Effect of Steam Explosion (SE) Pretreatment on the Contamination of Woody Biomass with Metallic Inhibitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Steam Explosion (SE)
2.3. Measurement of Heavy Metal Content
- -
- maximum power: 30 W
- -
- maximum current: 0.8 mA
- -
- maximum voltage: 50 kV
- -
- X-ray tube with air-cooled molybdenum anode
2.4. Statistical Analysis
3. Results
3.1. Weight Loss
3.2. Metal Content After Steam Explosion
3.3. Statistical Analysis
4. Discussion
4.1. Weight Loss
4.2. Metal Content After Steam Explosion
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szadkowski, J.; Radomski, A.; Antczak, A.; Szadkowska, D.; Lewandowska, A.; Marchwicka, M.; Kupczyk, A. The yield of model hydrolysis and fermentation in the technology of bioethanol production from poplar wood (Populus sp.). Przemysł Chem. 2017, 96, 518–520. [Google Scholar] [CrossRef]
- Roman, K.; Barwicki, J.; Hryniewicz, M.; Szadkowska, D.; Szadkowski, J. Production of Electricity and Heat from Biomass Wastes Using a Converted Aircraft Turbine AI-20. Processes 2021, 9, 364. [Google Scholar] [CrossRef]
- Segurado, R.; Pereira, S.; Correia, D.; Costa, M. Techno-economic analysis of a trigeneration system based on biomass gasification. Renewable and Sustainable Energy Reviews. April 2019, 103, 501–514. [Google Scholar] [CrossRef]
- Prosiński, S. Chemia Drewna; Państwowe Wydawnictwo Rolne i Leśne: Warszawa, Poland, 1984; pp. 50–54. [Google Scholar]
- Szadkowska, D.; Auriga, R.; Lesiak, A.; Szadkowski, J.; Marchwicka, M. Influence of Pine and Alder Woodchips Storage Method on the Chemical Composition and Sugar Yield in Liquid Biofuel Production. Polymers 2022, 14, 3495. [Google Scholar] [CrossRef]
- Roman, K. The Estimation of the Possibility of Bioethanol Production from Hemp Cellulose Using the HWE Method. Energies 2025, 18, 1441. [Google Scholar] [CrossRef]
- Marchwicka, M.; Antczak, A.; Drożdżek, M.; Akus-Szylberg, F.; Szadkowska, D.; Szadkowski, J.; Żmuda, E.; Radomski, A.; Zawadzki, J. Influence of selected treatment methods on the dry residue and sugars content extracted from wheat and rye bran. Ann. Wars. Univ. Life Sci. SGGW. For. Wood Technol. 2023, 124, 99–106. [Google Scholar] [CrossRef]
- Roman, K.; Dasiewicz, J.; Marchwicka, M. Impact of Hot Water Extraction on the Compaction Efficiency and Material Properties of Miscanthus giganteus in Pellet Production. Materials 2024, 17, 6137. [Google Scholar] [CrossRef] [PubMed]
- Krutul, D.; Szadkowski, J.; Výbohová, E.; Kučerová, V.; Čabalová, I.; Antczak, A.; Szadkowska, D.; Drożdżek, M.; Zawadzki, J. Effect of steam explosion pretreatment on chosen saccharides yield and cellulose structure from fast-growing poplar (Populus deltoides × maximowiczii) wood. Wood Sci. Technol. 2024, 58, 441–458. [Google Scholar] [CrossRef]
- Yildiz, S.; Gümüşkaya, E. The effects of thermal modification on crystalline structure of cellulose in soft and hardwood. Build. Environ. 2007, 42, 62–67. [Google Scholar] [CrossRef]
- Yang, B.; Dai, Z.; Ding, S.Y.; Wyman, C.E. Enzymatic hydrolysis of cellulosic biomass. Biofuels 2011, 2, 421–449. [Google Scholar] [CrossRef]
- Tomás-Pejó, E.; Alvira, P.; Ballesteros, M.; Negro, M.J. Chapter 7—Pretreatment Technologies for Lignocellulose-to-Bioethanol Conversion; Pandey, A., Larroche, C., Ricke, S.C., Dussap, C.-G., Gnansounou, E., Biofuels, Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 149–176. [Google Scholar] [CrossRef]
- Karimi, K.; Taherzadeh, M.J. A critical review of analytical methods in pretreatment of lignocelluloses: Composition, imaging, and crystallinity. Bioresour. Technol. 2016, 200, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Kačík, F.; Kačíková, D.; Jablonský, M.; Katuščák, S. Cellulose degradation in newsprint paper ageing. Polym. Degrad. Stab. 2009, 94, 1509–1514. [Google Scholar] [CrossRef]
