Photoluminescence Enhancement in Perovskite Nanocrystals via Compositional, Ligand, and Surface Engineering
Abstract
1. Introduction
1.1. Metal Halide Perovskite Nanocrystals
1.2. Instability Factors and Their Solutions for PL Enhancements of PeNCs
2. Engineering Strategies for Enhancing the PL Properties of PeNCs
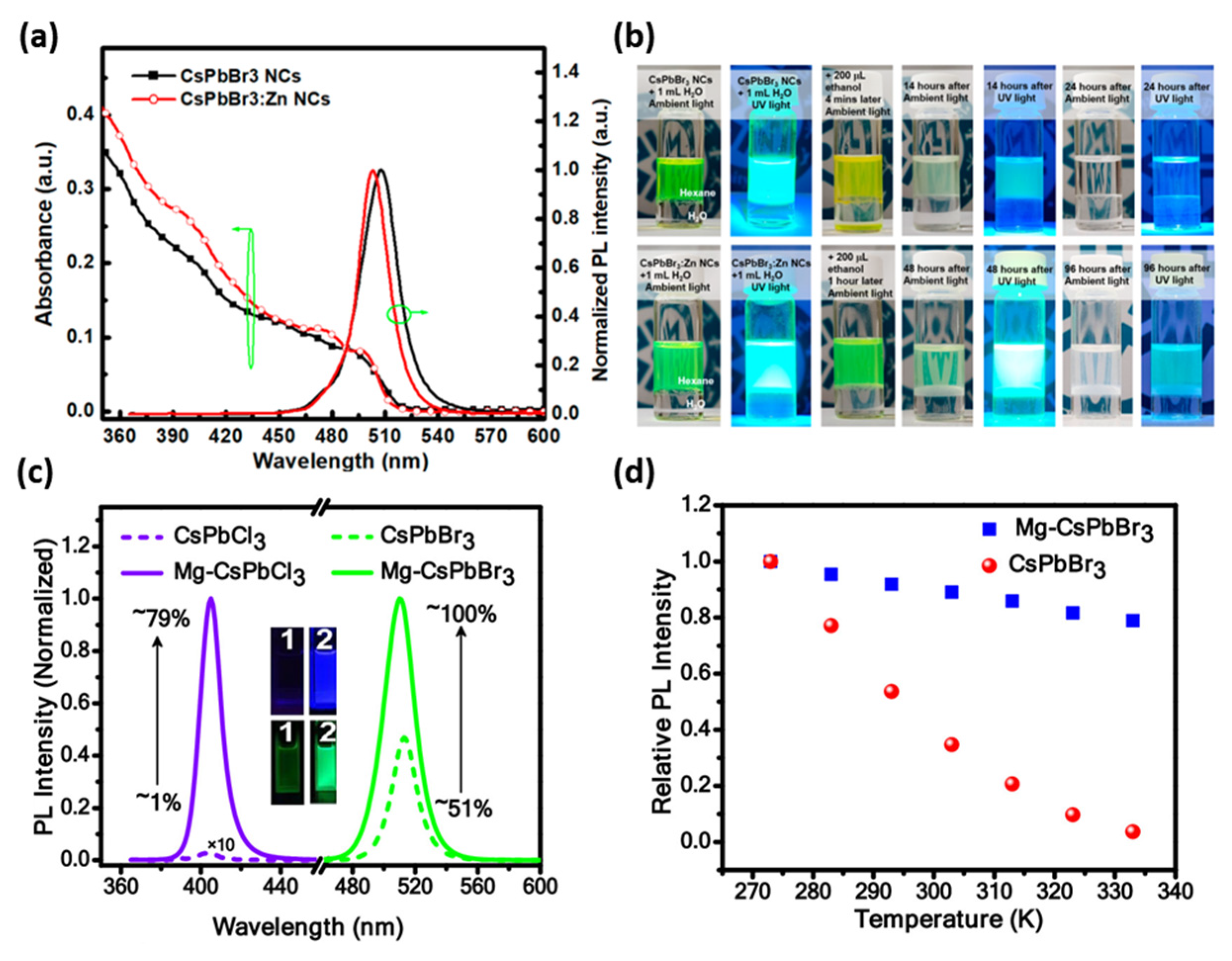
2.1. Compositional Engineering
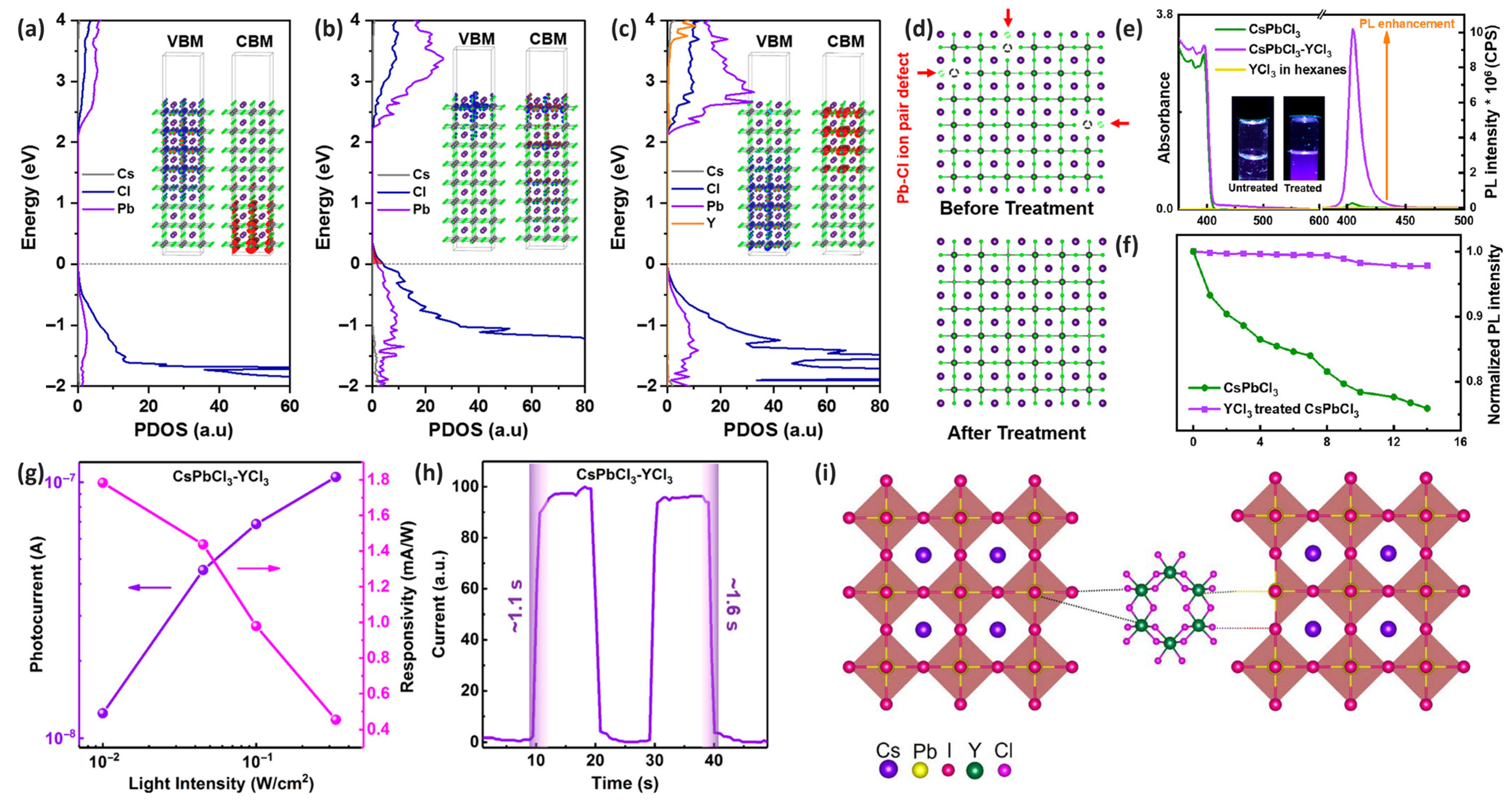
2.2. Surface Passivation
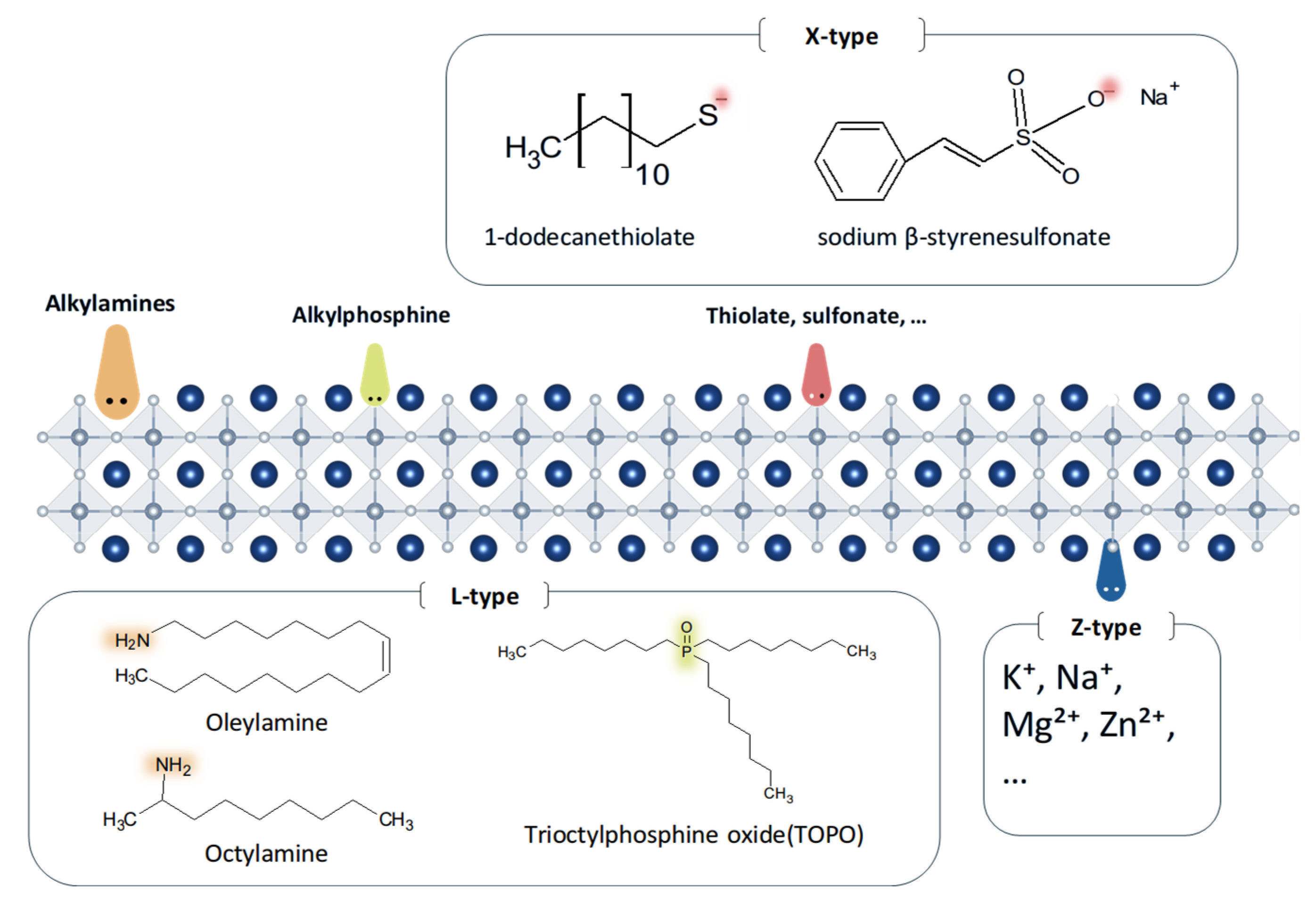
2.3. Ligand Engineering
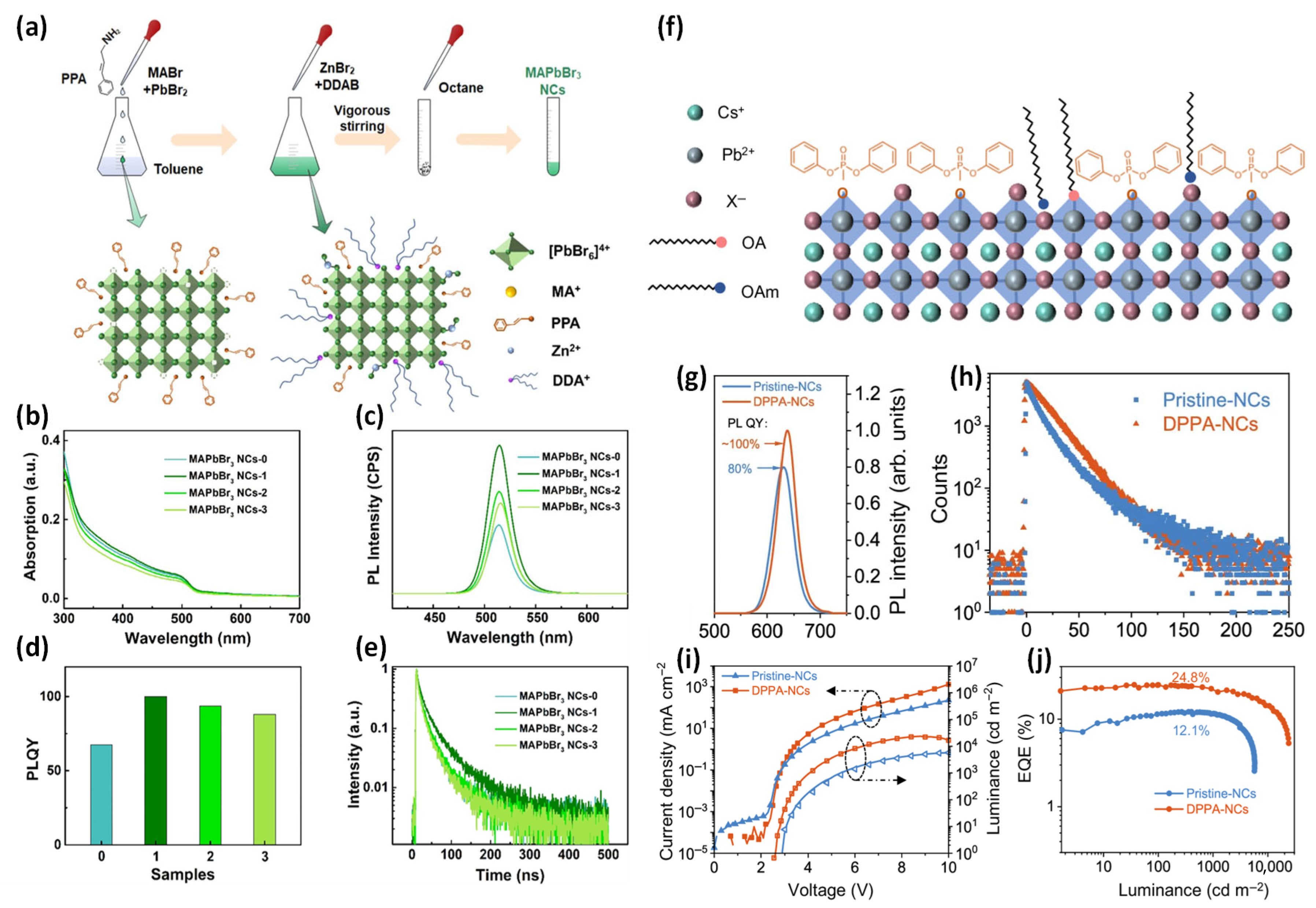
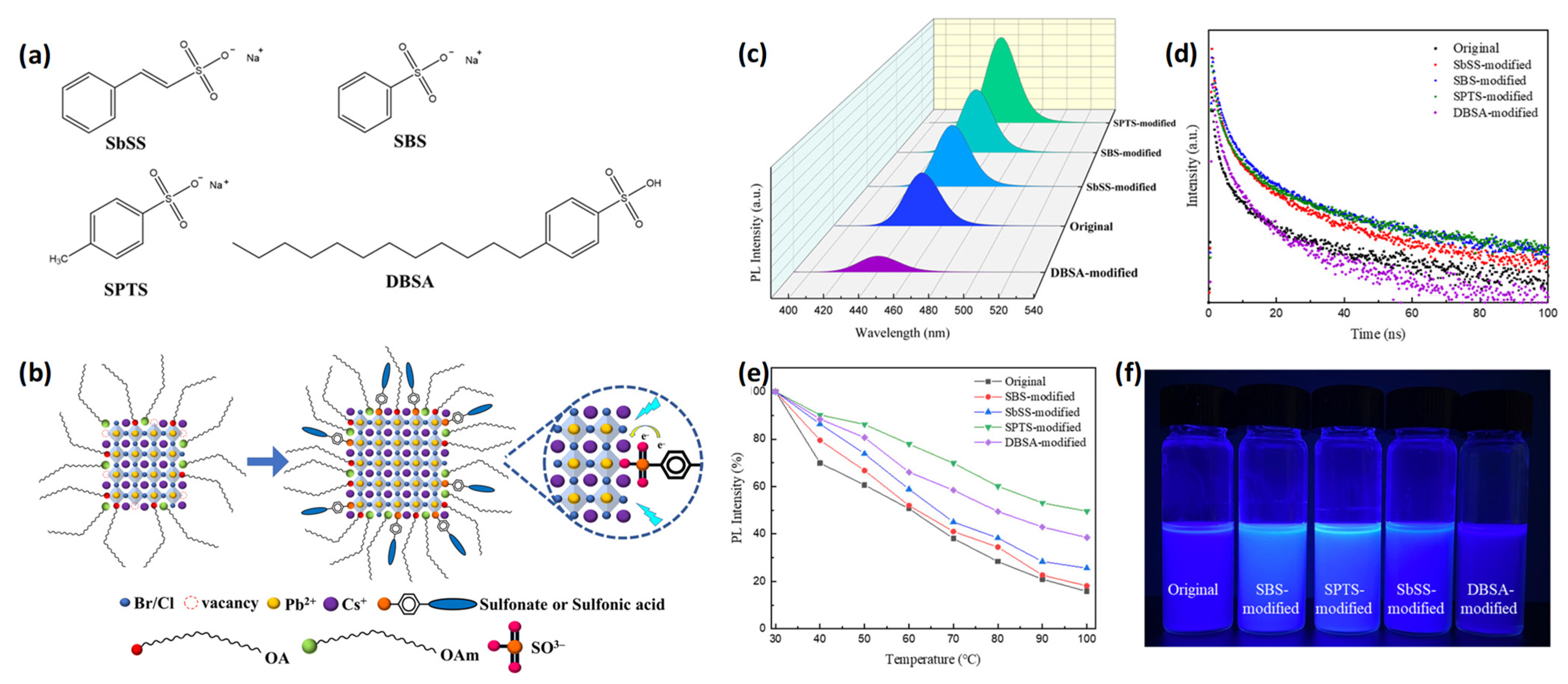
2.3.1. L-Type Ligands
2.3.2. X-Type Ligands
2.3.3. Z-Type Ligands
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-Performance Photovoltaic Perovskite Layers Fabricated through Intramolecular Exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef]
- Dong, H.; Ran, C.; Gao, W.; Li, M.; Xia, Y.; Huang, W. Metal Halide Perovskite for Next-Generation Optoelectronics: Progresses and Prospects. eLight 2023, 3, 3. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, G.; Li, Y.; Hu, R.; Zheng, J. Preparation of a Self-Healing Polyaniline-Based Gel and Its Application as a Healable All-in-One Capacitor. Chem. Eng. J. 2021, 420, 129790. [Google Scholar] [CrossRef]
- Feng, S.; Povilus, B.; Yang, S. Color Tuning Halide Perovskites: Optical Amplification and Lasing. Mater. Today Adv. 2023, 20, 100431. [Google Scholar] [CrossRef]
- Triana, M.A.; Hsiang, E.L.; Zhang, C.; Dong, Y.; Wu, S.T. Luminescent Nanomaterials for Energy-Efficient Display and Healthcare. ACS Energy Lett. 2022, 7, 1001–1020. [Google Scholar] [CrossRef]
- Chu, S.; Chen, W.; Fang, Z.; Xiao, X.; Liu, Y.; Chen, J.; Huang, J.; Xiao, Z. Large-Area and Efficient Perovskite Light-Emitting Diodes via Low-Temperature Blade-Coating. Nat. Commun. 2021, 12, 147. [Google Scholar] [CrossRef]
- Chouhan, L.; Ghimire, S.; Subrahmanyam, C.; Miyasaka, T.; Biju, V. Synthesis, Optoelectronic Properties and Applications of Halide Perovskites. Chem. Soc. Rev. 2020, 49, 2869–2885. [Google Scholar] [CrossRef]
- Savill, K.J.; Ulatowski, A.M.; Herz, L.M. Optoelectronic Properties of Tin-Lead Halide Perovskites. ACS Energy Lett. 2021, 6, 2413–2426. [Google Scholar] [CrossRef]
- Hoye, R.L.Z.; Hidalgo, J.; Jagt, R.A.; Correa-Baena, J.P.; Fix, T.; MacManus-Driscoll, J.L. The Role of Dimensionality on the Optoelectronic Properties of Oxide and Halide Perovskites, and Their Halide Derivatives. Adv. Energy Mater. 2022, 12, 2100499. [Google Scholar] [CrossRef]
- Huang, H.; Bodnarchuk, M.I.; Kershaw, S.V.; Kovalenko, M.V.; Rogach, A.L. Lead Halide Perovskite Nanocrystals in the Research Spotlight: Stability and Defect Tolerance. ACS Energy Lett. 2017, 2, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Caligiuri, V.; Wang, M.; Goldoni, L.; Prato, M.; Krahne, R.; De Trizio, L.; Manna, L. Benzoyl Halides as Alternative Precursors for the Colloidal Synthesis of Lead-Based Halide Perovskite Nanocrystals. J. Am. Chem. Soc. 2018, 140, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Levchuk, I.; Osvet, A.; Tang, X.; Brandl, M.; Perea, J.D.; Hoegl, F.; Matt, G.J.; Hock, R.; Batentschuk, M.; Brabec, C.J. Brightly Luminescent and Color-Tunable Formamidinium Lead Halide Perovskite FAPbX3 (X = Cl, Br, I) Colloidal Nanocrystals. Nano Lett. 2017, 17, 2765–2770. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, J.; Chen, X.; Chen, J.; Zhong, J. Grinding Synthesis of APbX3 (A = MA, FA, Cs; X = Cl, Br, I) Perovskite Nanocrystals. ACS Appl. Mater. Interfaces 2019, 11, 10059–10067. [Google Scholar] [CrossRef]
