazard ratios with a 95% confidence interval were 1.372 and p-value 0.0261 for osteoporotic fragility re-fracture with the lumbar spine. |
| Qu et al. [123] | DXA | 96 samples with OF and 107 samples with osteoporosis | All F, 74.8 ± 10.0 for OF and 71.5 ± 6.1 for non-fracture y | - | The findings demonstrated that the fracture sample’s BMD was smaller than the non-fracture sample. |
| Liu et al. [124] | μ-CT | 105 | 44 M and 61 F, 43–71 y | Minimum 2 | The result shows that the threshold value of BS/TV is 3.145, at which the surgeon can analyze the risk of fusion fracture in contrast to BMD evaluations. |
| Zellagui et al. [125] | Geometry + Mechanics Predictive Tool | 636 | -, 50–87 y | 3 years | The result of the fracture risk obtained using this novel predictive tool is (True positive rate = 78%, True negative rate = 81%), which is much better than that obtained using DXA-based BMD. |
| Holloway et al. [126] | FRAX | 591 | All M, 40–90 | - | There was no significant improvement in predicting fractures by MOF or hip FRAX with trabecular bone score modification. |
| Yang et al. [127] | DXA-based FEA | 324 Prior hip fracture patients and 655 non-fracture patients | All F, ≥65 | - | After changing for FRAX fractured hip possibility estimated using BMD, the increased hip danger of fracture was linked with lower FS and greater FRI. |
| Lee et al. [128] | μ-CT | 30 | -, - | - | The Tb.Th exhibited a slight difference irrespective of bone condition. |
| Liu et al. [129] | FRAX | 1975 | -, ≥40 | 6.8 ± 1.1 years | The measured to expected fractures proportion for MOF was 1.19 (95%CI 1.02–1.39) and for hip fractures it was 1.07 (95%CI 0.82–1.39) using the FRAX risk tool. |
| Li et al. [130] | QCT | 1166 | F, - | - | In older females with femoral neck fractures, osteoporosis may impact the association between proximal femur BMD and gluteus maximus muscle density. |
3. Integration of Multi-Scale Predictive Tools for Early Diagnosis of Pathologies
4. Translational Research and Clinical Applications
5. Conclusions
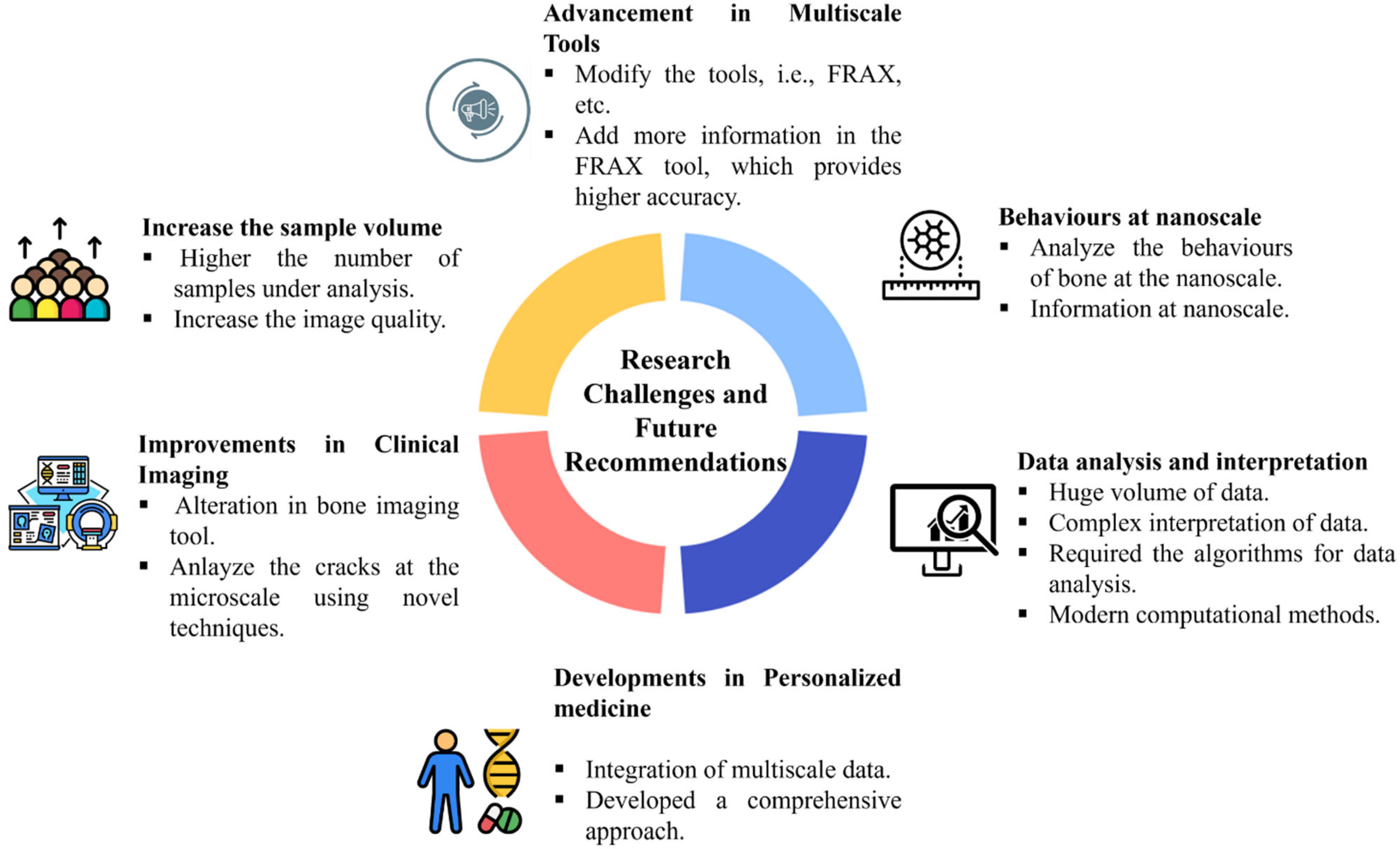
6. Current Challenges and Future Directions
6.1. Key Barriers
6.2. Potential Solutions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Acronym | Meaning |
| ANN | Artificial neural network |
| AI | Artificial intelligence |
| aBMD | Areal bone mineral density |
| AFM | Atomic force microscopy |
| BMD | Bone mineral density |
| BMC | Bone mineral content |
| BMI | Body mass index |
| BCT | Biomechanical computed tomography |
| BV/TV | Bone volume fraction |
| BSI | Bone strain index |
| CT | Computed tomography |
| COVID-19 | Coronavirus disease 2019 |
| CTX | Serum C-telopeptides of type I collagen |
| CTX-1 | Carboxy-terminal crosslinked telopeptide of type 1 collagen |
| CTRA | Computed tomography rigidity analysis |
| CFS | Clinical Frailty Scale |
| CNNs | Convolutional neural networks |
| DXA | Dual-energy X-ray absorptiometry |
| DL | Deep learning |
| FE | Finite element |
| Fx | Fracture prediction |
| FRAX | Fracture risk assessment tool |
| FEA | Finite element analysis |
| GWAS | Genome-wide association studies |
| HRpQCT | High-resolution peripheral quantitative computed tomography |
| HA | Hydroxyapatite |
| IBEX BH | IBEX bone health |
| LDCT | Low-dose chest CT |
| LCN | Lacuno-canalicular network |
| MOF | Major osteoporotic fractures |
| µ-CT | Micro-computed tomography |
| OJN | Osteonecrosis of the jaws |
| OI | Osseous infarction |
| OPG | Osteoprotegerin |
| OSTA | Osteoporosis self-assessment tool for Asians |
| P1NP | Procollagen type I N-terminal propeptide |
| P1CP | Procollagen type 1 C-terminal propeptide |
| pQCT | Peripheral quantitative computed tomography |
| PHPT | Primary hyperparathyroidism |
| PFFI | Proximal femur fracture index |
| QCT | Quantitative computed tomography |
| RANKL | Receptor-activator of nuclear factor kappa beta ligand |
| RANK | Receptor-activator of nuclear factor kappa beta |
| SNPs | Single-nucleotide polymorphisms |
| SAXS | Small-angle X-ray scattering |
| SMI | Structural model index |
| Tb.N | Trabecular number |
| Tb.Th | Trabecular Thickness |
| TBS | Trabecular bone score |
| XFEM | Extended finite element method |
| YAM | Young adult mean |
References
- El-Gazzar, A.; Högler, W. Mechanisms of Bone Fragility: From Osteogenesis Imperfecta to Secondary Osteoporosis. Int. J. Mol. Sci. 2021, 22, 625. [Google Scholar] [CrossRef]
- Burr, D.B. Changes in Bone Matrix Properties with Aging. Bone 2019, 120, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Marini, F.; Giusti, F.; Iantomasi, T.; Brandi, M.L. Congenital Metabolic Bone Disorders as a Cause of Bone Fragility. Int. J. Mol. Sci. 2021, 22, 10281. [Google Scholar] [CrossRef]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, Y.; Gao, Y.; Zhang, Z.; Qin, L.; Song, J.; Wang, H.; Wu, I.X. Prediction Models for Osteoporotic Fractures Risk: A Systematic Review and Critical Appraisal. Aging Dis. 2022, 13, 1215–1238. [Google Scholar] [CrossRef] [PubMed]
- Johnell, O.; Kanis, J.A. An Estimate of the Worldwide Prevalence and Disability Associated with Osteoporotic Fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Achenbach, S.J.; Atkinson, E.J.; Khosla, S.; Melton, L.J., III. Trends in Fracture Incidence: A Population-Based Study Over 20 Years. J. Bone Miner. Res. 2014, 29, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.-M.; Bisignano, C.; James, S.L.; Abady, G.G.; Abedi, A.; Abu-Gharbieh, E.; Alhassan, R.K.; Alipour, V.; Arabloo, J.; Asaad, M.; et al. Global, Regional, and National Burden of Bone Fractures in 204 Countries and Territories, 1990–2019: A Systematic Analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yu, P.; Wu, Y.; Wu, Y.; Tan, Z.; Ling, J.; Ma, J.; Zhang, J.; Zhu, W.; Liu, X. Sex Specific Global Burden of Osteoporosis in 204 Countries and Territories, from 1990 to 2030: An Age-Period-Cohort Modeling Study. J. Nutr. Health Aging 2023, 27, 767–774. [Google Scholar] [CrossRef]