- Gałązka, A.; Szadkowski, J. Enzymatic Hydrolysis of Fast-Growing Poplar Wood After Pretreatment by Steam Explosion. Cellul. Chem. Technol. 2021, 55, 637–647. [Google Scholar] [CrossRef]
- Czatzkowska, M.; Harnisz, M.; Korzeniewska, E.; Koniuszewska, I. Inhibitors of the methane fermentation process with particular emphasis on the microbiological aspect: A review. Energy Sci. Eng. 2020, 8, 1880–1897. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Zhao, Y.; Liu, L.-L.; Jia, B.; Zhao, F.; Huang, W.-D.; Zhan, J.-C. Copper Tolerance and Biosorption of Saccharomyces cerevisiae during Alcoholic Fermentation. PLoS ONE 2015, 10, e0128611. [Google Scholar] [CrossRef]
- Lindholm-Lehto, P.C. Biosorption of Heavy Metals by Lignocellulosic Biomass and Chemical Analysis. Biomass metal adsorption. BioResources 2019, 14, 4952–4995. Available online: https://bioresources.cnr.ncsu.edu/wp-content/uploads/2019/03/BioRes_14_2_Review_LindholmLehto_Biosorption_Heavy_Metals_Biomass_Chem_15030-1.pdf (accessed on 15 May 2025). [CrossRef]
- Milne, T.; Brennan, A.H.; Glenn, B.H. Sourcebook of Methods of Analysis for Biomass and Biomass Conversion Processes. In Elsevier Applied Sciences; Elsevier Science Publ.: London, UK; New York, NY, USA, 1990. [Google Scholar]
- Li, Y.; Zhou, L.W.; Wang, R.Z. Urban biomass and methods of estimating municipal biomass resources. Renew. Sustain. Energy Rev. 2017, 80, 1017–1030. [Google Scholar] [CrossRef]
- Pastircakova, K. Determination of trace metal concentrations in ashes from various biomass materials. Energy Educ. Sci. Technol. 2004, 13, 97–104. [Google Scholar]
- Debela, F.; Thring, R.W.; Arocena, J.M. Immobilization of Heavy Metals by Co-pyrolysis of Contaminated Soil with Woody Biomass. Water Air Soil Pollut. 2012, 223, 1161–1170. [Google Scholar] [CrossRef]
- Giudicianni, P.; Gargiulo, V.; Grottola, C.M.; Alfè, M.; Ferreiro, A.I.; Almeida Mendes, M.A.; Fagnano, M.; Ragucci, R. Inherent Metal Elements in Biomass Pyrolysis: A Review. Energy Fuels 2021, 35, 5407–5478. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J.J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Bauer, A.; Bosch, P.; Friedl, A.; Amon, T. Analysis of methane potentials of steam-exploded wheat straw and estimation of energy yields of combined ethanol and methane production. J. Biotechnol. 2009, 142, 50–55. [Google Scholar] [CrossRef]
- Holtzapple, M.T.; Humphrey, A.E.; Taylor, J.D. Energy requirements for the size reduction of poplar and aspen wood. Biotechnol. Bioenergy 1989, 33, 207–210. [Google Scholar] [CrossRef]
- Li, X.; Zhang, N.N.; Quyang, J.; Xu, Y.; Yong, Q.A.; Yu, S.Y. Optimization of steam-pretreatment conditions for corn stover using response surface methodology, Conference Paper. In Research Progress in Paper Industry and Biorefinery (4TH ISETPP); Sun, R.C., Fu, S.Y., Eds.; Poznań University of Life Sciences: Poznań, Poland, 2010; Volume 1e3, pp. 790–793. [Google Scholar]
- Kubicek, C.K. Fungi and Lignocellulosic Biomass; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; p. 304. [Google Scholar] [CrossRef]
- Zielenkiewicz, T. Nowe metody analizy instrumentalnej wybranych pierwiastków i związków chemicznych w drewnie i kompozytach drzewnych [EN: New methods of instrumental analysis of chosen elements and chemical compounds in wood and wood composites]. In Rozprawy Naukowe; Uniwersytet Przyrodniczy w Poznaniu: Poznań, Poland, 2015; Volume 475, pp. 1–87. Available online: https://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-4230b7c5-d55d-4a93-b700-f37deec515e4 (accessed on 10 January 2025).
