Research on Chromium-Free Passivation and Corrosion Performance of Pure Copper
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Passivation Formulations
2.2. Pre-Treatment and Passivation Methods
2.3. Testing and Characterization Instruments
2.4. Testing Methods
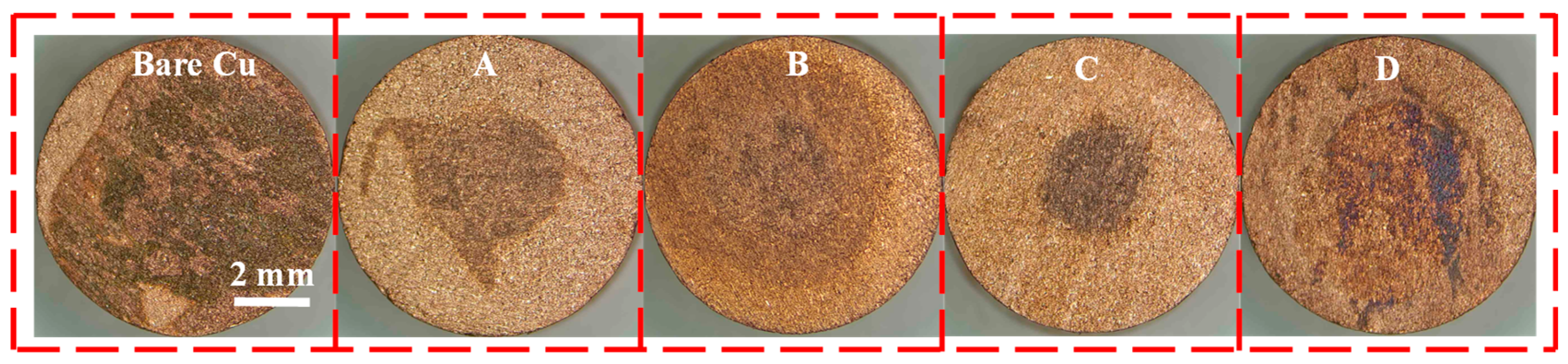
2.4.1. Nitric Acid Drop Test
2.4.2. Electrochemical Measurements
2.4.3. Salt-Spray Immersion Weight-Loss Test
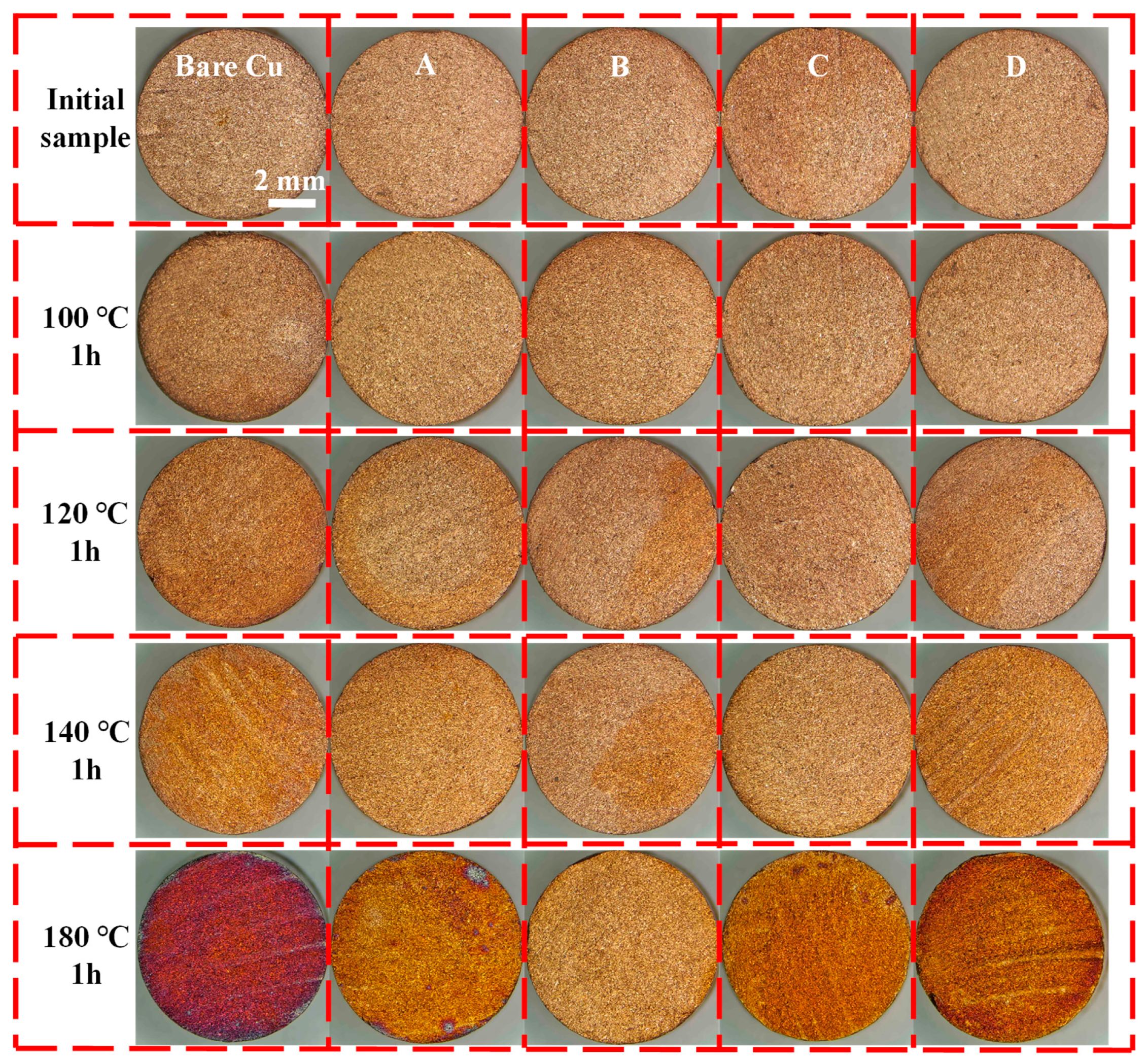
2.4.4. High-Temperature Oxidation Resistance Test
3. Results and Discussion
3.1. Corrosion Resistance at Room Temperature
3.2. Oxidation Resistance at High Temperatures
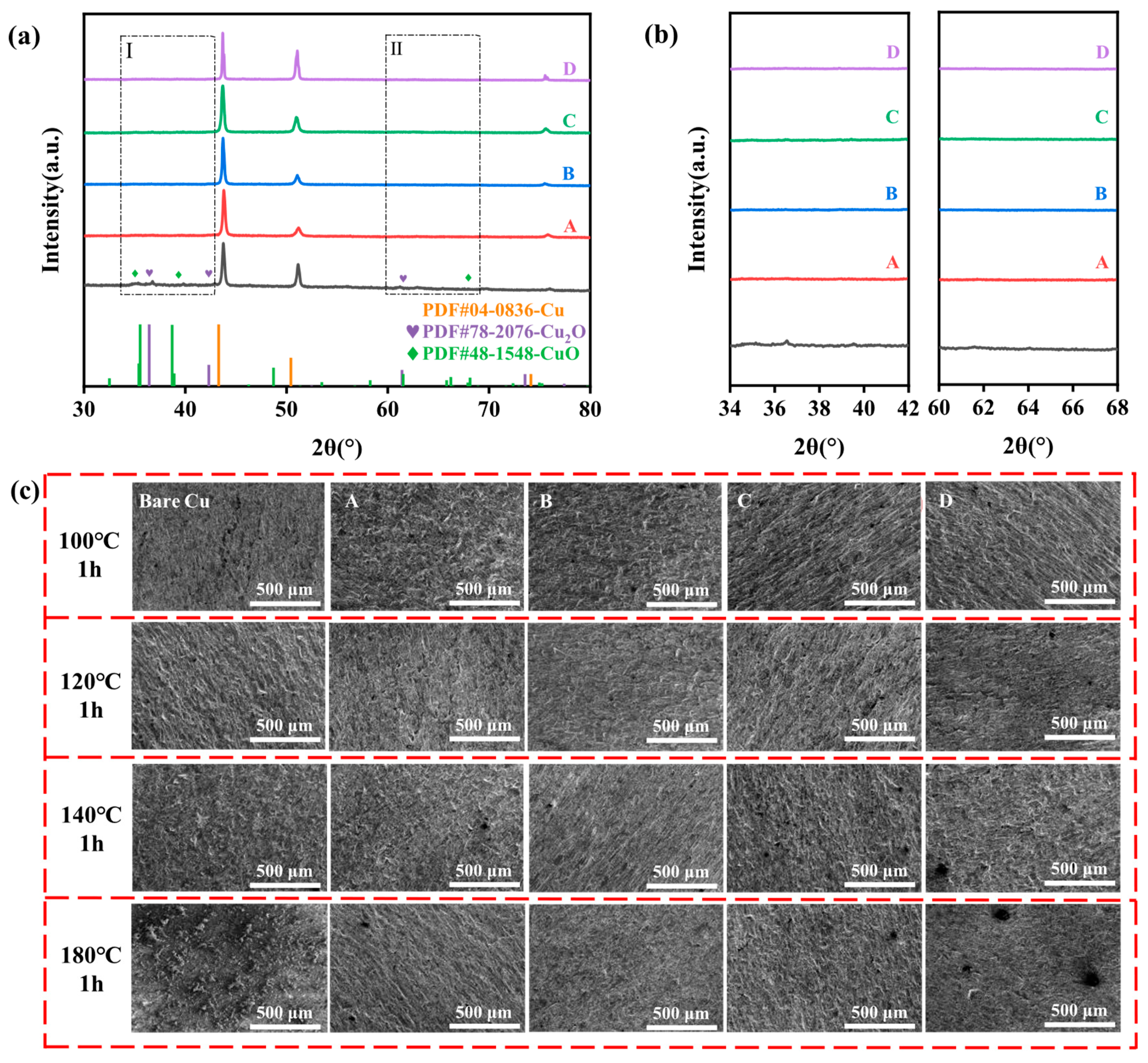
3.3. Component Analysis
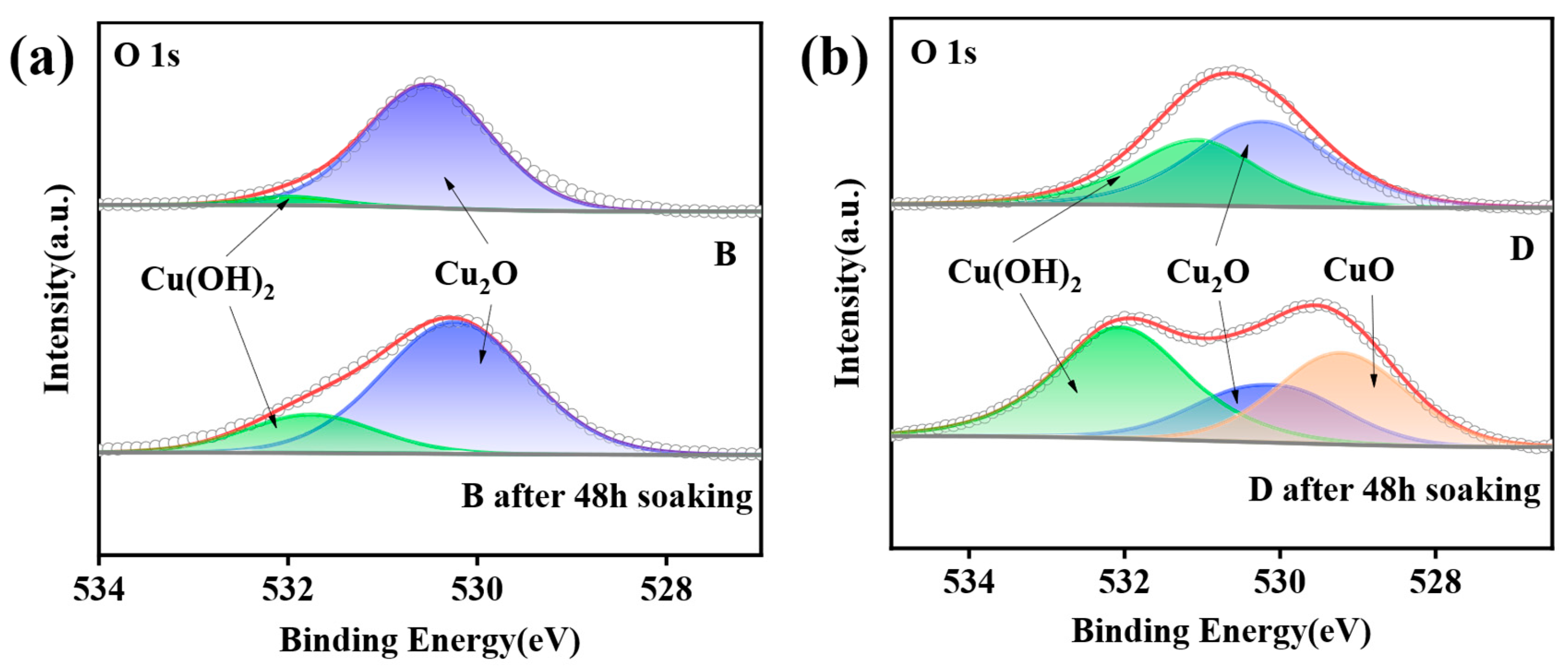
3.4. Passivation Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMT | 2-Amino-5-mercapto-1,3,4-thiadiazole |
| PMTA | 1-phenyl-5-mercapto tetrazolium |
| MBI | 2-mercaptobenzimidazole |
| CHS | Hexadecanethiol |
| SDS | Sodium dodecyl sulfate |
References
- Singh, G.; Haseeb, A.S.M.A. Effect of In-Situ Free Air Ball Laser Heating on Bonding Strength and Grain Structure for Copper Wire Bond. J. Mater. Sci. Mater. Electron. 2017, 28, 13750–13756. [Google Scholar] [CrossRef]
- Xu, H.; Qin, H.; Clauberg, H.; Chylak, B.; Acoff, V.L. Behavior of Palladium and Its Impact on Intermetallic Growth in Palladium-Coated Cu Wire Bonding. Acta Mater. 2013, 61, 79–88. [Google Scholar] [CrossRef]