- Borgström, F.; Karlsson, L.; Ortsäter, G.; Norton, N.; Halbout, P.; Cooper, C.; Lorentzon, M.; McCloskey, E.V.; Harvey, N.C.; Javaid, M.K.; et al. Fragility Fractures in Europe: Burden, Management and Opportunities. Arch. Osteoporos. 2020, 15, 59. [Google Scholar] [CrossRef]
- Singer, A.; McClung, M.R.; Tran, O.; Morrow, C.D.; Goldstein, S.; Kagan, R.; McDermott, M.; Yehoshua, A. Treatment Rates and Healthcare Costs of Patients with Fragility Fracture by Site of Care: A Real-World Data Analysis. Arch. Osteoporos. 2023, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johansson, H.; Harvey, N.C.; McCloskey, E.V. A Brief History of FRAX. Arch. Osteoporos. 2018, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Bregoli, C.; Biffi, C.A.; Tuissi, A.; Buccino, F. Effect of Trabecular Architectures on the Mechanical Response in Osteoporotic and Healthy Human Bone. Med. Biol. Eng. Comput. 2024, 62, 3263–3281. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Cauley, J.A.; Wagman, R.; Ensrud, K.; Fink, H.A.; Hillier, T.A.; Lui, L.-Y.; Cummings, S.R.; Schousboe, J.T.; Napoli, N. The Ability of a Single BMD and Fracture History Assessment to Predict Fracture over 25 Years in Postmenopausal Women: The Study of Osteoporotic Fractures. J. Bone Miner. Res. 2018, 33, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Rubin, C.; Turner, A.S.; Müller, R.; Mittra, E.; McLeod, K.; Lin, W.; Qin, Y. Quantity and Quality of Trabecular Bone in the Femur Are Enhanced by a Strongly Anabolic, Noninvasive Mechanical Intervention. J. Bone Miner. Res. 2002, 17, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Ru, K.; Swati, R.F.; Zeng, H.; Khan, Z.; Chen, Z.; Qian, A.; Hu, L. Chapter 3—The Whole Bone Mechanical Properties and Modeling Study. In Bone Cell Biomechanics, Mechanobiology and Bone Diseases; Qian, A., Hu, L., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 53–94. ISBN 978-0-323-96123-3. [Google Scholar]
- Ma, C.; Du, T.; Niu, X.; Fan, Y. Biomechanics and Mechanobiology of the Bone Matrix. Bone Res. 2022, 10, 1–17. [Google Scholar] [CrossRef]
- Jadžić, J.; Đurić, M. Structural Basis of Increased Bone Fragility in Aged Individuals: Multi-Scale Perspective. Med. Istraživanja 2024, 57, 67–74. [Google Scholar] [CrossRef]
- Vendrami, C.; Shevroja, E.; Gonzalez Rodriguez, E.; Gatineau, G.; Elmers, J.; Reginster, J.-Y.; Harvey, N.C.; Lamy, O.; Hans, D. Muscle Parameters in Fragility Fracture Risk Prediction in Older Adults: A Scoping Review. J. Cachexia Sarcopenia Muscle 2024, 15, 477–500. [Google Scholar] [CrossRef]
- Ravazzano, L.; Colaianni, G.; Tarakanova, A.; Xiao, Y.-B.; Grano, M.; Libonati, F. Multiscale and Multidisciplinary Analysis of Aging Processes in Bone. NPJ Aging 2024, 10, 1–18. [Google Scholar] [CrossRef]
- World Health Organization. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis: Report of a WHO Study Group [Meeting Held in Rome from 22 to 25 June 1992]; World Health Organization: Rome, Italy, 1994; ISBN 978-92-4-120843-7. [Google Scholar]
- Slart, R.H.J.A.; Punda, M.; Ali, D.S.; Bazzocchi, A.; Bock, O.; Camacho, P.; Carey, J.J.; Colquhoun, A.; Compston, J.; Engelke, K.; et al. Updated Practice Guideline for Dual-Energy X-Ray Absorptiometry (DXA). Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 539–563. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, C.; Pisano, A.A.; Vasta, M.; Fuschi, P. A Computed Tomography-Based Limit Analysis Approach to Investigate the Mechanical Behavior of the Human Femur Prone to Fracture. Meccanica 2024, 59, 1301–1313. [Google Scholar] [CrossRef]
- Nazarian, A.; Cory, E.; Müller, R.; Snyder, B.D. Shortcomings of DXA to Assess Changes in Bone Tissue Density and Microstructure Induced by Metabolic Bone Diseases in Rat Models. Osteoporos. Int. 2009, 20, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Schultz, K.; Wolf, J.M. Emerging Technologies in Osteoporosis Diagnosis. J. Hand Surg. 2019, 44, 240–243. [Google Scholar] [CrossRef]
- Rodriguez-Palomo, A.; Østergaard, M.; Birkedal, H. Bone Hierarchical Structure: Heterogeneity and Uniformity. Adv. Funct. Mater. 2024, 34, 2307026. [Google Scholar] [CrossRef]
- Buccino, F.; Colombo, C.; Vergani, L.M. A Review on Multiscale Bone Damage: From the Clinical to the Research Perspective. Mater. 2021, 14, 1240. [Google Scholar] [CrossRef]
- Dei Rossi, G.; Vergani, L.M.; Buccino, F. A Novel Triad of Bio-Inspired Design, Digital Fabrication, and Bio-Derived Materials for Personalised Bone Repair. Materials 2024, 17, 5305. [Google Scholar] [CrossRef]
- Buccino, F.; Aiazzi, I.; Casto, A.; Liu, B.; Sbarra, M.C.; Ziarelli, G.; Vergani, L.M.; Bagherifard, S. Down to the Bone: A Novel Bio-Inspired Design Concept. Materials 2021, 14, 4226. [Google Scholar] [CrossRef]
- Ulivieri, F.M.; Rinaudo, L. Beyond Bone Mineral Density: A New Dual X-Ray Absorptiometry Index of Bone Strength to Predict Fragility Fractures, the Bone Strain Index. Front. Med. 2021, 7, 590139. [Google Scholar] [CrossRef]
- Migliorini, F.; Giorgino, R.; Hildebrand, F.; Spiezia, F.; Peretti, G.M.; Alessandri-Bonetti, M.; Eschweiler, J.; Maffulli, N. Fragility Fractures: Risk Factors and Management in the Elderly. Medicina 2021, 57, 1119. [Google Scholar] [CrossRef]