- Zielenkiewicz, T.; Zawadzki, J.; Radomski, A. XRF spectrometer calibration for copper determination in wood. X-Ray Spectrom. 2012, 41, 371–373. [Google Scholar] [CrossRef]
- Tanase-Opedal, M.; Ghoreishi, S.; Hermundsgård, D.H.; Barth, T.; Moe, S.T.; Brusletto, R. Steam explosion of lignocellulosic residues for co-production of value-added chemicals and high-quality pellets. Biomass Bioenergy 2024, 181, 107037. [Google Scholar] [CrossRef]
- Akizuki, S.; Suzuki, H.; Fujiwara, M.; Toda, T. Impacts of steam explosion pretreatment on semi-continuous anaerobic digestion of lignin-rich submerged macrophyte. J. Clean. Prod. 2023, 385, 135377. [Google Scholar] [CrossRef]
- Ziegler-Devin, I.; Chrusciel, L.; Brosse, N. Steam Explosion Pretreatment of Lignocellulosic Biomass: A Mini-Review of Theorical and Experimental Approaches. Front. Chem. 2021, 9, 705358. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Guo, Y.; Chen, N.; Chen, B. A Review of the Efficient and Thermal Utilization of Biomass Waste. Sustainability 2024, 16, 9506. [Google Scholar] [CrossRef]
- Akinyele, O.S.; Shokunbi, I.O. Comparative analysis of dry ashing and wet digestion methods for the determination of trace and heavy metals in food samples. Food Chem. 2015, 173, 682–684. [Google Scholar] [CrossRef] [PubMed]
- Kalnicky, D.J.; Singhvi, R. Field portable XRF analysis of environmental samples. J. Hazard. Mater. 2001, 83, 93–122. [Google Scholar] [CrossRef]
- Trojek, T.; Dušková, A. Quantitative X-ray fluorescence micro-analysis of wood samples and visualization of tree rings. Radiat. Phys. Chem. 2024, 218, 111603. [Google Scholar] [CrossRef]
- Scharnweber, T.; Rocha, E.; González Arrojo, A.; Ahlgrimm, S.; Gunnarson, B.E.; Holzkämper, S.; Wilmking, M. To extract or not to extract? Influence of chemical extraction treatment of wood samples on element concentrations in tree-rings measured by X-ray fluorescence. Front. Environ. Sci. 2023, 11, 1031770. [Google Scholar] [CrossRef]
- Block, C.N.; Shibata, T.; Solo-Gabriele, H.M.; Townsend, T.G. Use of handheld X-ray fluorescence spectrometry units for identification of arsenic in treated wood. Environ. Pollut. 2007, 148, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Krutul, D.; Szadkowski, J.; Antczak, A.; Drożdżek, M.; Radomski, A.; Karpiński, S.; Zawadzki, J. The Concentration of Selected Heavy Metals in Poplar Wood Biomass and Liquid Fraction Obtained after High Temperature Pretreatment. Wood Res. 2021, 66, 39–48. [Google Scholar] [CrossRef]