- Wei, J.H.; Wang, X.D.; Liao, J.F.; Kuang, D. Bin High Photoluminescence Quantum Yield (>95%) of MAPbBr3 Nanocrystals via Reprecipitation from Methylamine-MAPbBr3 Liquid. ACS Appl. Electron. Mater. 2020, 2, 2707–2715. [Google Scholar] [CrossRef]
- Liu, Z.; Bekenstein, Y.; Ye, X.; Nguyen, S.C.; Swabeck, J.; Zhang, D.; Lee, S.T.; Yang, P.; Ma, W.; Alivisatos, A.P. Ligand Mediated Transformation of Cesium Lead Bromide Perovskite Nanocrystals to Lead Depleted Cs4PbBr6 Nanocrystals. J. Am. Chem. Soc. 2017, 139, 5309–5312. [Google Scholar] [CrossRef]
- Qu, J.; Xu, S.; Shao, H.; Xia, P.; Lu, C.; Wang, C.; Ban, D. Recent Progress of Copper Halide Perovskites: Properties, Synthesis and Applications. J. Mater. Chem. C 2023, 11, 6260–6275. [Google Scholar] [CrossRef]
- Gao, F.; Zhu, X.; Feng, Q.; Zhong, W.; Liu, W.; Xu, H.; Liu, Y. Deep-Blue Emissive Cs3Cu2I5 Perovskites Nanocrystals with 96.6% Quantum Yield via InI3-Assisted Synthesis for Light-Emitting Device and Fluorescent Ink Applications. Nano Energy 2022, 98, 107270. [Google Scholar] [CrossRef]
- Wu, Y.; Xiang, G.; Zhang, M.; Wei, D.; Cheng, C.; Leng, J.; Ma, H. Electronic Structures and Photoelectric Properties in Cs3Sb2X9 (X = Cl, Br, or I) under High Pressure: A First Principles Study. Nanomaterials 2022, 12, 2982. [Google Scholar] [CrossRef]
- Cai, T.; Shi, W.; Hwang, S.; Kobbekaduwa, K.; Nagaoka, Y.; Yang, H.; Hills-Kimball, K.; Zhu, H.; Wang, J.; Wang, Z.; et al. Lead-Free Cs4CuSb2Cl12 Layered Double Perovskite Nanocrystals. J. Am. Chem. Soc. 2020, 142, 11927–11936. [Google Scholar] [CrossRef]
- Shen, Y.; Yin, J.; Cai, B.; Wang, Z.; Dong, Y.; Xu, X.; Zeng, H. Lead-Free, Stable, High-Efficiency (52%) Blue Luminescent FA3Bi2Br9 Perovskite Quantum Dots. Nanoscale Horiz. 2020, 5, 580–585. [Google Scholar] [CrossRef]
- Xing, K.; Cao, S.; Yuan, X.; Zeng, R.; Li, H.; Zou, B.; Zhao, J. Thermal and Photo Stability of All Inorganic Lead Halide Perovskite Nanocrystals. Phys. Chem. Chem. Phys. 2021, 23, 17113–17128. [Google Scholar] [CrossRef] [PubMed]
- Almutlaq, J.; Yin, J.; Mohammed, O.F.; Bakr, O.M. The Benefit and Challenges of Zero-Dimensional Perovskites. J. Phys. Chem. Lett. 2018, 9, 4131–4138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fu, X.; Tang, Y.; Wang, H.; Zhang, C.; Yu, W.W.; Wang, X.; Zhang, Y.; Xiao, M. Phase Segregation Due to Ion Migration in All-Inorganic Mixed-Halide Perovskite Nanocrystals. Nat. Commun. 2019, 10, 1088. [Google Scholar] [CrossRef]
- Ghimire, S.; Klinke, C. Two-Dimensional Halide Perovskites: Synthesis, Optoelectronic Properties, Stability, and Applications. Nanoscale 2021, 13, 12394–12422. [Google Scholar] [CrossRef]
- Li, X.; Cao, F.; Yu, D.; Chen, J.; Sun, Z.; Shen, Y.; Zhu, Y.; Wang, L.; Wei, Y.; Wu, Y.; et al. All Inorganic Halide Perovskites Nanosystem: Synthesis, Structural Features, Optical Properties and Optoelectronic Applications. Small 2017, 13, 1603996. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, X.; Zhang, X.; Shen, X.; Lu, M.; Wu, J.; Shi, Z.; Colvin, V.L.; Hu, J.; Bai, X.; et al. Lead-Free Halide Perovskites for Light Emission: Recent Advances and Perspectives. Adv. Sci. 2021, 8, 2003334. [Google Scholar] [CrossRef]