- Thanapackiam, P.; Rameshkumar, S.; Subramanian, S.S.; Mallaiya, K. Electrochemical Evaluation of Inhibition Efficiency of Ciprofloxacin on the Corrosion of Copper in Acid Media. Mater. Chem. Phys. 2016, 174, 129–137. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, X.; Sun, H.; Li, Y.; Zhang, K.; Wu, Y. Corrosion Behavior of Copper at Elevated Temperature. Int. J. Electrochem. Sc. 2012, 7, 7902–7914. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Peng, Z.; Wang, R.; Liu, Z. Nanotwinned Microstructure Engineering of Electroplated Copper for Enhanced Anti-corrosion in Alkaline Medium. Met. Mater. Int. 2023, 30, 113. [Google Scholar] [CrossRef]
- Yang, T.; Chen, W.; Li, X.; Song, J.; Dong, L.; Fu, Y. Environment-friendly and Chromium-Free Passivation of Copper and Its Alloys. Mater. Today Commun. 2021, 29, 102826. [Google Scholar] [CrossRef]
- Peng, J.; Chen, B.; Wang, Z.; Guo, J.; Wu, B.; Hao, S.; Zhang, Q.; Gu, L.; Zhou, Q.; Liu, Z.; et al. Surface Coordination Layer Passivates Oxidation of Copper. Nature 2020, 586, 390–394. [Google Scholar] [CrossRef]
- Lei, Y.; Sheng, N.; Hyono, A.; Ueda, M.; Ohtsuka, T. Electrochemical synthesis of polypyrrole films on copper from phytic solution for corrosion protection. Corros. Sci. 2013, 76, 302–309. [Google Scholar] [CrossRef]
- Qafsaoui, W.; Kendig, M.W.; Perrot, H.; Takenouti, H. Coupling of electrochemical techniques to study copper corrosion inhibition in 0.5molL−1 NaCl by 1-pyrrolidine dithiocarbamate. Electrochim. Acta 2013, 87, 348–360. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chen, Y.; Lin, C.S. High-temperature Oxidation Resistance of Hot Stamping Steel with Chromium Coating Electroplated in Trivalent Chromium Bath. Mater. Today Commun. 2022, 33, 104663. [Google Scholar] [CrossRef]