- Mueller, R.F.; Steiner, A. Inhibition of Anaerobic Digestion Caused by Heavy Metals. Water Sci. Technol. 1992, 26, 835–846. [Google Scholar] [CrossRef]
- Chandel, A.K.; da Silva, S.S.; Singh, O.V. Detoxification of Lignocellulose Hydrolysates: Biochemical and Metabolic Engineering Toward White Biotechnology. Bioenergy Res. 2013, 6, 388–401. [Google Scholar] [CrossRef]
- Galvagno, S.; Gasciaro, G.; Casu, S.; Martino, M.; Mingazzini, C.; Russo, A.; Portofino, S. Steam gasification of tyre waste, poplar, and refuse-derived fuel: A comparative analysis. Waste Manag. 2009, 29, 678–689. [Google Scholar] [CrossRef]
- Xu, Q.; Li, X.; Ding, R.; Wang, D.; Liu, Y.; Wang, Q.; Zhao, J.; Chen, F.; Zeng, G.; Yang, Q.; et al. Understanding and mitigating the toxicity of cadmium to the anaerobic fermentation of waste activated sludge. Water Res. 2017, 124, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Fang, H.P. Inhibition of heavy metals on fermentative hydrogen production by granular sludge. Chemosphere 2007, 67, 668–673. [Google Scholar] [CrossRef]
- Zielenkiewicz, T.; Szadkowski, J.; Drożdżek, M.; Zielenkiewicz, A.; Kłosińska, T.; Antczak, A.; Zawadzki, J.; Gawron, J. Application of X-Ray Fluorescence Technique for Determination of Heavy Metals Uptake by Different Species of Poplar. Drewno 2016, 59, 113–126. [Google Scholar] [CrossRef]
- Krutul, D.; Zielenkiewicz, T.; Zawadzki, J.; Radomski, A.; Antczak, A.; Drożdżek, M. Influence of Urban Agglomeration Environmental Pollution on Content of Chosen Metals in Bark, Roots and Wood of Norway Maple (Acer Platanoides L.). Wood Res. 2018, 63, 741–754. Available online: https://www.woodresearch.sk/wr/201805/01.pdf (accessed on 8 February 2025).
- Zawadzki, J.; Zielenkiewicz, T.; Radomski, A.; Witomski, P.; Drożdżek, M. Testing Content of Copper in Scots Pine Wood (Pinus Sylvestris L.) after Preservative Treatment. Wood Res. 2010, 55, 91–100. Available online: https://www.woodresearch.sk/wr/201004/09.pdf (accessed on 5 April 2025).
- Rozporządzenie Ministra Zdrowia z Dnia 7 Grudnia 2017 r. w Sprawie Jakości Wody Przeznaczonej do Spożycia Przez Ludzi (Dz.U. 2017, poz. 2294). [Regulation of the Minister of Health of 7 December 2017 on the Quality of Water Intended for Human Consumption (Journal of Laws 2017, Item 2294)]. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20170002294/O/D20172294.pdf (accessed on 15 April 2025).
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption (Recast) (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020L2184 (accessed on 5 June 2025).