- Barekar, S.S.; Sarawade, S.S.; Kumar, N. A Survey on the Mechanical Properties of Bone. Multimed. Tools Appl. 2024, 84, 1359–1383. [Google Scholar] [CrossRef]
- Bioletto, F.; Barale, M.; Prencipe, N.; Berton, A.M.; Parasiliti-Caprino, M.; Gasco, V.; Ghigo, E.; Procopio, M.; Grottoli, S. Trabecular Bone Score as an Index of Bone Fragility in Patients with Acromegaly: A Systematic Review and Meta-Analysis. Neuroendocrinology 2023, 113, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Schileo, E.; Taddei, F. Finite Element Assessment of Bone Fragility from Clinical Images. Curr. Osteoporos. Rep. 2021, 19, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, J.B.H.; Lenchik, L.; Weaver, A.A.; Boutin, R.D.; Wuertzer, S. Opportunistic Screening of Bone Fragility Using Computed Tomography. Semin. Musculoskelet. Radiol. 2024, 28, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Bowman, L.; Loucks, A.B. In Vivo Assessment of Cortical Bone Fragility. Curr. Osteoporos. Rep. 2020, 18, 13–22. [Google Scholar] [CrossRef]
- Buccino, F.; Zagra, L.; Longo, E.; D’Amico, L.; Banfi, G.; Berto, F.; Tromba, G.; Vergani, L.M. Osteoporosis and Covid-19: Detected Similarities in Bone Lacunar-Level Alterations via Combined AI and Advanced Synchrotron Testing. Mater. Des. 2023, 231, 112087. [Google Scholar] [CrossRef]
- Mirzaali, M.J.; Libonati, F.; Ferrario, D.; Rinaudo, L.; Messina, C.; Ulivieri, F.M.; Cesana, B.M.; Strano, M.; Vergani, L. Determinants of Bone Damage: An Ex-Vivo Study on Porcine Vertebrae. PLoS ONE 2018, 13, e0202210. [Google Scholar] [CrossRef]
- Lewis, G.S.; Mischler, D.; Wee, H.; Reid, J.S.; Varga, P. Finite Element Analysis of Fracture Fixation. Curr. Osteoporos. Rep. 2021, 19, 403–416. [Google Scholar] [CrossRef]
- Bazyar, P.; Sheidaee, E. Design and Simulating Lattice Structures in the FE Analysis of the Femur Bone. Bioprinting 2024, 37, e00326. [Google Scholar] [CrossRef]
- Cen, H.; Gong, H.; Liu, H.; Jia, S.; Wu, X.; Fan, Y. A Comparative Study on the Multiscale Mechanical Responses of Human Femoral Neck Between the Young and the Elderly Using Finite Element Method. Front. Bioeng. Biotechnol. 2022, 10, 893337. [Google Scholar] [CrossRef]
- Ascenzi, M.-G.; Kawas, N.P.; Lutz, A.; Kardas, D.; Nackenhorst, U.; Keyak, J.H. Individual-Specific Multi-Scale Finite Element Simulation of Cortical Bone of Human Proximal Femur. J. Comput. Phys. 2013, 244, 298–311. [Google Scholar] [CrossRef]
- Barkaoui, A.; Hambli, R.; Tavares, J.M.R.S. Effect of Material and Structural Factors on Fracture Behaviour of Mineralised Collagen Microfibril Using Finite Element Simulation. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 1181–1190. [Google Scholar] [CrossRef]
- Ju, C.; Yang, K.; Yang, Q.; Mi, Y.; Wang, C.; Ji, H. Multiscale Dynamics Analysis of Lumbar Vertebral Cortical Bone Based on the Abaqus Submodel Finite Element Method. Sci. Rep. 2025, 15, 6861. [Google Scholar] [CrossRef] [PubMed]
- Cen, H.; Yao, Y.; Liu, H.; Jia, S.; Gong, H. Multiscale Mechanical Responses of Young and Elderly Human Femurs: A Finite Element Investigation. Bone 2021, 153, 116125. [Google Scholar] [CrossRef] [PubMed]
- Zysset, P.K.; Dall’Ara, E.; Varga, P.; Pahr, D.H. Finite Element Analysis for Prediction of Bone Strength. Bonekey Rep. 2013, 2, 386. [Google Scholar] [CrossRef] [PubMed]
- Ulivieri, F.M.; Piodi, L.P.; Rinaudo, L.; Scanagatta, P.; Cesana, B.M. Bone Strain Index in the Prediction of Vertebral Fragility Refracture. Eur. Radiol. Exp. 2020, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Libonati, F.; Rinaudo, L.; Bellazzi, M.; Ulivieri, F.M.; Vergani, L. A New Finite Element Based Parameter to Predict Bone Fracture. PLoS ONE 2019, 14, e0225905. [Google Scholar] [CrossRef]
- Messina, C.; Acquasanta, M.; Rinaudo, L.; Tortora, S.; Arena, G.; Albano, D.; Sconfienza, L.M.; Ulivieri, F.M. Short-Term Precision Error of Bone Strain Index, a New DXA-Based Finite Element Analysis Software for Assessing Hip Strength. J. Clin. Densitom. 2021, 24, 330–337. [Google Scholar] [CrossRef]
- Tabacco, G.; Naciu, A.M.; Messina, C.; Sanson, G.; Rinaudo, L.; Cesareo, R.; Falcone, S.; Manfrini, S.; Napoli, N.; Bilezikian, J.P.; et al. DXA-Based Bone Strain Index: A New Tool to Evaluate Bone Quality in Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2021, 106, 2304–2312. [Google Scholar] [CrossRef]
- Devlin, H.; Horner, K. Mandibular Radiomorphometric Indices in the Diagnosis of Reduced Skeletal Bone Mineral Density. Osteoporos. Int. 2002, 13, 373–378. [Google Scholar] [CrossRef]
- Drozdzowska, B.; Pluskiewicz, W.; Tarnawska, B. Panoramic-Based Mandibular Indices in Relation to Mandibular Bone Mineral Density and Skeletal Status Assessed by Dual Energy X-Ray Absorptiometry and Quantitative Ultrasound. Dentomaxillofac. Radiol. 2002, 31, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Calciolari, E.; Donos, N.; Park, J.C.; Petrie, A.; Mardas, N. Panoramic Measures for Oral Bone Mass in Detecting Osteoporosis: A Systematic Review and Meta-Analysis. J. Dent. Res. 2015, 94, 17S–27S. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Todo, M.; Umebayashi, D.; Yamamoto, Y. Risk Assessment of Vertebral Compressive Fracture Using Bone Mass Index and Strength Predicted by Computed Tomography Image Based Finite Element Analysis. Clin. Biomech. 2021, 85, 105365. [Google Scholar] [CrossRef] [PubMed]
- Qasim, M.; Farinella, G.; Zhang, J.; Li, X.; Yang, L.; Eastell, R.; Viceconti, M. Patient-Specific Finite Element Estimated Femur Strength as a Predictor of the Risk of Hip Fracture: The Effect of Methodological Determinants. Osteoporos. Int. 2016, 27, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Fleps, I.; Morgan, E.F. A Review of CT-Based Fracture Risk Assessment with Finite Element Modeling and Machine Learning. Curr. Osteoporos. Rep. 2022, 20, 309–319. [Google Scholar] [CrossRef]
