The Effect of H2O and CO2 on the Adsorption Behavior of H2 and CO on Hematite
Abstract
1. Introduction
2. Method and Experiment
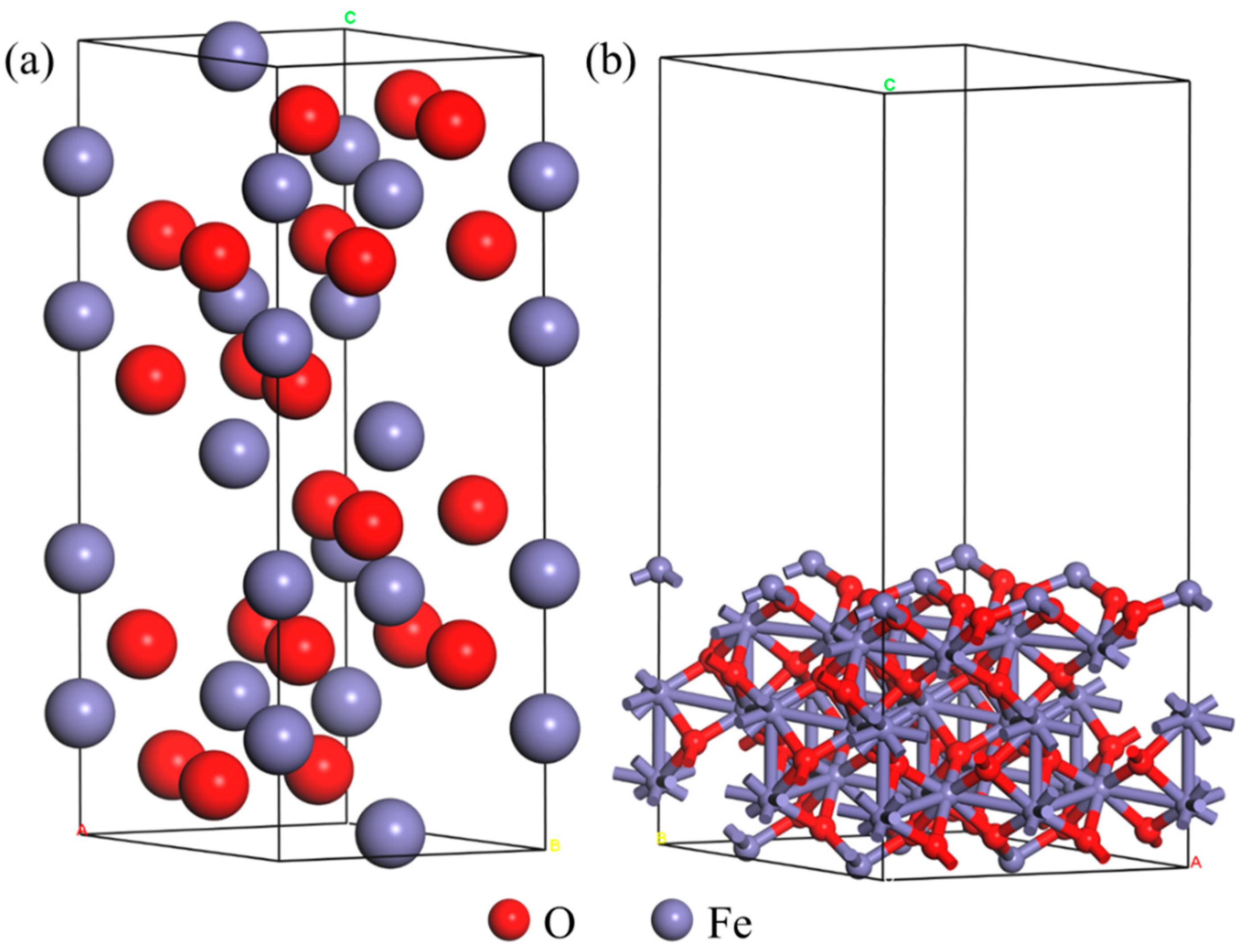
2.1. Computational Details
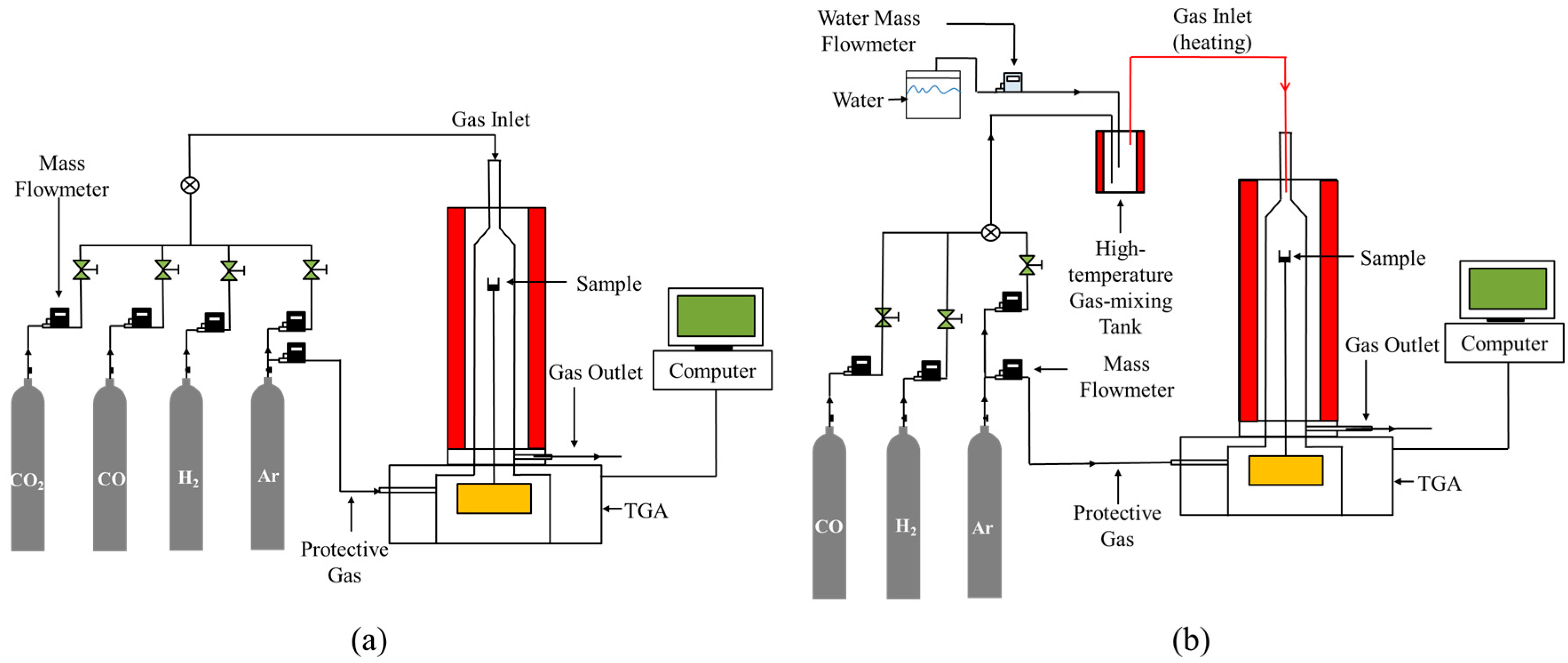
2.2. Experiment Procedure
