Self-Assembled Monolayers of Various Alkyl-Phosphonic Acids on Bioactive FHA Coating for Improving Surface Stability and Corrosion Resistance of Biodegradable AZ91D Mg Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Hydrothermal FHA Coatings and the SAM Treatment
2.2. Microstructure Characterization and Analysis
2.3. Electrochemical Corrosion Behavior and the Immersion Tests
3. Results and Discussion
3.1. Microstructural Features and Phase Compositions
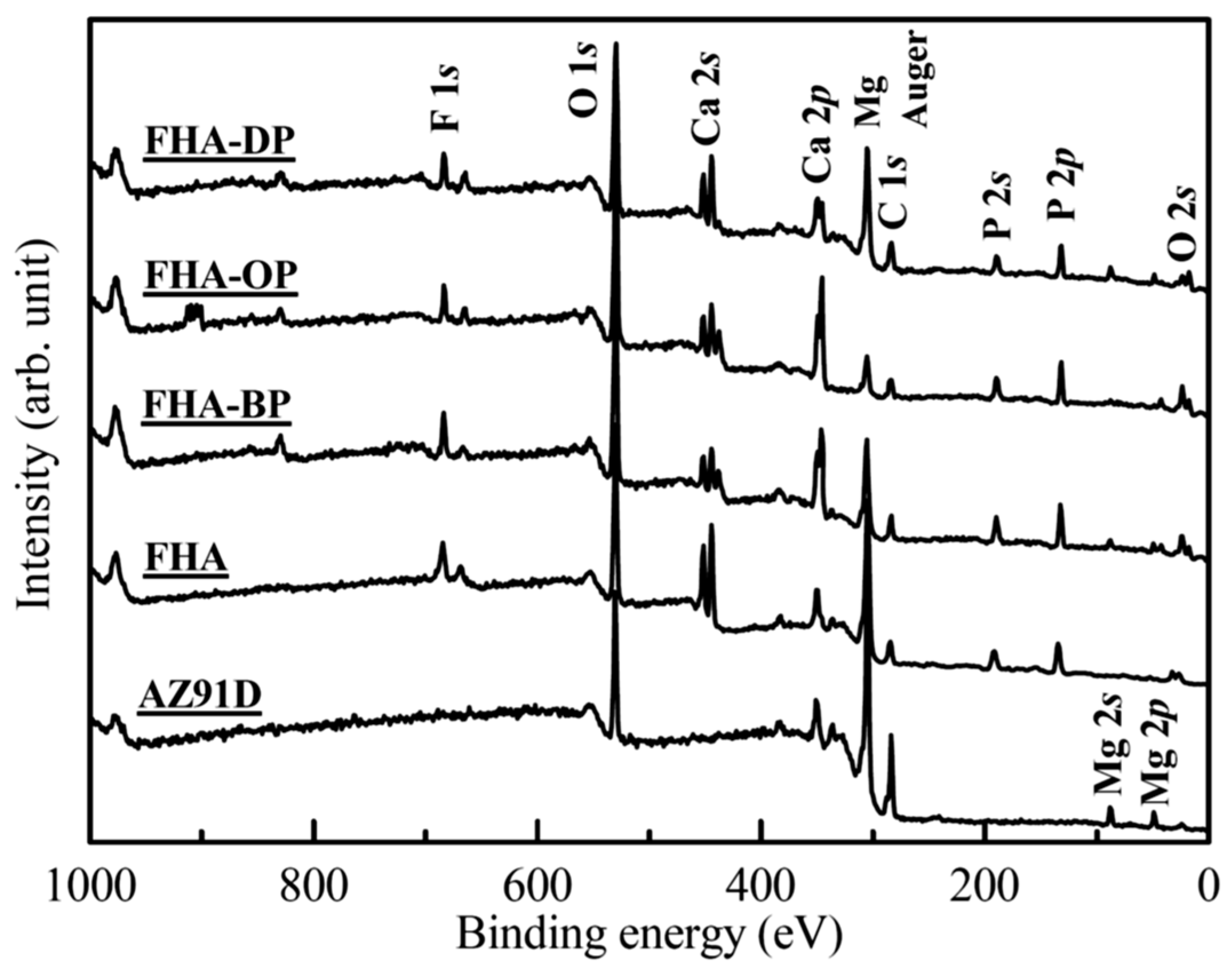
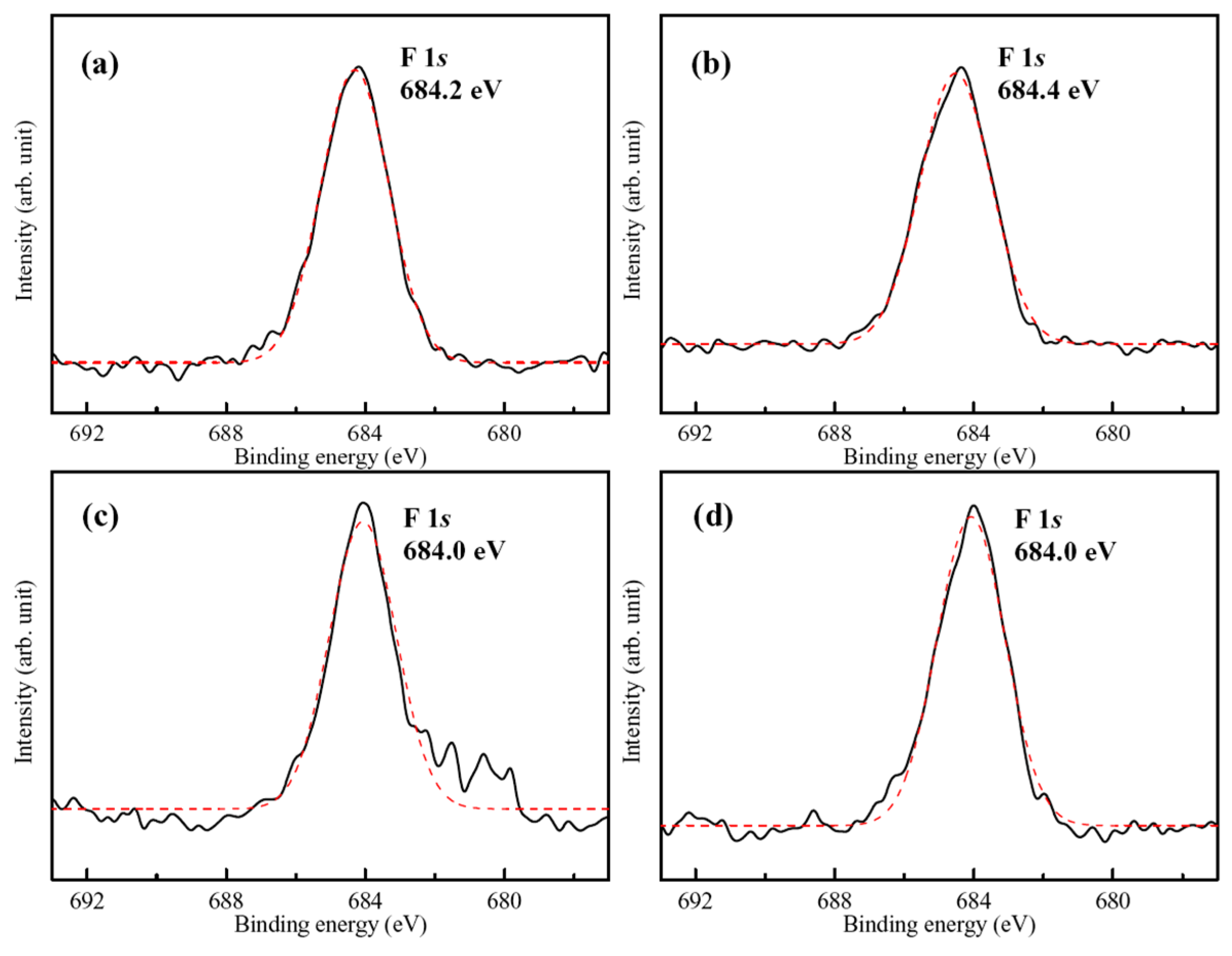