- Imai, K. Computed Tomography-Based Finite Element Analysis to Assess Fracture Risk and Osteoporosis Treatment. World J. Exp. Med. 2015, 5, 182–187. [Google Scholar] [CrossRef]
- Dall’Ara, E.; Luisier, B.; Schmidt, R.; Kainberger, F.; Zysset, P.; Pahr, D. A Nonlinear QCT-Based Finite Element Model Validation Study for the Human Femur Tested in Two Configurations In Vitro. Bone 2013, 52, 27–38. [Google Scholar] [CrossRef]
- Schermann, H.; Gortzak, Y.; Kollender, Y.; Dadia, S.; Trabelsi, N.; Yosibash, Z.; Sternheim, A. Patient-Specific Computed Tomography-Based Finite Element Analysis: A New Tool to Assess Fracture Risk in Benign Bone Lesions of the Femur. Clin. Biomech. 2020, 80, 105155. [Google Scholar] [CrossRef]
- Liebl, H.; Garcia, E.G.; Holzner, F.; Noel, P.B.; Burgkart, R.; Rummeny, E.J.; Baum, T.; Bauer, J.S. In-Vivo Assessment of Femoral Bone Strength Using Finite Element Analysis (FEA) Based on Routine MDCT Imaging: A Preliminary Study on Patients with Vertebral Fractures. PLoS ONE 2015, 10, e0116907. [Google Scholar] [CrossRef]
- Šustková, A.; Thomková, B.; Zikmund, T.; Kaiser, J.; Joukal, M.; Marcián, P. The Influence of Image Processing of μ-CT Images on Mechanical Behavior of Mandibular Trabecular Bone Structure Using Micro Finite Element Method. Procedia Struct. Integr. 2023, 43, 276–281. [Google Scholar] [CrossRef]
- Clark, D.P.; Badea, C.T. Micro-CT of Rodents: State-of-the-Art and Future Perspectives. Phys. Medica 2014, 30, 619–634. [Google Scholar] [CrossRef]
- Inai, R.; Nakahara, R.; Morimitsu, Y.; Akagi, N.; Marukawa, Y.; Matsushita, T.; Tanaka, T.; Tada, A.; Hiraki, T.; Nasu, Y.; et al. Bone Microarchitectural Analysis Using Ultra-High-Resolution CT in Tiger Vertebra and Human Tibia. Eur. Radiol. Exp. 2020, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Buccino, F.; Giuseppoli, F.; Kochetkova, T.; Schwiedrzik, J.; Vergani, L.M. Advances in Nanoscopic Mechanobiological Structure-Property Relationship in Human Bones for Tailored Fragility Prevention. Mater. Today Commun. 2024, 40, 110108. [Google Scholar] [CrossRef]
- Cheung, W.; Hung, V.W.; Cheuk, K.; Chau, W.; Tsoi, K.K.; Wong, R.M.; Chow, S.K.; Lam, T.; Yung, P.S.; Law, S.; et al. Best Performance Parameters of HR-pQCT to Predict Fragility Fracture: Systematic Review and Meta-Analysis. J. Bone Miner. Res. 2021, 36, 2381–2398. [Google Scholar] [CrossRef] [PubMed]
- Popp, K.L.; Hughes, J.M.; Martinez-Betancourt, A.; Scott, M.; Turkington, V.; Caksa, S.; Guerriere, K.I.; Ackerman, K.E.; Xu, C.; Unnikrishnan, G.; et al. Bone Mass, Microarchitecture and Strength Are Influenced by Race/Ethnicity in Young Adult Men and Women. Bone 2017, 103, 200–208. [Google Scholar] [CrossRef]
- Adams, A.L.; Fischer, H.; Kopperdahl, D.L.; Lee, D.C.; Black, D.M.; Bouxsein, M.L.; Fatemi, S.; Khosla, S.; Orwoll, E.S.; Siris, E.S.; et al. Osteoporosis and Hip Fracture Risk from Routine Computed Tomography Scans: The Fracture, Osteoporosis, and CT Utilization Study (FOCUS). J. Bone Miner. Res. 2018, 33, 1291–1301. [Google Scholar] [CrossRef]
- Yuan, C.; Yu, X.-T.; Wang, J.; Shu, B.; Wang, X.-Y.; Huang, C.; Lv, X.; Peng, Q.-Q.; Qi, W.-H.; Zhang, J.; et al. Multi-Modal Molecular Determinants of Clinically Relevant Osteoporosis Subtypes. Cell Discov. 2024, 10, 1–24. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, H.W.; Shen, H.; Ehrlich, M. Prioritization of Osteoporosis Associated Genome Wide Association Study (GWAS) Single Nucleotide Polymorphisms (SNPs) Using Epigenomics and Transcriptomics. JBMR Plus 2021, 5, e10481. [Google Scholar] [CrossRef]
- Ichikawa, S.; Koller, D.L.; Padgett, L.R.; Lai, D.; Hui, S.L.; Peacock, M.; Foroud, T.; Econs, M.J. Replication of Previous Genome-Wide Association Studies of Bone Mineral Density in Premenopausal American Women. J. Bone Miner. Res. 2010, 25, 1821–1829. [Google Scholar] [CrossRef]
- Kemp, J.P.; Morris, J.A.; Medina-Gomez, C.; Forgetta, V.; Warrington, N.M.; Youlten, S.E.; Zheng, J.; Gregson, C.L.; Grundberg, E.; Trajanoska, K.; et al. Identification of 153 New Loci Associated with Heel Bone Mineral Density and Functional Involvement of GPC6 in Osteoporosis. Nat. Genet. 2017, 49, 1468–1475. [Google Scholar] [CrossRef]
- Liaw, Y.-C.; Matsuda, K.; Liaw, Y.-P. Identification of an Novel Genetic Variant Associated with Osteoporosis: Insights from the Taiwan Biobank Study. JBMR Plus 2024, 8, ziae028. [Google Scholar] [CrossRef]
- Wright, H.L.; McCarthy, H.S.; Middleton, J.; Marshall, M.J. RANK, RANKL and Osteoprotegerin in Bone Biology and Disease. Curr. Rev. Musculoskelet. Med. 2009, 2, 56–64. [Google Scholar] [CrossRef]
- Peake, C.; Shah, K.; Solan, M.C. Bone Metabolism and the Receptor Activator of Nuclear Factor-κB Ligand (RANKL) Pathway: A Comprehensive Review. Orthop. Trauma 2021, 35, 297–304. [Google Scholar] [CrossRef]
- Ikebuchi, Y.; Aoki, S.; Honma, M.; Hayashi, M.; Sugamori, Y.; Khan, M.; Kariya, Y.; Kato, G.; Tabata, Y.; Penninger, J.M.; et al. Coupling of Bone Resorption and Formation by RANKL Reverse Signalling. Nature 2018, 561, 195–200. [Google Scholar] [CrossRef]
- Tourolle, D.C.; Dempster, D.W.; Ledoux, C.; Boaretti, D.; Aguilera, M.; Saleem, N.; Müller, R. Ten-Year Simulation of the Effects of Denosumab on Bone Remodeling in Human Biopsies. JBMR Plus 2021, 5, e10494. [Google Scholar] [CrossRef]
