3.3.2. Condensate
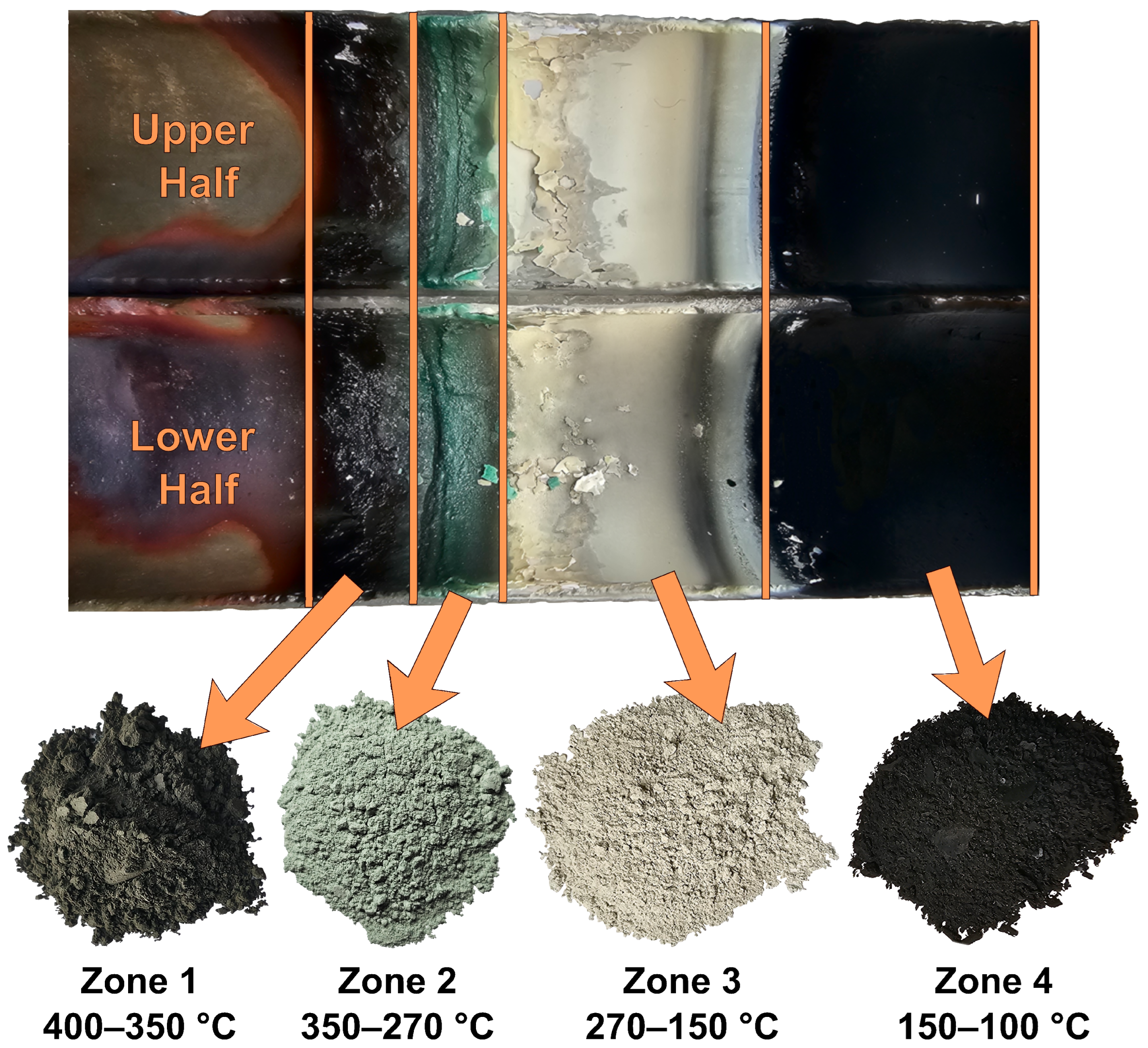
Condensate deposition occurred in the “cold” part of the reactor within the temperature range of 100 to 400 °C. Several deposition zones with different colors were observed on the condenser (
Figure 7) by the end of the process. Black condensate was deposited in the hot condensation zone (400–350 °C). As the temperature decreased, zones of dark green (350–270 °C) and beige (270–150 °C) were observed. Fine black condensate was deposited in the final condensation zone (150–100 °C).