3. Results and Discussion
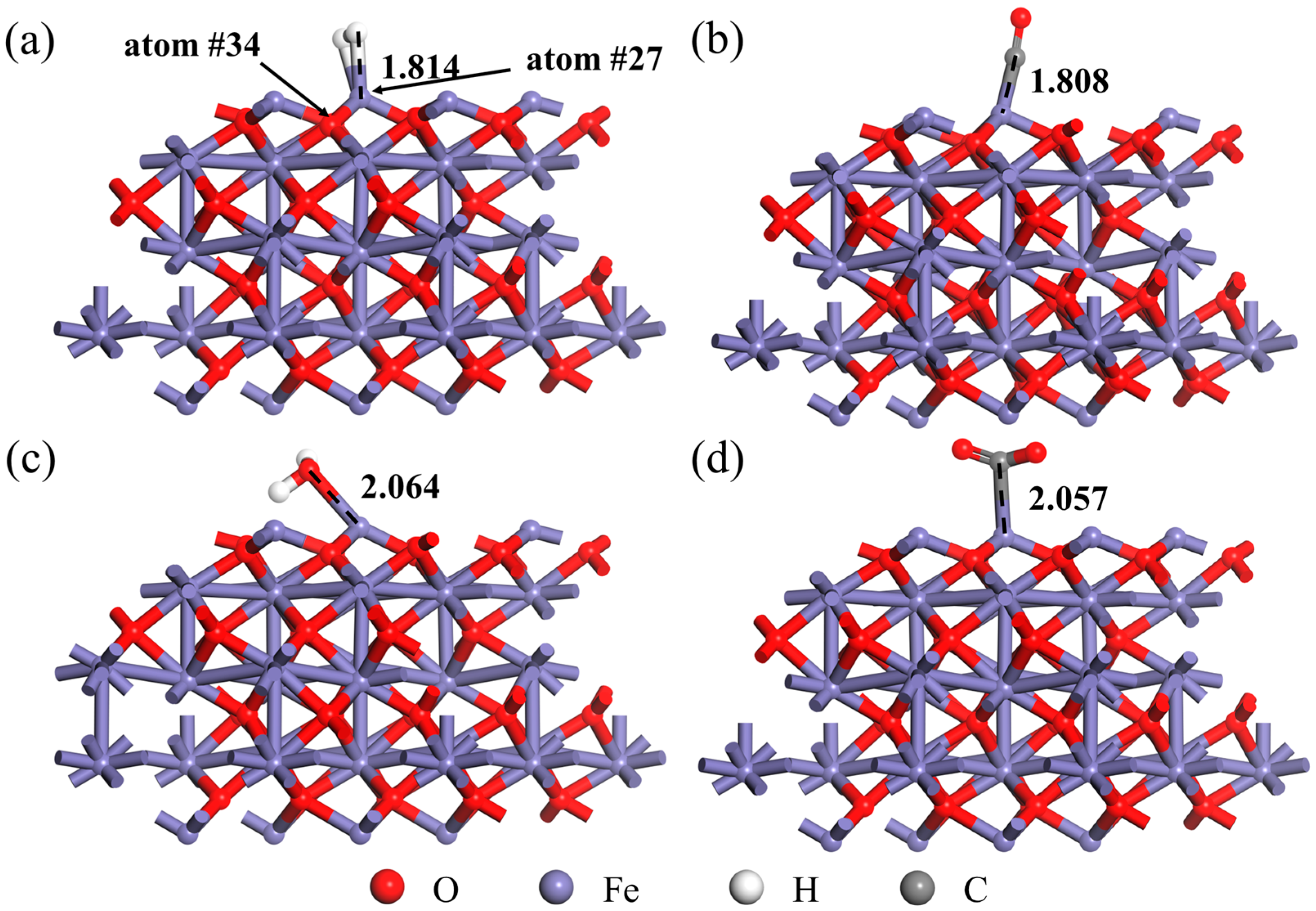
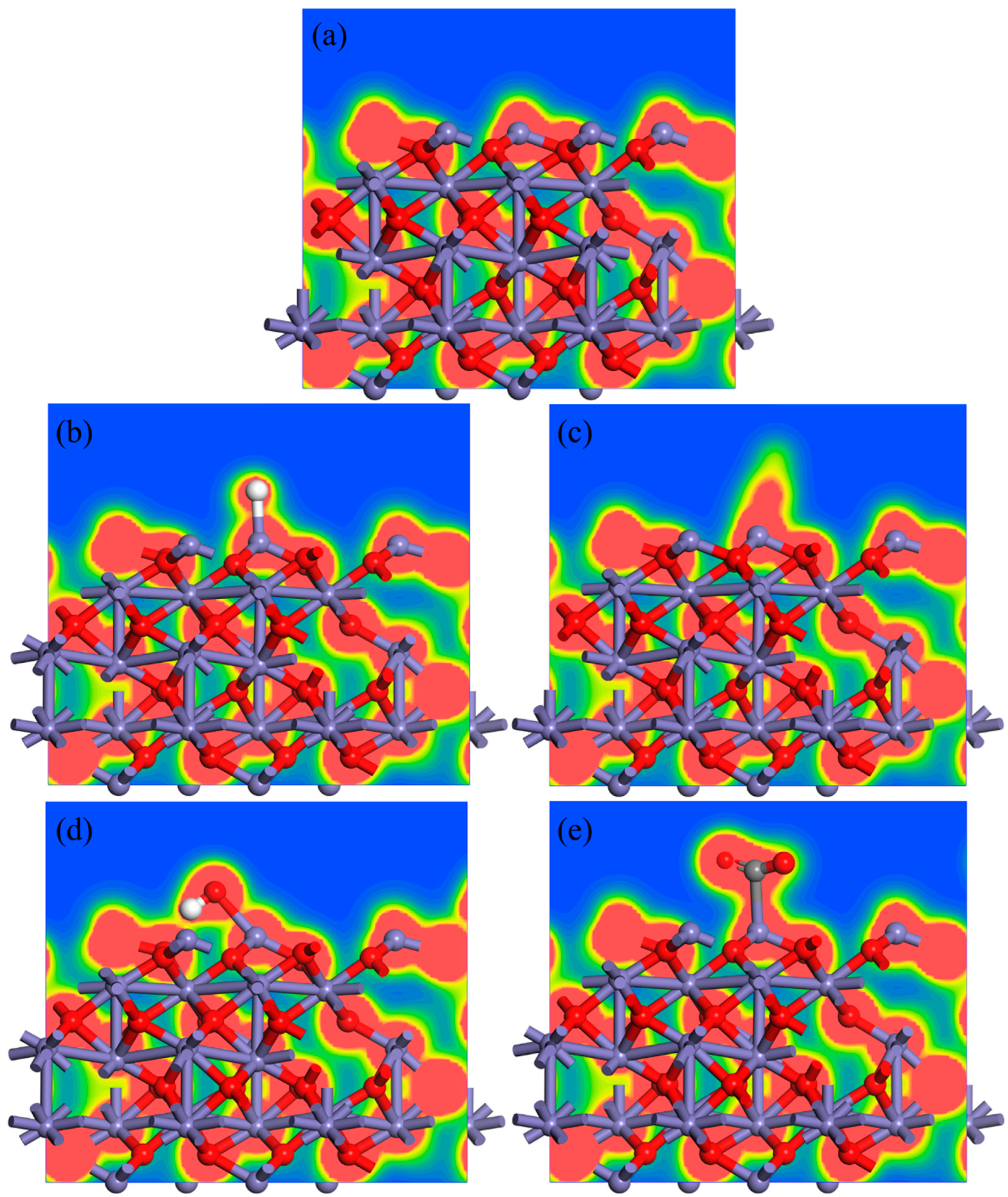
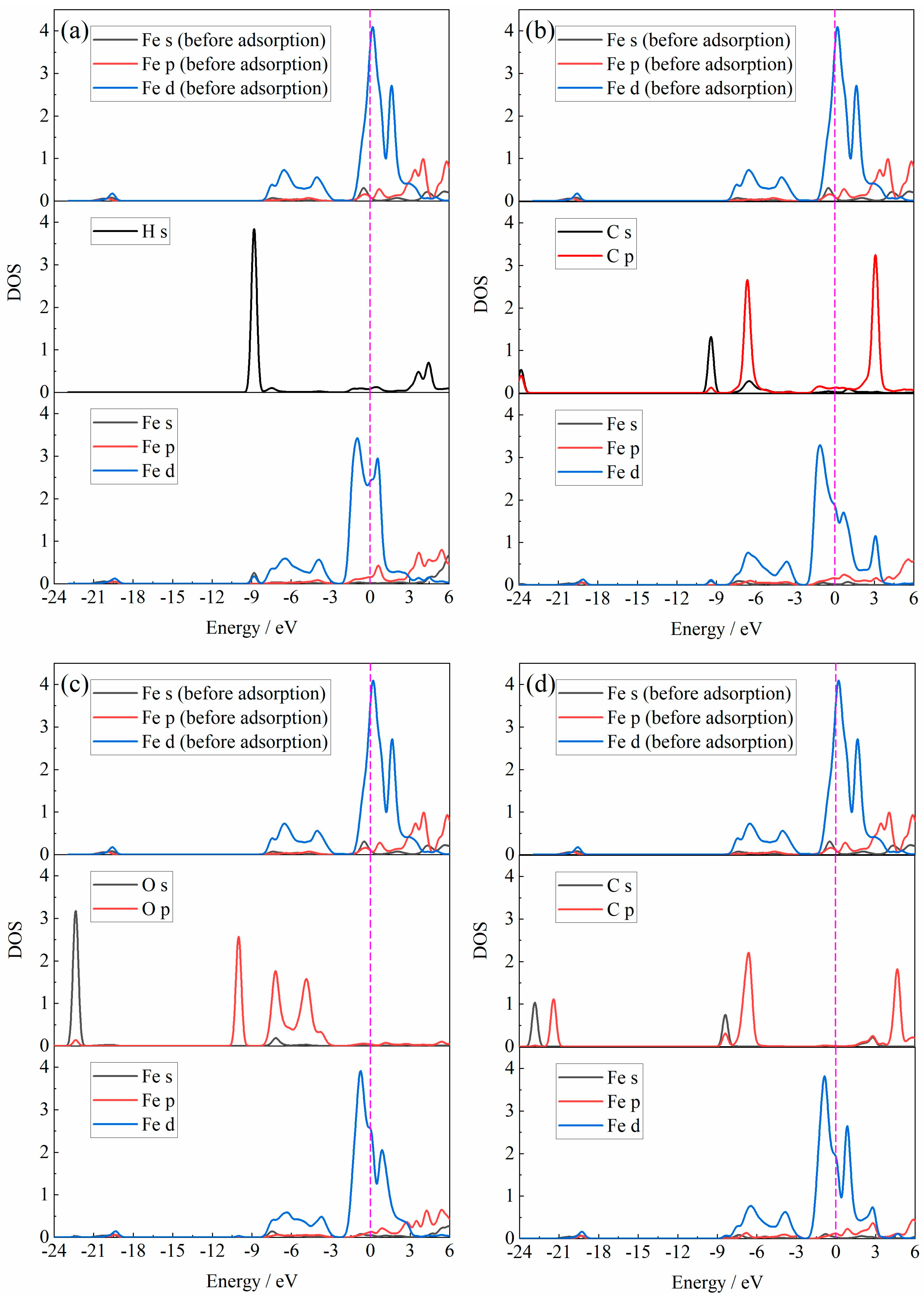
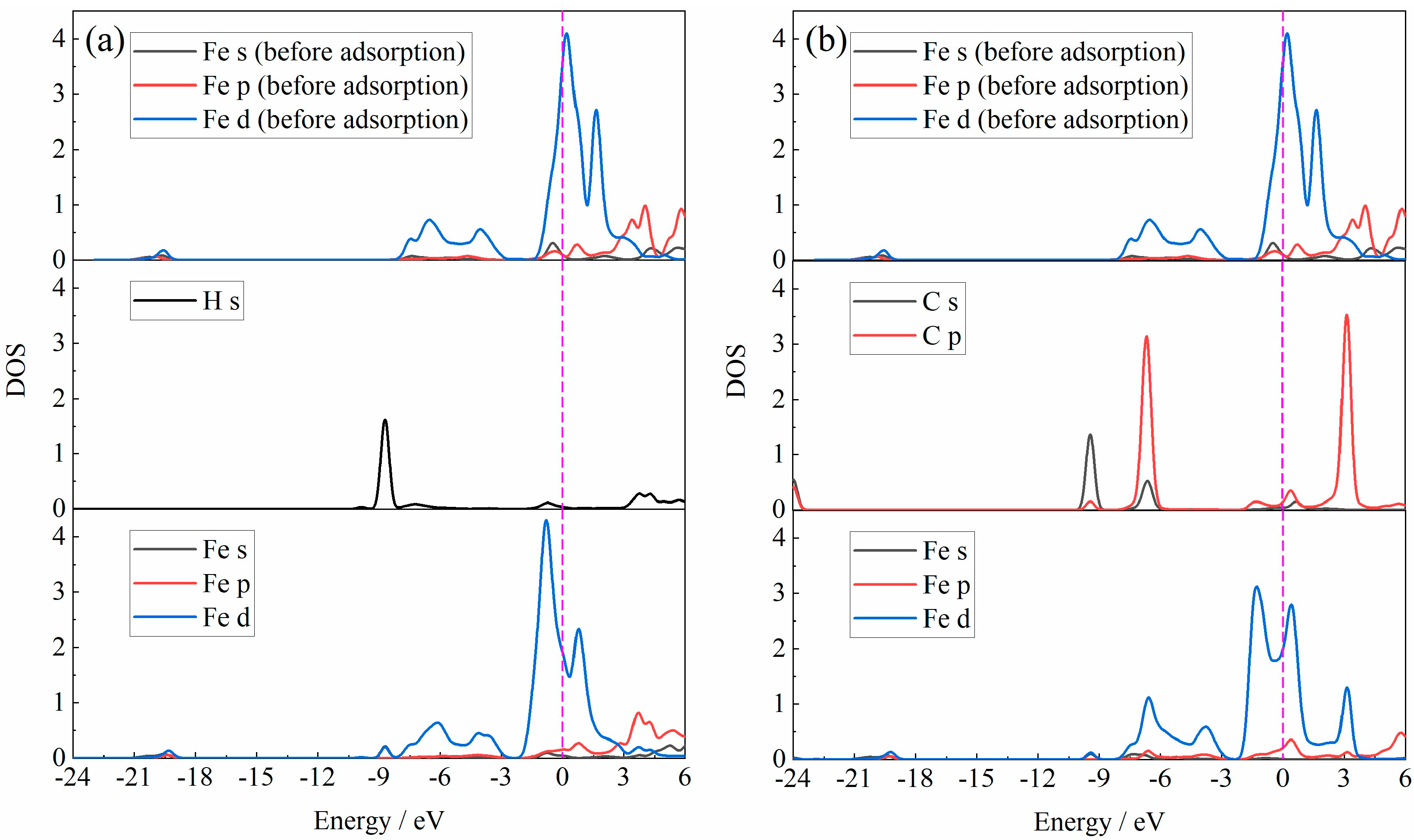
3.1. DFT Calculation
3.2. Reduction Experiment
4. Conclusions
- (1)
- The gas molecules (H2, CO, H2O and CO2) all adsorbed on the Fe atom of the Fe2O3 (001) surface rather than the O atom. In addition, the adsorption energy of the Fe2O3-CO adsorption system was −1.317 eV, which was smaller than the adsorption energy of the Fe2O3-H2 adsorption system (−0.013 eV), indicating that the Fe2O3-CO adsorption system was more stable than the Fe2O3-H2 adsorption system. And the adsorption energy of the Fe2O3-H2O adsorption system was −0.702 eV, indicating that its adsorption stability was also higher than that of the Fe2O3-H2 adsorption system.