- Lai, J.; Yang, H.; Huang, J.; He, L. Investigating the Impact of Wnt Pathway-Related Genes on Biomarker and Diagnostic Model Development for Osteoporosis in Postmenopausal Females. Sci. Rep. 2024, 14, 2880. [Google Scholar] [CrossRef] [PubMed]
- Hart, N.; Newton, R.; Tan, J.; Rantalainen, T.; Chivers, P.; Siafarikas, A.; Nimphius, S. Biological Basis of Bone Strength: Anatomy, Physiology and Measurement. J. Musculoskelet. Neuronal Interact. 2020, 20, 347. [Google Scholar] [PubMed]
- Watts, N.B. Clinical Utility of Biochemical Markers of Bone Remodeling. Clin. Chem. 1999, 45, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Szulc, P. Bone Turnover: Biology and Assessment Tools. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 725–738. [Google Scholar] [CrossRef]
- Schini, M.; Vilaca, T.; Gossiel, F.; Salam, S.; Eastell, R. Bone Turnover Markers: Basic Biology to Clinical Applications. Endocr. Rev. 2023, 44, 417–473. [Google Scholar] [CrossRef]
- Allende-Vigo, M.Z. The Use of Biochemical Markers of Bone Turnover in Osteoporosis. P. R. Health Sci. J. 2007, 26, 91–95. [Google Scholar]
- Takada, J.; Dinavahi, R.; Miyauchi, A.; Hamaya, E.; Hirama, T.; Libanati, C.; Nakamura, Y.; Milmont, C.E.; Grauer, A. Relationship between P1NP, a Biochemical Marker of Bone Turnover, and Bone Mineral Density in Patients Transitioned from Alendronate to Romosozumab or Teriparatide: A Post Hoc Analysis of the STRUCTURE Trial. J. Bone Miner. Metab. 2020, 38, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.-R.; Chen, C.-H. Bone Biomarker for the Clinical Assessment of Osteoporosis: Recent Developments and Future Perspectives. Biomark. Res. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.S.; Suzuki, J.B. CTX Biochemical Marker of Bone Metabolism. Is It a Reliable Predictor of Bisphosphonate-Associated Osteonecrosis of the Jaws After Surgery? Part II: A Prospective Clinical Study. Implant. Dent. 2010, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Altınsoy, K.E. The Role of Bone Turnover Markers in Diagnosis, Monitoring, and Pathological Fractures of Osteoporosis. Ulus. Travma Acil Cerrahi Derg. 2024, 30, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Ganti, L. The Treatment and Monitoring of Osteoporosis Using Bone Turnover Markers. Orthop. Rev. 2025, 17, 127772. [Google Scholar] [CrossRef]
- Silva, B.C.; Boutroy, S.; Zhang, C.; McMahon, D.J.; Zhou, B.; Wang, J.; Udesky, J.; Cremers, S.; Sarquis, M.; Guo, X.-D.E.; et al. Trabecular Bone Score (TBS)—A Novel Method to Evaluate Bone Microarchitectural Texture in Patients with Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2013, 98, 1963–1970. [Google Scholar] [CrossRef]
- Gama, E.M.F.; Mendonça, L.M.C.; Paranhos-Neto, F.P.; Vieira Neto, L.; Madeira, M.; Farias, M.L.F. TBS Correlates with Bone Density and Microstructure at Trabecular and Cortical Bone Evaluated by HR-pQCT. J. Bone Miner. Metab. 2024, 42, 352–360. [Google Scholar] [CrossRef]
- Leslie, W.D.; Hans, D.; Silva, B.C. Fracture Prediction from Trabecular Bone Score Is Unaffected by Anti-Resorptive Treatment: A Registry-Based Cohort Study. J. Clin. Densitom. 2023, 26, 10–15. [Google Scholar] [CrossRef]
- Gatineau, G.; Hind, K.; Shevroja, E.; Gonzalez-Rodriguez, E.; Lamy, O.; Hans, D. Advancing Trabecular Bone Score (TBS): Clinical Performance of TBS Version 4.0 with Direct Correction for Soft Tissue Thickness—The Osteolaus Study. Osteoporos. Int. 2025, 36, 715–724. [Google Scholar] [CrossRef]
- Yeh, O.C.; Keaveny, T.M. Biomechanical Effects of Intraspecimen Variations in Trabecular Architecture: A Three-Dimensional Finite Element Study. Bone 1999, 25, 223–228. [Google Scholar] [CrossRef]
- Albert, C.; Jameson, J.; Toth, J.M.; Smith, P.; Harris, G. Bone Properties by Nanoindentation in Mild and Severe Osteogenesis imperfecta. Clin. Biomech. 2013, 28, 110–116. [Google Scholar] [CrossRef]
- Katsamenis, O.L.; Jenkins, T.; Thurner, P.J. Toughness and Damage Susceptibility in Human Cortical Bone Is Proportional to Mechanical Inhomogeneity at the Osteonal-Level. Bone 2015, 76, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Gehweiler, D.; Schultz, M.; Schulze, M.; Riesenbeck, O.; Wähnert, D.; Raschke, M.J. Material Properties of Human Vertebral Trabecular Bone under Compression Can Be Predicted Based on Quantitative Computed Tomography. BMC Musculoskelet. Disord. 2021, 22, 709. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhou, B.; Wang, J.; Liu, T.; Wu, X.; Fang, R.; Kang, Y.; Dai, R. Microstructural Properties of Trabecular Bone Autografts: Comparison of Men and Women with and without Osteoporosis. Arch. Osteoporos. 2018, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Op Den Buijs, J.; Dragomir-Daescu, D. Validated Finite Element Models of the Proximal Femur Using Two-Dimensional Projected Geometry and Bone Density. Comput. Methods Programs Biomed. 2011, 104, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Villamor, E.; Monserrat, C.; Del Río, L.; Romero-Martín, J.A.; Rupérez, M.J. Prediction of Osteoporotic Hip Fracture in Postmenopausal Women through Patient-Specific FE Analyses and Machine Learning. Comput. Methods Programs Biomed. 2020, 193, 105484. [Google Scholar] [CrossRef]
- Hsieh, C.-I.; Zheng, K.; Lin, C.; Mei, L.; Lu, L.; Li, W.; Chen, F.-P.; Wang, Y.; Zhou, X.; Wang, F.; et al. Automated Bone Mineral Density Prediction and Fracture Risk Assessment Using Plain Radiographs via Deep Learning. Nat. Commun. 2021, 12, 5472. [Google Scholar] [CrossRef]
- Sornay-Rendu, E.; Duboeuf, F.; Ulivieri, F.M.; Rinaudo, L.; Chapurlat, R. The Bone Strain Index Predicts Fragility Fractures. The OFELY Study. Bone 2022, 157, 116348. [Google Scholar] [CrossRef]