- Dutt, V.G.V.; Akhil, S.; Mishra, N. Surface Passivation Strategies for Improving Photoluminescence and Stability of Cesium Lead Halide Perovskite Nanocrystals. ChemNanoMat 2020, 6, 1730–1742. [Google Scholar] [CrossRef]
- Shao, Y.; Fang, Y.; Li, T.; Wang, Q.; Dong, Q.; Deng, Y.; Yuan, Y.; Wei, H.; Wang, M.; Gruverman, A.; et al. Grain Boundary Dominated Ion Migration in Polycrystalline Organic-Inorganic Halide Perovskite Films. Energy Environ. Sci. 2016, 9, 1752–1759. [Google Scholar] [CrossRef]
- Kamat, P.V.; Kuno, M. Halide Ion Migration in Perovskite Nanocrystals and Nanostructures. Acc. Chem. Res. 2021, 54, 520–531. [Google Scholar] [CrossRef]
- Han, T.H.; Tan, S.; Xue, J.; Meng, L.; Lee, J.W.; Yang, Y. Interface and Defect Engineering for Metal Halide Perovskite Optoelectronic Devices. Adv. Mater. 2019, 31, 1803515. [Google Scholar] [CrossRef]
- Aftabuzzaman, M.; Hong, Y.; Jeong, S.; Levan, R.; Lee, S.J.; Choi, D.H.; Lee, K. Colloidal Perovskite Nanocrystals for Blue-Light-Emitting Diodes and Displays. Adv. Sci. 2025, 12, 2409736. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ren, J.; Zhou, X.; Zhang, G. Stability Improvements of Metal Halide Perovskite Nanocrystals and Their Optoelectrical Applications. Mater. Chem. Front. 2023, 7, 2175–2207. [Google Scholar] [CrossRef]
- Aristidou, N.; Sanchez-Molina, I.; Chotchuangchutchaval, T.; Brown, M.; Martinez, L.; Rath, T.; Haque, S.A. The Role of Oxygen in the Degradation of Methylammonium Lead Trihalide Perovskite Photoactive Layers. Angew. Chem. 2015, 127, 8326–8330. [Google Scholar] [CrossRef]
- Konidakis, I.; Karagiannaki, A.; Stratakis, E. Advanced Composite Glasses with Metallic, Perovskite, and Two-Dimensional Nanocrystals for Optoelectronic and Photonic Applications. Nanoscale 2022, 14, 2966–2989. [Google Scholar] [CrossRef]
- Wang, S.; Yousefi Amin, A.A.; Wu, L.; Cao, M.; Zhang, Q.; Ameri, T. Perovskite Nanocrystals: Synthesis, Stability, and Optoelectronic Applications. Small Struct. 2021, 2, 2000124. [Google Scholar] [CrossRef]
- Otero-Martínez, C.; Fiuza-Maneiro, N.; Polavarapu, L. Enhancing the Intrinsic and Extrinsic Stability of Halide Perovskite Nanocrystals for Efficient and Durable Optoelectronics. ACS Appl. Mater. Interfaces 2022, 14, 34291–34302. [Google Scholar] [CrossRef]
- Becker, M.; Klüner, T.; Wark, M. Formation of Hybrid ABX3 Perovskite Compounds for Solar Cell Application: First-Principles Calculations of Effective Ionic Radii and Determination of Tolerance Factors. Dalt. Trans. 2017, 46, 3500–3509. [Google Scholar] [CrossRef]
- Travis, W.; Glover, E.N.K.; Bronstein, H.; Scanlon, D.O.; Palgrave, R.G. On the Application of the Tolerance Factor to Inorganic and Hybrid Halide Perovskites: A Revised System. Chem. Sci. 2016, 7, 4548–4556. [Google Scholar] [CrossRef]
- Burger, S.; Ehrenreich, M.G.; Kieslich, G. Tolerance Factors of Hybrid Organic-Inorganic Perovskites: Recent Improvements and Current State of Research. J. Mater. Chem. A 2018, 6, 21785–21793. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, Z.; Nam, S.H.; Chen, Q.; Wang, J.; Zeng, Z.; Ge, C.; Li, Y.; Wang, J.; Kim, Y.H.; et al. Spin Lifetime in Hybrid Organic-Inorganic Perovskites: Mechanisms, Measurements, and Prospects for Spintronic Applications. J. Phys. Chem. Lett. 2025, 16, 5109–5120. [Google Scholar] [CrossRef] [PubMed]