- Zhu, Y.; Shen, X.; Zhang, X.; Xu, Q. Corrosion Resistance Behavior of Zinc Substrate Treated with Rare Earth Salt Passivation. JOM 2024, 76, 3979–3993. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, J.; Shen, J.; Wang, X.; Fan, X.; Liu, Z.; Bao, M.; Wang, B.; Feng, Y. Study on Corrosion Resistance of Galvanised Steel Passivated by a Silanised Cerium-Tannic Acid Solution. Surf. Coat. Tech. 2024, 494, 131483. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Y.; Zhou, Y.; Yan, F.; Nie, G. Electrochemical Investigation of the Synergistic Effect Between Molybdate and Tungstate on Surface Passivation of Carbon Steel. Int. J. Electrochem. Sc. 2021, 16, 151027. [Google Scholar] [CrossRef]
- Wu, M.; Ma, H.; Shi, J. Beneficial and Detrimental Effects of Molybdate As an Inhibitor on Reinforcing Steels in Saturated Ca(OH)2 Solution: Spontaneous Passivation. Cem. Concr. Comp. 2021, 116, 103887. [Google Scholar] [CrossRef]
- Xu, Q.; Zhou, G.; Wang, H.; Cai, W. Electrochemical Studies of Polyaspartic Acid and Sodium Tungstate As Corrosion Inhibitors for Brass and Cu30Ni Alloy in Simulated Cooled Water Solutions. Anti-Corros. Methods Mater. 2006, 53, 207–211. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Gong, Y.; Gao, F.; Luo, Z.; Zhang, S.; Li, H. Water Soluble Corrosion Inhibitors for Copper in 3.5 Wt% Sodium Chloride Solution. Corros. Sci. 2017, 123, 339–350. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, H.; He, S.; Weng, W.; Tan, W.; Zhong, S. Construction of Chromium-Free Passivation Film of Pomelo Peel Extract on the Surface of Lithium-Ion Battery Copper Foil and Study on Anti-Corrosion Mechanism. J. Mol. Liq. 2024, 412, 125846. [Google Scholar] [CrossRef]