- (2)
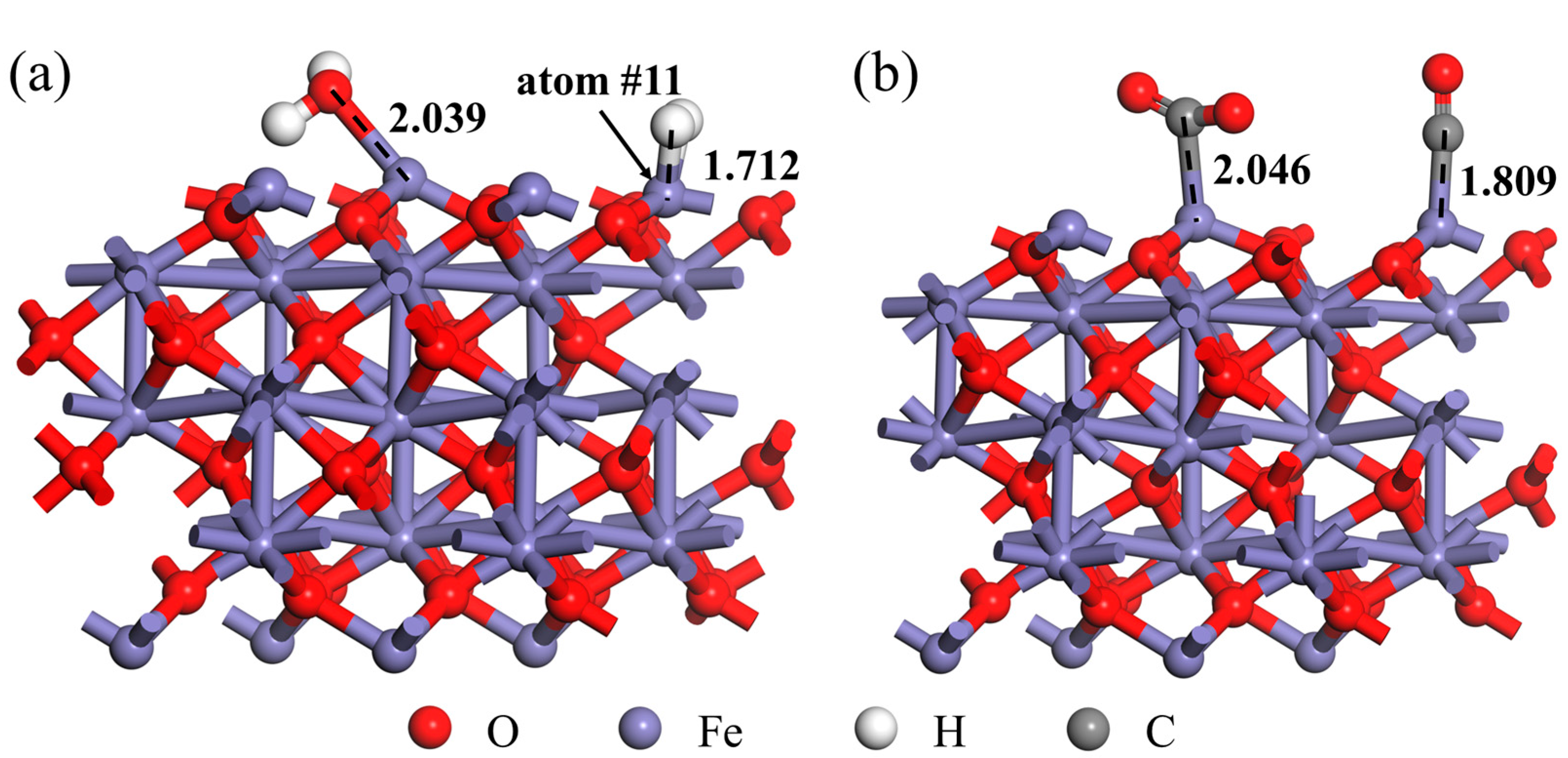
- The H2O molecule pre-adsorption was beneficial to the H2 molecule adsorption, but the H2O molecule pre-adsorption affected the further reduction behavior of Fe2O3 by H2. However, the adsorption energy of the CO molecule adsorbed to the Fe2O3-CO2 adsorption system was larger than that of the CO molecule directly adsorbed to Fe2O3, indicating that the stability of the Fe2O3-CO adsorption system was relatively stronger. Considering the adsorption energy alone, compared with the H2O molecule, CO2 had relatively less influence on the subsequent reduction behavior of CO with Fe2O3.