- Livakas, N.; Toso, S.; Ivanov, Y.P.; Das, T.; Chakraborty, S.; Divitini, G.; Manna, L. CsPbCl3 → CsPbI3 Exchange in Perovskite Nanocrystals Proceeds through a Jump-the-Gap Reaction Mechanism. J. Am. Chem. Soc. 2023, 145, 20442–20450. [Google Scholar] [CrossRef]
- Van der Stam, W.; Geuchies, J.J.; Altantzis, T.; Van Den Bos, K.H.W.; Meeldijk, J.D.; Van Aert, S.; Bals, S.; Vanmaekelbergh, D.; De Mello Donega, C. Highly Emissive Divalent-Ion-Doped Colloidal CsPb1-XMxBr3 Perovskite Nanocrystals through Cation Exchange. J. Am. Chem. Soc. 2017, 139, 4087–4097. [Google Scholar] [CrossRef] [PubMed]
- Amat, A.; Mosconi, E.; Ronca, E.; Quarti, C.; Umari, P.; Nazeeruddin, M.K.; Grätzel, M.; De Angelis, F. Cation-Induced Band-Gap Tuning in Organohalide Perovskites: Interplay of Spin-Orbit Coupling and Octahedra Tilting. Nano Lett. 2014, 14, 3608–3616. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Bogachuk, D.; Suo, J.; Wagner, L.; Kim, H.; Lim, J.; Hinsch, A.; Boschloo, G.; Nazeeruddin, M.K.; Hagfeldt, A. Strain Effects on Halide Perovskite Solar Cells. Chem. Soc. Rev. 2022, 51, 7509–7530. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Kumar, S.; Bär, J.; Bertolotti, F.; Masciocchi, N.; Guagliardi, A.; Grotevent, M.; Shorubalko, I.; Bodnarchuk, M.I.; et al. Dismantling the “Red Wall” of Colloidal Perovskites: Highly Luminescent Formamidinium and Formamidinium-Cesium Lead Iodide Nanocrystals. ACS Nano 2017, 11, 3119–3134. [Google Scholar] [CrossRef]
- Serafini, P.; Villanueva-Antolí, A.; Adhikari, S.D.; Masi, S.; Sánchez, R.S.; Rodriguez-Pereira, J.; Pradhan, B.; Hofkens, J.; Gualdrón-Reyes, A.F.; Mora-Seró, I. Increasing the Performance and Stability of Red-Light-Emitting Diodes Using Guanidinium Mixed-Cation Perovskite Nanocrystals. Chem. Mater. 2023, 35, 3998–4006. [Google Scholar] [CrossRef]
- Vashishtha, P.; Veldhuis, S.A.; Dintakurti, S.S.H.; Kelly, N.L.; Griffith, B.E.; Brown, A.A.M.; Ansari, M.S.; Bruno, A.; Mathews, N.; Fang, Y.; et al. Investigating the Structure-Function Relationship in Triple Cation Perovskite Nanocrystals for Light-Emitting Diode Applications. J. Mater. Chem. C 2020, 8, 11805–11821. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.Y.; Domanski, K.; Correa-Baena, J.P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-Containing Triple Cation Perovskite Solar Cells: Improved Stability, Reproducibility and High Efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef]
- Ko, P.K.; Chen, D.; Li, C.H.A.; Chan, C.C.S.; Sergeev, A.; Ding, P.; Lam, D.; Ouyang, B.; Guo, L.; Wong, K.S.; et al. Color Tuning in Cesium Lead Halide Nanocrystals via A-Site Substitution as an Alternative Method for Achieving Spectrally Stable Blue Perovskite Nanocrystal Light-Emitting Diodes. Chem. Mater. 2024, 36, 3735–3744. [Google Scholar] [CrossRef]
- Lu, Y.; Alam, F.; Shamsi, J.; Abdi-Jalebi, M. Doping Up the Light: A Review of A/B-Site Doping in Metal Halide Perovskite Nanocrystals for Next-Generation LEDs. J. Phys. Chem. C 2024, 128, 10084–10107. [Google Scholar] [CrossRef]