| Steam Explosion Temperature [°C] | Average Weight of Dry Samples Before SE [g] | Mass of Wet Chips After SE [g] | Dry Shavings Weight After Draining [g] | Material Weight Loss [g] | Material Weight Loss [%] |
|---|---|---|---|---|---|
| 160 | 18.988 | 42.82 | 17.984 | 1.003 | 5.3 |
| 175 | 18.892 | 27.83 | 16.976 | 1.915 | 10.1 |
| 190 | 18.926 | 32.23 | 16.760 | 2.166 | 11.4 |
| 205 | 18.894 | 22.3 | 16.725 | 2.169 | 11.5 |
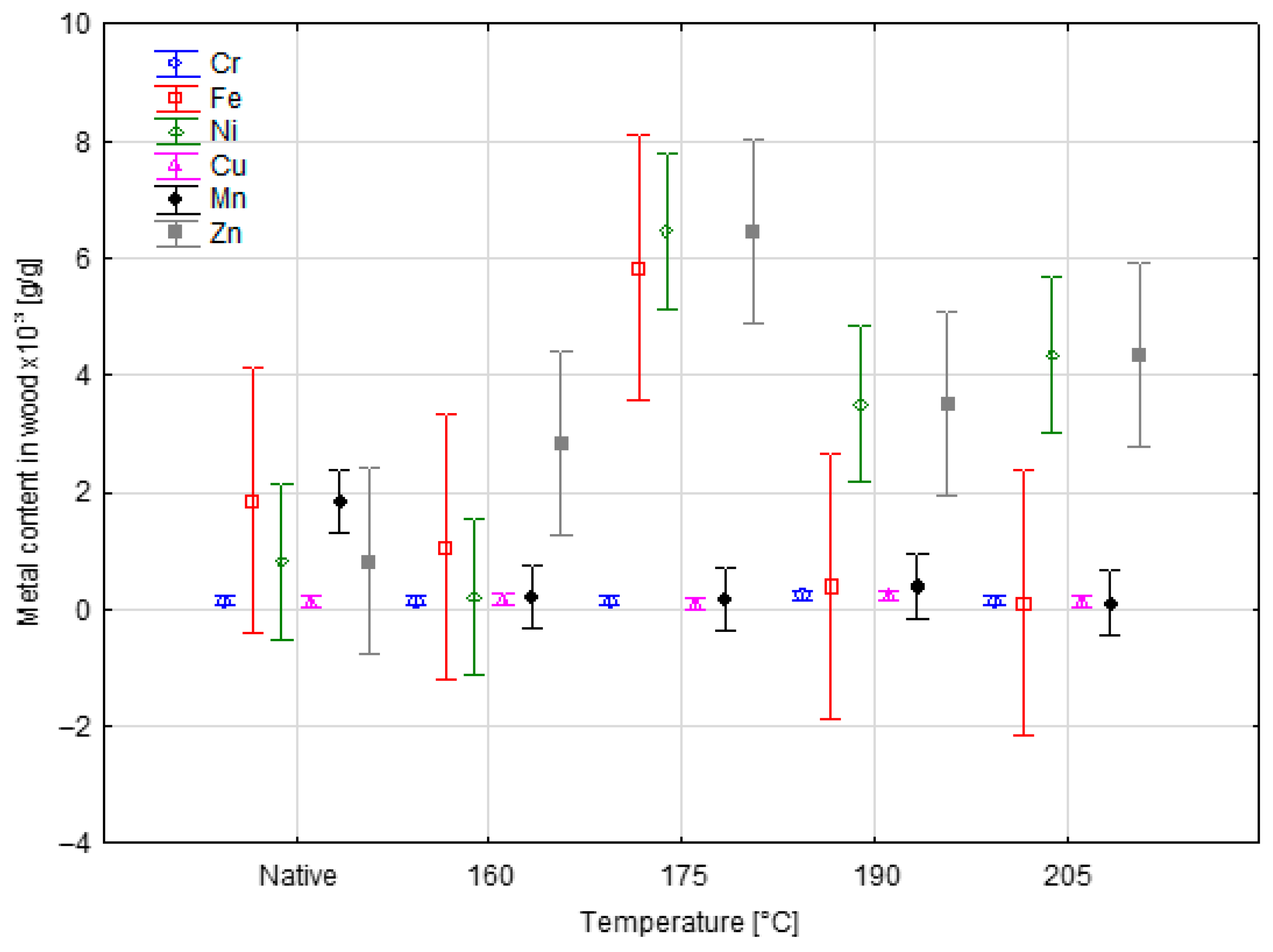
| Temperature [°C] | Sample | Metal Content in Wood × 103 [g/g] | |||||
|---|---|---|---|---|---|---|---|
| Cr | Fe | Ni | Cu | Mn | Zn | ||
| 160 | Average | 0.14392 | 1.07192 | 0.20548 | 0.16069 | 0.19723 | 2.84197 |
| Standard deviation (SD) | 0.06235 | 0.40234 | 0.07452 | 0.05627 | 0.03717 | 1.47619 | |
| 175 | Average | 0.14569 | 5.83317 | 6.46632 | 0.09413 | 0.15745 | 6.46632 |
| SD | 0.05953 | 3.79896 | 1.03646 | 0.10620 | 0.01812 | 1.03646 | |