- (3)
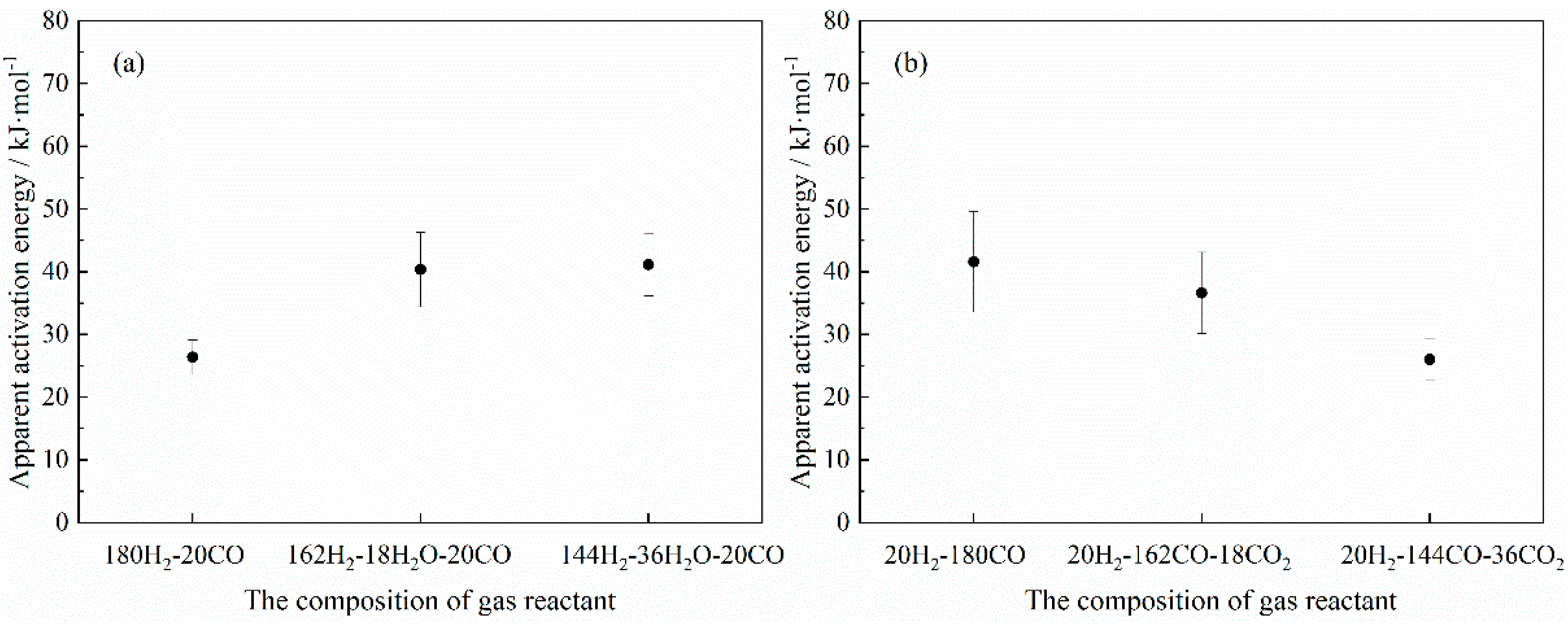
- With the addition of H2O in the H2-CO gas mixture, the apparent activation energy of the reduction reaction increased with its content, which meant that the addition of H2O mainly influenced the occurrence of the interfacial chemical reaction. However, the apparent activation energy presented a decreasing trend as the CO2 content in the H2-CO gas mixture increased, suggesting that the possible rate-controlling step changed to a combined gas diffusion and interfacial chemical reaction biased towards gas diffusion. Therefore, on the whole, CO2 had a greater impact on the gas diffusion, while H2O had a greater impact on the interfacial chemical reaction (gas adsorption), which was consistent with the DFT calculation results.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.; Sovacool, B.K.; Bazilian, M.; Griffiths, S.; Lee, J.; Yang, M.; Lee, J. Decarbonizing the iron and steel industry: A systematic review of sociotechnical systems, technological innovations, and policy options. Energy Res. Soc. Sci. 2022, 89, 102565. [Google Scholar] [CrossRef]
- World Steel Association. World Steel in Figures 2025. Available online: https://worldsteel.org/data/world-steel-in-figures/world-steel-in-figures-2025/ (accessed on 29 July 2025).
- Birat, J.P. Society, Materials, and the Environment: The Case of Steel. Metals 2020, 10, 331. [Google Scholar] [CrossRef]
- Raabe, D. The Materials Science behind Sustainable Metals and Alloys. Chem. Rev. 2023, 123, 2436–2608. [Google Scholar] [CrossRef]
- Wenceslao, J.; Samane, M. Sustainability in steelmaking. Curr. Opin. Green Sust. 2020, 24, 42–47. [Google Scholar] [CrossRef]
- Raabe, D.; Tasan, C.C.; Olivetti, E.A. Strategies for improving the sustainability of structural metals. Nature 2019, 575, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Jiang, J.; Zhang, Z.; Manovic, V. Inherent potential of steelmaking to contribute to decarbonisation targets via industrial carbon capture and storage. Nat. Commun. 2018, 9, 4422. [Google Scholar] [CrossRef]
- Wolfinger, T.; Spreitzer, D.; Schenk, J. Analysis of the Usability of Iron Ore Ultra-Fines for Hydrogen-Based Fluidized Bed Direct Reduction—A Review. Materials 2022, 15, 2687. [Google Scholar] [CrossRef]
- Heidari, A.; Niknahad, N.; Iljana, M.; Fabritius, T. A Review on the Kinetics of Iron Ore Reduction by Hydrogen. Materials 2021, 14, 7540. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zuo, H. Review of hydrogen-rich ironmaking technology in blast furnace. Ironmak. Steelmak. 2021, 48, 749–768. [Google Scholar] [CrossRef]
- Sun, M.; Pang, K.; Barati, M.; Meng, X. Hydrogen-Based Reduction Technologies in Low-Carbon Sustainable Ironmaking and Steelmaking: A Review. J. Sustain. Metall. 2024, 10, 10–25. [Google Scholar] [CrossRef]
- Turkdogan, E.T.; Vinters, J.V. Reducibility of iron ore pellets and effect of additions. Can. Metall. Quart. 1973, 12, 9–21. [Google Scholar] [CrossRef]
- Yadav, U.S.; Pandey, B.D.; Das, B.K.; Jena, D.N. Influence of magnesia on sintering characteristics of iron ore. Ironmak. Steelmak. 2002, 29, 91–95. [Google Scholar] [CrossRef]
- Seth, B.; Ross, H.U. The Effect of Lime on The Reducibility of Iron-Oxide Agglomerates. Can. Metall. Quart. 1963, 2, 15–30. [Google Scholar] [CrossRef]
- Salucci, E.; D’Angelo, A.; Russo, V.; Grénman, H.; Saxén, H. Review on the reduction kinetics of iron oxides with hydrogen-rich gas: Experimental investigation and modeling approaches. Ind. Eng. Chem. Res. 2025, 64, 1–35. [Google Scholar] [CrossRef]
- Lyu, B.; Wang, G.; Yang, F.; Zuo, H.; Xue, Q.; Wang, J. Kinetic Analysis of Isothermal and Non-Isothermal Reduction of Iron Ore Fines in Hydrogen Atmosphere. Metals 2022, 12, 1754. [Google Scholar] [CrossRef]
- Zakeri, A.; Coley, K.S.; Tafaghodi, L. Hydrogen-Based Direct Reduction of Iron Oxides: A Review on the Influence of Impurities. Sustainability 2023, 15, 13047. [Google Scholar] [CrossRef]
- Jozwiak, W.K.; Kaczmarek, E.; Maniecki, T.P.; Ignaczak, W.; Maniukiewicz, W. Reduction behavior of iron oxides in hydrogen and carbon monoxide atmospheres. Appl. Catal. A Gen. 2007, 326, 17–27. [Google Scholar] [CrossRef]