- Luo, B.; Li, F.; Xu, K.; Guo, Y.; Liu, Y.; Xia, Z.; Zhang, J.Z. B-Site Doped Lead Halide Perovskites: Synthesis, Band Engineering, Photophysics, and Light Emission Applications. J. Mater. Chem. C 2019, 7, 2781–2808. [Google Scholar] [CrossRef]
- Zeng, Y.-T.; Li, Z.-R.; Chang, S.-P.; Ansay, A.; Wang, Z.-H.; Huang, C.-Y. Bright CsPbBr3 Perovskite Nanocrystals with Improved Stability by In-Situ Zn-Doping. Nanomaterials 2022, 12, 759. [Google Scholar] [CrossRef]
- Das, S.; De, A.; Samanta, A. Ambient Condition Mg2+ Doping Producing Highly Luminescent Green-and Violet-Emitting Perovskite Nanocrystals with Reduced Toxicity and Enhanced Stability. J. Phys. Chem. Lett. 2020, 11, 1178–1188. [Google Scholar] [CrossRef]
- Kim, H.; Bae, S.R.; Lee, T.H.; Lee, H.; Kang, H.; Park, S.; Jang, H.W.; Kim, S.Y. Enhanced Optical Properties and Stability of CsPbBr3 Nanocrystals Through Nickel Doping. Adv. Funct. Mater. 2021, 31, 2102770. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, B.; Yuan, J.; Tang, N.; Lian, L.; Qin, L.; Zhu, L.; Zhang, J.; Chen, R.; Zang, J. Cu2+-Doped CsPbI3 Nanocrystals with Enhanced Stability for Light-Emitting Diodes. J. Phys. Chem. Lett. 2021, 12, 3038–3045. [Google Scholar] [CrossRef]
- Guvenc, C.M.; Yalcinkaya, Y.; Ozen, S.; Sahin, H.; Demir, M.M. Gd3+-Doped α-CsPbI3 Nanocrystals with Better Phase Stability and Optical Properties. J. Phys. Chem. C 2019, 123, 24865–24872. [Google Scholar] [CrossRef]
- Ten Brinck, S.; Zaccaria, F.; Infante, I. Defects in Lead Halide Perovskite Nanocrystals: Analogies and (Many) Differences with the Bulk. ACS Energy Lett. 2019, 4, 2739–2747. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; Guan, H.; Zhao, S.; Cao, S.; Chen, C.; Liu, M.; Zang, Z. Highly Stable CsPbBr3 Quantum Dots by Silica-Coating and Ligand Modification for White Light-Emitting Diodes and Visible Light Communication. Chem. Eng. J. 2021, 419, 129551. [Google Scholar] [CrossRef]
- Cao, W.; Liu, P.; Chen, W.; Wang, W.; Xu, B.; Wu, D.; Hao, J.; Cao, W.; Fang, F.; Li, Y.; et al. Halide-Rich Synthesized Cesium Lead Bromide Perovskite Nanocrystals for Light-Emitting Diodes with Improved Performance. Chem. Mater. 2017, 29, 5168–5173. [Google Scholar] [CrossRef]
- Ahmed, G.H.; El-Demellawi, J.K.; Yin, J.; Pan, J.; Velusamy, D.B.; Hedhili, M.N.; Alarousu, E.; Bakr, O.M.; Alshareef, H.N.; Mohammed, O.F. Giant Photoluminescence Enhancement in CsPbCl3 Perovskite Nanocrystals by Simultaneous Dual-Surface Passivation. ACS Energy Lett. 2018, 3, 2301–2307. [Google Scholar] [CrossRef]
- Yang, D.; Li, X.; Zeng, H. Surface Chemistry of All Inorganic Halide Perovskite Nanocrystals: Passivation Mechanism and Stability. Adv. Mater. Interfaces 2018, 5, 1701662. [Google Scholar] [CrossRef]
- Shamsi, J.; Urban, A.S.; Imran, M.; De Trizio, L.; Manna, L. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.I.; Katware, A.; Amin, A.; Jung, S.H.; Lee, J.H. YCl3-Substituted CsPbI3 Perovskite Nanorods for Efficient Red-Light-Emitting Diodes. Nanomaterials 2023, 13, 1366. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Seth, S.; Samanta, A. Boosting the Photoluminescence of CsPbX3 (X = Cl, Br, I) Perovskite Nanocrystals Covering a Wide Wavelength Range by Postsynthetic Treatment with Tetrafluoroborate Salts. Chem. Mater. 2018, 30, 3633–3637. [Google Scholar] [CrossRef]