3.2. XPS Analysis
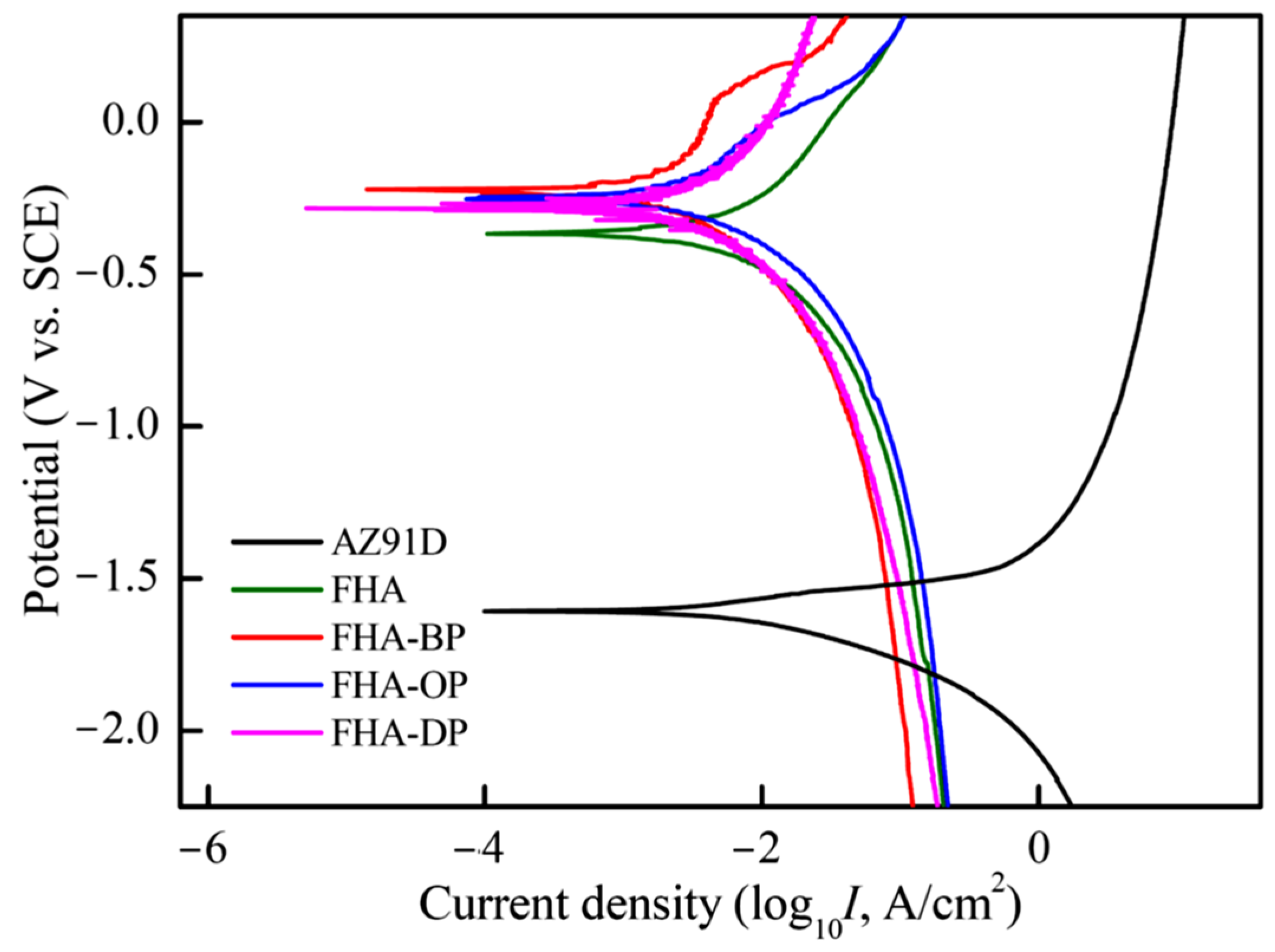
3.3. Electrochemical Performances and Dissolution Behaviors in DMEM
4. Conclusions
- (1)
- The FHA coating exhibits a super-hydrophilic surface chemical state with a static contact angle of less than 2° for the DMEM solution.