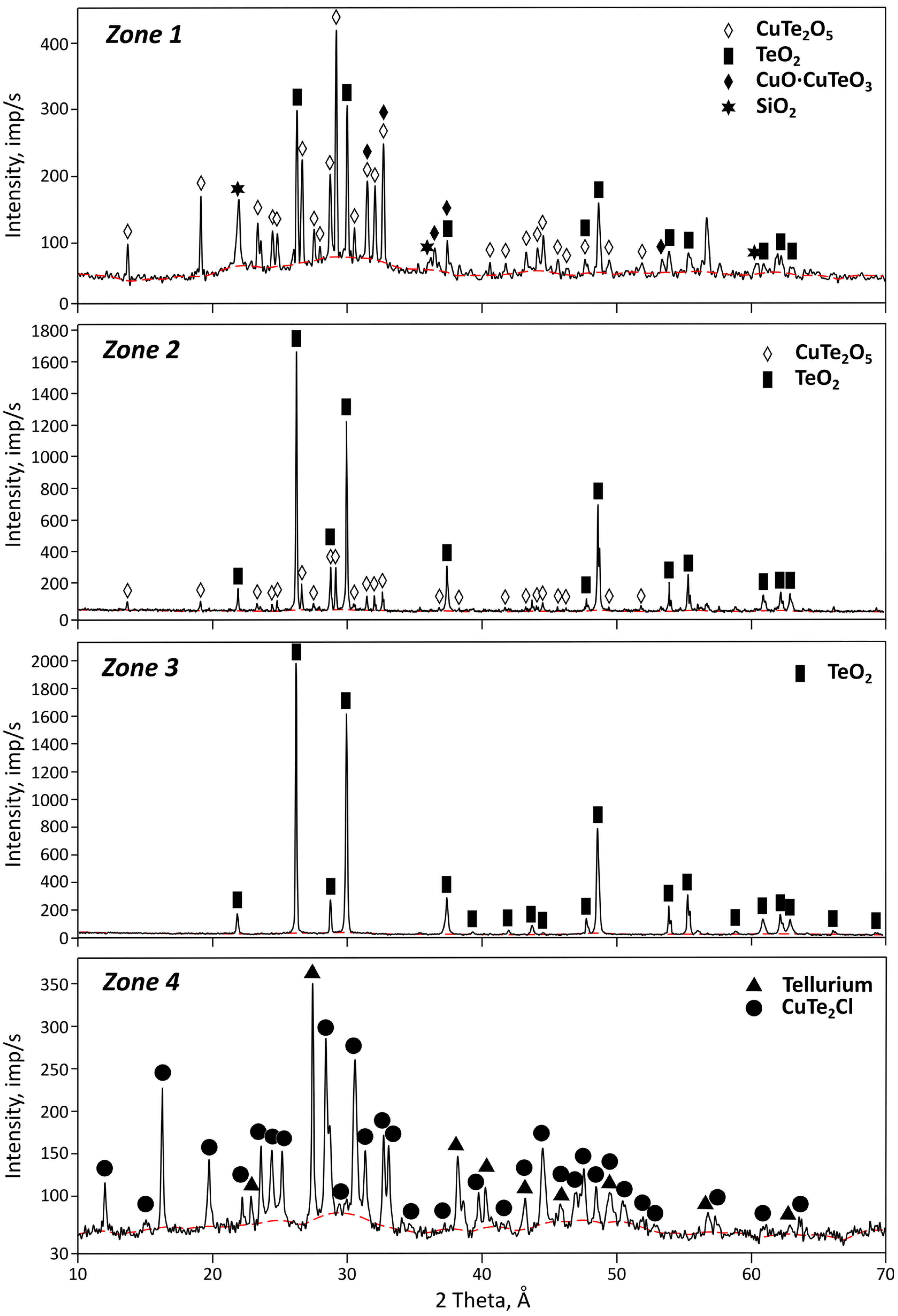
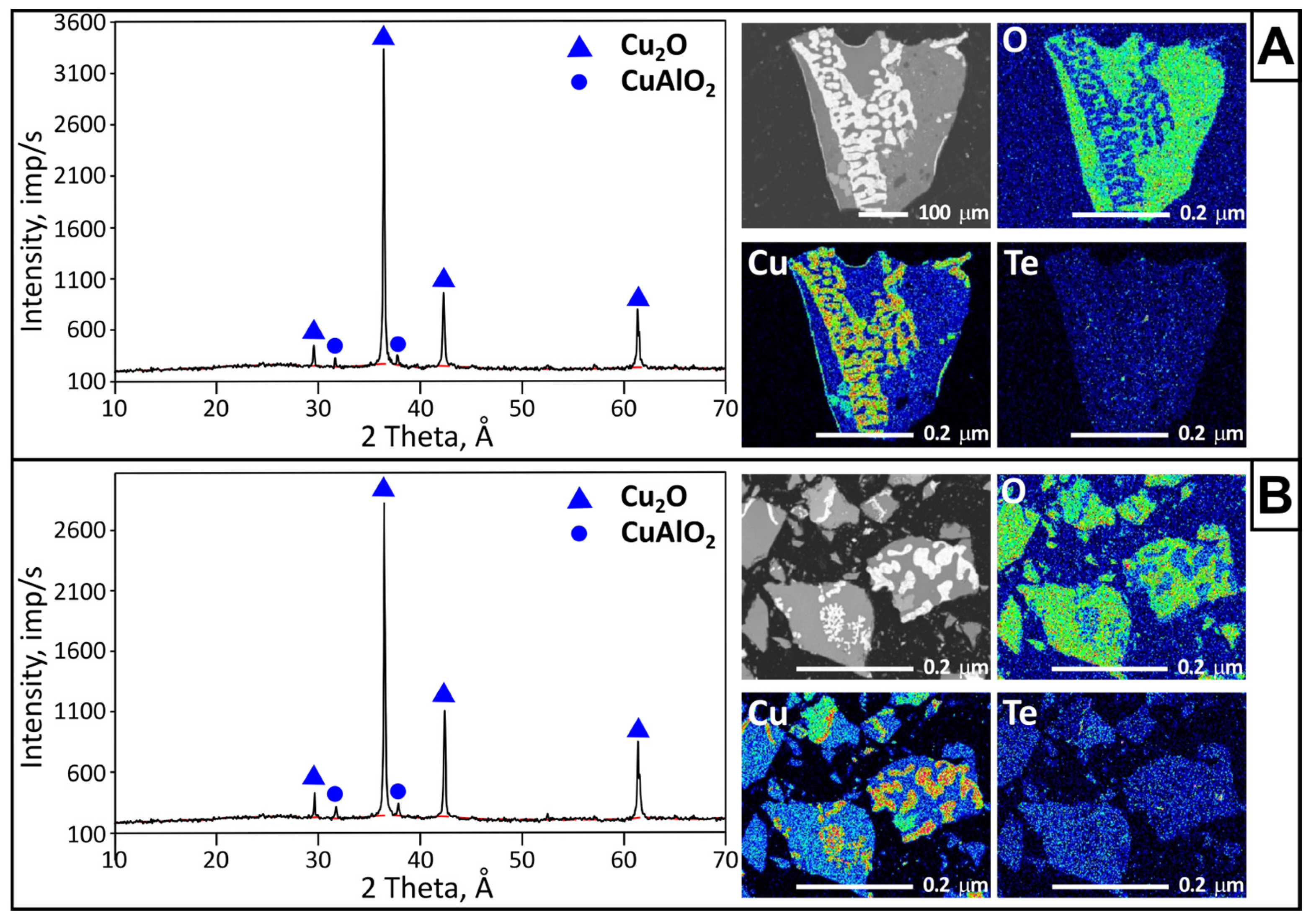
According to the X-ray phase analysis results, the overall condensate sample consisted of the following phases: TeO2 (PDF 01-074-0269)—60%; Te3Cu2O7 (PDF 00-035-1083)—24%; and CuTe2O5 (PDF 00-033-0494)—16%. X-ray fluorescence analysis showed that the main elemental composition (wt.%) was Te—62.066; Cu—8.449; and O—24.887. Silicon (3.022 wt.%) was also present, introduced during sample collection from the condenser surface. Minor amounts (up to 0.4 wt.%) of aluminum, sulfur, chlorine, potassium, chromium, selenium, and arsenic were detected.
For a more detailed analysis, condensate samples were collected from four zones separated by color. The samples were subjected to X-ray phase analysis (
Table 3,
Figure 8), X-ray fluorescence analysis (
Table 4), and electron probe microanalysis (
Figure 9,
Figure 10,
Figure 11 and
Figure 12,
Table 5,
Table 6,
Table 7 and
Table 8). During interpretation of the point EDS analysis results, the presence of carbon was disregarded, as it is related to sample preparation.