- Roux, J.-P.; Duboeuf, F.; Sornay-Rendu, E.; Rinaudo, L.; Ulivieri, F.M.; Wegrzyn, J.; Chapurlat, R. The Relationship between Bone Strain Index, Bone Mass, Microarchitecture and Mechanical Behavior in Human Vertebrae: An Ex Vivo Study. Osteoporos. Int. 2024, 35, 1069–1075. [Google Scholar] [CrossRef]
- Cao, X.; Keyak, J.H.; Sigurdsson, S.; Zhao, C.; Zhou, W.; Liu, A.; Lang, T.F.; Deng, H.-W.; Gudnason, V.; Sha, Q. A New Hip Fracture Risk Index Derived from FEA-Computed Proximal Femur Fracture Loads and Energies-to-Failure. Osteoporos. Int. 2024, 35, 785–794. [Google Scholar] [CrossRef]
- Landi, F.; Onder, G.; Carpenter, I.; Cesari, M.; Soldato, M.; Bernabei, R. Physical Activity Prevented Functional Decline among Frail Community-Living Elderly Subjects in an International Observational Study. J. Clin. Epidemiol. 2007, 60, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Porto, J.M.; Pieruccini-Faria, F.; Bandeira, A.C.L.; Bôdo, J.S.; de Abreu, D.C.C. Physical Activity Components Associated with Gait Parameters in Community-Dwelling Older Adults. J. Bodyw. Mov. Ther. 2024, 38, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Hans, D.; Cooper, C.; Baim, S.; Bilezikian, J.P.; Binkley, N.; Cauley, J.A.; Compston, J.E.; Dawson-Hughes, B.; El-Hajj Fuleihan, G.; et al. Interpretation and Use of FRAX in Clinical Practice. Osteoporos. Int. 2011, 22, 2395–2411. [Google Scholar] [CrossRef] [PubMed]
- Middleton, R.G.; Shabani, F.; Uzoigwe, C.E.; Shoaib, A.; Moqsith, M.; Venkatesan, M. FRAX and the Assessment of the Risk of Developing a Fragility Fracture. J. Bone Jt. Surg. Br. Vol. 2012, 94-B, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Watts, N.B. The Fracture Risk Assessment Tool (FRAX®): Applications in Clinical Practice. J. Women’s Health 2011, 20, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Zanardo, M.; Mennini, C.; Glielmo, P.; Fusco, S.; Albano, D.; Messina, C. Dual Energy X-Ray Absorptiometry: Radiographer’S Role in Assessing Fracture Risk Assessment Tool (FRAX) Questionnaire Variables. J. Clin. Densitom. 2024, 27, 101458. [Google Scholar] [CrossRef] [PubMed]
- Leslie, W.D.; Lix, L.M.; Johansson, H.; Oden, A.; McCloskey, E.; Kanis, J.A. Independent Clinical Validation of a Canadian FRAX Tool: Fracture Prediction and Model Calibration. J. Bone Miner. Res. 2010, 25, 2350–2358. [Google Scholar] [CrossRef]
- Sucharitpongpan, W. The Optimal Cut-off Values of FRAX without BMD for Predicting Osteoporosis Fracture Risk in the Older Adults at Nan, Thailand. Osteoporos. Sarcopenia 2024, 10, 11–15. [Google Scholar] [CrossRef]
- Cheung, E.; Cheung, C.-L.; Kung, A.W.C.; Tan, K.C.B. Possible FRAX-Based Intervention Thresholds for a Cohort of Chinese Postmenopausal Women. Osteoporos. Int. 2014, 25, 1017–1023. [Google Scholar] [CrossRef]
- Clark, P.; Denova-Gutiérrez, E.; Zerbini, C.; Sanchez, A.; Messina, O.; Jaller, J.J.; Campusano, C.; Orces, C.H.; Riera, G.; Johansson, H.; et al. FRAX-Based Intervention and Assessment Thresholds in Seven Latin American Countries. Osteoporos. Int. 2018, 29, 707–715. [Google Scholar] [CrossRef]
- Lesnyak, O.; Zakroyeva, A.; Babalyan, V.; Cazac, V.; Gabdulina, G.; Ismailov, S.; Lobanchenko, O.; Rudenka, E.; Tsagareli, M.; Johansson, H.; et al. FRAX-Based Intervention Thresholds in Eight Eurasian Countries: Armenia, Belarus, Georgia, Kazakhstan, the Kyrgyz Republic, Moldova, the Russian Federation, and Uzbekistan. Arch. Osteoporos. 2021, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Naseri, A.; Bakhshayeshkaram, M.; Salehi, S.; Heydari, S.T.; Dabbaghmanesh, M.H.; Dabbaghmanesh, M.M. FRAX-Derived Intervention and Assessment Thresholds for Osteoporosis in Ten Middle Eastern Countries. Arch. Osteoporos. 2024, 19, 41. [Google Scholar] [CrossRef] [PubMed]
- Pluskiewicz, W.; Werner, A.; Bach, M.; Adamczyk, P.; Drozdzowska, B. Fracture Risk Prediction in Postmenopausal Women from GO Study: The Comparison between FRAX, Garvan, and POL-RISK Algorithms. Arch. Osteoporos. 2024, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.-H.; Wu, T.-Y.; Liaw, C.-K.; Hsiao, S.-H.; Kuo, K.-L.; Tsai, C.-Y. Real World Fracture Prediction of Fracture Risk Assessment Tool (FRAX), Osteoporosis Self-Assessment Tool for Asians (OSTA) and One-Minute Osteoporosis Risk Test: An 11-Year Longitudinal Study. Bone Rep. 2024, 20, 101742. [Google Scholar] [CrossRef]
- El Miedany, Y. FRAX: Re-Adjust or Re-Think. Arch. Osteoporos. 2020, 15, 150. [Google Scholar] [CrossRef]
- Garnero, P.; Vergnaud, P.; Hoyle, N. Evaluation of a Fully Automated Serum Assay for Total N-Terminal Propeptide of Type I Collagen in Postmenopausal Osteoporosis. Clin. Chem. 2008, 54, 188–196. [Google Scholar] [CrossRef]
- Chalhoub, D.; Orwoll, E.S.; Cawthon, P.M.; Ensrud, K.E.; Boudreau, R.; Greenspan, S.; Newman, A.B.; Zmuda, J.; Bauer, D.; Cummings, S.; et al. Areal and Volumetric Bone Mineral Density and Risk of Multiple Types of Fracture in Older Men. Bone 2016, 92, 100–106. [Google Scholar] [CrossRef]
- McLean, R.R.; Kiel, D.P.; Berry, S.D.; Broe, K.E.; Zhang, X.; Cupples, L.A.; Hannan, M.T. Lower Lean Mass Measured by Dual-Energy X-Ray Absorptiometry (DXA) Is Not Associated with Increased Risk of Hip Fracture in Women: The Framingham Osteoporosis Study. Calcif. Tissue Int. 2018, 103, 16–23. [Google Scholar] [CrossRef]
- Messina, C.; Rinaudo, L.; Cesana, B.M.; Maresca, D.; Piodi, L.P.; Sconfienza, L.M.; Sardanelli, F.; Ulivieri, F.M. Prediction of Osteoporotic Fragility Re-Fracture with Lumbar Spine DXA-Based Derived Bone Strain Index: A Multicenter Validation Study. Osteoporos. Int. 2021, 32, 85–91. [Google Scholar] [CrossRef]