- Mao, X.; Hu, X.; Fan, Y.; Chou, K. Effect of water vapor on the reduction kinetics of hematite powder by hydrogen-water vapor in different stages. J. Min. Metall. B 2023, 59, 65–76. [Google Scholar] [CrossRef]
- Hammam, A.; Nasr, M.I.; Elsadek, M.H. Studies on the Reduction Behavior of Iron Oxide Pellet Fines with Hydrogen Gas: Mechanism and Kinetic Analysis. J. Sustain. Metall. 2023, 9, 1289–1302. [Google Scholar] [CrossRef]
- Spreitzer, D.; Schenk, J. Iron Ore Reduction by Hydrogen Using a Laboratory Scale Fluidized Bed Reactor: Kinetic Investigation—Experimental Setup and Method for Determination. Metall. Mater. Trans. B 2019, 50, 2471–2484. [Google Scholar] [CrossRef]
- Guo, X.; Sasaki, Y.; Kashiwaya, Y. Microreaction mechanism in reduction of magnetite to wustite. Metall. Mater. Trans. B 2004, 35, 517–522. [Google Scholar] [CrossRef]
- Daniel, S.; Johannes, S. Reduction of Iron Oxides with Hydrogen—A Review. Steel Res. Int. 2019, 90, 1900108. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Li, Y.W.; Wang, J.; Jiao, H. DFT+U Study of Molecular and Dissociative Water Adsorptions on the Fe3O4(110) Surface. J. Phys. Chem. C 2013, 117, 7648–7655. [Google Scholar] [CrossRef]
- Wang, K.; Han, T.; Chen, X.; Rushimisha, I.E. Insights into behavior and mechanism of tetracycline adsorption on virgin and soil-exposed microplastics. J. Hazard. Mater. 2022, 440, 129770. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Wei, X.; Maldonado, A.J.H.; Chen, Z. Unveiling Adsorption Mechanisms of Organic Pollutants onto Carbon Nanomaterials by Density Functional Theory Computations and Linear Free Energy Relationship Modeling. Environ. Sci. Technol. 2017, 51, 11820–11828. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, Z.; Zhang, T. Exploring Mechanism of H2 Adsorption on Surfaces of Iron Oxides by Density Functional Theory Calculation. JOM 2025, 77, 144–155. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z. A DFT-based microkinetic theory for Fe2O3 reduction by CO in chemical looping. P. Combust. Inst. 2023, 39, 4447–4455. [Google Scholar] [CrossRef]
- Adam, F.; Dupré, B.; Gleitzer, C. The role of water in the low-temperature hydrogen reduction of hematite into iron, and the role of the surface oxygen chemical potential in double reactions. React. Solids 1989, 7, 383–397. [Google Scholar] [CrossRef]
- Steffen, R.; Tacke, K.; Pluschkell, W. Grundlagenuntersuchungen zur Umweltfreundlichen Reduktion von Eisenerz mit Wasserstoff oder Wasserstoffreichen Gemischen, 1st ed.; Amt für amtliche Veröffentlichungen der Europäischen Gemeinschaften: Luxembourg, 1998; pp. 56–60. [Google Scholar]
- Mao, X. Fundamental Study on the Reaction Behavior of Iron Oxide and H2-CO Mixture Gas. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2023. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys.-Condens. Mat. 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Wang, Y.; Perdew, J.P.; Chevary, J.A. Exchange potentials in density-functional theory. Phys. Rev. A 1990, 41, 78–86. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892. [Google Scholar] [CrossRef]
- Fletcher, R. A new approach to variable metric algorithms. Comput. J. 1970, 13, 317–322. [Google Scholar] [CrossRef]
- Tian, X.G.; Zhang, Y.; Yang, T.S. First-principles study of H2 dissociative adsorption reactions on WO3 surfaces. Acta Phys. Chim. Sin. 2012, 28, 1063–1069. [Google Scholar] [CrossRef]
- Ho, Q.D.; Rauls, E. Cavity size effects on the adsorption of CO2 on pillar[n]arene structures: A density functional theory study. ChemistrySelect 2023, 8, e202302266. [Google Scholar] [CrossRef]
- Ho, Q.D.; Rauls, E. Investigations of functional groups effect on CO2 adsorption on pillar[5]arenes using density functional theory calculations. ChemistrySelect 2024, 9, e202401490. [Google Scholar] [CrossRef]
- Ho, Q.D.; Rauls, E. Ab initio study: Investigating the adsorption behaviors of polarized greenhouse gas molecules on pillar[5]arenes. Mater. Today Commun. 2023, 36, 106875. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, Q.; Lei, W. Origin of tunable photocatalytic selectivity of well-defined α-Fe2O3 nanocrystals. Small 2014, 10, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Y. Research on Reduction Behavior of Fluxed Pellets Under Hydrogen-Rich Conditions in Blast Furnace. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2022. [Google Scholar] [CrossRef]