- Liu, Y.; Mo, Y.; Zhong, H.; Cao, Z. Construction of Al-BTA Passivation Film on the Surface of Electrolytic Copper Foil and Study of Corrosion Resistance Mechanism. Colloid Surf. A 2023, 676, 132179. [Google Scholar] [CrossRef]
- Zhao, W.; Li, F.; Lv, X.; Chang, J.; Shen, S.; Dai, P.; Xia, Y.; Cao, Z. Research Progress of Organic Corrosion Inhibitors in Metal Corrosion Protection. Crystals 2023, 13, 1329. [Google Scholar] [CrossRef]
- Xiong, S.; Wu, H.; Liu, Z. N-containing Heterocyclic Benzotriazole Derivatives As New Corrosion Inhibitor for Mild Steel Contained in Emulsion. Anti-Corros. Methods Mater. 2022, 69, 183–193. [Google Scholar] [CrossRef]
- Li, Y.; Lu, X.; Wu, K.; Yang, L.; Zhang, T.; Wang, F. Exploration the Inhibition Mechanism of Sodium Dodecyl Sulfate on Mg Alloy. Corros. Sci. 2020, 168, 108559. [Google Scholar] [CrossRef]
- Nagy, Z.K. DC Electrochemical Techniques for the Measurement of Corrosion Rates. In Modern Aspects of Electrochemistry; Springer: Berlin/Heidelberg, Germany, 1993; Volume 25, pp. 135–190. [Google Scholar] [CrossRef]
- Li, Q.; Ma, A.; Chen, Y.; Zhang, Q.; Wang, W.; Liu, H.; Zhao, W.; Zhou, H. Anticorrosive Coating from Mussel-Inspired Hyperbranched Epoxy Resin with High Mechanical Properties and Corrosion Resistance. J. Mater. Sci. 2024, 59, 21129–21143. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, Y.; Lamichhane, B.; Regmi, B.; Lee, Y.; Yang, S.; Kim, S.J.; Jung, M.; Jang, J.H.; Jeong, H.Y.; et al. An Impermeable Copper Surface Monolayer with High-Temperature Oxidation Resistance. Nat. Commun. 2025, 16, 1462. [Google Scholar] [CrossRef]