- Qu, X.; Zheng, B.; Chen, T.; Cao, Z.; Qu, B.; Jiang, T. Bone Turnover Markers and Bone Mineral Density to Predict Osteoporotic Fractures in Older Women: A Retrospective Comparative Study. Orthop. Surg. 2020, 12, 116–123. [Google Scholar] [CrossRef]
- Liu, P.; Zhou, B.; Chen, F.; Dai, Z.; Kang, Y. Effect of Trabecular Microstructure of Spinous Process on Spinal Fusion and Clinical Outcomes After Posterior Lumbar Interbody Fusion: Bone Surface/Total Volume as Independent Favorable Indicator for Fusion Success. World Neurosurg. 2020, 136, e204–e213. [Google Scholar] [CrossRef]
- Zellagui, S.; Hivet, A.; El Mouss, M.; Hambli, R. Prediction of Proximal Femur Fracture Risk from DXA Images Based on Novel Fracture Indexes. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2021, 9, 205–216. [Google Scholar] [CrossRef]
- Holloway, K.L.; Mohebbi, M.; Betson, A.G.; Hans, D.; Hyde, N.K.; Brennan-Olsen, S.L.; Kotowicz, M.A.; Pasco, J.A. Prediction of Major Osteoporotic and Hip Fractures in Australian Men Using FRAX Scores Adjusted with Trabecular Bone Score. Osteoporos. Int. 2018, 29, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Luo, Y.; Yang, L.; Dall’Ara, E.; Eastell, R.; Goertzen, A.L.; McCloskey, E.V.; Leslie, W.D.; Lix, L.M. Comparison of Femoral Strength and Fracture Risk Index Derived from DXA-Based Finite Element Analysis for Stratifying Hip Fracture Risk: A Cross-Sectional Study. Bone 2018, 110, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, H.-J.; Yun, J.-H. Three-Dimensional Microstructure of Human Alveolar Trabecular Bone: A Micro-Computed Tomography Study. J. Periodontal Implant Sci. 2017, 47, 20–29. [Google Scholar] [CrossRef]
- Liu, I.-T.; Liang, F.-W.; Li, C.-C.; Chang, Y.-F.; Sun, Z.-J.; Lu, T.-H.; Chang, C.-S.; Wu, C.-H. Validation of the Taiwan FRAX® Calculator for the Prediction of Fracture Risk. Arch. Osteoporos. 2022, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Lu, J.; Sun, H.; Li, Y. Relationship between Muscle and Subcutaneous Adipose Tissue Size and Density and Proximal Femur Bone in Elderly Women with Hip Fracture. Aging Clin. Exp. Res. 2024, 36, 130. [Google Scholar] [CrossRef]
- Buchwald, T.; Kozielski, M.; Szybowicz, M. Determination of Collagen Fibers Arrangement in Bone Tissue by Using Transformations of Raman Spectra Maps. J. Spectrosc. 2012, 27, 261487. [Google Scholar] [CrossRef]
- Farlay, D.; Falgayrac, G.; Ponçon, C.; Rizzo, S.; Cortet, B.; Chapurlat, R.; Penel, G.; Badoud, I.; Ammann, P.; Boivin, G. Material and Nanomechanical Properties of Bone Structural Units of Cortical and Trabecular Iliac Bone Tissues from Untreated Postmenopausal Osteoporotic Women. Bone Rep. 2022, 17, 101623. [Google Scholar] [CrossRef]
- Zheng, K.; Zhong, J.; Hu, J.; Nebbiolo, E.; Sanchez-Weatherby, J.; Tang, T.; Landis, W.J.; Chen, J.; Winlove, P.; Sherlock, B.E.; et al. Effects of Mineralization on the Hierarchical Organization of Collagen—A Synchrotron X-Ray Scattering and Polarized Second Harmonic Generation Study. Interface Focus 2024, 14, 20230046. [Google Scholar] [CrossRef]
- Mondal, A.; Nguyen, C.; Ma, X.; Elbanna, A.E.; Carlson, J.M. Network Models for Characterization of Trabecular Bone. Phys. Rev. E 2019, 99, 042406. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Chang, Y.-F.; Chen, C.-H.; Lewiecki, E.M.; Wüster, C.; Reid, I.; Tsai, K.-S.; Matsumoto, T.; Mercado-Asis, L.B.; Chan, D.-C.; et al. Consensus Statement on the Use of Bone Turnover Markers for Short-Term Monitoring of Osteoporosis Treatment in the Asia-Pacific Region. J. Clin. Densitom. 2021, 24, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Zeng, X.; Li, Y.; Li, M.; Pu, B.; Zhi, B.; Wang, Y.; Qu, H. A Study on Whether Deep Learning Models Based on CT Images for Bone Density Classification and Prediction Can Be Used for Opportunistic Osteoporosis Screening. Osteoporos. Int. 2024, 35, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Wang, S.; Zhang, J.; Fan, Y.; Liu, Y.; Wei, W. Automatic Osteoporosis Screening System Using Radiomics and Deep Learning from Low-Dose Chest CT Images. Bioengineering 2024, 11, 50. [Google Scholar] [CrossRef]
- Kim, Y.W.; Kim, J.H.; Yoon, S.H.; Lee, J.H.; Lee, C.-H.; Shin, C.S.; Park, Y.S. Vertebral Bone Attenuation on Low-Dose Chest CT: Quantitative Volumetric Analysis for Bone Fragility Assessment. Osteoporos. Int. 2017, 28, 329–338. [Google Scholar] [CrossRef]
- Meertens, R.; Lopez, B.; Crone, B.; Gundry, M.; Metcalfe-Smith, E.; Gibbard, W.; Jubb, T.; Manning, F.; Scott, P.; McWilliam, R. Development of an Opportunistic Diagnostic Prediction Algorithm for Osteoporosis and Fragility Fracture Risk Estimates from Forearm Radiographs (The OFFER1 Study). JBMR Plus 2024, 8, ziae020. [Google Scholar] [CrossRef]
- Hung, W.-C.; Lin, Y.-L.; Cheng, T.-T.; Chin, W.-L.; Tu, L.-T.; Chen, C.-K.; Yang, C.-H.; Wu, C.-H. Establish and Validate the Reliability of Predictive Models in Bone Mineral Density by Deep Learning as Examination Tool for Women. Osteoporos. Int. 2024, 35, 129–141. [Google Scholar] [CrossRef]
- Ong, W.; Liu, R.W.; Makmur, A.; Low, X.Z.; Sng, W.J.; Tan, J.H.; Kumar, N.; Hallinan, J.T.P.D. Artificial Intelligence Applications for Osteoporosis Classification Using Computed Tomography. Bioengineering 2023, 10, 1364. [Google Scholar] [CrossRef]
- New FRAXplus® (Beta Version) Illustrates Potential of Refined Risk Factor Information Entered to the World’s Most Widely Used Fracture Risk Assessment Tool|International Osteoporosis Foundation. Available online: https://www.osteoporosis.foundation/news/new-fraxplusr-beta-version-illustrates-potential-refined-risk-factor-information-entered (accessed on 21 August 2024).