- (2)
- The static contact angle of FHA coating surface is significantly increased after applying the SAM treatment with different alkyl chains of alkyl-phosphonic acids. SAM-treated FHA-coated AZ91D Mg alloy obviously display a stable surface chemistry with highly hydrophobicity resulted from the methyl terminal groups (–CH3) of the alkyl chains.
- (3)
- XPS analysis results demonstrate that organic and hydrophobic 1-butylphosphonic (BP), 1-octylphosphonic (OP), and dodecylphosphate (DP) acids self-assembled monolayers (SAM) can be directly grafted to the hydrothermally synthesized FHA through the T-BAG method without affecting FHA surface morphologies.
- (4)
- Both of bioactive FHA coating and SAM-treated FHA-BP, FHA-OP, and FHA-DP specimens significantly enhances the electrochemical corrosion resistance of AZ91D alloy in the DMEM solution.
- (5)
- As a result of the immersion tests in the DMEM solution, the self-assembled various alkyl chains of alkyl-phosphonic acids hydrophobic monolayer on the FHA coating surface can inhibit the penetrative corrosion effect and decrease the weight loss after immersion. Longer alkyl-chains of the dodecylphosphonic acid (DP) monolayer display superior effect on improving corrosion resistance in the DMEM solution.
- (6)
- Hydrophobic SAM-treated FHA surface can provide better corrosion resistance than the hydrophilic raw FHA-coated AZ91D specimens, which will be beneficial for cell attachment and proliferation in the further cell culture experiments.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Waizy, H.; Seitz, J.M.; Reifenrath, J.; Weizbauer, A.; Bach, F.W.; Meyer-Lindenberg, A.; Denkena, B.; Windhagen, H. Biodegradable magnesium implants for orthopedic applications. J. Mater. Sci. 2013, 48, 39–50. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Bai, J.; Yang, Y.; Wang, Y.; Zhou, Y.; Jiang, W.; Wang, J.; Zhu, J.; Zhu, C.; et al. Magnesium-based biomaterials for coordinated tissue repair: A comprehensive overview of design strategies, advantages, and challenges. J. Magnes. Alloys 2024, 12, 3025–3061. [Google Scholar] [CrossRef]
- Persaud-Sharma, D.; McGoron, A. Biodegradable magnesium alloys: A review of material development and applications. J. Biomim. Biomater. Tissue Eng. 2012, 12, 25–39. [Google Scholar] [CrossRef]
- Uppal, G.; Thakur, A.; Chauhan, A.; Bala, S. Magnesium based implants for functional bone tissue regeneration—A review. J. Magnes. Alloys 2022, 10, 356–386. [Google Scholar] [CrossRef]
- He, M.; Chen, L.; Ying, M.; Xu, S.; Liang, Z. Review on magnesium and magnesium-based alloys as biomaterials for bone immobilization. J. Mater. Res. Technol. 2023, 23, 4396–4419. [Google Scholar] [CrossRef]
- Kumar, G.; Preetam, S.; Pandey, A.; Birbilis, N.; Al-Saadi, S.; Pasbakhsh, P.; Zheludkevich, M.; Balan, P. Advances in magnesium-based bioresorbable cardiovascular stents: Surface engineering and clinical prospects. J. Magnes. Alloys 2025, 13, 948–981. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Q.; Shao, H.; Shan, Z.; Xu, J.; Tong, W.; Wong, R.M.Y.; Qin, L. Magnesium as an emerging bioactive material for orthopedic applications: Beside needs lead the way from innovation to clinical translation. Regen. Biomater. 2025, 12, rbaf032. [Google Scholar] [CrossRef]