- Han, G.; Chen, X.F.; Zhao, T.X.; Li, C.C.; Weng, X. Effect of alloying element doping on hydrogen adsorption and corrosion behavior of Fe2O3 (001) via high throughput method. Anti-Corros. Methods Mater. 2025, 72, 490–496. [Google Scholar] [CrossRef]
- Han, G.; Zhao, T.X.; Chen, X.F.; Li, C.C.; Weng, X. Effect of hydrogen on surface structure of Fe2O3 (001) containing point defects. Anti-Corros. Methods Mater. 2025, 75, 664–671. [Google Scholar] [CrossRef]
- Mao, X.; Garg, P.; Hu, X. Kinetic analysis of iron ore powder reaction with hydrogen—Carbon monoxide. Int. J. Miner. Metall. Mater. 2022, 29, 1882–1890. [Google Scholar] [CrossRef]
- Nasr, M.I.; Omar, A.A.; Khedr, M.H.; El-Geassy, A.A. Effect of Nickel Oxide Doping on the Kinetics and Mechanism of Iron Oxide Reduction. ISIJ Int. 1995, 35, 1043–1049. [Google Scholar] [CrossRef]

| H2:(CO + CO2) | CO:CO2 | No. | Temperature/K | Flow Rate/(mL/min) | ||
| H2 | CO | CO2 | ||||
| 1:9 | 10:0 | 1 | 1023 | 20 | 180 | 0 |
| 2 | 1173 | 20 | 180 | 0 | ||
| 3 | 1273 | 20 | 180 | 0 | ||
| 4 | 1373 | 20 | 180 | 0 | ||
| 9:1 | 5 | 1023 | 20 | 162 | 18 | |
| 6 | 1173 | 20 | 162 | 18 | ||
| 7 | 1273 | 20 | 162 | 18 | ||
| 8 | 1373 | 20 | 162 | 18 | ||
| 8:2 | 9 | 1023 | 20 | 144 | 36 | |
| 10 | 1173 | 20 | 144 | 36 | ||
| 11 | 1273 | 20 | 144 | 36 | ||
| 12 | 1373 | 20 | 144 | 36 | ||
| (H2 + H2O):CO | H2:H2O | No. | Temperature/K | Flow Rate/(mL/min) | ||
| H2 | CO | H2O | ||||
| 9:1 | 10:0 | 13 | 1023 | 180 | 20 | 0 |
| 14 | 1173 | 180 | 20 | 0 | ||
| 15 | 1273 | 180 | 20 | 0 | ||
| 16 | 1373 | 180 | 20 | 0 | ||
| 9:1 | 17 | 1023 | 162 | 20 | 18 | |
| 18 | 1173 | 162 | 20 | 18 | ||
| 19 | 1273 | 162 | 20 | 18 | ||
| 20 | 1373 | 162 | 20 | 18 | ||
| 8:2 | 21 | 1023 | 144 | 20 | 36 | |
| 22 | 1173 | 144 | 20 | 36 | ||
| 23 | 1273 | 144 | 20 | 36 | ||
| 24 | 1373 | 144 | 20 | 36 | ||
| Adsorption System | Fe2O3-H2 | Fe2O3-CO | Fe2O3-H2O | Fe2O3-CO2 |
|---|---|---|---|---|
| adsorption energy/eV | −0.013 | −1.317 | −0.702 | −0.076 |
| Adsorption System | Fe2O3 (Initial) | Fe2O3-H2 | Fe2O3-CO | Fe2O3-H2O | Fe2O3-CO2 |
|---|---|---|---|---|---|
| dFe-adsorption atom/Å | - | 1.814 | 1.808 | 2.064 | 2.057 |
| dFe-O/Å | 1.716 | 1.746 | 1.772 | 1.758 | 1.746 |
| net charge of Fe/e | 0.75 | 0.95 | 0.76 | 0.9 | 0.96 |
| bond order of Fe-O | 0.54 | 0.49 | 0.52 | 0.47 | 0.5 |
| Adsorption System | Fe2O3-H2 | Fe2O3-H2O-H2 | Fe2O3-CO | Fe2O3-CO2-CO |
|---|---|---|---|---|
| dFe-adsorption atom/Å | 1.814 | 1.712 | 1.808 | 1.809 |
| dFe-O/Å | 1.746 | 1.763 | 1.772 | 1.772 |
| net charge of Fe/e | 0.95 | 0.93 | 0.76 | 0.82 |
| bond order of Fe-O | 0.49 | 0.48 | 0.52 | 0.49 |
| adsorption energy/eV | −0.013 | −0.132 | −1.317 | −1.203 |
| NO. | Apparent Activation Energy (kJ/mol) | Possible Rate-Controlling Step |
|---|---|---|
| 1 | 8~16 | Gas diffusion |
| 2 | 29~42 | Combined gas diffusion and interfacial chemical reaction |
| 3 | 60~67 | Interfacial chemical reaction |
| 4 | >90 | Solid-state diffusion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, X.; Zhou, B.; Deng, H.; Zeng, Q.; Li, J.; Chen, J.; Xiao, Y.; Chou, K. The Effect of H2O and CO2 on the Adsorption Behavior of H2 and CO on Hematite. Materials 2025, 18, 4175. https://doi.org/10.3390/ma18174175
Mao X, Zhou B, Deng H, Zeng Q, Li J, Chen J, Xiao Y, Chou K. The Effect of H2O and CO2 on the Adsorption Behavior of H2 and CO on Hematite. Materials. 2025; 18(17):4175. https://doi.org/10.3390/ma18174175
Chicago/Turabian StyleMao, Xudong, Baoqing Zhou, Hui Deng, Qiong Zeng, Jingbo Li, Jie Chen, Yiyu Xiao, and Kuochih Chou. 2025. "The Effect of H2O and CO2 on the Adsorption Behavior of H2 and CO on Hematite" Materials 18, no. 17: 4175. https://doi.org/10.3390/ma18174175
APA StyleMao, X., Zhou, B., Deng, H., Zeng, Q., Li, J., Chen, J., Xiao, Y., & Chou, K. (2025). The Effect of H2O and CO2 on the Adsorption Behavior of H2 and CO on Hematite. Materials, 18(17), 4175. https://doi.org/10.3390/ma18174175