- Lin, C.H.; Duh, J.G. Corrosion behavior of (Ti–Al–Cr–Si–V)xNy coatings on mild steels derived from RF magnetron sputtering. Surf. Coat. Technol. 2008, 203, 558–561. [Google Scholar] [CrossRef]
- Park, I.; Kim, S. Determination of Corrosion Protection Current Density Requirement of Zinc Sacrificial Anode for Corrosion Protection of AA5083-H321 in Seawater. Appl. Surf. Sci. 2020, 509, 145346. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, G.; Zou, S.; Zhou, W.; Fu, X.; Shi, S. Electrochemical Impedance Spectroscopy (EIS) Explanation of Single Crystal Cu(100)/Cu(111) in Different Corrosion Stages. Materials 2023, 16, 1740. [Google Scholar] [CrossRef]
- Jabbarzare, S.; Bakhsheshi-Rad, H.R.; Nourbakhsh, A.A.; Ahmadi, T.; Berto, F. Effect of Graphene Oxide on the Corrosion, Mechanical and Biological Properties of Mg-based Nanocomposite. Int. J. Min. Met. Mater. 2021, 29, 305–319. [Google Scholar] [CrossRef]
- Diaz-Droguett, D.E.; Espinoza, R.; Fuenzalida, V.M. Copper Nanoparticles Grown under Hydrogen: Study of the Surface Oxide. Appl. Surf. Sci. 2011, 257, 4597–4602. [Google Scholar] [CrossRef]
- Akgul, F.A.; Akgul, G.; Yildirim, N.; Unalan, H.E.; Turan, R. Influence of thermal annealing on microstructural, morphological, optical properties and surface electronic structure of copper oxide thin films. Mater. Chem. Phys. 2014, 147, 987–995. [Google Scholar] [CrossRef]