- Zheng, K.; Žídek, K.; Abdellah, M.; Messing, M.E.; Al-Marri, M.J.; Pullerits, T. Trap States and Their Dynamics in Organometal Halide Perovskite Nanoparticles and Bulk Crystals. J. Phys. Chem. C 2016, 120, 3077–3084. [Google Scholar] [CrossRef]
- Huang, H.; Raith, J.; Kershaw, S.V.; Kalytchuk, S.; Tomanec, O.; Jing, L.; Susha, A.S.; Zboril, R.; Rogach, A.L. Growth Mechanism of Strongly Emitting CH3NH3PbBr3 Perovskite Nanocrystals with a Tunable Bandgap. Nat. Commun. 2017, 8, 996. [Google Scholar] [CrossRef]
- Tan, Y.; Zou, Y.; Wu, L.; Huang, Q.; Yang, D.; Chen, M.; Ban, M.; Wu, C.; Wu, T.; Bai, S.; et al. Highly Luminescent and Stable Perovskite Nanocrystals with Octylphosphonic Acid as a Ligand for Efficient Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 3784–3792. [Google Scholar] [CrossRef]
- De Roo, J.; Ibáñez, M.; Geiregat, P.; Nedelcu, G.; Walravens, W.; Maes, J.; Martins, J.C.; Van Driessche, I.; Kovalenko, M.V.; Hens, Z. Highly Dynamic Ligand Binding and Light Absorption Coefficient of Cesium Lead Bromide Perovskite Nanocrystals. ACS Nano 2016, 10, 2071–2081. [Google Scholar] [CrossRef]
- Ravi, V.K.; Santra, P.K.; Joshi, N.; Chugh, J.; Singh, S.K.; Rensmo, H.; Ghosh, P.; Nag, A. Origin of the Substitution Mechanism for the Binding of Organic Ligands on the Surface of CsPbBr3 Perovskite Nanocubes. J. Phys. Chem. Lett. 2017, 8, 4988–4994. [Google Scholar] [CrossRef]
- Wu, L.; Zhong, Q.; Yang, D.; Chen, M.; Hu, H.; Pan, Q.; Liu, H.; Cao, M.; Xu, Y.; Sun, B.; et al. Improving the Stability and Size Tunability of Cesium Lead Halide Perovskite Nanocrystals Using Trioctylphosphine Oxide as the Capping Ligand. Langmuir 2017, 33, 12689–12696. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Z.; Gao, H.; Shao, J.; Zou, H.; Yao, D.; Liu, Y.; Zhang, H.; Yang, B. One-Step Preparation of Cesium Lead Halide CsPbX3 (X = Cl, Br, and I) Perovskite Nanocrystals by Microwave Irradiation. ACS Appl. Mater. Interfaces 2017, 9, 42919–42927. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, Z.; Zhang, X.; Liao, K.; Wang, G. Synergistic Passivation Inducing Long-Term Stability for Fluorescent CsPbI3 Perovskite Nanocrystals. Adv. Opt. Mater. 2024, 12, 2302193. [Google Scholar] [CrossRef]
- Hills-Kimball, K.; Yang, H.; Cai, T.; Wang, J.; Chen, O. Recent Advances in Ligand Design and Engineering in Lead Halide Perovskite Nanocrystals. Adv. Sci. 2021, 8, 2100214. [Google Scholar] [CrossRef]
- Zu, Y.; Zhang, T.; Jing, L.; Gao, H.; Wang, W.; Yang, K.; Zeng, B.; Gan, R.; Gong, D.; Liu, P.; et al. Bifunctional Ligand Passivation Enables Robust-Stability Perovskite Nanocrystals for Backlit Display. Dyes Pigment. 2025, 242, 112983. [Google Scholar] [CrossRef]
- Pradhan, J.; Moitra, P.; Umesh; Das, B.; Mondal, P.; Kumar, G.S.; Ghorai, U.K.; Acharya, S.; Bhattacharya, S. Encapsulation of CsPbBr3 Nanocrystals by a Tripodal Amine Markedly Improves Photoluminescence and Stability Concomitantly via Anion Defect Elimination. Chem. Mater. 2020, 32, 7159–7171. [Google Scholar] [CrossRef]
- Yuan, L.; Chen, D.; He, K.; Xu, J.; Xu, K.; Hu, J.; Liang, S.; Zhu, H. Advancing Microarray Fabrication: One-Pot Synthesis and High-Resolution Patterning of UV-Crosslinkable Perovskite Quantum Dots. Nano Res. 2024, 17, 8600–8609. [Google Scholar] [CrossRef]