- Mathieu, S.; Rapin, C.; Hazan, J.; Steinmetz, P. Corrosion behaviour of high pressure die-cast and semi-solid cast AZ91D alloys. Corro. Sci. 2002, 44, 2737–2756. [Google Scholar] [CrossRef]
- Ding, Y.; Wen, C.; Hodgson, P.; Li, Y. Effect of alloying elements on the corrosion behavior and biocompatibility of degradable magnesium alloys: A review. J. Mater. Chem. B 2014, 2, 1912–1933. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef]
- Zhao, S.; Tayyebi, M.; Yarigarravesh, M.; Hu, G. A review of magnesium corrosion in bio-applications: Mechanism, classfication, modeling, in-vitro, and in vivo experimental testing, and tailoring Mg corrosion rate. J. Mater. Sci. 2023, 58, 12158–12181. [Google Scholar] [CrossRef]
- Martinez, D.C.; Borkam-Schuster, A.; Helmholz, H.; Dobkowska, A.; Luthringer-Feyerabend, B.; Płocinski, T.; Willumeit-Römer, R.; Swieszkowski, W. Bone cells influence the degradation interface of pure Mg and WE43 materials: Insights from multimodal in vitro analysis. Acta Biomater. 2024, 187, 471–490. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Gilbert, J.L.; Lv, W.W.; Du, P.; Pan, H. Reduction reactions dominate the interactions between Mg alloys and cells: Understanding the mechanisms. Bioact. Mater. 2025, 45, 363–387. [Google Scholar] [CrossRef]
- Schwartz, Z.; Boyan, B.D. Underlying mechanisms at the bone-biomaterial interface. J. Cell. Biochem. 1994, 56, 340–347. [Google Scholar] [CrossRef]
- Yin, Z.Z.; Qi, W.C.; Zeng, R.C.; Chen, X.B.; Gu, C.D.; Guan, S.K.; Zheng, Y.F. Advances in coatings on biodegradable magnesium alloys. J. Magnes. Alloys 2020, 8, 42–65. [Google Scholar] [CrossRef]
- Guo, X.; Hu, Y.; Yuan, K.; Qiao, Y. Review of the effect of surface coating modification on magnesium alloy biocompatibility. Materials 2022, 15, 3291. [Google Scholar] [CrossRef]
- Chai, H.; Guo, L.; Wang, X.; Gao, X.; Liu, K.; Fu, Y.; Guan, J.; Tan, L.; Yang, K. In vitro and in vivo evaluations on osteogenesis and biodegradability of a β-tricalcium phosphate coated magnesium alloy. J. Biomed. Mater. Res. A 2012, 100, 293–304. [Google Scholar] [CrossRef]
- Li, M.; He, P.; Wu, Y.; Zhang, Y.; Xia, H.; Zheng, Y.; Han, Y. Stimulatory effects of the degradation products from Mg-Ca-Sr alloy on the osteogenesis through regulating ERK signaling pathway. Sci. Rep. 2016, 6, 32323. [Google Scholar] [CrossRef] [PubMed]
- Chandra, D.; Chauhan, N.R. Surface protective coatings on Mg alloys—A review. J. Matpr. 2021, 47, 3819–3822. [Google Scholar] [CrossRef]
- Johari, N.A.; Alias, J.; Zanurin, A.; Mohamed, N.S.; Alang, N.A.; Zain, M.Z.M. Anti-corrosive coatings of magnesium: A review. J. Matpr. 2022, 48, 1842–1848. [Google Scholar] [CrossRef]