Figure 8.
X-ray diffractograms of condensates collected from different temperature zones.
Figure 8.
X-ray diffractograms of condensates collected from different temperature zones.
Table 3.
Phase composition of the condensates by condensation zone.
Table 3.
Phase composition of the condensates by condensation zone.
| Zone | Deposition Temperature, °C | CuTe2O5 | TeO2 | CuO·CuTeO3 | SiO2 | CuTe2Cl | Te |
|---|
| 1 | 400–350 | 54.8 | 19.5 | 16.6 | 9.1 | – | – |
| 2 | 350–270 | 57.7 | 42.3 | – | – | – | – |
| 3 | 270–150 | – | 100 | – | – | – | – |
| 4 | 150–100 | – | – | – | – | 85.5 | 14.5 |
As can be seen from the presented data, the condensate from the first zone consists of phases of tellurium-containing compounds (CuTe
2O
5, TeO
2, and CuO·CuTeO
3) and silica. According to [
35], evaporation of silicon oxide is possible only at temperatures above 1700 °C. Therefore, its presence in the condensate as a result of evaporation can be excluded. Its presence in the sample is instead attributed to the collection of condensate from the surface of the quartz condenser. Direct evaporation of CuTe
2O
5 and CuO·CuTeO
3 at the experimental temperature is also unlikely, as these compounds are thermally unstable and decompose with the release of volatile TeO
2 at lower temperatures. Thus, the presence of tellurites in the condensate can be explained by the intensive evaporation of tellurium dioxide, which mechanically entrains copper oxide molecules. Their deposition occurs together with tellurium oxide in the cold part of the reactor, where secondary formation of the discussed phases is possible.
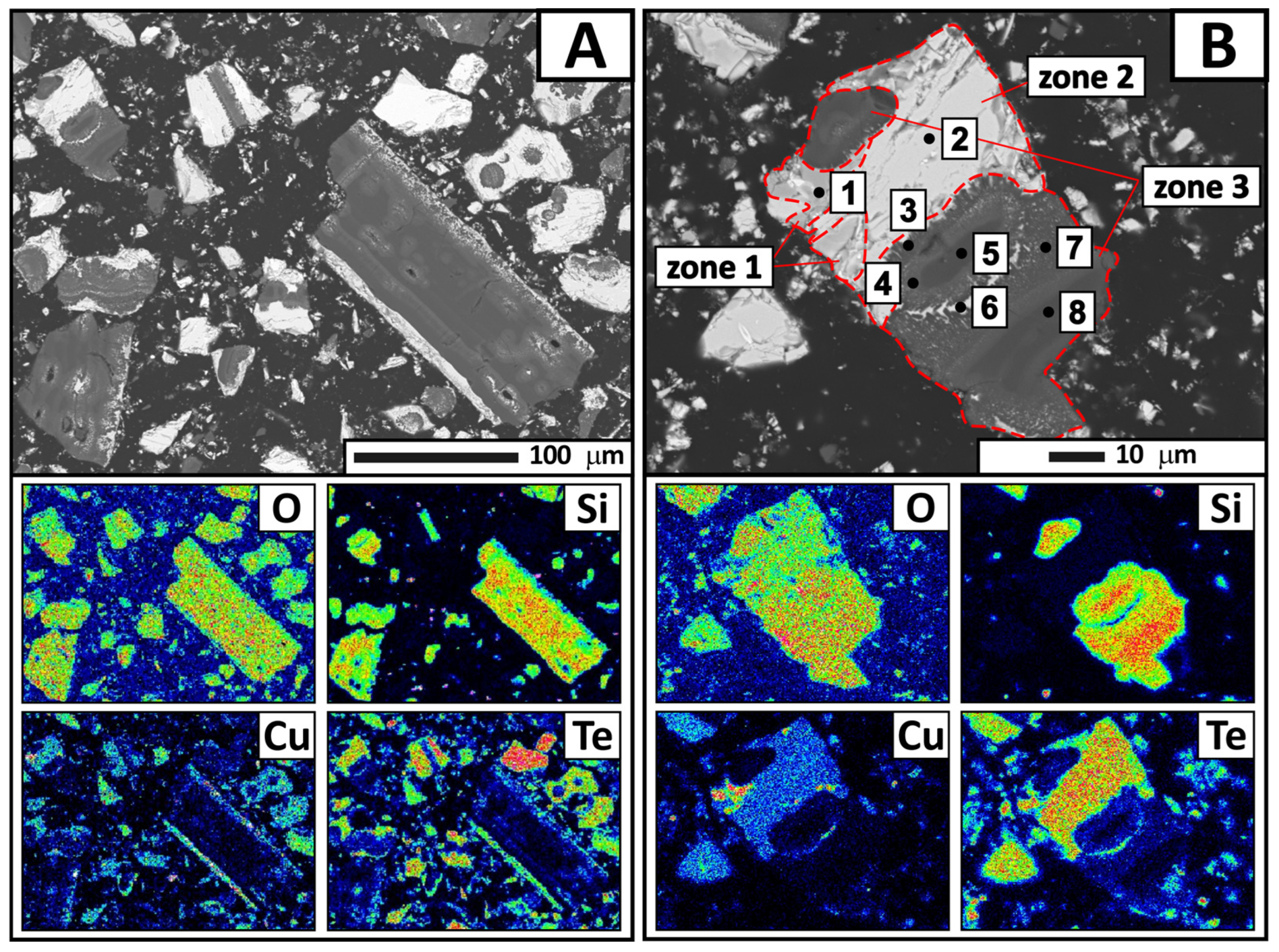
The electron probe microanalysis results revealed individual condensate grains with heterogeneous microstructure (
Figure 9). As shown in the figure, a grain consists of three main zones: light gray (zone 2) and gray (zone 1) zones with a cracked structure, as well as a dark gray zone (zone 3) exhibiting a needle-like structure at the interface with the gray zone.