| 190 | Average | 0.22880 | 0.39063 | 3.50958 | 0.22880 | 0.39063 | 3.50958 |
| SD | 0.06827 | 0.06474 | 1.05307 | 0.06827 | 0.06474 | 1.05307 | |
| 205 | Average | 0.14129 | 0.11287 | 4.36040 | 0.14129 | 0.11287 | 4.36040 |
| SD | 0.05123 | 0.02576 | 1.68736 | 0.05123 | 0.02576 | 1.68736 | |
| Nativ | Average | 0.14271 | 1.84917 | 0.82471 | 0.14271 | 1.84917 | 0.82471 |
| SD | 0.06428 | 0.93937 | 0.55677 | 0.06428 | 0.93937 | 0.55677 | |
| Group 1—correlation occurs to a large extent—close correlation | |||||||
| Group 2—moderate correlation | |||||||
| Group 3—correlation occurs to a small extent | |||||||
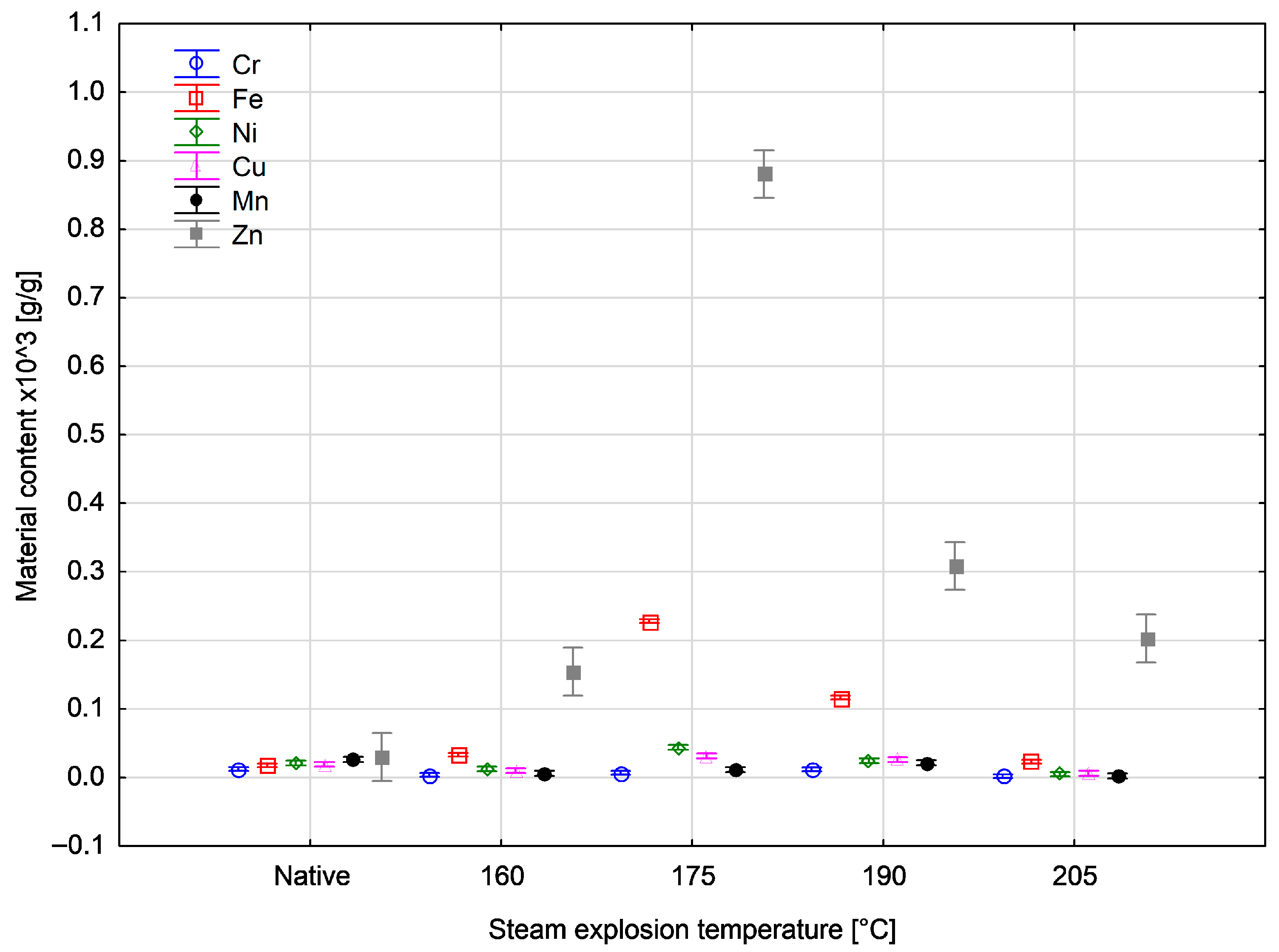
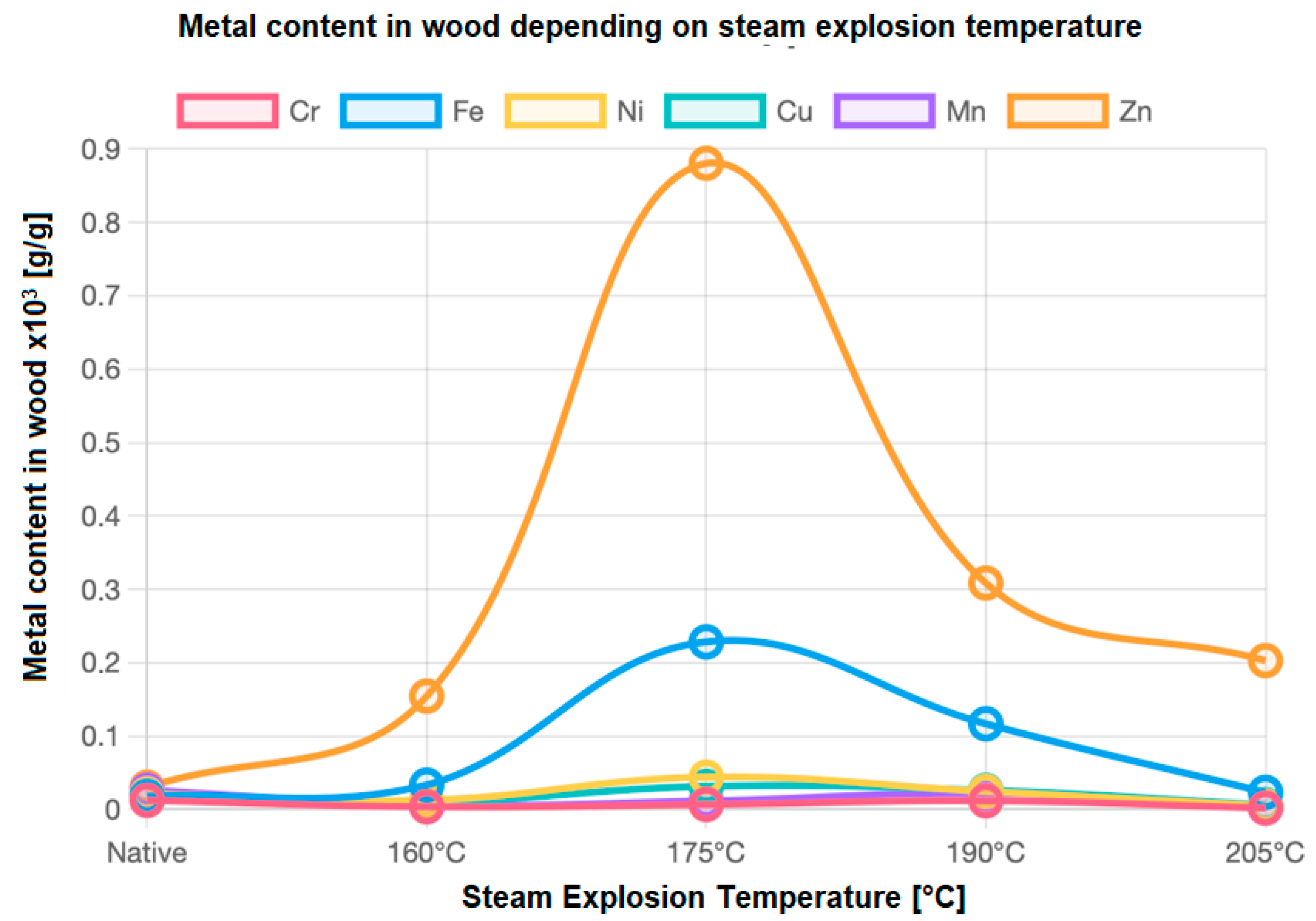
| Temperature [°C] | Sample | Metal Content in Wood × 103 [g/g] | |||||
|---|---|---|---|---|---|---|---|
| Cr | Fe | Ni | Cu | Mn | Zn | ||