- Chang, L.; Tian, L.; Liu, W.; Duan, X. Formation of dicalcium phosphate dihydrate on magnesium alloy by micro-arc oxidation coupled with hydrothermal treatment. Corros. Sci. 2013, 72, 118–124. [Google Scholar] [CrossRef]
- Guo, Y.; Su, Y.; Gu, R.; Zhang, Z.; Li, G.; Lian, J.; Ren, L. Enhanced corrosion resistance and biocompatibility of biodegradable magnesium alloy modified by calcium phosphate/collagen coating. Surf. Coat. Technol. 2020, 401, 126318. [Google Scholar] [CrossRef]
- Wang, T.; Jia, S.; Xu, Y.; Dong, Y.; Guo, Y.; Huang, Z.; Li, G.; Lian, J. Improving the corrosion resistance and biocompatibility of magnesium alloy via composite coatings of calcium phosphate/carbonate induced by silane. Prog. Org. Coat. 2022, 163, 106653. [Google Scholar] [CrossRef]
- Drevet, R.; Faure, J.; Benhayoune, H. Bioactive calcium phosphate coatings for bone implant applications: A review. Coatings 2023, 13, 1091. [Google Scholar] [CrossRef]
- Yao, W.; Tan, Y.; Lu, Q.; Yi, H.; Cheng, C.; Wu, L.; Saji, V.S.; Pan, F. Recent advances in protective coatings and surface modifications for corrosion protection of Mg alloys. J. Mater. Res. Technol. 2024, 31, 3238–3254. [Google Scholar] [CrossRef]
- Gao, J.; Su, Y.; Qin, Y.X. Calcium phosphate coatings enhance biocompatibility and degradation resistance of magnesium alloy: Correlating in vitro and in vivo studies. Bioact. Mater. 2021, 6, 1223–1229. [Google Scholar] [CrossRef]
- Shen, S.; Cai, S.; Bao, X.; Xu, P.; Li, Y.; Jiang, S.; Xu, G. Biomimetic fluoridated hydroxyapatite coating with micro/nano-topography on magnesium alloy for orthopaedic application. Chem. Eng. J. 2018, 339, 7–13. [Google Scholar] [CrossRef]
- Yilmaz, B.; Alshemary, A.Z.; Evis, Z. Co-doped hydroxyapatites as potential materials for biomedical applications. Microchem. J. 2019, 144, 443–453. [Google Scholar] [CrossRef]
- Kubiak-Mihkelsoo, Z.; Kostrzebska, A.; Blaszczyszyn, A.; Pitulaj, A.; Dominiak, M.; Gedrange, T.; Nawrot-Hadzik, I.; Matys, J.; Hadzik, J. Ionic doping of hydroxyapatite for bone regeneration: Advances in structure and properties over two decades—A narrative review. Appl. Sci. 2025, 15, 1108. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Tayebi, L.; Vashaee, D. Biodegradable magnesium bone implants coated with a novel bioceramic nanocomposite. Materials 2020, 13, 1315. [Google Scholar] [CrossRef]
- Wang, S.H.; Yang, C.W.; Lee, T.M. Evaluation of microstructural features and in vitro biocompatibility of hydrothermally coated fluorohydroxyapatite on AZ80 Mg alloy. Ind. Eng. Chem. Res. 2016, 55, 5207–5215. [Google Scholar] [CrossRef]
- Wang, S.H.; Lee, S.P.; Yang, C.W.; Lo, C.M. Surface modification of biodegradable Mg-based scaffolds for human mesenchymal stem cell proliferation and osteogenic differentiation. Materials 2021, 14, 441. [Google Scholar] [CrossRef] [PubMed]
- Toworfe, G.K.; Bhattacharyya, S.; Composto, R.J.; Adams, C.S.; Shapiro, I.M.; Ducheyne, P. Effect of functional end groups of silane self assembled monolayer surfaces on apatite formation, fibronectin adsorption and osteoblast cell function. J. Tissue Eng. Regen. Med. 2009, 3, 26–36. [Google Scholar] [CrossRef]