Point EDS analysis was performed to determine the chemical composition of these specific areas in detail. The results (
Table 5) showed that the gray zone corresponds to copper oxide (point 1). The light gray zone contains a significant amount of tellurium (65.02 wt.%), along with copper and oxygen (point 2). This composition indicates the presence of a complex system. The empirical formula calculation yielded a compound of CuTe
2.25O
5.62, which is close to CuTe
2O
5. The dark gray region consists of oxygen, silicon, tellurium, and copper in varying proportions (points 3–5 and 7–8). The tellurium content ranges from 0.66 to 10.99 wt.%, decreasing as the analysis points move away from the boundaries of the region. It appears that gaseous tellurium-containing compounds interact with amorphous quartz (quartz glass) at condensation temperatures of 350–400 °C. This process is likely accompanied by surface migration of tellurium oxide into the condenser material. Cracking and delamination of the quartz surface layer occur during subsequent crystallization of CuTe
2O
5. These processes are apparently responsible for the quartz impurity in the tellurium-containing condensate.
According to the X-ray phase analysis results, the second condensation zone consists of CuTe
2O
5 and TeO
2 phases, with tellurium oxide being the dominant phase. The X-ray fluorescence analysis revealed a significant increase in tellurium content (up to 65.72 wt.%) and a decrease in copper and quartz (down to 5.54 and 0.36 wt.%, respectively). The electron probe analysis results confirmed the X-ray phase analysis data and provided additional information on the presence of quartz (
Figure 10,
Table 6). CuTe
2O
5 occurs both as small inclusions at the boundaries of tellurium oxide grains and as larger isolated formations.