- Wang, Y.; Im, J.; Soares, J.W.; Steeves, D.M.; Whitten, J.E. Thiol Adsorption on and Reduction of Copper Oxide Particles and Surfaces. Langmuir 2016, 32, 3848–3857. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Watts, J.F. XPS Study of Non-rinse Chromate Treatments. Surf. Interface Anal. 1993, 20, 379–384. [Google Scholar] [CrossRef]
- Rossin, A.J.; Grillo, F.; Francis, S.M.; Miller, D.N.; Rossall, A.K.; van den Berg, J.A.; Hunt, G.J.; Baddeley, C.J. Understanding the Passivation Layer Formed by Tolyltriazole on Copper, Bronze, and Brass Surfaces. Appl. Surf. Sci. 2024, 669, 160585. [Google Scholar] [CrossRef]
- Liu, M.; Yin, D.; Tan, B.; Yang, F.; Sun, X.; Gao, P.; Zhang, S.; Wang, Y. Toward Understanding the Adsorption and Inhibition Mechanism of Cu-MBTA Passivation Film on Copper Surface: A Combined Experimental and DFT Investigation. Electron. Mater. Lett. 2020, 17, 109–118. [Google Scholar] [CrossRef]
- Gar, M.F.; Ev, I.M. Inhibition of copper corrosion by 1,2,3-benzotriazole: A review. Corros. Sci. 2010, 52, 2737–2749. [Google Scholar] [CrossRef]
- Qiu, J.; Gao, X.; Feng, K.; Ma, H. Modification copper surface by micron thickness film via thiol-based click reaction. Corros. Sci. 2023, 221, 111344. [Google Scholar] [CrossRef]
- Aliyeva, V.A.; Gurbanov, A.V.; Mahmoud, A.G.; Gomila, R.M.; Frontera, A.; Mahmudov, K.T.; Pombeiro, A.J.L. Chalcogen Bonding in Copper(ii)-Mediated Synthesis. Faraday Discuss. 2023, 244, 77–95. [Google Scholar] [CrossRef] [PubMed]




| Number | Solution Composition |
|---|---|
| 1 | 0.15 g/L AMT + 0.5 g/L PMTA |