- Torres, N.; Oh, S.; Appleford, M.; Dean, D.D.; Jorgensen, J.H.; Ong, J.L.; Agrawal, C.M.; Mani, G. Stability of antibacterial self-assembled monolayers on hydroxyapatite. Acta Biomater. 2010, 6, 3242–3255. [Google Scholar] [CrossRef] [PubMed]
- Nakaoka, R.; Yamakoshi, Y.; Isama, K.; Tsuchiya, T. Effects of surface chemistry prepared by self-assembled monolayers on osteoblast behavior. J. Biomed. Mater. Res. A 2010, 94, 524–532. [Google Scholar] [CrossRef]
- Mahapatro, A.; Matos Negrón, T.D.; Bonner, C.; Abdel-Fattah, T.M. Nanolayers on magnesium (Mg) alloy for metallic bone tissue engineering scaffolds. J. Biomater. Tissue Eng. 2013, 3, 196–204. [Google Scholar] [CrossRef]
- Mahapatro, A. Bio-functional nano-coatings on metallic biomaterials. Mater. Sci. Eng. C 2015, 55, 227–251. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. II. Liquids. J. Am. Chem. Soc. 1917, 39, 1848–1906. [Google Scholar] [CrossRef]
- Hanson, E.L.; Schwartz, J.; Nickel, B.; Koch, N.; Danisman, M.F. Bonding self-assembled, compact organophosphonate monolayers to the native oxide surface of silicon. J. Am. Chem. Soc. 2003, 125, 16074–16080. [Google Scholar] [CrossRef]
- Ishizaki, T.; Teshima, K.; Masuda, Y.; Sakamoto, M. Liquid phase formation of alkyl- and perfluoro-phosphonic acid derived monolayers on magnesium alloy AZ31 and their chemical properties. J. Colloid. Interf. Sci. 2011, 360, 280–288. [Google Scholar] [CrossRef]
- Ishizaki, T.; Okido, M.; Masuda, Y.; Saito, N.; Sakamoto, M. Corrosion resistant performances of alkanoic and phosphonic acids derived self-assembled monolayers on magnesium alloy AZ31 by vapor-phase method. Langmuir 2011, 27, 6009–6017. [Google Scholar] [CrossRef]
- Mrksich, M.; Whitesides, G.M. Using self-assembled monolayers to understand the interactions of man-made surfaces with proteins and cells. Annu. Rev. Biophys. Biomol. Struct. 1996, 25, 55–78. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.W.; Liu, C.; Lin, D.J.; Yeh, M.L.; Lee, T.M. Hydrothermal treatment and butylphosphonic acid derived self-assembled monolayres for improving the surface chemistry and corrosion resistance of AZ61 magnesium alloy. Sci. Rep. 2017, 7, 16910. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Nanosized and nanocrystalline calcium orthophosphates. Acta Biomater. 2010, 6, 715–734. [Google Scholar] [CrossRef] [PubMed]
- Hoveidaei, A.H.; Sadat-Shojai, M.; Mosalamiaghili, S.; Salarikia, S.R.; Roghani-shahraki, H.; Ghaderpanah, R.; Ersi, M.H.; Conway, J.D. Nano-hydroxyapatite structures for bone regenerative medicine: Cell-material interaction. Bone 2024, 179, 116956. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wang, G.; Feng, L.; Liu, B.; Ma, Y.; Jiang, L.; Zhu, D. Reversible switching between superhydrophilicity and superhydrophobicity. Angew. Chem. Int. Ed. 2004, 43, 357–360. [Google Scholar] [CrossRef]