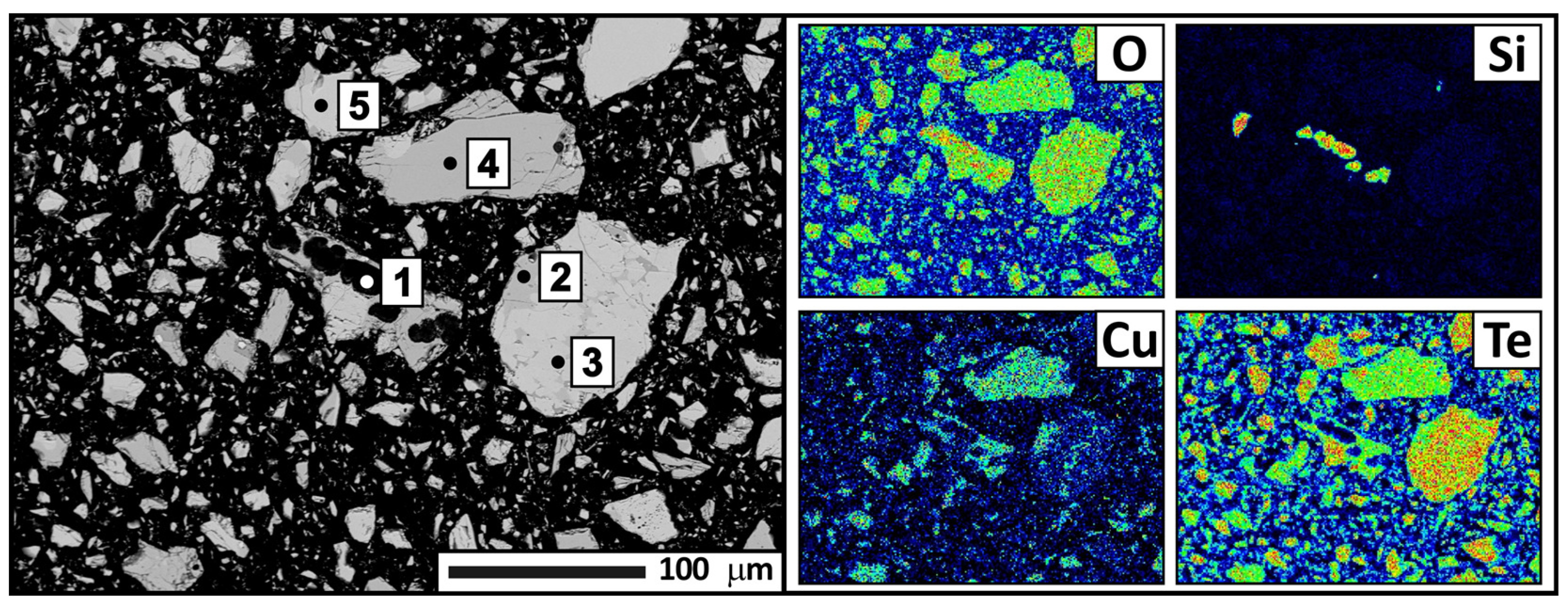
The third zone is represented by a single phase of tellurium oxide with a small amount of copper impurity. The copper content decreases to 0.58 wt.%, while the oxygen and tellurium contents remain almost unchanged (27.058 and 67.202 wt.%, respectively). The sample is relatively homogeneous, with pores and voids of various shapes and sizes observed in the structure (
Figure 11,
Table 7). Small, round pores are fairly evenly distributed, while larger elongated pores are grouped in straight lines. This defect is associated with layer-by-layer condensation of tellurium oxide during sample preparation and likely resulted from shrinkage of the condensate film upon cooling.
The condensate from the fourth zone is a fine powder, easily detached from the condenser surface. The X-ray phase analysis data indicate that the material mainly contains the CuTe
2Cl phase with a small amount of tellurium. However, elemental tellurium was not detected in subsequent SEM analysis, likely due to its low content. The main elements forming the condensate are tellurium (35.16 wt.%), copper (23.03 wt.%), oxygen (9.46 wt.%), and chlorine (7.49 wt.%). Electron probe analysis of individual condensate fragments (
Figure 12,
Table 8) showed that, in addition to the main elements, trace elements from the original sample are also deposited in this zone. The presence of copper in the condensate is most likely associated with the intensive evaporation of volatile tellurium tetrachloride.
Based on the analysis, the following mechanism for the formation of tellurium-containing condensate can be hypothesized. Tellurium chloride and elemental tellurium are initially rapidly evaporated from tellurium-containing middlings. Copper or its chloride compounds are then drawn into the vapor-gas phase, and their combined condensation at low temperatures (150–100 °C) leads to formation of the chlorine-containing CuTe2Cl phase. As phase transformations occur in the tellurium-containing middlings, tellurium dioxide is released, and its rapid evaporation is accompanied by the capture of copper oxide molecules. As the vapor-gas phase passes through the first (400–350 °C) and second (350–270 °C) temperature condensation zones, they co-precipitate to form CuTe2O5 and CuO CuTeO3 in the solid phase: Te2O + CuO = CuTe2O5 and Te2O + CuO = CuO CuTeO3. In the third zone (270-150 °C), residual traces of copper are detected, and the condensate is represented by a single phase of TeO2, indicating complete precipitation of copper oxide in the higher-temperature condensation zones.