- Oh, B.M.; Jeong, Y.; Zheng, J.; Cho, N.Y.; Song, M.; Choi, J.W.; Kim, J.H. Simple One-Pot Synthesis and High-Resolution Patterning of Perovskite Quantum Dots Using a Photocurable Ligand. Chem. Commun. 2021, 57, 12824–12827. [Google Scholar] [CrossRef] [PubMed]
- Wächtler, E.; Gericke, R.; Brendler, E.; Gerke, B.; Langer, T.; Pöttgen, R.; Zhechkov, L.; Heine, T.; Wagler, J. Group 10-Group 14 Metal Complexes [E-TM]IV: The Role of the Group 14 Site as an L, X and Z-Type Ligand. Dalt. Trans. 2016, 45, 14252–14264. [Google Scholar] [CrossRef]
- Sun, W.; Yun, R.; Liu, Y.; Zhang, X.; Yuan, M.; Zhang, L.; Li, X. Ligands in Lead Halide Perovskite Nanocrystals: From Synthesis to Optoelectronic Applications. Small 2023, 19, 2205950. [Google Scholar] [CrossRef]
- Zhao, C.; Dai, J.; Zhu, C.; Liu, X.; Dong, H.; Yuan, F.; Jiao, B.; Yu, Y.; Wu, Z. Complementary Triple-Ligand Engineering Approach to Methylamine Lead Bromide Nanocrystals for High-Performance Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2022, 14, 10508–10516. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, X.; Zhang, D.; Gao, Y.; Feng, Y.; Ma, Z.; Huang, J.; He, H.; Ye, Z.; Dai, X. Thermal Management towards Ultra-Bright and Stable Perovskite Nanocrystal-Based Pure Red Light-Emitting Diodes. Nat. Commun. 2024, 15, 6561. [Google Scholar] [CrossRef]
- Ruan, L.; Shen, W.; Wang, A.; Zhou, Q.; Zhang, H.; Deng, Z. Stable and Conductive Lead Halide Perovskites Facilitated by X-Type Ligands. Nanoscale 2017, 9, 7252–7259. [Google Scholar] [CrossRef] [PubMed]
- Kuan, C.H.; Yang, S.H. Surface Ligand Engineering of Perovskite Nanocrystals with a Conjugated Sulfonate Ligand for Light-Emitting Applications. Mater. Adv. 2022, 3, 7824–7832. [Google Scholar] [CrossRef]
- Huang, S.H.; Yang, S.H.; Tsai, W.C.; Hsu, H.C. Enhancing Optical and Thermal Stability of Blue-Emitting Perovskite Nanocrystals through Surface Passivation with Sulfonate or Sulfonic Acid Ligands. Nanomaterials 2024, 14, 1049. [Google Scholar] [CrossRef]
- Uddin, M.A.; Hossain, T.; Kothalawala, N.L.; Joy, S.; Kim, D.Y.; Graham, K.R. Multifunctional Thiol-Containing Additives for Improved Photoluminescence and Photovoltaic Performance of Cs0.15FA0.85PbI3 Perovskites. ACS Appl. Electron. Mater. 2022, 4, 903–909. [Google Scholar] [CrossRef]
- Fiuza-Maneiro, N.; Sun, K.; López-Fernández, I.; Gómez-Graña, S.; Müller-Buschbaum, P.; Polavarapu, L. Ligand Chemistry of Inorganic Lead Halide Perovskite Nanocrystals. ACS Energy Lett. 2023, 8, 1152–1191. [Google Scholar] [CrossRef]
- Zhang, N.; Xia, K.; He, Q.; Pan, J. Recent Progress in the Stability of Red-Emissive Perovskite Nanocrystals for Light-Emitting Diodes. ACS Mater. Lett. 2022, 4, 1233–1254. [Google Scholar] [CrossRef]
- Behera, R.K.; Dutta, A.; Ghosh, D.; Bera, S.; Bhattacharyya, S.; Pradhan, N. Doping the Smallest Shannon Radii Transition Metal Ion Ni(II) for Stabilizing α-CsPbI3 Perovskite Nanocrystals. J. Phys. Chem. Lett. 2019, 10, 7916–7921. [Google Scholar] [CrossRef]