- Schini, M.; Johansson, H.; Harvey, N.C.; Lorentzon, M.; Kanis, J.A.; McCloskey, E.V. An Overview of the Use of the Fracture Risk Assessment Tool (FRAX) in Osteoporosis. J. Endocrinol. Investig. 2024, 47, 501–511. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, J.; Teng, F. Role of Artificial Intelligence in Medical Image Analysis: A Review of Current Trends and Future Directions. J. Med. Biol. Eng. 2024, 44, 231–243. [Google Scholar] [CrossRef]
- Hamzat, A.K.; Murad, M.S.; Kanan, M.; Asmatulu, R. Comparative Analysis of Deep Learning Models for Fracture Detection and Classification in X-Ray Images. Adv. Eng. Tec. Appl. 2024, 13, 109–115. [Google Scholar]
- Popoviciu, M.S.; Kaka, N.; Sethi, Y.; Patel, N.; Chopra, H.; Cavalu, S. Type 1 Diabetes Mellitus and Autoimmune Diseases: A Critical Review of the Association and the Application of Personalized Medicine. J. Pers. Med. 2023, 13, 422. [Google Scholar] [CrossRef]
- Panwar, P.; Lamour, G.; Mackenzie, N.C.W.; Yang, H.; Ko, F.; Li, H.; Brömme, D. Changes in Structural-Mechanical Properties and Degradability of Collagen during Aging-Associated Modifications. J. Biol. Chem. 2015, 290, 23291–23306. [Google Scholar] [CrossRef]



| Tools/Methods | Employed Scales | Predictive Indicators/Parameters | Effectiveness/Strength | Limitations | References |
|---|---|---|---|---|---|
| FEA | Cellular, tissue, organ, and whole scales, etc. | Maximum stress, Maximum strain, etc. | Simulates the complex loading conditions. Predict the entire model. Inexpensive relative to experiments. | FEA makes it more susceptible to misunderstandings, execution, and interpretation mistakes. | [40,41,42,43,44,45,46,47] |
| DXA + FEA | Organ and whole scale, etc. | Bone strain index (BSI) | Provide a better risk assessment of osteoporotic patients. BSI is suitable for irregular and complex structures. | Because of the characteristics of DXA images, the model reduces a 3D problem to 2D, which results in errors from thickness variability. | [48,49,50,51] |
| Panoramic radiography (Radiomorphometric indices) | Tissue/Organ | Mandibular cortical index (MCI), Mental index (MI), Panoramic mandibular index (PMI) | Widely available, low-cost, opportunistic use in dental practice | Limited standardization, variability in observer interpretation | [52,53,54] |
| CT-image based FEA | Tissue, organ, and whole scale, etc. | Stress (Maximum and minimum principal stress), strain (maximum and minimum principal strains), fractures, load, and sites, etc. | Vertebral fracture risk can be more precisely evaluated using new osteoporotic indices. | Routine clinical CT scans have limited resolution and higher noise, reducing the accuracy of bone geometry and material properties in finite element models. | [55,56,57,58,59,60,61,62] |
| µ-CT | Cellular and tissue scale, etc. | Trabecular Thickness (Tb.Th), Trabecular Number (Tb.N), Trabecular Separation, Trabecular Bone Volume Fraction (BV/TV), etc. | High-resolution morphological data are provided by CT. High resolution, relatively low cost, and scanning efficiency. | µ-CT has a low contrast sensitivity. | [63,64] |
| Peripheral quantitative CT (pQCT) and HR-pQCT | Tissue and organ scale, etc. | Volumetric Bone Mineral Density (vBMD), Cortical Thickness, etc. | Easy accessibility. | For the spatial resolution, only a few transversal data are available. | [65,66,67] |
| Biomechanical computed tomography (BCT) | Organ and whole scale, etc. | Hip BMD T-score, Femoral strength, Fragile bone strength, etc. | BCT analysis of routine abdominal or pelvic CT scans is as effective as DXA in identifying patients at high risk of hip fractures (BCT can identify patients at high risk of hip fractures). | BCT accuracy may be limited for scans acquired at lower kVp (e.g., 80 kVp) or reconstructed with sharp/nonstandard kernels. | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ateeq, M.; Vergani, L.M.; Buccino, F. Integration of Multi-Scale Predictive Tools of Bone Fragility: A Structural and Material Property Perspective. Materials 2025, 18, 4639. https://doi.org/10.3390/ma18194639
Ateeq M, Vergani LM, Buccino F. Integration of Multi-Scale Predictive Tools of Bone Fragility: A Structural and Material Property Perspective. Materials. 2025; 18(19):4639. https://doi.org/10.3390/ma18194639
Chicago/Turabian StyleAteeq, Muhammad, Laura Maria Vergani, and Federica Buccino. 2025. "Integration of Multi-Scale Predictive Tools of Bone Fragility: A Structural and Material Property Perspective" Materials 18, no. 19: 4639. https://doi.org/10.3390/ma18194639
APA StyleAteeq, M., Vergani, L. M., & Buccino, F. (2025). Integration of Multi-Scale Predictive Tools of Bone Fragility: A Structural and Material Property Perspective. Materials, 18(19), 4639. https://doi.org/10.3390/ma18194639