- Vishnu, J.; Manivasagam, V.K.; Gopal, V.; Garcia, C.B.; Hameed, P.; Manivasagam, G.; Webster, T.J. Hydrothermal treatment of etched titanium: A potential surface nano-modification technique for enhanced biocompatibility. Nanomed. NBM 2019, 20, 102016. [Google Scholar] [CrossRef]
- Yang, C.W.; Lui, T.S. Kinetics of hydrothermal crystallization under saturated steam pressure and the self-healing effect by nanocrystallite for hydroxyapatite coatings. Acta Biomater. 2009, 5, 2728–2737. [Google Scholar] [CrossRef]
- Yang, C.W.; Wang, G.K. Effect of hydrothermal (Sr)-hydroxyapatite coatings on the corrosion resistance and Mg2+ ion release to enhance osteoblastic cell responses of AZ91D alloy. Materials 2020, 13, 591. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, D.; Liu, Q.; Yin, X.; Zhang, Y.; Jing, X.; Zhang, M. Fabrication of hydrophobic surface with hierarchical structure on Mg alloy and its corrosion resistance. Electrochim. Acta 2010, 55, 6897–6906. [Google Scholar] [CrossRef]
- Zhang, L.C.; Xu, M.; Hu, Y.D.; Gao, F.; Gong, T.; Liu, T.; Li, X.; Pan, C.J. Biofunctionization of biodegradable magnesium alloy to improve the in vitro corrosion resistance and biocompatibility. Appl. Surf. Sci. 2018, 451, 20–31. [Google Scholar] [CrossRef]






| Na+ | Cl− | K+ | Ca2+ | Mg2+ | HCO3− | HPO42− | SO42− | pH | |
|---|---|---|---|---|---|---|---|---|---|
| DMEM | 155.3 | 115.7 | 5.3 | 1.8 | 0.8 | 44.1 | 0.9 | 0.8 | 7.4 |
| Blood plasma | 142 | 103 | 5 | 2.5 | 1.5 | 27 | 1 | 0.5 | 7.35–7.45 |
| FHA | FHA-BP | FHA-OP | FHA-DP | |
|---|---|---|---|---|
| Ra (μm) † | 7.3 ± 0.2 | 7.1 ± 0.7 | 6.7 ± 0.6 | 6.9 ± 0.3 |
| Static contact angle (δ) | <2° | 90° | 88° | 86° |
| Ecorr. (V vs. SCE) | Icorr. (μA/cm2) | Rp (kΩ cm2) | Pi (mm/year) | |
|---|---|---|---|---|
| AZ91D | –1.61 | 98.3 | 0.66 | 2.25 |
| FHA | –0.37 | 3.80 | 17.04 | 0.09 |
| FHA-BP | –0.23 | 1.62 | 48.36 | 0.04 |
| FHA-OP | –0.25 | 1.97 | 32.58 | 0.05 |
| FHA-DP | –0.28 | 0.51 | 136.3 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-W.; Li, P.-H. Self-Assembled Monolayers of Various Alkyl-Phosphonic Acids on Bioactive FHA Coating for Improving Surface Stability and Corrosion Resistance of Biodegradable AZ91D Mg Alloy. Materials 2025, 18, 4633. https://doi.org/10.3390/ma18194633
Yang C-W, Li P-H. Self-Assembled Monolayers of Various Alkyl-Phosphonic Acids on Bioactive FHA Coating for Improving Surface Stability and Corrosion Resistance of Biodegradable AZ91D Mg Alloy. Materials. 2025; 18(19):4633. https://doi.org/10.3390/ma18194633
Chicago/Turabian StyleYang, Chung-Wei, and Peng-Hsiu Li. 2025. "Self-Assembled Monolayers of Various Alkyl-Phosphonic Acids on Bioactive FHA Coating for Improving Surface Stability and Corrosion Resistance of Biodegradable AZ91D Mg Alloy" Materials 18, no. 19: 4633. https://doi.org/10.3390/ma18194633
APA StyleYang, C.-W., & Li, P.-H. (2025). Self-Assembled Monolayers of Various Alkyl-Phosphonic Acids on Bioactive FHA Coating for Improving Surface Stability and Corrosion Resistance of Biodegradable AZ91D Mg Alloy. Materials, 18(19), 4633. https://doi.org/10.3390/ma18194633