| 160 | Average | 0.00369 | 0.03320 | 0.01274 | 0.01017 | 0.00634 | 0.15440 |
| SD | 0.00026 | 0.00039 | 0.00034 | 0.00021 | 0.00014 | 0.00156 | |
| 175 | Average | 0.00675 | 0.22837 | 0.04405 | 0.03150 | 0.01153 | 0.88052 |
| SD | 0.00135 | 0.00282 | 0.00240 | 0.00242 | 0.00196 | 0.00519 | |
| 190 | Average | 0.01181 | 0.11675 | 0.02461 | 0.02615 | 0.02179 | 0.30847 |
| SD | 0.00366 | 0.00249 | 0.00358 | 0.00246 | 0.00488 | 0.06003 | |
| 205 | Average | 0.00189 | 0.02330 | 0.00483 | 0.00635 | 0.00254 | 0.20284 |
| SD | 0.00039 | 0.00129 | 0.00089 | 0.00085 | 0.00069 | 0.00446 | |
| Nativ | Average | 0.01246 | 0.01803 | 0.02128 | 0.01939 | 0.02629 | 0.03029 |
| SD | 0.00277 | 0.00254 | 0.00415 | 0.00482 | 0.00377 | 0.00536 | |
| correlation occurs to a large extent—close correlation | |||||||
| no labelling, no correlation observed | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szadkowski, J.; Gałązka, A.; Wardal, W.J. Effect of Steam Explosion (SE) Pretreatment on the Contamination of Woody Biomass with Metallic Inhibitors. Materials 2025, 18, 4536. https://doi.org/10.3390/ma18194536
Szadkowski J, Gałązka A, Wardal WJ. Effect of Steam Explosion (SE) Pretreatment on the Contamination of Woody Biomass with Metallic Inhibitors. Materials. 2025; 18(19):4536. https://doi.org/10.3390/ma18194536
Chicago/Turabian StyleSzadkowski, Jan, Anna Gałązka, and Witold Jan Wardal. 2025. "Effect of Steam Explosion (SE) Pretreatment on the Contamination of Woody Biomass with Metallic Inhibitors" Materials 18, no. 19: 4536. https://doi.org/10.3390/ma18194536
APA StyleSzadkowski, J., Gałązka, A., & Wardal, W. J. (2025). Effect of Steam Explosion (SE) Pretreatment on the Contamination of Woody Biomass with Metallic Inhibitors. Materials, 18(19), 4536. https://doi.org/10.3390/ma18194536