- Navas, J.; Sánchez-Coronilla, A.; Gallardo, J.J.; Cruz Hernández, N.; Piñero, J.C.; Alcántara, R.; Fernández-Lorenzo, C.; De Los Santos, D.M.; Aguilar, T.; Martín-Calleja, J. New Insights into Organic-Inorganic Hybrid Perovskite CH3NH3PbI3 Nanoparticles. An Experimental and Theoretical Study of Doping in Pb2+ Sites with Sn2+, Sr2+, Cd2+ and Ca2+. Nanoscale 2015, 7, 6216–6229. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Cao, S.; Xing, K.; Ning, M.; Zeng, R.; Wang, Y.; Zhao, J. Mg2+-Assisted Passivation of Defects in CsPbI3Perovskite Nanocrystals for High-Efficiency Photoluminescence. J. Phys. Chem. Lett. 2021, 12, 11090–11097. [Google Scholar] [CrossRef]
- Choe, H.; Jeon, D.; Lee, S.J.; Cho, J. Mixed or Segregated: Toward Efficient and Stable Mixed Halide Perovskite-Based Devices. ACS Omega 2021, 6, 24304–24315. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Nan, M.; He, Y.; Lu, S.; Shen, W.; Cheng, G.; Chen, S.; Huang, W. Z-Type Ligand Enables Efficient and Stable Deep-Blue Perovskite Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2024, 16, 22139–22146. [Google Scholar] [CrossRef] [PubMed]
- Sakhatskyi, K.; John, R.A.; Guerrero, A.; Tsarev, S.; Sabisch, S.; Das, T.; Matt, G.J.; Yakunin, S.; Cherniukh, I.; Kotyrba, M.; et al. Assessing the Drawbacks and Benefits of Ion Migration in Lead Halide Perovskites. ACS Energy Lett. 2022, 7, 3401–3414. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Man, Z.; Rao, S.; Zhao, J.; Wang, S.; Zhang, R.; Teng, F.; Tang, A. Rigid Crosslinker-Assisted Nondestructive Direct Photolithograph for Patterned QLED Displays. Light Sci. Appl. 2025, 14, 251. [Google Scholar] [CrossRef]




| Materials | PL Peak (nm) | Synthesis Method | PLQY (%) | Reference |
|---|---|---|---|---|
| CsPbCl3 | 408 | Hot injection | 65 | [11] |
| CsPbBr3 | 512 | Hot injection | 92 | [11] |
| CsPbI3 | 691 | Hot injection | 58 | [11] |
| Cs4PbBr6 | 375 | two-step dissolution–recrystallization mechanism | - | [15] |
| FAPbCl3 | 415 | LARP | 1 | [12] |
| FAPbCl1.5Br1.5 | 455 | LARP | 26 | [12] |
| FAPbBr3 | 526 | LARP | 84 | [12] |
| FAPbBr1.5I1.5 | 630 | LARP | 21 | [12] |
| FAPbI3 | 740 | LARP | 55 | [12] |
| MAPbCl3 | 400 | Wet grinding | 5 | [13] |
| MAPbBr3 | 522 | Wet grinding | 75 | [13] |
| MAPbI3 | 780 | Wet grinding | 55 | [13] |
| CsCu2I3 | 561 | Hot injection | 11 | [16] |
| Cs3Cu2I5 | 440 | Hot injection | 96.6 | [17] |
| K3SbCl6 | 440 | Hot injection | 22.3 | [18] |
| Cs2AgBiBr6 | 625 | Hot injection, LARP | - | [19] |
| FA3Bi2Br9 | 437 | LARP | 52 | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-M.; Jeong, E.-H.; Kim, H.-S.; Choi, S.-Y.; Park, M.-H. Photoluminescence Enhancement in Perovskite Nanocrystals via Compositional, Ligand, and Surface Engineering. Materials 2025, 18, 4195. https://doi.org/10.3390/ma18174195
Lee C-M, Jeong E-H, Kim H-S, Choi S-Y, Park M-H. Photoluminescence Enhancement in Perovskite Nanocrystals via Compositional, Ligand, and Surface Engineering. Materials. 2025; 18(17):4195. https://doi.org/10.3390/ma18174195
Chicago/Turabian StyleLee, Chae-Mi, Eun-Hoo Jeong, Ho-Seong Kim, Seo-Yeon Choi, and Min-Ho Park. 2025. "Photoluminescence Enhancement in Perovskite Nanocrystals via Compositional, Ligand, and Surface Engineering" Materials 18, no. 17: 4195. https://doi.org/10.3390/ma18174195
APA StyleLee, C.-M., Jeong, E.-H., Kim, H.-S., Choi, S.-Y., & Park, M.-H. (2025). Photoluminescence Enhancement in Perovskite Nanocrystals via Compositional, Ligand, and Surface Engineering. Materials, 18(17), 4195. https://doi.org/10.3390/ma18174195