| 2 | 0.25 g/L MBI + 1 g/L PMTA |
| 3 | 10 g/L CHS + 7.5 g/L SDS |
| 4 | 5 g/L CrO3 + 0.5 g/L H2SO4 |
| Sample | Bare Cu | A | B | C | D |
|---|---|---|---|---|---|
| Drip results (s) | 8 | 16 | 18 | 14 | 16 |
| Sample | Bare Cu | A | B | C | D |
|---|---|---|---|---|---|
| Ecorr (V vs. SCE) | −1.314 | −1.212 | −1.261 | −1.205 | −1.247 |
| icorr (A·cm−2) | 8.917 × 10−4 | 3.719 × 10−4 | 2.442 × 10−4 | 3.444 × 10−4 | 3.543 × 10−4 |
| Sample | Bare Cu | A | B | C | D |
|---|---|---|---|---|---|
| Rct (Ω) | 654 | 1464 | 2096 | 1027 | 749 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Xue, Z.; Chen, H.; Li, W.; Li, H.; Hu, J.; Zhang, J.; Chen, Q.; Hou, G.; Tang, Y. Research on Chromium-Free Passivation and Corrosion Performance of Pure Copper. Materials 2025, 18, 4585. https://doi.org/10.3390/ma18194585
Yu X, Xue Z, Chen H, Li W, Li H, Hu J, Zhang J, Chen Q, Hou G, Tang Y. Research on Chromium-Free Passivation and Corrosion Performance of Pure Copper. Materials. 2025; 18(19):4585. https://doi.org/10.3390/ma18194585
Chicago/Turabian StyleYu, Xinghan, Ziye Xue, Haibo Chen, Wei Li, Hang Li, Jing Hu, Jianli Zhang, Qiang Chen, Guangya Hou, and Yiping Tang. 2025. "Research on Chromium-Free Passivation and Corrosion Performance of Pure Copper" Materials 18, no. 19: 4585. https://doi.org/10.3390/ma18194585
APA StyleYu, X., Xue, Z., Chen, H., Li, W., Li, H., Hu, J., Zhang, J., Chen, Q., Hou, G., & Tang, Y. (2025). Research on Chromium-Free Passivation and Corrosion Performance of Pure Copper. Materials, 18(19), 4585. https://doi.org/10.3390/ma18194585








